Abstract
Background
New direct-acting antiviral agents (DAAs) approved for the treatment of patients infected by Hepatitis C virus (HCV) are well tolerated and increase sustained virological response (SVR) rate. We summarize current evidence on the efficacy and safety from comparative randomized controlled trials (RCTs) of DAAs.
Methods
We systematically searched MEDLINE, Embase, Scopus, CENTRAL, and Lilacs as well as a list of reference literature. We included RCTs comparing DAAs with placebo or active control and reporting response rates and adverse events according to antiviral regimens. Risk ratios (RRs) were pooled as appropriate. We assessed the risk of bias of included studies and graded the quality of evidence according to the GRADE method.
Results
We included 28 RCTs, enrolling more than 7000 patients. The quality of evidence was generally low. Twelve-week treatment with DAAs in naïve patients significantly increased SVR12 and SVR24 compared with placebo (RR 1.4, 95% CI 1.3–1.6; RR 1.5, 95% CI 1.4–1.6, respectively). This means that for every 1000 patients, 240 or 260 more patients experienced SVR12 or SVR24 if treated with any DAAs. We could not find RCTs assessing progression of liver disease or development of hepatocellular carcinoma. DAAs were not associated with higher incidence of serious adverse events or discontinuation due to adverse events.
Conclusions
This systematic review confirms that new DAAs are more effective in inducing SVR than placebo. Outside clinical trials, in real word, HCV cure with DAA regimens occurs in less than 90% of patients, so further comparative evaluations are needed to establish their long-term effects.
Keywords: hepatitis C, liver, meta-analysis, outcome research, systematic review
Abbreviations: AE, adverse event; CI, confidence interval; DAA, direct-acting antiviral agent; HCC, hepatocellular carcinoma; HCV, Hepatitis C virus; NNPIs, nonnucleoside polymerase inhibitors; NPIs, nucleoside polymerase inhibitors; PEG-IFN, pegylated interferon; PrIs, protease inhibitors; RAVs, resistance-associated variants; RBV, Ribavirin; RCT, randomized controlled trial; RR, risk ratio; SAEs, serious adverse events; SE, standard error; SVR, sustained virological response
Hepatitis C virus (HCV) infection is a public health problem, with global estimates of 130–150 million people chronically infected, defined as detectable virus present at 6 months from disease detection.1, 2 Between 55% and 85% of acute HCV infections evolve to chronicity and, in a significant proportion of patients, may lead to liver fibrosis and cirrhosis. Severe consequences of this disease include decompensated cirrhosis and hepatocellular carcinoma (HCC) leading to liver transplantation or an estimated 700,000 deaths per year worldwide.3, 4, 5 HCV exists in 7 genotypes, with different geographical distributions, and is responsible for different responses to treatment.6, 7
The aim of antiviral treatment is to eradicate HCV infection, thus preventing disease progression. The evolution of HCV treatment has been very fast in the last decade, with increasing rates of patients achieving sustained virological response (SVR), defined as undetectable HCV RNA 12 or 24 weeks after the end of treatment.4 SVR rate is the most commonly used endpoint in clinical trials for HCV as undetectable HCV RNA translates into a significant risk reduction for overall mortality, HCC development, and liver transplantation.8 SVR12 is now accepted as a primary study endpoint by most regulatory bodies.9
Until 2011, standard treatment for HCV-infected patients was a combination of pegylated interferon (PEG-IFN) alpha (α) and Ribavirin (RBV), leading to SVR in about 50% of treated patients. The introduction of protease inhibitors (PrIs), telaprevir or boceprevir, for treatment of genotype 1 increased the number of patients achieving SVR to 70–80% in this population, despite a high rate of adverse events (AEs).10, 11 New direct-acting antivirals (DAAs), specifically designed to inhibit 3 viral proteins, are now available. These drugs can be grouped into 4 different classes according to their mechanism of action: NS3/4A protease inhibitors, NS5A protein inhibitors, NS5B nucleoside (NPIs), and nonnucleoside (NNPIs) polymerase inhibitors (Table 1).9, 12, 13, 14 DAAs do not have equal antiviral activity across all HCV genotypes; for genotype 1 and 4, all DAA regimes are active; for genotype 2, sofosbuvir is indicated; and for genotype 3, sofosbuvir, daclatasvir, and ledipasvir are indicated. Two combination regimens (velpatasvir/sofosbuvir and asunaprevir/daclatasvir/beclabuvir) are used to treat genotypes 5 and 6 (Table 1).
Table 1.
Characteristics of DAAs Approved by the European Medicines Agency (EMA).a
| Active substance (commercial name) | Year of approval | Dosage | Administration | Genotype |
|---|---|---|---|---|
| Sofosbuvir (Sovaldi) | 2014 | 400 mg | 12 wks (SOF + PegIFN/RBV) | 1, 2, 3, and 4 |
| 12 wks (SOF + RBV) | ||||
| 24 wks (SOF + RBV) | ||||
| Until the transplantation 24 wks (SOF + RBV) | ||||
| Until the transplantation 48 wks (SOF + RBV) | ||||
| 12 wks (SOF + SIM + RBV) | ||||
| 24 wks (SOF + SIM + RBV) | ||||
| Simeprevir (Olysio) | 2015 | 150 mg | 12 wks (SIM + PegIFN/RBV) + 12 wks (PegIFN/RBV) | 1 and 4 |
| 12 wks (SIM + PegIFN/RBV) + 36 wks (PegIFN/RBV) | ||||
| 12 wks (SIM + SOF) | ||||
| 12 wks (SIM + SOF) + 12 wks (SIM + SOF) | ||||
| Daclatasvir (Daklinza) | 2015 | 60 mg | 12 wks (DACL + SOF ± RBV) | 1, 3, and 4 |
| 24 wks (DACL + SOF ± RBV) | ||||
| 24 wks (DACL + PegIFN + RBV) + 24 wks (PegIFN/RBV) | ||||
| Ledipasvir/sofosbuvir (Harvoni) | 2014 | 90 mg/400 mg | 8 wks (Led/SOF) | 1,3, and 4 |
| 12 wks (Led/SOF ± RBV) | ||||
| 24 wks (Led/SOF ± RBV) | ||||
| Ombitasvir/paritaprevir/ritonavir (Viekirax) | 2015 | 12.5 mg/75 mg/250 mg | 12 wks (ombitasvir/paritaprevir/ritonavir + RBV) | 1 and 4 |
| 24 wks (ombitasvir/paritaprevir/ritonavir + RBV) | ||||
| Dasabuvir (Exviera) | 2015 | 50 mg | 12 wks (ombitasvir/paritaprevir/ritonavir + DAS) | 1 and 4 |
| 24 wks (ombitasvir/paritaprevir/ritonavir + DAS) | ||||
| 12 wks (ombitasvir/paritaprevir/ritonavir + DAS + RBV) | ||||
| 24 wks (ombitasvir/paritaprevir/ritonavir + DAS + RBV) | ||||
| Grazoprevir/elbasvir (Zepatier) | 2016 | 100 mg/50 mg | 8 wks (GRA + ELB) | 1 and 4 |
| 12 wks (GRA + ELB ± RBV) | ||||
| Velpatasvir/sofosbuvir (Epclusa) | 2016 | 100 mg/400 mg | 12 wks (Valp + SOF ± RBV) | 1, 2, 3, 4, 5, and 6 |
| Asunaprevir/daclatasvir/beclabuvir | Soon be available | 1, 2, 3, 4, and 5 |
Wks, weeks; SOF, sofosbuvir; RBV, ribavirin; IFN, interferon; SIM, simeprevir; DACL, daclatasvir; Led, ledipasvir; DAS, dasabuvir; GRA, grazoprevir; ELB, elbasvir; Valp, velpatasvir.
Faldaprevir 120 mg was withdrawn during the marketing authorization procedure.
These new antiviral agents appear to be better tolerated than first-generation PrIs and claim to be associated with SVR rates of up to 90–100%.11, 15 Initially, they were used as add-on therapies to standard treatments (PEG-IFNα/RBV) and then in IFN-free regimens, intended to reduce the toxicity of treatments in naïve patients and offer an option to PEG-IFNα nonresponders.14 Currently, they are also being used in different combinations including 2 or more DAAs (Supplementary Table I) with or without RBV.
The availability of new DAAs completely changed the recommendations for HCV treatment, transforming HCV infection into a potentially curable condition in most patients. Comprehensive overviews of evidence could be useful to this aim and inform the on-going discussion about the economic burden of these drugs, which is a significant barrier to their affordability.16, 17
We aimed to review the available evidence from RCTs of new licensed DAAs or DAAs under development for the treatment of chronic HCV. The present review focuses on the first regimens of DAAs developed and takes into account only comparative randomized trials addressing the net effect of these drugs as compared to placebo or active comparators (e.g. first-generation PrIs).
Methods
The review protocol was registered in PROSPERO, the International Prospective Register of Systematic Reviews (PROSPERO 2015:CRD42015020290). We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (Prisma) guideline.18
Criteria for Considering Studies for This Review
We included RCTs conducted in adult patients of both genders, affected by chronic HCV infection (any genotype). We included both treatment-naïve patients and patients previously treated with PEG-IFNα and RBV. Several protease inhibitors, polymerase inhibitors, and NS5A inhibitors have already been approved in the European Union and/or United States, including several fixed-dose combinations. Moreover, it is estimated that about 30 agents are in clinical development.19
To be included, RCTs had to test one of the following DAAs:
-
1.
NS3/4A inhibitors: simeprevir, paritaprevir, asunaprevir, vaniprevir
-
2.
NS5A inhibitors: daclatasvir, ledipasvir, ombitasvir
-
3.
NS5B nucleoside polymerase inhibitors (NPIs): sofosbuvir, faldaprevir
-
4.
NS5B nonnucleoside polymerase inhibitors (NNPIs): dasabuvir, filibuvir
-
5.
Other experimental DAAs not yet approved by regulatory agencies at the time of study planning.
We included RCTs comparing the DAAs with placebo, no treatment, or first-generation PrIs. Trials inclusion required >12 weeks DAA treatment, irrespective of the study follow-up duration. We excluded single-arm trials, trials testing different regimens of the same DAAs (i.e. different dosage, length of treatment), trials including acute HCV infection patients only, patients with HIV coinfection, or those undergoing liver transplantation, and trials that did not report SVR data.
