Abstract
Nonalcoholic fatty liver disease (NAFLD) is currently the most common chronic liver disease worldwide, affecting a quarter of the global adult population. Nonalcoholic steatohepatitis, the more active form of NAFLD with active hepatic necroinflammation and faster fibrosis progression, has become one of the leading indications for liver transplantation and an important cause of hepatocellular carcinoma in Western countries. Epidemiological studies suggest that NAFLD is almost equally prevalent in Asia as in the West, but severe liver complications appear to be less common. In this article, we review the epidemiology, clinical characteristics, risk factors and clinical outcomes of NAFLD in Asia. We highlight the issue of NAFLD in the nonobese population and discuss whether it is a unique phenomenon in Asia. Because of the rapidly changing epidemiology and natural history, future studies should continue to monitor the magnitude of NAFLD in Asia and define the best policy to control this new epidemic.
Keywords: non-alcoholic steatohepatitis, obesity, metabolic syndrome, Asia, epidemiology
Abbreviations: BMI, body mass index; HCC, hepatocellular carcinoma; HSD17B13, hydroxysteroid 17-beta dehydrogenase 13; NAFL, nonalcoholic fatty liver; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; PNPLA3, patatin-like phospholipase domain–containing protein 3; TM6SF2, transmembrane 6 superfamily 2
Nonalcoholic fatty liver disease (NAFLD) is currently the most common chronic liver disease worldwide, affecting a quarter of the global adult population.1 NAFLD is the umbrella term covering patients with a range of disease activities. Nonalcoholic fatty liver (NAFL), also commonly known as simple steatosis or bland steatosis, is the relatively benign form of NAFLD with fat accumulation in the liver but little necroinflammation or tissue injury.2 In contrast, nonalcoholic steatohepatitis (NASH) is characterized by not only fat accumulation in the liver but also lobular inflammation and hepatocyte ballooning. While patients with NAFL can progress to NASH with time and develop fibrosis progression,3 hepatocellular carcinoma (HCC) and cirrhotic complications rarely develop in patients with NAFL at the baseline. Importantly, NASH and liver fibrosis can also improve with lifestyle changes and weight reduction, suggesting that the natural history of NAFLD is highly dynamic.
Long considered a disease of the West, NAFLD is increasingly recognized in Asia. According to a recent meta-analysis, NAFLD appears to be as common in Asia as in the West.1 However, while NASH has become a leading cause of cirrhosis and HCC in the United States, the same has not occurred in Asia yet.4 In this article, we review the epidemiology, clinical characteristics, risk factors and clinical outcomes of NAFLD in Asia.
Epidemiology of NAFLD in Asia
Younossi et al.1 reviewed data from 86 studies and reported a global prevalence of NAFLD as 25.2% (95% confidence interval [CI] 22.1–28.7%). The prevalence was highest in the Middle East (31.8%) and South America (30.5%) and lowest in Africa (13.5%). The prevalence of NAFLD in Asia was 27.4% (95% CI 23.3–31.9%), which was not lower than that in Europe (23.7%) and North American (24.1%).
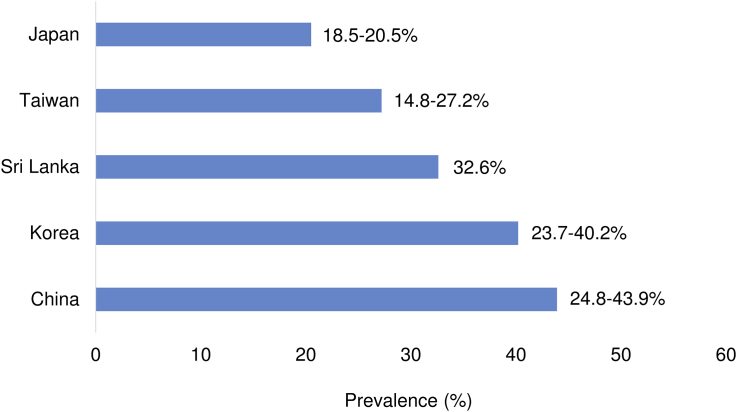
Asia is a vast continent including countries with different lifestyle habits and economic development. In the same meta-analysis by Younossi et al., the prevalence of NAFLD in Asia ranged from 14.8% in Taiwan to 43.9% in China (Figure 1).1 It is important to remember that the included studies differ in research setting, study population, the method of diagnosis and the year of investigation. Studies at hospital clinics tend to report a higher prevalence of NAFLD than true general population studies. The prevalence was also higher in recent than old studies. The latter suggests that NAFLD is increasingly common in Asia. In one of the few secular trend studies, Kojima et al.5 reported that the prevalence increased from 12.6% in 1989 to 30.3% in 1998 in Japan. Similarly, a meta-analysis of studies from China also showed an increase in NAFLD prevalence from 18.2% in 2000–2006 to 20.7% in 2010–2013.6
Figure 1.