Outcome Measures
Primary Outcomes
-
•
Rate of SVR, defined as HCV RNA <15 IU/ml 12 (SVR12) or 24 (SVR24) weeks after the end of treatment according to the definition in each study.
-
•
All-cause mortality and progression of liver disease.
Secondary Outcomes
-
•
Rate of nonresponse (treatment failure), defined as HCV 15 IU/ml at the end of treatment.
-
•
Rate of virological breakthrough during treatment, defined as the reappearance of HCV RNA while still on therapy in patients with viral suppression in their early course of therapy.
-
•
Rate of relapse, defined as the reappearance of HCV RNA after the end of treatment.
-
•
Discontinuation due to AEs (as defined in each study).
-
•
Rate and types of AEs (serious and most frequent according to each drug).
Search Strategy
We systematically searched electronic databases: MEDLINE, Embase, Scopus, Web of Sciences, Cochrane Central Register of Controlled Trials (CENTRAL), and Lilacs. Search strategy adopted was similar across the databases, and it was developed using keywords including “hepatitis C”, “Simeprevir”, “Asunaprevir”, “Daclatasvir”, “Ledipasvir”, “Ombitasvir”, “Sofosbuvir”, “Dasabuvir”, “Paritaprevir”, “Grazoprevir”, “Danoprevir”, “Filibuvir”, and “Faldaprevir”. We limited the search to studies in humans published in English, Italian, or Spanish from January 2012 until July 2017. We screened relevant reference lists of articles and also searched for on-going clinical trials in www.clinicaltrials.gov.
Study Selection and Data Collection
Two authors independently screened the abstracts retrieved through the database searches and selected the studies for inclusion according to eligibility criteria. From each of the included trials, two authors independently extracted the following data: 1) characteristics of participants: age, gender, race, number of randomized patients and those who completed the study, HCV genotype, IL28B polymorphisms, degree of fibrosis, and presence of cirrhosis; 2) characteristics of the study: year of publication, trial name and phase, experimental drug and comparator, and treatment duration and length of follow-up; 3) efficacy outcomes: SVR12, SVR24, death, progression of liver disease (patients developing cirrhosis or HCC at the follow-up), rates of nonresponders, and viral breakthrough and relapse; and 4) safety outcomes: rate of discontinuation due to AEs and type of AEs.
Risk of Bias Assessment
Two reviewers independently evaluated the risk of bias of each study following the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions.20 The following domains were considered: random sequence generation, allocation concealment, blinding of investigators, participants and outcome assessors, incomplete data outcome, and selective outcome reporting bias. We also evaluated the risk of bias items specific to AEs: 1) definition of serious AEs (SAEs) (i.e. death, hospitalization, etc.), if specified, and 2) method of AEs assessment, (i.e. whether the researchers actively monitored AEs or simply provided spontaneous reporting of AEs that arose during the study). Each domain was classified as at “high” or “low” risk of bias. If the information reported in the article was insufficient, the domain was defined as “unclear”.
Data Analysis
We pooled trials according to the DAA used (e.g. sofosbuvir) and length of treatment (e.g. 12 or 24 weeks). Meta-analyses were performed separately for naïve patients and previously treated patients. When different doses of DAAs were tested in the same trial, we pooled data of the dose approved by regulatory agencies for marketed drugs or the one tested in more than one study for drugs not yet on the market. We used the risk ratio (RR) with a 95% confidence interval (CI) to estimate the relative efficacy and safety of new DAAs. We assessed the presence of heterogeneity using the I-squared statistics (I2), which estimates the percentage of variation between study results, due to heterogeneity rather than sampling error. The I2 statistics indicates the percentage of the overall variability that is due to between-study (or interstudy) variability, as opposed to within-study (or intrastudy) variability. I2 value less than 25% reveals low heterogeneity, I2 between 25% and 75% indicates moderate heterogeneity, and I2 greater than 75% expresses substantial heterogeneity. In the presence of heterogeneity between studies, we pooled data using the random-effects model,21 otherwise we combined the studies using Mantel–Haenszel methods for a fixed-effects model.22 P-value lower than 0.05 was considered statistically significant. Publication bias was assessed graphically using a funnel plot23 and statistically evaluated by the regression symmetry test described by Egger et al.24 Analyses were performed using the RevMan 5.3 and Stata 11 software.
Evidence Profile
We evaluated the evidence using the GRADE approach and produced a “Summary of findings” table for studies comparing DAAs with placebo, outlining the main outcome results (SVR12, SVR24, mortality, discontinuation due to AEs and SAEs). RCTs were initially considered of high quality but were downgraded according to their risk of bias, directness of evidence (generalizability), consistency, and precision of results across all trials that measured a given specific outcome. Directness refers to the extent to which trial participants, interventions, and outcome measures considered in the included trials are relevant to the review question. Consistency concerns the degree of homogeneity (direction and magnitude) of results across the different studies. Precision describes the grade of uncertainty around the effect estimate, in other words the width of estimated CI. We determined the quality of the evidence for each outcome considering each of these factors as “high”, “moderate”, “low”, or “very low”.25 The overall quality of evidence corresponds to the lowest GRADE score defined for each outcome.
Results
Study Selection
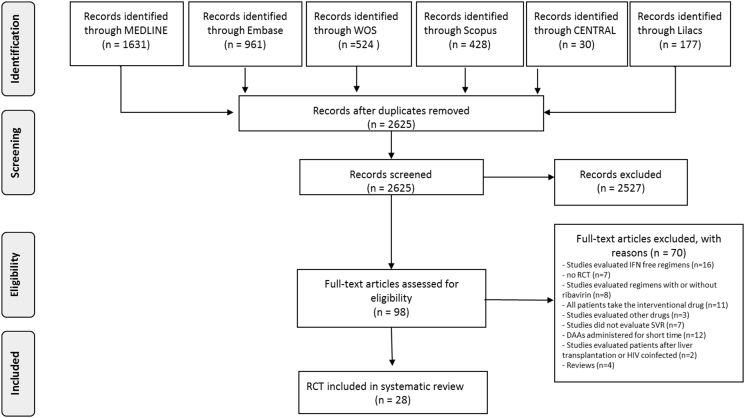
The database searches identified 2625 records. After initial screening, we excluded 2527 articles that did not meet inclusion criteria. A total of 98 studies were considered for eligibility, and full-text studies were analyzed. We excluded 70 studies because they (i) evaluated IFN-free DAA regimens (n = 16); (ii) compared DAA regimens with or without RBV (n = 8); (iii) did not test DAAs (n = 3); (iv) were not RCTs (n = 7); (v) compared different regimens of the same DAAs (i.e. all patients took the experimental drug, n = 11); (vi) tested DAAs that were administered for only few days (n = 12); (vii) did not report data on SVR rate (n = 7); (viii) were review articles (n = 4); and (ix) included patients after liver transplantation or HIV coinfection (n = 2) (Supplementary Table II reports the list of excluded trials). Finally, we included 28 trials26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53 enrolling 7710 patients (Figure 1). The search in clinicaltrials.gov revealed 15 ongoing studies (last update May 2017) that met the inclusion criteria.
Figure 1.
Literature flow diagram.
Characteristics of Included Studies
The number of participants ranged from 47 to 763. The median age of participants was 50 years (range 18–75 years). Most trials enrolled patients infected with HCV subtypes 1a or 1b. Two trials enrolled patients infected with HCV genotypes 2 or 3, and three with genotypes 4. Twenty-one trials included naïve patients, and 7 included patients previously treated with PEG-IFNα/RBV. Half of the trials reported the number of patients who had cirrhosis at randomization (15%, 581 of 3915 patients). Nine trials evaluated simeprevir; 5 trials: daclatasvir; 3 trials: vaniprevir; 2 trials: sofosbuvir, faldaprevir, danoprevir, or asunaprevir; and 1 trials: filibuvir, grazoprevir, or ombitasvir in combination with paritaprevir. Twenty-two trials compared one DAA in combination with PEG-IFNα/RBV to placebo. Five had an active-comparator design and considered sofosbuvir alone, telaprevir, or boceprevir as the comparator. We included 17 phase II and 10 phase III trials, and one trial did not report information about its phase. The main features of the 28 RCTs are summarized in Table 2.
Table 2.
Characteristics of Included Studies.
| Author | Study | Experimental group | Control group | HCV gen | Patients | Age |
Men |
Race, N (%) | Cirrhosi |
IL28B genotype, N (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | Phase | Drug | Dose (mg) | Treatment duration (weeks) | N patients | Drugc | N patients | Median (range) | No (%) | White | Black | Others | No (%) | CC | CT | TT | |||
| Pearlman 2015 | – | 2 | SIM + SOF | 150 + 400 | 12 | 58 | SOF + PEG-IFNα-2b/RBV | 24 | 1a | Naive and previously treated | 56.5b | 53 (64.6) | 38 (46) | 39 (47) | 5 (6) | 82 (100) | No CC: 71 (86.6) | ||
| Reddy 2015 | ATTAIN | 3 | SIM + placebo + Peg-IFNα2a/RBV | 150 + 750 | 12 | 379 | Tel + placebo + PEG-IFNα2a/RBV | 384 | 1a 1b |
Previously treated | 51 (18–69) | 466 (61.1) | 719 (94.2) | 38 (5) | 6 (0.8) | 163 (21.4) | 33 (4.3) | 472 (61.8) | 223 (29.2) |
| Zeuzem 2014 | ASPIRE | 2b | SIM + PEG-IFN/RBV | 100 | 12 | 66 | Placebo + PEG-IFN/RBV | 61b6 | 1a 1b |
Previously treated | 50 (20–69) | 311 (67.3) | 428 (92) | 34 (7) | Na | 83 (18.2) | 58 (17.7) | 212 (64.6) | 58 (17.7) |
| 100 | 24 | 65 | |||||||||||||||||
| 100 | 48 | 66 | |||||||||||||||||
| 150 | 12 | 66 | |||||||||||||||||
| 150 | 24 | 68 | |||||||||||||||||
| 150 | 48 | 65 | |||||||||||||||||
| Hayashi 2014 | DRAGON | Nr | SIM + PEG-IFNα-2a/RBV | 50 | 12 | 27 | PEG-IFNα-2a/RBV | 13 | 1b | Naive | 54 (20–69) | 43 (47) | Na | Na | 92 (100) | 0 (0) | nr | nr | nr |
| 50 | 24 | 13 | |||||||||||||||||
| 100 | 12 | 26 | |||||||||||||||||
| 100 | 24 | 13 | |||||||||||||||||
| Forns 2014 | PROMISE | 3 | SIM + PEG-IFNα-2a/RBV | 150 | 12 | 260 | Placebo + PEG-IFNα-2a/RBV | 133 | 1a 1b |
Previously treated | 52 (20–71) | 258 (65.6) | 371 (94.4) | 11 (2.8) | Na | 0 (0) | 96 (24.4) | 250 (63.6) | 47 (12) |
| Hayashi 2014 | CONCERTO-I | 3 | SIM + PEG-IFNα-2a/RBV | 100 | 12 | 123 | Placebo + PEG-IFNα-2a/RBV | 60 | 1a 1b |
Naive | 55 (23–69) | 63 (34.4) | Nr | Nr | Nr | 0(0) | 121 (66) | 62 (34) | Na |
| Jacobson 2014 | QUEST-I | 3 | SIM + PEG-IFNα-2a/RBV | 100 | 12 | 264 | Placebo + PEG-IFNα-2a/RBV | 130 | 1a 1b |
Naive | 48 (36–54) | 222 (56.3) | 349 (88.6) | 31 (7.8) | 8 (2) | Na | 114 (28.9) | 226 (57.3) | 54 (13.7) |
| Manns 2014 | QUEST-II | 3 | SIM + PEG-IFNα-2a or 2b/RBV | 150 | 12 | 257 | Placebo + PEG-IFNα/RBV | 134 | 1a 1b |
Naive | 45 (18–73) | 217 (55.5) | 360 (92) | 26 (6.6) | 5 (1.2) | 32 (8) | 117 (30) | 213 (54.5) | 61 (15.6) |
| Fried 2013 | PILLAR | 2b | SIM + PEG-IFNα-2a | 75 | 12 | 78 | Placebo + PEG-IFNα-2a | 77 | 1a 1b |
Naive | 47 (18–69) | 213 (55.2) | 362 (93.8) | 13 (3.4) | 11 (2.8) | Na | 78 (20.2) | 152 (39.4) | 32 (8.3) |
| 75 | 24 | 75 | |||||||||||||||||
| 150 | 12 | 77 | |||||||||||||||||
| 150 | 24 | 79 | |||||||||||||||||
| Jacobson 2013 | POSITRONa | 3 | SOF + RBV | 400 | 12 | 207 | Placebo + RBV | 71 | 2; 3 | Previously treated | 52b (21–75) | 151 (54.3) | 254 (91.4) | 13 (4.6) | 11 (6.2) | 44 (15.8) | 126 (45.3) | 120 (43) | 32 (11.5) |
| Lawitz 2013 | – | 2 | SOF + PEG-IFNα-2a/RBV | 200 | 12 | 48 | Placebo + PEG-IFNα-2a/RBV | 26 | 1a 1b |
Naive | 48.4 | 73 (60.3) | 97 (80) | 18 (14.9) | 6 (5) | Na | 50 (41.3) | 54 (44.6) | 17 (14) |
| 400 | |||||||||||||||||||
| Dore 2015 | – | 2b | DACL + PEG-IFNα-2a/RBV | 60 | 12 | 50 | Placebo + PEG-IFNα-2a/RBV | 51 | 2; 3 | Naive | 48.6 (20–67) | 96 (63.6) | 129 (85.4) | 4 (2.6) | 18 (11.9) | 19 (12.6) | 56 (37) | 76 (50.3) | 17 (11.3) |
| 16 | 51 | ||||||||||||||||||
| Hezodè 2015 | COMMAND-1 | 2b | DACL + PEG-IFNα-2a/RBV | 20 | 12 | 159 | Placebo + PEG-IFNα-2a/RBV | 78 | 1a 1b 4 |
Naive | 50.5 (18–70) | 265 (67) | 319 (80.7) | 45 (11.4) | 31 (7.8) | 29 (7.3) | 120 (30.4) | 206 (52) | 46 (11.6) |
| 60 | 12 | 158 | |||||||||||||||||
| Kumada 2016 | – | 3b | DACL + ASU | 60 + 100 | 24 | 119 | Tel + placebo + PEG-IFNα2a/RBV | 111 | 1b | Naive | 56 (20–70) | 102 (44) | Nr | Nr | Nr | Na | 153 (66.5) | 70 (30.4) | 5 (2.2) |
| Izumi 2014 | – | 2a | DACL + PEG-IFNα-2a/RBV | 10 | 24 | 9 | Placebo + PEG-IFNα-2a/RBV | 8 | 1a 1b |
Naïve | 56 (28–67) | 9 (36) | Nr | Nr | Nr | 0 (0) | 19 (76) | 5 (20) | 0(0) |
| 60 | 24 | 8 | |||||||||||||||||
| Pol 2012 | – | 2a | DACL + PEG-IFNα-2a/RBV | 3 | 12 | 12 | Placebo + PEG-IFNα-2a/RBV | 12 | 1a 1b |
Naive | 51 (28–68) | 32 (66.6) | 35 (72.9) | 9 (18.7) | 4 (8.3) | Na | 13 (27) | 18 (37.5) | 5 (10.4) |
| 10 | 12 | ||||||||||||||||||
| 60 | 12 | ||||||||||||||||||
| Bronowicki 2014 | – | 2b | ASU + PEG-IFNα-2a/RBV | 200 | 24 | 177 | Placebo + PEG-IFNα-2a/RBV | 61 | 1a 1b 4 |
Naive | 47.8 | 153 (64.3) | 199 (83.6) | 20 (8.4) | 19(8) | 25 (10.5) | 66 (27.7) | 128 (53.7) | 36 (15.1) |
| Bronowicki 2013 | – | 2a | ASU + PEG-IFNα-2a/RBV | 200 | 48 | 12 | Placebo + PEG-IFNα-2a/RBV | 11 | 1a 1b |
Naive | 48 | 35 (74.4) | 38 (80.8) | 8 (17) | 1 (2) | Na | 13 (27.6) | 24 (51) | 10 (21) |
| 600 twice d | |||||||||||||||||||
| 600 once d | |||||||||||||||||||
| Rodriguez-Torres 2014 | – | 2b | FIL + PEG-IFNα-2a/RBV | 300 | 24 | 96 | Placebo + PEG-IFNα-2a/RBV | 96 | 1 | Naive | 47.8 (10) | 153 (53) | 227 (78.8). | 18 (6.2) | 43 (14.9)- | Na | 63 (21.8) | 106 (368) | 27 (9.3) |
| 600 | |||||||||||||||||||
| Manns 2014 | – | 2 | GRAZ (MK-5172) + PEG-IFNα-2b/RBV | 100 | 12 | 66 | Placebo + BOC + PEG-IFNα-2b/RBV | 66 | 1b | Naive | 51b (18–72) | 191 (57.5) | 272 (81.9) | 44 (13.2) | 16 (4.8) | Na | Nr | Nr | Nr |
| 200 | 68 | ||||||||||||||||||
| 400 | 67 | ||||||||||||||||||
| 800 | 65 | ||||||||||||||||||
| Sulkowski 2013 | SILENC-1 | 2b | FAL + PEG-IFNα -2a/RBV | 120 | 24 | 69 | Placebo + PEG-IFNα-2a/RBV | 71 | 1a 1b |
Naive | 46b (SD 10.5) | 234 (54) | 356 (82.9) | 10 (2.3) | 63 (14.6) | Na | 60 (14) | no CC 163 (38) | |
| 240 | 143 | ||||||||||||||||||
| 240 | 146 | ||||||||||||||||||
| Ferenci 2015 | STARTVerson 1 | 3 | FAL + PEG-IFNα -2a/RBV | 120 | 12 | 259 | Placebo + PEG-IFNa2a/RBV | 132 | 1a 1b |
Naive | 47,6b | 342 (52.4) | 511 (78.4) | 9 (1.4) | 132 (20.2) | 30 (6) | 254 (38.9) | 316 (48.4) | 81 (12.4) |
| 240 | 261 | ||||||||||||||||||
| Marcellin 2013 | ATLAS | 2 | DAN +PEG-IFNα-2a/RBV | 300 | 12 | 72 | Placebo + PEG-IFNα-2a/RBV | 31 | 1a 1b |
Naive | 48.3 | 135 (60) | 193 (85.7) | 22 (9.7) | 10 (4.4) | Na | 56 (24.8) | Nr | Nr |
| 600 | 72 | ||||||||||||||||||
| 900 | 50 | ||||||||||||||||||
| Everson 2015 | DAUPHINE | 2 | DAN/r + PEG-IFNα-a/RBV | 200/100 | 24 | 92 | Placebo + PEG-IFNα-2a/RBV | 44 | 1a 1b 4 |
Naive | 52.5 (19–73) | 202 (62.5) | 259 (80) | 34 (10.5) | 30 (9.3) | Na | 94 (29.1) | No CC 229 (70.9) | |
| 100/100 | 93 | ||||||||||||||||||
| 50/100 | 94 | ||||||||||||||||||
| Hayashi 2015 | – | 3 | VAN + PEG-IFNα-a/RBV | 300 | 12 | 98 | Placebo + PEG-IFNα-2b/RBV | 98 | 1a 1b |
Naive | Nr | 137 (46.6) | Nr | Nr | Nr | Na | 198 (67.3) | 92 (31.3) | 4 (1.4) |
| 24 | 98 | ||||||||||||||||||
| Lawitz 2013 | – | 2b | VAN + PEG-IFNα-a/RBV | 300 | 48 | 41 | Placebo + PEG-IFNα-2a/RBV | 42 | 1a 1b |
Previously treated | 51 (23–65) | 82 (67.2) | 95 (77.8) | 7 (5.7) | 23 (18.9) | 0 (0) | Nr | Nr | Nr |
| 600 | 42 | ||||||||||||||||||
| Rodriguez-torres 2014 | – | 2b | VAN + PEG-IFNα-2a/RBV | 300 | 24 | 13 | Placebo + PEG-IFNα-2a/RBV | 14 | 1a 1b |
Previously treated | 55.5 (38–65) | 49 (66.2) | 65 (87.8) | 4 (5.4) | 5(6.7) | 74 (100) | nr | nr | nr |
| 300 | 48 | 13 | |||||||||||||||||
| 600 | 24 | 15 | |||||||||||||||||
| 600 | 48 | 15 | |||||||||||||||||
| Dore 2016 | MALACHITE I/II | 3b | OBV/PTV/r + DAS + RBV | 25 | 12 or 16 | 337 | Tel + placebo + PEG-IFNα2a/RBV | 122 | 1a 1b |
Naive and previously treated | 46b | 243 (52.9) | 440 (95.9) | (0) | 59 (12.8) | Nr | 79 (17.9) | no CC 380 (82.8) | |
| 100 | |||||||||||||||||||
| 150 | |||||||||||||||||||
| 250 | |||||||||||||||||||
ASU, asunaprevir; BOC, boceprevir; DACL, daclatasvir; DAN, danoprevir; DAS, dasabuvir; FAL, faldaprevir; FIL, filibuvir; GRAZ, grazoprevir; IFN, interferone; n, number; nr, not reported; na, not available; OBV/PTV/r, ombitasvir/paritaprevir, ritonavir; RBV, ribavirin; SIM, simeprevir; SOF, sofosbuvir; Tel, telaprevir; VAN, vanoprevir.