Prevalence of NAFLD in Asian countries. The figures were modified from Younossi et al.1
Even within the same country, there is marked rural–urban divide in the epidemiology of NAFLD. For instance, the coastal cities of China have enjoyed greater economic growth than inland areas. Accordingly, the reported prevalence of NAFLD in Hong Kong (27%) and Shanghai (15%) was higher than that in Chengdu (6%).4 In urban areas, overnutrition and sedentary lifestyle are conducive to the development of obesity and NAFLD.
Similar to data from the West, NAFLD is more common in men than in women in Asia.7 The prevalence of NAFLD increases with age. In particular, the prevalence is low (10–20%) in premenopausal women but increases exponentially after the age of 50 years.
Few studies have looked at the incidence of NAFLD in Asia. In a population study in Hong Kong using paired proton magnetic resonance spectroscopy at an interval of 3–5 years, the annual incidence of NAFLD was 3.4%.8 Central obesity and dyslipidaemia were major factors associated with incident NAFLD.
Severity of NAFLD in Asia
Because of the invasiveness of liver biopsy, histological studies suffer from selection bias and cannot reflect the severity of NAFLD in the community. In the Gut and Obesity Asia initiative study comprising histological data of 1008 patients from eight Asian countries, 46.1–97.4% patients who underwent liver biopsy for the assessment of NAFLD were found to have NASH, and 6.6–31.6% had advanced (F3-4) fibrosis.9
Studies using non-invasive tests of fibrosis allow inclusion of an unbiased population and better reflect the population of interest. In a study of 922 participants in the general population of Hong Kong, 3.7% of those with fatty liver had increased liver stiffness by transient elastography, suggestive of advanced fibrosis.7
Risk Factors of NAFLD
Lifestyle factors
The association between metabolic syndrome and its risk factors with NAFLD has been widely reported. In view of the increasing prevalence of NAFLD in Asia over recent decades, there may be several more lifestyle factors brought by urbanization and globalization that contribute to NAFLD development.
Diet
Apart from the well-known association of high fat, high sugar and high calorie intake with higher metabolic risk and NAFLD,10 the dietary pattern may also contribute to its development. In general, consuming a more Western type of diet consisting of more red meat and high-fat dairy products, compared with a more traditional Asian or Mediterranean type of diet that consists of more vegetables, legumes, fruits and fish, may each be independently associated with increased metabolic outcomes.11, 12 This possibly explains why NAFLD used to be less prevalent in Asia before the effects of urbanization and globalization. However, most studies are cross-sectional in nature, and patients who consume healthier diets are more likely to have other healthy habits, and it is not easy to differentiate these factors. More studies focused on this subject, with more reliable methods are still required to explore a clearer relationship between dietary patterns and NAFLD. It would be highly beneficial as dietary nutritional management is an important component in preventing and treating NAFLD.10, 13
Sedentary Lifestyle
The association between reduced overall physical activities below the recommended level and metabolic syndrome has been well established.14 However, the risk of sedentary behaviour may not be negated by compensatory physical activity. A large prospective study across multiple states in the US involving 240,819 adults found that individuals with >7 h of sedentary behaviour daily, such as watching the television, still had a 50% increased risk of death from all causes, despite of 7 h of moderate to vigorous physical activity per week.15 As a growing proportion of the working population has prolonged sitting in offices daily, the long-term impact on health deserves attention.