Authors reported also the FUSION study results, administering sofosbuvir in all study arms.
Results reported as mean.
Dosage of PEG-IFNα/RBV was 180 μg/1000–1200 mg in all studies included.
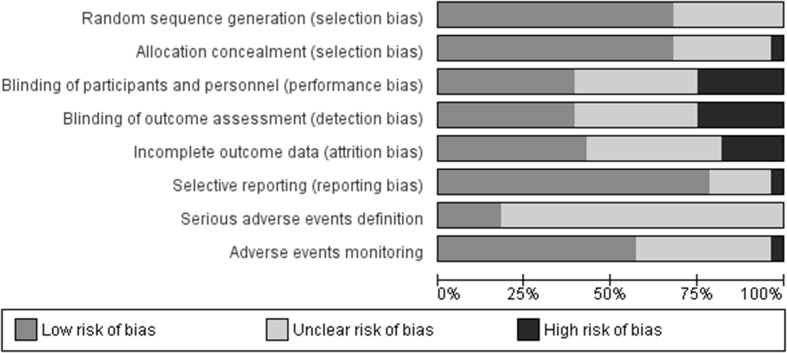
Risk of Bias Assessment
The overall risk of bias of the 28 trials included is reported in Figure 2. We judged most of the studies at low risk of selection bias as they adequately generated and concealed (20 studies, 71%) the randomization list. Trials were more heterogeneous in terms of risk of performance and detection bias. Only 11 trials were judged as adequate in terms of blinding of personnel, participants, and outcome assessors. Seven trials were open label. Twelve trials were judged at low risk of attrition bias and 22 at low risk of reporting bias. Sixteen trials (57%) monitored participants for signs and symptoms possibly related to AEs, but only 5 trials appeared to have implemented appropriate, prespecified definition of SAEs.
Figure 2.
Risk of bias graphic.
Quality of Evidence
We assessed the quality of the evidence for all the DAAs using the GRADE approach. Although this systematic review included only RCTs, the quality of evidence was generally low. The main reasons for downgrading were the risk of bias because three studies were at high risk for performance and detection bias and one for attrition bias and inconsistency because some meta-analyses showed moderate heterogeneity and imprecision. However, the meta-analyses did not suffer from serious indirectness. The overall quality of evidence was low for SVR24, very low for SVR12, mortality, and discontinuation due to SAEs (Table 3). The overall quality of evidence was low for danoprevir, faldaprevir, sofosbuvir, and vaniprevir and very low for the other drugs (data not shown). Thus, the certainty around the estimates of benefits and harms of DAAs remains uncertain.
Table 3.
Summary of Findings for DAAs vs Placebo According to Outcomes.
| DAAs compared with placebo for HCV-naive patients | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Certainty assessment |
Summary of findings |
|||||||||||
| No. of participants (studies) Follow-up |
Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Overall certainty of evidence | Study event rates (%) | Relative effect (95% CI) | Anticipated absolute effects | |||
| With placebo | With DAAs | Risk with placebo | Risk difference with DAAs | |||||||||
| SVR12 (follow-up: mean 12 weeks; assessed with: undetectable HCV RNA level 12 weeks after treatment discontinuation, measured with PCR) | ||||||||||||
| 2497 (12 RCTs) | Very seriousa | Seriousb | Not serious | Not serious | None | ⊕○○○ Very low |
467/868 (53.8%) | 1272/1629 (78.1%) | RR 1.44 (1.31–1.59) | 538 per 1.000 | 237 more per 1.000 (167 more to 317more) | |
| SVR24 (follow-up: mean 24 weeks; assessed with: undetectable HCV RNA level 24 weeks after treatment discontinuation) | ||||||||||||
| 1509 (10 RCTs) | Very seriousa | Not serious | Not serious | Not serious | None | ⊕⊕○○ Low |
254/476 (53.4%) | 815/1033 (78.9%) | RR 1.50 (1.36–1.64) | 534 per 1.000 | 267 more per 1.000 (192 more to 342 more) | |
| Mortality (follow-up: mean 48 weeks) | ||||||||||||
| 3203 (13 RCTs) | Very seriousa | Not serious | Seriousc | Seriousd | None | ⊕○○○ Very low |
0/894 (0.0%) | 9/2309 (0.4%) | RR 1.31 (0.34–5.06) | 0 per 1.000 | 0 fewer per 1.000 (0 fewer to 0 fewer) | |
| Discontinuation due to adverse events (follow-up: mean 48 weeks) | ||||||||||||
| 3545 (17 RCTs) | Very seriousa | Not serious | Not serious | Seriousd | None | ⊕○○○ Very low |
70/1089 (6.4%) | 133/2456 (5.4%) | RR 0.76 (0.58–1.01) | 64 per 1.000 | 15 fewer per 1.000 (27 fewer to 1 more) | |
| Serious adverse events (follow-up: mean 48 weeks) | ||||||||||||
| 3819 (17 RCTs) | Very seriousa | Not serious | Not serious | Seriousd | None | ⊕○○○ Very low |
65/1107 (5.9%) | 168/2712 (6.2%) | RR 0.92 (0.70–1.21) | 59 per 1.000 | 5 fewer per 1.000 (18 fewer to 12 more) | |
Three studies were at high risk of performance and detection bias. One study was at high risk of attrition bias.
Substantial heterogeneity.
The length of follow-up does not allow any reliable evaluation of this outcome.
The estimate confidence interval includes the null value.
Effect of DAAs Compared to Placebo
Primary Outcomes
Sustained virological response
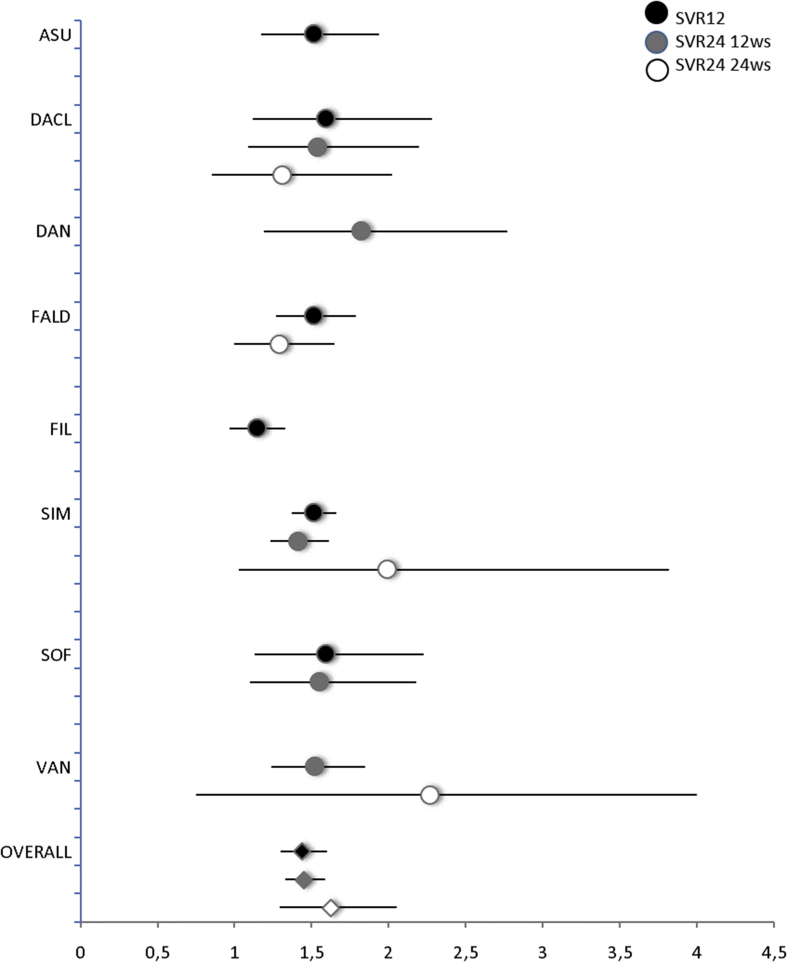
Our analyses showed that among naïve patients treated with DAAs, in combination with PEG-IFNα/RBV, 78% achieved SVR12 compared with 54% in the group treated by placebo with PEG-IFNα/RBV (RR 1.4; 95% CI 1.3–1.6, I2 = 46%; n = 12; Table 4; Figure 3). For every 1000 patients, 240 more experienced SVR12 if treated with any DAAs compared with placebo, ranging from 90 with filibuvir to 330 with sofosbuvir. The quality of evidence ranges from high to very low and overall was judged as very low (Table 4). In one study30 evaluating SVR12 (RR 2.2; 95% CI 1.7–2.8) in previously treated patients, 79% achieved SVR12 compared with 36% in the control group.
Table 4.