Sarcopenia
Skeletal muscle plays a primary role in insulin-mediated glucose disposal; thus, sarcopenia may lead to insulin resistance and NAFLD. Several studies in Korea and Japan have confirmed an association between sarcopenia and NAFLD.16, 17 The association may even be independent of the body mass index (BMI) and insulin resistance.18 In a Korean liver biopsy cohort, sarcopenia was further associated with histological NASH and significant fibrosis.19 Because both aerobic exercise and resistance training can improve NAFLD and improvement in NAFLD can be achieved with modest weight reduction,20 advice on physical activities should be part of the standard care of NAFLD.
Genetic Factors
Association studies have long shown that NAFLD is more common among first-degree relatives, and a twin study showed that both hepatic steatosis and fibrosis have around 50% heritability.21 Compared with other common metabolic disorders such as diabetes and obesity, knowledge on the genetic determinants of NAFLD is limited. Nonetheless, a genome-wide association study and an exome-wide association study identified the patatin-like phospholipase domain–containing protein 3 (PNPLA3 rs738409, encoding I148M) and transmembrane 6 superfamily 2 (TM6SF2 rs58542926, encoding E167K) as genetic determinants of hepatic steatosis, respectively.22, 23 Since then, both genetic markers have been validated across different countries and ethnicities, including the Asian population.24, 25, 26
The population prevalence of PNPLA3 gene polymorphism nicely explains why NAFLD is more common and severe among Hispanics and less common among Africans.22 Interestingly, the at-risk PNPLA3 G allele is more common in East Asians than in Caucasians.27, 28 In particular, the proportion of patients carrying the G allele is particularly high in nonobese patients with NAFLD .28, 29 This may explain why the prevalence of NAFLD is similarly high in Asia and Western countries, even though the former has a lower metabolic burden. In contrast, while PNPLA3 remains strongly associated with NAFLD in India, the minor allele frequency is rather low.30 The apolipoprotein C3 gene variants are associated with NAFLD in Indian men.31
PNPLA3 and TM6SF2 polymorphisms are also associated with histological severity of NAFLD and the risk of HCC.32 On the other hand, while the PNPLA3 polymorphism increases the risk of NAFLD, patients carrying the at-risk allele also have a greater improvement in hepatic steatosis after lifestyle intervention.33
Recently, exome sequence data from several large cohorts have identified hydroxysteroid 17-beta dehydrogenase 13 (HSD17B13 rs72613567) as a protective factor against NAFLD, histological NASH and alcohol-related liver disease.34 Data from Asia are eagerly awaited.
Demographic Factors
In general, NAFLD is more common in men than in women, and the prevalence increases with age.7 The prevalence of NAFLD increases steeply after the age of 50 years in women, suggesting the potential influence of menopause. A similar pattern is seen in diabetes, obesity and ischaemic heart disease.
Comorbidities
NAFLD is strongly associated with metabolic syndrome. It is particularly prevalent in patients with obesity and type 2 diabetes. Studies from Asia suggest that NAFLD is present in more than 50% of diabetic patients and up to 10–20% may have advanced fibrosis.35 As discussed in the following section, although NAFLD in patients with relatively normal BMI is well recognized in Asia, few such patients are completely metabolically healthy. Central obesity is often at play in such cases.
Obstructive sleep apnoea syndrome is also common among patients with NAFLD.36 This may be due to shared risk factors of obesity. However, chronic intermittent hypoxia is mechanistically linked to NAFLD and hepatic necroinflammation.37 An ongoing randomized controlled trial in Hong Kong is examining the impact of continuous positive airway pressure on hepatic steatosis and stiffness in patients with NAFLD and obstructive sleep apnoea (ClinicalTrials.gov Identifier: NCT02728765).
Clinical outcomes
Despite a similar prevalence of NAFLD in Asia and Western countries, severe liver complications still appear to be relatively uncommon in Asia. In a retrospective study of 6508 patients with NAFLD at a public hospital in Japan, only 0.25% developed HCC during a mean follow-up of 5.6 years.38 Other liver biopsy and radiological cohorts from Asian have reported similarly low incidence of HCC.39, 40 Similarly, chronic viral hepatitis and alcohol-related liver disease remain the leading indications for liver transplantation in Asia. However, if the aetiologies of end-stage liver disease are still changing rapidly in the United States and Europe,1 it would be too early to conclude if NASH will be an important indication for transplantation in Asia. Furthermore, because patients with NASH tend to have more comorbidities, the influence of the latter on the feasibility and decision on liver transplantation remains to be seen.
One possible explanation for the low incidence of severe liver complications is the relatively short disease duration in patients with NAFLD in Asia. As economic growth in most Asian countries only began in the 1980s or even after 2000, many patients with NAFLD became obese during adulthood. With childhood obesity emerging in Asia, the natural history of NAFLD in this region will likely change overtime. That being said, the proportion of patients with nonviral hepatitis-related HCC has already more than doubled from 1991 to 2010 in Japan, suggesting that the change is taking place.41
While cardiovascular disease remains the leading cause of death in patients with NAFLD, the relative importance of various complications depends on the severity of NAFLD. In a multicentre cohort of 458 NAFLD patients with F3-4 fibrosis from Spain, Cuba, Australia and Hong Kong, liver complications accounted for half of the deaths in F3 patients and all deaths in cirrhotic patients.42
Early reports from Japan suggest that almost half of the patients with NASH-related HCC were noncirrhotic.43 Although this may be partly due to misclassification with NASH-related cirrhosis counted as cryptogenic cirrhosis, subsequent studies confirmed that noncirrhotic HCC appeared to be more common in patients with NASH or idiopathic HCC than those with other liver diseases.44 This means that the current recommendations to perform HCC screening only in patients with NASH-related cirrhosis will miss a significant proportion of NASH-related HCC.2 However, given the low absolute incidence of HCC in the noncirrhotic population, routine HCC screening in all patients with NAFLD cannot be justified. Future studies should better define HCC risk factors other than cirrhosis in the NASH population.
The disease burden of NAFLD in Asia is not fully understood. In a recent modelling study of eight countries, the NASH population in China is projected to increase 48% to 48 million cases from 2015 to 2030.45 Decompensated cirrhosis and liver-related deaths secondary to NASH are expected to double during the same period.
Nonobese NAFLD
Although NAFLD is conventionally associated with obesity, 7–19% of patients with NAFLD are found to have a BMI of <25 kg/m2 across multiple Asian and American studies (Table 1), giving rise to the term nonobese NAFLD. However, it is important to bear in mind that BMI is an imperfect surrogate for adiposity. According to the World Health Organization, obesity and overweight are the abnormal or excessive fat accumulation that may impair health. BMI as a simple index of weight-for-height, thus, does not take into account the body fat composition and distribution and may be insufficient alone to classify obese patients.
Table 1.
Prevalence of NAFLD in Nonobese and Obese Populations in Asia.
| Study | Country/region | N | BMI <25 kg/m2 | BMI ≥25 kg/m2 |
|---|---|---|---|---|
| Fan 200550 | China | 3175 | 8% | 65% |
| Das 201051 | India | 1911 | 7% | 32% |
| Kwon 201252 | Korea | 29,994 | 13% | 50% |
| Nishioji 201453 | Japan | 3271 | 15% | 69% |
| Wei 2015a,29 | Hong Kong | 911 | 19% | 61% |
BMI, body mass index.
Based on proton magnetic resonance spectroscopy (the other studies were based on abdominal ultrasonography).