Meta-analysis of DAAs vs Placebo. Outcome: SVR12, SVR24, and Mortality According to the Drug Administered.
| Drug | N studies | RR | 95% CI | I2 (%) | Quality of evidence | Explanation for downgrading |
|---|---|---|---|---|---|---|
| SVR12 in naïve patientsa | ||||||
| ASU | 2 | 1.51 | 1.18–1.95 | 0 | ⊕⊕⊕○ Moderate | Risk of biasc |
| DACL | 3 | 1.59 | 1.12–2.28 | 57 | ⊕○○○ Very Low | Risk of bias (−2),d inconsistencye |
| FALD | 1 | 1.51 | 1.27–1.79 | n.a. | ⊕⊕⊕⊕ High | |
| FIL | 1 | 1.14 | 0.97–1.33 | n.a. | ⊕⊕○○ Low | Risk of bias,f imprecisiong |
| SIM | 4 | 1.46 | 1.28–1.67 | 49 | ⊕⊕⊕⊕ High | |
| SOF | 1 | 1.59 | 1.13–2.23 | n.a. | ⊕⊕○○ Low | Imprecision,h publication bias |
| All DAAs | 12 | 1.44 | 1.31–1.59 | 46 | ⊕○○○ Very Low | |
| SVR24 in naïvebpatients after 12 weeks of treatment | ||||||
| DACL | 3 | 1.54 | 1.25–1.89 | 56 | ⊕⊕○○ Low | Risk of bias (−2),d inconsistencye |
| DAN | 1 | 1.82 | 1.19–2.77 | n.a. | ⊕⊕⊕○ Moderate | Risk of biasi |
| SIM | 4 | 1.41 | 1.23–1.61 | 0 | ⊕⊕⊕○ Moderate | Risk of biasj |
| SOF | 1 | 1.55 | 1.10–2.18 | n.a. | ⊕⊕⊕○ Moderate | Imprecisionh |
| VAN | 1 | 1.52 | 1.24–1.85 | n.a. | ⊕⊕⊕⊕ High | |
| All DAAs | 10 | 1.50 | 1.36–1.64 | 3 | ⊕⊕○○ Low | |
| Mortality in naïve and previously treated patients | ||||||
| ASU | 2 | 2.02 | 0.1–41.6 | n.a. | ⊕⊕○○ Low | Indirectness,k imprecisiong |
| DACL | 2 | 0.74 | 0.03–18.07 | n.a. | ⊕○○○ Very Low | Risk of bias (−2),d indirectness,k imprecisiong |
| DAN | 2 | 0.65 | 0.07–5.73 | 0 | ⊕⊕○○ Low | Indirectness,k imprecisiong |
| FIL | 1 | Not estimable | ||||
| SIM | 7 | 1.79 | 0.21–15.22 | 0 | ⊕○○○ Very Low | Risk of bias,j indirectness,k imprecisiong |
| VAN | 3 | Not estimable | ||||
| All DAAs | 17 | 0.99 | 0.35–2.81 | 0 | ⊕○○○ Very Low | |
ASU, asunaprevir; DACL, daclatasvir; DAN, danoprevir; FALD, faldaprevir; FIL, filibuvir; SIM, simeprevir; SOF, sofosbuvir; VAN, vaniprevir; n.a., not applicable.
One study on SIM (Forns 2014 30), not included in the meta-analyses, enrolled participants previously treated with PEG-INFα/RIB: RR 2.20 (95% CI 1.74–2.78).
One study on SIM (Zeuzeum 2014 28), not included in the meta-analyses, enrolled participants previously treated with PEG-INFα/RIB RR 2.93 (95% CI 1.82–4.72).
One study was at high risk of performance and detection bias.
One study was at high risk of performance and detection bias. One study was at unclear risk of performance and detection bias. One study was at high risk of of attrition bias.
Substantial heterogeneity.
One study was at unclear risk of selection and performance bias and at high risk of detection bias.
The estimate confidence interval includes the null value.
The study involves a small sample size, which is not sufficient to have the needed power.
One study was at unclear risk of performance and detection bias.
One study was at unclear risk of selection bias and at high risk of performance and detection bias.
The length of follow up does not allow any reliable evaluation of this outcome.
Figure 3.
Meta-analysis of DAAs vs placebo. Outcome: SVR12 and SVR24 for 12 and 24 weeks of treatment in naive patients according to the drug. DAAs, direct-acting antiviral agents.
Naïve patients treated with DAAs for 12 weeks had more SVR24 (79%) than placebo (53%) controls (RR 1.5, 95% CI 1.36–1.64, I2 = 3%; n = 10; Table 4; Figure 3). For every 1000 patients, 260 more experienced SVR24 if treated with any DAAs than with placebo, ranging from 240 with simeprevir to 350 with danoprevir. The quality of evidence ranges from high to low and overall was judged as low (Table 4). One study28 evaluated SVR24 (RR 2.9; 95% CI 1.82–4.72) in previously treated patients, of whom 67% achieved SVR24 compared with 23% in the control group.
Trials evaluating daclatasvir, simeprevir, and sofosbuvir reported data for both SVR12 and SVR24, and there was no difference in terms of response rate.
Overall, patients treated with DAAs had higher SVR24 (80%) than controls (50%) after 24 weeks of treatment (RR 1.69 95% CI 1.35–2.12, I2 = 71%; n = 8, Figure 3). Similar results were achieved after 48 weeks of treatment (data not shown).
All-cause mortality and progression of liver disease
Seventeen placebo-controlled trials for a total of 3211 participants reported data on mortality. Overall, these studies reported 9 deaths out of 2309 participants (0.4%) in the experimental treatment arm and none in the control group (Table 4). The quality of evidence ranges from low to very low and overall was judged as very low (Table 4).
We could not find studies assessing the progression of liver disease (i.e., development of cirrhosis) after treatment discontinuation or development of HCC.
Secondary Outcomes
Nonresponse, relapse, and virological breakthrough
The rates of nonresponses and relapses were significantly lower in the intervention group than in the placebo group (RR: 0.1, 95% CI: 0.05–0.2, I2 = 54%, n = 8; RR: 0.47, 95% CI: 0.35–0.62, I2 = 61%, n = 18, respectively). On the contrary, we found that the virological breakthrough rate was higher in the DAAs treatment arms, although the difference was not statistically significant (RR: 1.2, 95% CI: 0.8–1.9, I2 = 36%, n = 13).
Safety
The rate of treatment discontinuation due to AEs was similar for patients enrolled in DAAs and placebo arms (RR: 0.85, 95% CI: 0.66–1.11, I2 = 15%, n = 20; Supplementary Table III), and the discontinuation rate was lower in DAAs compared to placebo in naïve patients (RR: 0.77, 95% CI: 0.58–1.01, I2 = 9%, n = 17) but higher than placebo in previously treated patients (RR: 1.78, 95% CI: 0.75–4.19, I2 = 0%, n = 3).
Overall, 6% of patients experienced SAEs (268 out of 4558 patients). Our analysis shows no significant difference between DAAs regimens and placebo (RR: 1.06, 95% CI: 0.82–1.37, I2 = 21%, n = 20) both in naïve and in previously treated patients (Supplementary Table IV). Pooled results showed that DAAs therapies did not increase the risk of AEs reported, except for pruritus and vomiting (Supplementary Table IV).
Evaluation of publication bias
A funnel plot was generated, with the studies reporting SVR12, SVR24, and mortality, to assess the possibility of publication bias or small study effects. The standard error (SE) was plotted against the estimated RR of each study. Trials reporting SVR12 were at the top of the plot and displayed a slightly asymmetrical pattern (Supplementary Figure 1a). For SVR24, the figure showed a slightly asymmetrical plot, which suggests possible publication bias (Supplementary Figure 1b). For mortality, the funnel plot showed small studies at the bottom of the plot, and it was symmetrical (Supplementary Figure 1c). No definitive conclusion about publication bias could be drawn.
Effect of DAAs with Respect to Different Active Comparators
Primary Outcomes
Sustained virological response
In five trials26, 27, 39, 45, 53 that considered active comparators (e.g. first-generation PrIs), patients treated with DAAs had a statistically significant better SVR12 rate (RR: 1.26, 95% CI: 1.08–1.48, I2 = 80%). Only one study45 evaluated SVR24 in patients treated with grazoprevir with respect to boceprevir showing a significantly better response rate for patients in the treatment arm.
All-cause mortality
Three studies26, 27, 45 reported data on mortality. Of these, two studies reported no death in the treatment group, and one study registered four deaths in the control group.
Secondary Outcomes
Safety
The rate of discontinuation due to AEs was significantly lower in patients treated with DAAs with respect to active control (RR: 0.34, 95% CI: 0.14–0.82, I2 = 70%, n = 5). Pooled results showed a significantly higher rate of AEs in patients treated with DAAs than the active comparators group (data not shown).
Discussion
This systematic review with meta-analyses summarizes published data from RCTs evaluating DAA treatments. When compared to placebo, DAAs plus PEG-IFNα/RBV increased SVR12 and SVR24 rates from 54% to 78% and from 53% to 79%, respectively, in naive patients. In treatment-experienced patients, the difference in the rate of therapy success was higher, which may be due to a lower response to PEG-IFNα/RBV. All drugs evaluated, except filibuvir, significantly improved SVR12 and SVR24 compared with PEG-IFNα/RBV alone, as also confirmed in two recent network meta-analyses.54, 55 In the few trials comparing DAAs with active controls, SVR12 also increased from 60% to 78%. The little information available from RCTs with active control groups did not allow the identification of the optimum DAA in this context.
It has been reported that patients achieving SVR show regression of fibrosis and reduced risk of HCC.56 However, we could not find RCTs assessing the progression of liver disease or cancer development. Data on mortality were too sparse to provide reliable evidence of an effect. New DAAs were not associated with higher incidence of SAEs or discontinuation due to AEs. The overall rate of AEs did not differ between DAAs and placebo. Instead, discontinuations due to AEs were fewer in patients treated with DAAs (3%) than active comparators (10%). Although DAA therapies led to significantly increased response rates, 4% of patients did not respond, and approximately 18% of previously treated and 11% of naive patients relapsed as compared to 25% of controls.
The main finding of this review is that the average measure of SVR12 or SVR24 estimated in comparative RCTs is around 80%. Although smaller than generally reported, this finding represents an absolute increase in response of about 25% from the introduction of DAAs. According to the GRADE evaluation, the certainty of this estimate is not optimal given some limitations in the design of several trials. However, this effect is so large that it could be considered as a benchmark of the benefit of these classes of drugs.