In Asia, it is common to see the “metabolically obese” phenotype, referring to individuals with a normal BMI but increased rate of metabolic complications such as diabetes and impaired glucose tolerance. Asians are more prone to central obesity, greater amount of visceral adipose tissue, lower muscle mass, and increased insulin resistance compared with their Western counterparts.46 In the Rotterdam study, waist circumference was suggested to be a better adiposity marker than BMI in detecting overweight in elderly population, to account for the diminished body height and decreased fat-free mass during ageing.47
Whether NAFLD in nonobese patients has greater severity and overall mortality still remains inconclusive. A retrospective multicentre cohort study of 1090 patients with NAFLD found more severe lobular inflammation and higher overall mortality compared with the obese group during a follow-up period of more than 12 years.48 On the other hand, a prospective cohort study of 307 patients with NAFLD from Hong Kong did not find a significant difference in the rate of adverse events among the two groups, after a median follow-up of 49 months.40 While the nonobese group had a lower median steatosis grade and fibrosis stage, the nonobese and obese groups had similar prevalence of NASH (44% vs 52%) and advanced fibrosis (26% vs 28%).
Furthermore, a population study in Hong Kong showed that nonobese patients with NAFLD had lower liver stiffness and serum keratin-18 fragment levels than their obese counterparts, suggesting that nonobese patients had less severe liver fibrosis and were less likely to have NASH.29
It has been suggested but remains uncertain that the rate of weight gain, changes in the waist circumference and serum triglyceride, as well as reduced insulin sensitivity may be related to new development of NAFLD in nonobese people. On the other hand, lifestyle intervention is effective in improving NAFLD in nonobese patients, and such patients can often achieve improvement in hepatic steatosis with relatively little weight reduction.49 More research is still needed in understanding the occurrence of NAFLD in patients with a BMI of <25 kg/m2, as well as searching for better markers of adiposity.
Conclusions
Epidemiological studies suggest that at least a quarter of the adult population in Asia has NAFLD, and there is marked difference across regions and between urban and rural areas. Because of westernization of lifestyle, the incidence of NAFLD and NASH is increasing. While HCC and cirrhotic complications from NASH remain uncommon, they are on the rise and will be fuelled by the emergence of childhood obesity in Asia. Nonobese NAFLD is more common in Asia, likely due to a difference in the definition of normal BMI and the tendency to develop central obesity. Future studies should continue to monitor the magnitude of NAFLD in Asia and define the best policy to control this new epidemic.
Conflicts of interest
Vincent Wong has served as a consultant or advisory board member for AbbVie, Allergan, Gilead Sciences, Janssen, Perspectium Diagnostics and Pfizer; and received lecture fees from Bristol-Myers Squibb, Echosens, Gilead Sciences and Merck.
Acknowledgment
Vincent Wong is supported by the General Research Fund (Project ref 14108916) from the Hong Kong SAR Government to conduct NAFLD research.
References
- 1.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Wong V.W., Chan W.K., Chitturi S. Asia-pacific working party on non-alcoholic fatty liver disease guidelines 2017-Part 1: definition, risk factors and assessment. J Gastroenterol Hepatol. 2018;33:70–85. doi: 10.1111/jgh.13857. [DOI] [PubMed] [Google Scholar]
- 3.Wong V.W., Wong G.L., Choi P.C. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010;59:969–974. doi: 10.1136/gut.2009.205088. [DOI] [PubMed] [Google Scholar]
- 4.Fan J.G., Kim S.U., Wong V.W. New trends on obesity and NAFLD in Asia. J Hepatol. 2017;67:862–873. doi: 10.1016/j.jhep.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Kojima S., Watanabe N., Numata M., Ogawa T., Matsuzaki S. Increase in the prevalence of fatty liver in Japan over the past 12 years: analysis of clinical background. J Gastroenterol. 2003;38:954–961. doi: 10.1007/s00535-003-1178-8. [DOI] [PubMed] [Google Scholar]
- 6.Li Z., Xue J., Chen P., Chen L., Yan S., Liu L. Prevalence of nonalcoholic fatty liver disease in mainland of China: a meta-analysis of published studies. J Gastroenterol Hepatol. 2014;29:42–51. doi: 10.1111/jgh.12428. [DOI] [PubMed] [Google Scholar]
- 7.Wong V.W., Chu W.C., Wong G.L. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectroscopy and transient elastography. Gut. 2012;61:409–415. doi: 10.1136/gutjnl-2011-300342. [DOI] [PubMed] [Google Scholar]
- 8.Wong V.W., Wong G.L., Yeung D.K. Incidence of non-alcoholic fatty liver disease in Hong Kong: a population study with paired proton-magnetic resonance spectroscopy. J Hepatol. 2015;62:182–189. doi: 10.1016/j.jhep.2014.08.041. [DOI] [PubMed] [Google Scholar]
- 9.Chan W.K., Treeprasertsuk S., Imajo K. Clinical features and treatment of nonalcoholic fatty liver disease across the Asia Pacific region-the GO ASIA initiative. Aliment Pharmacol Ther. 2018;47:816–825. doi: 10.1111/apt.14506. [DOI] [PubMed] [Google Scholar]
- 10.Fan J.G., Cao H.X. Role of diet and nutritional management in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2013;28(suppl 4):81–87. doi: 10.1111/jgh.12244. [DOI] [PubMed] [Google Scholar]
- 11.Chan R., Wong V.W., Chu W.C. Diet-quality scores and prevalence of nonalcoholic fatty liver disease: a population study using proton-magnetic resonance spectroscopy. PLoS One. 2015;10 doi: 10.1371/journal.pone.0139310. e0139310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang C.Q., Shu L., Wang S. Dietary patterns modulate the risk of non-alcoholic fatty liver disease in Chinese adults. Nutrients. 2015;7:4778–4791. doi: 10.3390/nu7064778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong V.W., Chan R.S., Wong G.L. Community-based lifestyle modification programme for non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol. 2013;59:536–542. doi: 10.1016/j.jhep.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Ryu S., Chang Y., Jung H.S. Relationship of sitting time and physical activity with non-alcoholic fatty liver disease. J Hepatol. 2015;63:1229–1237. doi: 10.1016/j.jhep.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Matthews C.E., George S.M., Moore S.C. Amount of time spent in sedentary behaviors and cause-specific mortality in US adults. Am J Clin Nutr. 2012;95:437–445. doi: 10.3945/ajcn.111.019620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim S., Kim J.H., Yoon J.W. Sarcopenic obesity: prevalence and association with metabolic syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA) Diabetes Care. 2010;33:1652–1654. doi: 10.2337/dc10-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong H.C., Hwang S.Y., Choi H.Y. Relationship between sarcopenia and nonalcoholic fatty liver disease: the Korean Sarcopenic Obesity Study. Hepatology. 2014;59:1772–1778. doi: 10.1002/hep.26716. [DOI] [PubMed] [Google Scholar]
- 18.Lee Y.H., Kim S.U., Song K. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: nationwide surveys (KNHANES 2008-2011) Hepatology. 2016;63:776–786. doi: 10.1002/hep.28376. [DOI] [PubMed] [Google Scholar]