The indisputably improved SVR to DAAs should be put in a wider perspective in which several open questions call for a systematic comparative approach through RCTs.
First, however large, the SVR rate we observed for coadministration of DAAs and PEG-IFNα/RBV is consistent with the rates usually reported13, 56, 57, 58, 59 but smaller than the rate reported for the IFN-free regimens based on DAAs.15 This may be due to the fact that the response rates we found come from RCTs comparing DAAs with other controls to highlight the net effect of DAAs. SVR12 rates of up to 90–100% reported in other reviews come from pooling data also from cohort studies or uncontrolled trials or trials comparing different regimens of the same drug.15, 55, 58 These studies are usually prone to generate larger treatment effects, so they should be interpreted with great caution.60
One second aspect to consider is the increasing use of various combinations of DAAs (Supplementary Table I)57, 61 which reportedly achieve even higher SVR, making the finding of the present review possibly disappointing, although possibly more reliable. Ideally, the introduction of regimens combining two or more DAAs should be driven by RCTs testing any new single component of the combinations as an add-on compared to placebo. Different combinations should be compared to each other in RCTs too. Uncontrolled trials would provide limited information on the respective contribution of each DAA to the efficacy and the place in therapy of a given combination. Uncontrolled studies would also poorly contribute to systematic reviews and meta-analyses. Despite many registered clinical trials evaluating oral DAA combinations reporting exciting results of effectiveness, outcomes in real-world patients are lower and suggest that treatment may fail in around 10–20% of patients. This is likely to mainly occur in special patient populations, such as HIV-positive persons, transplant recipients, and patients with advanced cirrhosis or renal insufficiency, hepatitis B or D coinfection, and prior DAA failures.62, 63, 64, 65 Several IFN-free regimens are currently available with a favorable safety profile and report SVR12 rates of treatment in more than 90% of HCV patients with genotype 3, and up to 100% in patients with genotype 1, 2, or 4, and so will other combinations in the future.66, 67 It is worth noting that the role of RBV in the different treatment regimens is still debated. Many studies included treatment arms with or without RBV, and some of these showed a clear benefit, whereas others did not.68
The third question to consider is that whatever the realistic estimate of the effect of DAAs is and their combinations in terms of SVR, we still ignore how far this translates into long-term benefits.
Besides the eradication of the HCV infection, the goal of the treatment with DAAs should be preventing or delaying liver cirrhosis and its consequences, such as liver transplant and HCC. Given the recent introduction of these drugs and the short follow-up of trials, data about recurrence, liver disease progression, and liver complications are scarce. Future epidemiological studies may prove the hoped-for reduction in mortality and HCC incidence.69 A rigorous and well-organized prospective comparative research plan can contribute reliable data to those studies.
Furthermore, DAAs offer shorter treatment duration and easier disease management also for cirrhosis or transplanted patients and nonresponders than previous therapies.70
One further open issue regards the subsets of infected patients who will best benefit from DAAs. The correct determination of the HCV genotype is important in guiding the choice of the most appropriate antiviral regimen based on PEG/IFNα-RBV.71, 72 Now, new DAAs, being genotype-dependent treatment regimens, ensure large treatment response rates for all infected patients.73 It is important to underline that patients with HCV genotype 1b have more treatment options than patients with others genotypes.14
The studies included in our review explored several baseline predictors of response to interferon-based regimens and reported that those factors influencing the response included IL28b polymorphism, older age, high viral load, ethnicity, and advanced fibrosis stage. Only one study35 assessed the statistical association of pretreatment factors and SVR and showed that HCV genotype 3 infection was significantly associated with reduced rates of SVR12 as compared with HCV genotype 2 (P < 0.001).
A further issue that deserve future investigation is the development of resistance-associated variants (RAVs). It is still to be determined if RAV exists before treatment initiation, as a naturally occurring variant, or if RAV is acquired at infection or during DAA therapy. RAV prevalence seems to depend on HCV genotype and could induce treatment failure.74, 75
Several types of RAVs are described in the literature. For NS3/4A protease inhibitors, RAVs sometimes disappear gradually after DAA therapy is stopped, whereas for NS5A inhibitors and NS5B polymerase inhibitors, RAVs tend to persist for more than 2 years.76 Although no longer a component of most preferred regimens, PEG/IFN is active in patients with RAV and therefore remains a viable adjunctive option for many treatment-experienced patients.77
Twenty studies included in our systematic reviews examined the role of several RAVs on DAAs response, for instance, Q80K, R155, D168 I170V, Y56F, and V1321 for the NS3 gene and 158S, R30Q, and L31M for the NS5A gene. In seven studies, the presence of the Q80K polymorphism at baseline was associated with a lower SVR12 rate in patients treated with simeprevir (data not shown).
Resistance affects DAAs response, but its impact is difficult to quantify given the concomitant use of different types of sequencing technologies to detect RAV, which could in turn hamper the comparability.
Response to DAAs may also be affected by drug–drug interactions, especially in patients with comorbidities or treated with multiple medications. The interactions regarding cytocrome (CYP) P450 isoenzyme pathway involved in DAA metabolism are of particular interest. For instance, simeprevir and daclatasvir are metabolized by CYP450 isoform 3A4, and concomitant use of CYP3A4 inducers decrease drug exposure, potentially compromising their therapeutic effect. Ledipasvir and elbasvir are inhibitors of p-glycoprotein and breast cancer receptor protein. Drug–drug interaction are particularly relevant for combination regimens including ritonavir, given its potent CYP3A4 enzyme inhibitory effect that can increase the plasma concentration of other coadministered drugs, e.g. paritaprevir14, 70, 77
The question of treatment affordability is imposed by the high costs that disappointingly limit the clinical adoption of DAAs in some countries. Current guidelines recommend treating patients with a life expectancy of more than a year, and recently some governments have made treatment available to a large group of patients, including those with cirrhosis. Nevertheless, the treatment of patients with limited or no fibrosis could help reduce HCV transmission.78 Comparative data on robust clinical endpoints are needed to address these questions too because RCTs can better define the value of the various DAAs, their combinations and regimens, and their respective cost-effectiveness in different patients subgroups.
Other systematic reviews have previously published on DAAs efficacy and safety.15, 54, 55, 57, 58 A recent Cochrane systematic review by Jakobsen et al79 evaluating the benefits and harms of DAAs, highlights the limited data on clinical effects of these drugs on the management of patients infected by HCV. Considering hepatitis C–related morbidity and serious adverse events as primary outcomes, the authors conclude that there was no evidence of significant effects on the risk of SAEs and that there was not enough information to confirm that these drugs had any clinically relevant effects. As in our review, trials included in the Cochrane systematic review contribute few data regarding mortality, which along with the short follow-up did not allow us and our colleagues from the Cochrane Collaboration to correctly evaluate this outcome. Our results are also aligned with the findings reported by Jakobsen et al79 in that DAAs seems to increase the SVR rate in patients enrolled in the experimental group with respect to those enrolled in the control group. However, the Cochrane colleagues put into question the SVR as a nonvalidated surrogate outcome. Similarly, our meta-analyses showed that 12-week treatment with DAAs and PEG/IFNα-RBV in naïve patients increased SVR12 and SVR24 compared with controls, but the quality of evidence was very low and low, respectively. The large number of patients and studies increased the power of Jakobsen's meta-analysis, whereas both our studies conclude the uncertain relevance of the clinical effect of DAAs. Most of the trials included in our systematic review reported short-term results, so it is difficult to evaluate long-term effects of DAAs, such as HCC. Overall, the quality of evidence for all outcomes considered was generally very low, in agreement with the assessment of Jakobsen et al. The Cochrane review differs from this current systematic review in terms of participants and interventions considered for inclusion. Trial eligibility for our review included patients with HCV treated with DAAs and PEG/IFNα-RBV, whereas the review of Jakobsen et al also included trials evaluating different DAAs regimens or combinations (e.g. IFN-free regimens, short-term treatment, or combination of two DAAs), different drugs (e.g. telaprevir or boceprevir, or drugs recognized by molecule names), or drugs not equally distributed between intervention and control groups (e.g. all patients taking the interventional drug) and patients with comorbidities (e.g. coinfected by HIV). Not all of these trials met our inclusion criteria, as reported in the Methods section and in S2 Table, and therefore were not included in the present review.
This systematic review has a number of limitations that call for caution in result interpretation. Our meta-analysis included few trials testing DAA add-ons to PEG-IFNα/RBV compared with placebo or first-generation PrIs. However, because recent studies are mainly cohort or uncontrolled studies focusing on IFN-free DAA combinations, the studies included in our meta-analysis are likely to remain the only RCTs evaluating DAAs for anti-HCV treatment. Our systematic review is also limited by the rapid evolution of the treatment strategy, as shown by the number of ongoing trials we identified. Although most RCTs are homogeneous in terms of study design and patients characteristics, moderate-to-high heterogeneity among trials afflicts our meta-analysis, possibly due to the small number of RCTs included and differences in sample size and study phases (phase 2 and 3). In addition, the effect estimates for each DAA were similar to the overall effects because only single comparisons were available for most of the cases. Moreover, included trials enrolled selected populations, and the duration of the interventions were short, limiting the assessment of long-term effects, which usually develop after many years, and consequently limiting the impact of results. The funnel plot on SVR12 showed a slight asymmetry, suggesting possible publication bias. Finally, it should be considered that all the studies were funded by the pharmaceutical industry.
In conclusion, new DAAs are reportedly associated with better SVR12 and SVR24 rates, possibly leading to clinical benefits, but some included studies were at high risk of detection and attrition bias, and the overall quality of evidence was very low. Our systematic review, even if RCTs comparing DAAs plus PEG-IFNα/RBV with placebo or active comparators are included only, highlights these great results. We selected this approach as we believe comparative efficacy is the most suitable means to establish the respective efficacy and place in therapy of single DAAs and their combinations. The inclusion criteria of our review are a likely explanation for the lower SVR rates detected than those of nonrandomized studies, which generally amplify treatment effects.60 RCTs included in our systematic review did not report the risk of developing liver disease or HCC. These long-term effects, along with any possible survival benefit, need to be investigated further. As long-lasting RCTs addressing these issues are hardly feasible, epidemiological studies are more likely to answer these questions. However, future systematic reviews should preferably rely on comparative RCTs to inform clinical and regulatory decisions with regard to DAAs combination, regimens, and cost-effectiveness.
Conceived as a first step of a long, continuous review process, the present work provides essential information on single pieces of a complex puzzle and suggests the suitable methodological approach to contribute to the overall picture of therapeutic strategies against HCV.
Author contributions
V.P., R.B., R.D.A., E.C., V.B., and T.T. conceived and designed the systematic review. V.P., R.B., and R.D.A. performed the systematic review. V.P., R.B., and R.D.A. analyzed the data. V.P., R.B., E.C., V.B., and J.C. wrote the article. All authors performed the critical revision of the intellectual content of the manuscript.
Conflicts of interest
The authors have none to declare.
Acknowledgments
The authors wish to thank Professor Silvio Garattini for helpful suggestions and scientific evaluation of this review.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jceh.2018.07.004.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.http://www.who.int/mediacentre/factsheets/fs164/en/..
- 2.Gower E., Estes C., Blach S., Razavi-Shearer K., Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61(1 suppl):S45–S57. doi: 10.1016/j.jhep.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 3.GBD 2013 Mortality and Causes of Death Collaborators Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Webster D.P., Klenerman P., Dusheiko G.M. Hepatitis C. Lancet. 2015;385:1124–1135. doi: 10.1016/S0140-6736(14)62401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lauer G.M., Walker B.D. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- 6.Messina J.P., Humphreys I., Flaxman A. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;61:77–87. doi: 10.1002/hep.27259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petruzziello A., Marigliano S., Loquercio G., Cozzolino A., Cacciapuoti C. Global epidemiology of hepatitis C virus infection: an up-date of the distribution and circulation of hepatitis C virus genotypes. World J Gastroenterol. 2016;22:7824–7840. doi: 10.3748/wjg.v22.i34.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pearlman B.L., Traub N. Sustained virologic response to antiviral therapy for chronic hepatitis C virus infection: a cure and so much more. Clin Infect Dis. 2011;52:889–900. doi: 10.1093/cid/cir076. [DOI] [PubMed] [Google Scholar]
- 9.Chen J., Florian J., Carter W. Earlier sustained virologic response end points for regulatory approval and dose selection of hepatitis C therapies. Gastroenterology. 2013;144:1450–1455.e2. doi: 10.1053/j.gastro.2013.02.039. [DOI] [PubMed] [Google Scholar]
- 10.Pecoraro V., Cariani E., Villa E., Trenti T. Optimisation of triple therapy for patients with chronic hepatitis C: a systematic review. Eur J Clin Invest. 2016;46:737–748. doi: 10.1111/eci.12656. [DOI] [PubMed] [Google Scholar]
- 11.Zoulim F., Liang T.J., Gerbes A.L. Hepatitis C virus treatment in the real world: optimising treatment and access to therapies. Gut. 2015;64:1824–1833. doi: 10.1136/gutjnl-2015-310421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seifert L.L., Perumpail R.B., Ahmed A. Update on hepatitis C: direct-acting antivirals. World J Hepatol. 2015;7:2829–2833. doi: 10.4254/wjh.v7.i28.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feeney E.R., Chung R.T. Antiviral treatment of hepatitis C. BMJ. 2014;348:g3308. doi: 10.1136/bmj.g3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez Grande R., Jimenez Perez M., Gonzalez Arjona C., Mostazo Torres J. New approaches in the treatment of hepatitis C. World J Gastroenterol. 2016;22:1421–1432. doi: 10.3748/wjg.v22.i4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falade-Nwulia O., Suarez-Cuervo C., Nelson D.R., Fried M.W., Segal J.B., Sulkowski M.S. Oral direct-acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med. 2017;166:637–648. doi: 10.7326/M16-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DNDi . 2016. An Alternative Research and Development Strategy to Deliver Affordable Treatments for Hepatitis C Patients.https://www.dndi.org/wp-content/uploads/2016/04/AlternativeRDStrategyHepC.pdf Available on. [Google Scholar]
- 17.Iyengar S., Tay-Teo K., Vogler S. Prices, costs, and affordability of new Medicines for hepatitis C in 30 countries: an economic analysis. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liberati A., Altman D.G., Tetzlaff J. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.HCV Advocate. 2017; 19(1). http://hcvadvocate.org/news/NewsUpdates_pdf/Advocate_2017/advocate0117.pdf#AASLD.
- 20.Higgins J.P.T., Green S. The Cochrane Collaboration; 2011. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. [Google Scholar]
- 21.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 22.Mantel N., Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 23.Sterne J.A.C., Egger M. Funnel plots for detecting bias in meta analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 24.Egger M., Smith G.D., Scheider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guyatt G., Oxman A.D., Akl E.A. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 26.Pearlman B.L., Ehleben C., Perrys M. The combination of simeprevir and sofosbuvir is more effective than that of peginterferon, ribavirin, and sofosbuvir for patients with hepatitis C-related child's class a cirrhosis. Gastroenterology. 2015;148:762–770.e2. doi: 10.1053/j.gastro.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 27.Reddy K.R., Zeuzem S., Zoulim F. Simeprevir versus Telaprevir with peginterferon and ribavirin in previous null or partial responders with chronic hepatitis C virus genotype 1 infection (ATTAIN): a randomised, double-blind, non-inferiority phase 3 trial. Lancet Infect Dis. 2015;15:27–35. doi: 10.1016/S1473-3099(14)71002-3. [DOI] [PubMed] [Google Scholar]
- 28.Zeuzem S., Berg T., Gane E. Simeprevir increases rate of sustained virologic response among treatment-experienced patients with HCV genotype-1 infection: a phase IIb trial. Gastroenterology. 2014;146:430–441.e6. doi: 10.1053/j.gastro.2013.10.058. [DOI] [PubMed] [Google Scholar]
- 29.Hayashi N., Seto C., Kato M., Komada Y., Goto S. Once-daily simeprevir (TMC435) with peginterferon/ribavirin for treatment-naive hepatitis C genotype 1-infected patients in Japan: the DRAGON study. J Gastroenterol. 2014;49:138–147. doi: 10.1007/s00535-013-0875-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forns X., Lawitz E., Zeuzem S. Imeprevir with peginterferon and ribavirin leads to high rates of SVR in patients with HCV genotype 1 who relapsed after previous therapy: a phase 3 trial. Gastroenterology. 2014;146:1669–1679.e3. doi: 10.1053/j.gastro.2014.02.051. [DOI] [PubMed] [Google Scholar]
- 31.Hayashi N., Izumi N., Kumada H. Simeprevir with peginterferon/ribavirin for treatment-naïve hepatitis C genotype 1 patients in Japan: CONCERTO-1, a phase III trial. J Hepatol. 2014;61:219–227. doi: 10.1016/j.jhep.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Jacobson I.M., Dore G.J., Foster G.R. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2014;384:403–413. doi: 10.1016/S0140-6736(14)60494-3. [DOI] [PubMed] [Google Scholar]
- 33.Manns M., Marcellin P., Poordad F. Simeprevir with pegylated interferonalfa 2a or 2b plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-2): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2014;384:414–426. doi: 10.1016/S0140-6736(14)60538-9. [DOI] [PubMed] [Google Scholar]
- 34.Fried M.W., Buti M., Dore G.J. Once-daily simeprevir (TMC435) with pegylated interferon and ribavirin in treatment-naïve genotype 1 hepatitis C: the randomized PILLAR study. Hepatology. 2013;58:1918–1929. doi: 10.1002/hep.26641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacobson I.M., Gordon S.C., Kowdley K.V. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368:1867–1877. doi: 10.1056/NEJMoa1214854. [DOI] [PubMed] [Google Scholar]
- 36.Lawitz E., Lalezari J., Hassanein T. Sofosbuvir in combination with peginterferon alfa-2a and ribavirin for non-cirrhotic, treatment-naive patients with genotypers 1, 2, and 3 hepatitis C infection: a randomised, double-blind, phase 2 trial. Lancet Infect Dis. 2013;13:401–408. doi: 10.1016/S1473-3099(13)70033-1. [DOI] [PubMed] [Google Scholar]
- 37.Dore G.J., Lawitz E., Hézode C. Daclatasvir plus peginterferon and ribavirin is noninferior to peginterferon and ribavirin alone, and reduces the duration of treatment for HCV genotype 2 or 3 infection. Gastroenterology. 2015;148:355–366.e1. doi: 10.1053/j.gastro.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Hézode C., Hirschfield G.M., Ghesquiere W. Daclatasvir plus peginterferon alfa and ribavirin for treatment-naive chronic hepatitis C genotype 1 or 4 infection: a randomised study. Gut. 2015;64:948–956. doi: 10.1136/gutjnl-2014-307498. [DOI] [PubMed] [Google Scholar]
- 39.Kumada H., Suzuki F., Suzuki Y. Randomized comparison of daclatasvir + asunaprevir versus telaprevir + peginterferon/ribavirin in Japanese hepatitis C virus patients. J Gastroenterol Hepatol. 2016;31:14–22. doi: 10.1111/jgh.13073. [DOI] [PubMed] [Google Scholar]
- 40.Izumi N., Yokosuka O., Kawada N. Daclatasvir combined with peginterferon alfa-2a and ribavirin in Japanese patients infected with hepatitis C genotype 1. Antivir Ther. 2014;19:501–510. doi: 10.3851/IMP2731. [DOI] [PubMed] [Google Scholar]
- 41.Pol S., Ghalib R.H., Rustgi V.K. Daclatasvir for previously untreated chronic hepatitis C genotype-1 infection: a randomised, parallel-group, double-blind, placebo-controlled, dose-finding, phase 2a trial. Lancet Infect Dis. 2012;12:671–677. doi: 10.1016/S1473-3099(12)70138-X. [DOI] [PubMed] [Google Scholar]