- 19.Koo B.K., Kim D., Joo S.K. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J Hepatol. 2017;66:123–131. doi: 10.1016/j.jhep.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 20.Hashida R., Kawaguchi T., Bekki M. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: a systematic review. J Hepatol. 2017;66:142–152. doi: 10.1016/j.jhep.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 21.Loomba R., Schork N., Chen C.H. Genetics of NiTC. Heritability of hepatic fibrosis and steatosis based on a prospective twin study. Gastroenterology. 2015;149:1784–1793. doi: 10.1053/j.gastro.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romeo S., Kozlitina J., Xing C. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kozlitina J., Smagris E., Stender S. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2014;46:352–356. doi: 10.1038/ng.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sookoian S., Pirola C.J. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011;53:1883–1894. doi: 10.1002/hep.24283. [DOI] [PubMed] [Google Scholar]
- 25.Pirola C.J., Sookoian S. The dual and opposite role of the TM6SF2-rs58542926 variant in protecting against cardiovascular disease and conferring risk for nonalcoholic fatty liver: a meta-analysis. Hepatology. 2015;62:1742–1756. doi: 10.1002/hep.28142. [DOI] [PubMed] [Google Scholar]
- 26.Wong V.W., Wong G.L., Tse C.H., Chan H.L. Prevalence of the TM6SF2 variant and non-alcoholic fatty liver disease in Chinese. J Hepatol. 2014;61:708–709. doi: 10.1016/j.jhep.2014.04.047. [DOI] [PubMed] [Google Scholar]
- 27.Kitamoto T., Kitamoto A., Yoneda M. Genome-wide scan revealed that polymorphisms in the PNPLA3, SAMM50, and PARVB genes are associated with development and progression of nonalcoholic fatty liver disease in Japan. Hum Genet. 2013;132:783–792. doi: 10.1007/s00439-013-1294-3. [DOI] [PubMed] [Google Scholar]
- 28.Shen J., Wong G.L., Chan H.L. PNPLA3 gene polymorphism accounts for fatty liver in community subjects without metabolic syndrome. Aliment Pharmacol Ther. 2014;39:532–539. doi: 10.1111/apt.12609. [DOI] [PubMed] [Google Scholar]
- 29.Wei J.L., Leung J.C., Loong T.C. Prevalence and severity of nonalcoholic fatty liver disease in non-obese patients: a population study using proton-magnetic resonance spectroscopy. Am J Gastroenterol. 2015;110:1306–1314. doi: 10.1038/ajg.2015.235. quiz 1315. [DOI] [PubMed] [Google Scholar]
- 30.Kanth V.V., Sasikala M., Rao P.N., Steffie Avanthi U., Rao K.R., Nageshwar Reddy D. Pooled genetic analysis in ultrasound measured non-alcoholic fatty liver disease in Indian subjects: a pilot study. World J Hepatol. 2014;6:435–442. doi: 10.4254/wjh.v6.i6.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petersen K.F., Dufour S., Hariri A. Apolipoprotein C3 gene variants in nonalcoholic fatty liver disease. N Engl J Med. 2010;362:1082–1089. doi: 10.1056/NEJMoa0907295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raksayot M., Chuaypen N., Khlaiphuengsin A. Independent and additive effects of PNPLA3 and TM6SF2 polymorphisms on the development of non-B, non-C hepatocellular carcinoma. J Gastroenterol. 2018 Nov 30 doi: 10.1007/s00535-018-01533-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Shen J., Wong G.L., Chan H.L. PNPLA3 gene polymorphism and response to lifestyle modification in patients with nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2015;30:139–146. doi: 10.1111/jgh.12656. [DOI] [PubMed] [Google Scholar]
- 34.Abul-Husn N.S., Cheng X., Li A.H. A protein-truncating HSD17B13 variant and protection from chronic liver disease. N Engl J Med. 2018;378:1096–1106. doi: 10.1056/NEJMoa1712191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwok R., Choi K.C., Wong G.L. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut. 2016;65:1359–1368. doi: 10.1136/gutjnl-2015-309265. [DOI] [PubMed] [Google Scholar]
- 36.Bhatt S.P., Guleria R., Vikram N.K., Nandhan S.V., Singh Y., Gupta A.K. Association of inflammatory genes in obstructive sleep apnea and non alcoholic fatty liver disease in Asian Indians residing in north India. PLoS One. 2018;13 doi: 10.1371/journal.pone.0199599. e0199599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aron-Wisnewsky J., Minville C., Tordjman J. Chronic intermittent hypoxia is a major trigger for non-alcoholic fatty liver disease in morbid obese. J Hepatol. 2012;56:225–233. doi: 10.1016/j.jhep.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 38.Kawamura Y., Arase Y., Ikeda K. Large-scale long-term follow-up study of Japanese patients with non-alcoholic Fatty liver disease for the onset of hepatocellular carcinoma. Am J Gastroenterol. 2012;107:253–261. doi: 10.1038/ajg.2011.327. [DOI] [PubMed] [Google Scholar]
- 39.Wong V.W., Wong G.L., Yeung J.C. Long-term clinical outcomes after fatty liver screening in patients undergoing coronary angiogram: a prospective cohort study. Hepatology. 2016;63:754–763. doi: 10.1002/hep.28253. [DOI] [PubMed] [Google Scholar]
- 40.Leung J.C., Loong T.C., Wei J.L. Histological severity and clinical outcomes of nonalcoholic fatty liver disease in nonobese patients. Hepatology. 2017;65:54–64. doi: 10.1002/hep.28697. [DOI] [PubMed] [Google Scholar]
- 41.Tateishi R., Okanoue T., Fujiwara N. Clinical characteristics, treatment, and prognosis of non-B, non-C hepatocellular carcinoma: a large retrospective multicenter cohort study. J Gastroenterol. 2015;50:350–360. doi: 10.1007/s00535-014-0973-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vilar-Gomez E., Calzadilla-Bertot L., Wong V.W. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi-national cohort study. Gastroenterology. 2018;155:443–457 e417. doi: 10.1053/j.gastro.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 43.Yasui K., Hashimoto E., Komorizono Y., Japan Nash Study Group MoHL, Welfare of J Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2011;9:428–433. doi: 10.1016/j.cgh.2011.01.023. quiz e450. [DOI] [PubMed] [Google Scholar]
- 44.Mittal S., El-Serag H.B., Sada Y.H. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2016;14:124–131. doi: 10.1016/j.cgh.2015.07.019. e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Estes C., Anstee Q.M., Arias-Loste M.T. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69:896–904. doi: 10.1016/j.jhep.2018.05.036. [DOI] [PubMed] [Google Scholar]
- 46.Chan J.C., Malik V., Jia W. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. J Am Med Assoc. 2009;301:2129–2140. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 47.Visscher T.L., Seidell J.C., Molarius A., van der Kuip D., Hofman A., Witteman J.C. A comparison of body mass index, waist-hip ratio and waist circumference as predictors of all-cause mortality among the elderly: the Rotterdam study. Int J Obes Relat Metab Disord. 2001;25:1730–1735. doi: 10.1038/sj.ijo.0801787. [DOI] [PubMed] [Google Scholar]
- 48.Dela Cruz A.C., Bugianesi E., George J. Characteristics and long-term prognosis of lean patients with nonalcoholic fatty liver disease. Gastroenterology. 2014;146 S-909. [Google Scholar]
- 49.Wong V.W., Wong G.L., Chan R.S. Beneficial effects of lifestyle intervention in non-obese patients with non-alcoholic fatty liver disease. J Hepatol. 2018;69:1349–1356. doi: 10.1016/j.jhep.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 50.Fan J.G., Zhu J., Li X.J. Prevalence of and risk factors for fatty liver in a general population of Shanghai, China. J Hepatol. 2005;43:508–514. doi: 10.1016/j.jhep.2005.02.042. [DOI] [PubMed] [Google Scholar]
- 51.Das K., Das K., Mukherjee P.S. Nonobese population in a developing country has a high prevalence of nonalcoholic fatty liver and significant liver disease. Hepatology. 2010;51:1593–1602. doi: 10.1002/hep.23567. [DOI] [PubMed] [Google Scholar]
- 52.Kwon Y.M., Oh S.W., Hwang S.S., Lee C., Kwon H., Chung G.E. Association of nonalcoholic fatty liver disease with components of metabolic syndrome according to body mass index in Korean adults. Am J Gastroenterol. 2012;107:1852–1858. doi: 10.1038/ajg.2012.314. [DOI] [PubMed] [Google Scholar]
- 53.Nishioji K., Sumida Y., Kamaguchi M. Prevalence of and risk factors for non-alcoholic fatty liver disease in a non-obese Japanese population, 2011-2012. J Gastroenterol. 2015;50:95–108. doi: 10.1007/s00535-014-0948-9. [DOI] [PubMed] [Google Scholar]