- 42.Bronowicki J.P., Ratziu V., Gadano A. Randomized trial of asunaprevir plus peginterferon alfa and ribavirin for previously untreated genotype 1 or 4 chronic hepatitis C. J Hepatol. 2014;61:1220–1227. doi: 10.1016/j.jhep.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 43.Bronowicki J.P., Pol S., Thuluvath P.J. Randomized study of asunaprevir plus pegylated interferon-α and ribavirin for previously untreated genotype 1 chronic hepatitis C. Antivir Ther. 2013;18:885–893. doi: 10.3851/IMP2660. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez-Torres M., Yoshida E.M., Marcellin P. A phase 2 study of filibuvir in combination with pegylated IFN alfa and ribavirin for chronic HCV. Ann Hepatol. 2014;13:364–375. [PubMed] [Google Scholar]
- 45.Manns M.P., Vierling J.M., Bacon B.R. The combination of MK-5172, peginterferon, and ribavirin is effective in treatment-naive patients with hepatitis C virus genotype 1 infection without cirrhosis. Gastroenterology. 2014;147:366–376.e6. doi: 10.1053/j.gastro.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 46.Sulkowski M.S., Asselah T., Lalezari J. Faldaprevir combined with pegylated interferon alfa-2a and ribavirin in treatment-naïve patients with chronic genotype 1 HCV: SILEN-C1 trial. Hepatology. 2013;57:2143–2154. doi: 10.1002/hep.26276. [DOI] [PubMed] [Google Scholar]
- 47.Ferenci P., Asselah T., Foster G.R. STARTVerso1: a randomized trial of faldaprevir plus pegylated interferon/ribavirin for chronic HCV genotype-1 infection. J Hepatol. 2015;62:1246–1255. doi: 10.1016/j.jhep.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 48.Marcellin P., Cooper C., Balart L. Randomized controlled trial of danoprevir plus peginterferon alfa-2a and ribavirin in treatment-naïve patients with hepatitis C virus genotype 1 infection. Gastroenterology. 2013;145:790–800.e3. doi: 10.1053/j.gastro.2013.06.051. [DOI] [PubMed] [Google Scholar]
- 49.Everson G., Cooper C., Hezode C. DAUPHINE: a randomized phase II study of danoprevir/ritonavir plus peginterferon alpha-2a/ribavirin in HCV genotypes 1 or 4. Liver Int. 2015;35:108–119. doi: 10.1111/liv.12471. [DOI] [PubMed] [Google Scholar]
- 50.Hayashi N., Mobashery N., Izumi N. Vaniprevir plus peginterferon alfa-2a and ribavirin in treatment-experienced Japanese patients with hepatitis C virus genotype 1 infection: a randomized phase II study. J Gastroenterol. 2015;50:238–248. doi: 10.1007/s00535-014-0979-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lawitz E., Rodriguez-Torres M., Stoehr A. A phase 2B study of MK-7009 (vaniprevir) in patients with genotype 1 HCV infection who have failed previous pegylated interferon and ribavirin treatment. J Hepatol. 2013;59:11–17. doi: 10.1016/j.jhep.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 52.Rodriguez-Torres M., Stoehr A., Gane E.J. Combination of vaniprevir with peginterferon and ribavirin significantly increases the rate of SVR in treatment-experienced patients with chronic HCV genotype 1 infection and cirrhosis. Clin Gastroenterol Hepatol. 2014;12:1029–1037.e5. doi: 10.1016/j.cgh.2013.09.067. [DOI] [PubMed] [Google Scholar]
- 53.Dore G.J., Conway B., Luo Y. Efficacy and safety of ombitasvir/paritaprevir/r and dasabuvir compared to IFN-containing regimens in genotype 1 HCV patients: the MALACHITE-I/II trials. J Hepatol. 2016;64:19–28. doi: 10.1016/j.jhep.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 54.Suwanthawornkul T., Anothaisintawee T., Sobhonslidsuk A., Thakkinstian A., Teerawattananon Y. Efficacy of second generation direct-acting antiviral agents for treatment naive hepatitis C genotype 1: a systematic review and network meta-analysis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0145953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu G.Q., Zou Z.L., Zheng J.N. Systematic review and network meta-analysis of randomized controlled trials: comparative effectiveness and safety of direct-acting antiviral agents for treatment-naive hepatitis C genotype 1. Medicine (Baltimore) 2016;95:e3004. doi: 10.1097/MD.0000000000003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith Palmer J., Cerri K., Valentine W. Achieving sustained virologic response in hepatitis C: a systematic review of the clinical, economic and quality of life benefit. BMC Infect Dis. 2015;15:19. doi: 10.1186/s12879-015-0748-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arends J.E., Kracht P.A., Hoepelman A.I., ESGVH Performance of hepatitis C virus (HCV) direct-acting antivirals in clinical trials and daily practice. Clin Microbiol Infect. 2016;22:846–852. doi: 10.1016/j.cmi.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 58.Kohli A., Shaffer A., Sherman A., Kottilil S. Treatment of hepatitis C: a systematic review. J Am Med Assoc. 2014;312:631–640. doi: 10.1001/jama.2014.7085. [DOI] [PubMed] [Google Scholar]
- 59.Belousova V., Abd-Rabou A.A., Mousa S.A. Recent advances and future directions in the management of hepatitis C infections. Pharmacol Ther. 2015;145:92–102. doi: 10.1016/j.pharmthera.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 60.Ioannidis J.P., Haidich A.B., Pappa M. Comparison of evidence of treatment effects in randomized and nonrandomized studies. J Am Med Assoc. 2001;286:821–830. doi: 10.1001/jama.286.7.821. [DOI] [PubMed] [Google Scholar]
- 61.Li G., De Clercq E. Current therapy for chronic hepatitis C: the role of direct-acting antivirals. Antiviral Res. 2017;142:83–122. doi: 10.1016/j.antiviral.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soriano V., Labarga P., de Mendoza C. New hepatitis C therapies for special patient populations. Expet Opin Pharmacother. 2016;17:217–229. doi: 10.1517/14656566.2016.1112790. [DOI] [PubMed] [Google Scholar]
- 63.Jensen D., O'Leary J., Pockros P. Safety and efficacy of sofosbuvir-containing regimens for hepatitis C: real-world experience in a diverse, longitudinal observational cohort. Hepatology. 2014;60(suppl):219A. AASLD 2014, Boston, MA. November 7-11 2014 [abstract 45] 25. [Google Scholar]
- 64.Dieterich D., Bacon B., Flamm S. Evaluation of sofosbuvir and simeprevir-based regimens in the TRIO network: academic and community treatment of a real world, heterogeneous population. Hepatology. 2014;60(suppl):220A. AASLD 2014, Boston, MA. November 7-11 2014 [abstract 46] [Google Scholar]
- 65.Benítez-Gutiérrez L., Barreiro P., Labarga P. Prevention and management of treatment failure to new oral hepatitis C drugs. Expert Opin Pharmacother. 2016;17:1215–1223. doi: 10.1080/14656566.2016.1182156. [DOI] [PubMed] [Google Scholar]
- 66.Asselah T., Boyer N., Saadoun D., Martinot Peignoux M., Marcellin P. Direct-acting ntivirals for the treatment of hepatitis C virus infection: optimizing current IFN-free treatment and future perspectives. Liver Int. 2016;(suppl 1):47–57. doi: 10.1111/liv.13027. [DOI] [PubMed] [Google Scholar]
- 67.Ferreira V.L., Assis Jarek N.A., Tonin F.S., Borba H.H., Wiens A., Pontarolo R. Safety of interferon-free therapies for chronic hepatitis C: a network meta-analysis. J Clin Pharm Ther. 2016;41:478–485. doi: 10.1111/jcpt.12426. [DOI] [PubMed] [Google Scholar]
- 68.Hézode C., Bronowicki J.P. Ideal oral combinations to eradicate HCV: the role of ribavirin. J Hepatol. 2016;64:215–225. doi: 10.1016/j.jhep.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 69.Ponziani F.R., Mangiola F., Binda C. Future of liver disease in the era of direct acting antivirals for the treatment of hepatitis C. World J Hepatol. 2017;9:352–367. doi: 10.4254/wjh.v9.i7.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Banerjee D., Reddy K.R. Review article: safety and tolerability of direct-acting anti-viral agents in the new era of hepatitis C therapy. Aliment Pharmacol Ther. 2016;43:674–696. doi: 10.1111/apt.13514. [DOI] [PubMed] [Google Scholar]
- 71.Berg T., Sarrazin C., Herrmann E. Prediction of treatment outcome in patients with chronic hepatitis C: significance of baseline parameters and viral dynamics during therapy. Hepatology. 2003;37:600–609. doi: 10.1053/jhep.2003.50106. [DOI] [PubMed] [Google Scholar]
- 72.Cavalcante L.N., Lyra A.C. Predictive factors associated with hepatitis cantiviral therapy response. World J Hepatol. 2015;7:1617–1631. doi: 10.4254/wjh.v7.i12.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lynch S.M., Wu G.Y. Hepatitis C virus: a review of treatment guidelines, cost-effectiveness, and access to Therapy. J Clin Transl Hepatol. 2016;4:310–319. doi: 10.14218/JCTH.2016.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cuypers L., Ceccherini-Silberstein F., Van Laethem K., Li G., Vandamme A.M., Rockstroh J.K. Impact of HCV genotype on treatment regimens and drug resistance: asnapshot in time. Rev Med Virol. 2016;26:408–434. doi: 10.1002/rmv.1895. [DOI] [PubMed] [Google Scholar]
- 75.Weisberg I.S., Jacobson I.M. Primer on hepatitis C virus resistance to direct-acting antiviral treatment: a practical approach for the treating physician. Clin Liver Dis. 2017;21:659–672. doi: 10.1016/j.cld.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 76.Buti M., Esteban R. Management of direct antiviral agent failures. Clin Mol Hepatol. 2016;22(4):432–438. doi: 10.3350/cmh.2016.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bidell M.R., McLaughlin M., Faragon J., Morse C., Patel N. Desirable characteristics of hepatitis C treatment regimens: a review of what we have and what we need. Infect Dis Ther. 2016;5:299–312. doi: 10.1007/s40121-016-0118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chua J.V., Kottilil S. Are we nearing the end in the fight against hepatitis C? Expert Rev Gastroenterol Hepatol. 2017:1–2. doi: 10.1080/17474124.2017.1309287. [DOI] [PubMed] [Google Scholar]
- 79.Jakobsen J.C., Nielsen E.E., Feinberg J. Direct-acting antivirals for chronic hepatitis C. Cochrane Database Syst Rev. 2017;6 doi: 10.1002/14651858.CD012143.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.