Abstract
Background
Liver failure (LF) is a serious complication of transarterial chemoembolization (TACE) in patients with hepatocellular carcinoma (HCC). This could be influenced by the hemodynamic and functional status of the underlying cirrhotic liver. We evaluated baseline hepatic venous pressure gradient (HVPG) and indocyanine green (ICG) clearance as predictive factors for the development of LF in patients with liver cirrhosis undergoing TACE for HCC.
Methods
Forty-two patients with cirrhosis and HCC, referred for TACE, were clinically evaluated including the assessment of Child Turcotte Pugh score (CTP), Model for End-Stage Liver Disease (MELD), HVPG measurement, and ICG retention test. Predictors of development of hepatic failure after TACE were determined.
Results
In our study population, the mean age of the patients was 58 years, with mean CTP of 6.60 ± 1.149 and mean MELD score of 9.57 ± 2.923. The mean HVPG and ICG retention at 15 min was 13.57 ± 4.64 mmHg and 21.571 ± 12.434, respectively. Post-TACE Liver Failure (PTLF within 1 month after TACE) developed in 23.80% patients, whereas 76.19% patients did not have PTLF. The statistically significant preprocedure variables that might predict hepatic failure after TACE using univariate analysis were found to be high CTP, MELD score, ICG retention, HVPG, serum bilirubin, serum creatinine, alfa-fetoprotein levels, large tumor size, and low baseline serum albumin. On multivariate analysis, ICG was an independent factor predictive of hepatic failure after TACE.
Conclusion
Pretreatment evaluation of routine liver function is of fundamental importance before TACE. Baseline ICG retention test (ICG-R15) is a marker indicating the state of liver function in patients undergoing TACE and is an independent predictor for PTLF. Our study concludes that with a cutoff of 25, ICG-R15 has 92.9% accuracy, 90% sensitivity, and 87.5% specificity to predict hepatic failure after TACE.
Keywords: TACE, HVPG, ICG clearance test
Abbreviations: BCLC, Barcelona Clinic Liver Cancer; HCC, Hepatocellular Carcinoma; HVPG, Hepatic Venous Pressure Gradient; ICG, Indocyanine green; IRB, Institutional Review Board; LF, Liver Failure; MELD, Model for End-Stage Liver Disease; PHT, Portal Hypertension; PTLF, Post-TACE liver failure; RFA, Radiofrequency Ablation; ROC, Receiver operating characteristic curve; TACE, Transarterial chemoembolization
Hepatocellular carcinoma (HCC) is being increasingly diagnosed at earlier stages, with advances in imaging technologies; hence, many treatment options such as liver transplantation, resection, and percutaneous ablation are available. Liver transplantation is the best curative option for HCC; however, it has limitations such as availability of donors. Resection is possible in only up to 10–20% of cases because of associated comorbidities and advanced cirrhosis. HCC has many options for interventional management; some of them are complicated by development of portal hypertension (PHT).1
Percutaneous ablation remains the only curative option for this large group of patients with Child's A and B cirrhosis and single nodular–type HCC <5 cm or <3 HCC lesions, each smaller than 3 cm, not amenable to resection or transplantation, and RFA is one of the most popular local ablative therapies for inoperable HCC. Clinical implications of percutaneous radiofrequency ablation (RFA) in the treatment of early-stage HCC have expanded, and currently, RFA is recognized as a curative modality for early-stage HCC whose outcomes of which are comparable with those of surgery, as evidenced by many studies.2
TACE is used widely in the treatment of unresectable HCC and for those who are not suitable for management by RFA. Lipiodol and gelatin sponge particles are commonly used embolic materials for TACE.3 An important event in patients with liver cirrhosis is the presence of portal hypertension (PHT), which is defined by an increase in the portal pressure above 5 mmHg, as measured by the hepatic vein pressure gradient (HVPG). PHT is considered clinically significant when the HVPG is more than 10 mmHg. This pressure is likely to be associated with the development of complications, such as ascites, varices, and hepatic encephalopathy. PHT may acutely increase the following procedures such as TACE for HCC, which adds to complications after these methods of intervention.1, 4 TACE can influence portal pressure by increasing portal blood flow compensatory to reduction of arterial blood flow after embolization. In fact, increased arterial inflow from the left gastric artery into esophageal varices has been reported.4 TACE could also inflict severe endothelial dysfunction in hepatic vasculature due to the post-TACE ischemia and resultant increase in the vascular tone.4 The increase of portal blood flow after TACE could also add to the rapid increase in variceal pressure.4
The outcome of cirrhotic patients with HCC could be influenced by the severity of PTH and HVPG. The correlation between portal hemodynamics and the risk of variceal hemorrhage in PTH is well described.5 In patients with cirrhosis and HCC, a rapid rise in portal pressure could lead to rapid decompensation in the form of ascites, variceal bleed, or liver failure (LF). In fact, patients with HVPG greater than 10 mmHg are not considered suitable for hepatic resection because of increased risk of postoperative clinical decompensation. Whether the same context is applicable for patients undergoing TACE is not well studied.
Dynamic tests to evaluate liver function measure the clearance of tracer substances and provide considerably more information than static tests. Indocyanine green (ICG) is a synthetic dye that is eliminated by the liver without extrahepatic metabolism and excretion, and its blood clearance has been used to determine the operative risk before hepatectomy6 and to evaluate donor liver function in transplantation.7, 8 LF after resection has a poor prognosis and is a major complication. Insufficient reserve in the residual parenchyma leads to a reduced liver function, lack of ability to regenerate, and occurrence of LF.6 Similar to the role of ICG in surgical patients, pre-TACE assessment of liver function by ICG test may be most beneficial in the routine stratification of risk, thus enabling both patient consent to be obtained and TACE procedure to be performed, with prior understanding of risk of post-TACE liver failure (PTLF). ICG clearance test has also been found to be useful for assessment of prognosis of patients with cirrhosis.9 As 80% of patients with HCC have liver cirrhosis, there is an important role of ICG in prognostication of patients with HCC undergoing TACE procedure.
We investigated the influence, if any, of the baseline HVPG and ICG clearance on the development of PTLF.
Materials and methods
Study population
Between January 2016 and April 2017, 42 patients with cirrhosis and HCC, who were referred for TACE, were included in this study. Patients with liver cirrhosis and multinodular HCC or those with liver cirrhosis and solitary HCC greater than 3 cm were included in this study. The exclusion criteria were as follows: patients younger than 18 years and those older than 75 years, poor performance status, Child–Pugh score of more than 9, refractory ascites/hepatic encephalopathy/main portal vein thrombosis as well as branch portal vein thrombosis (these patients have already compromised hepatic function), cardiomyopathy (chemotherapeutic drugs used in TACE may further cause cardiomyopathy and precipitate cardiac failure), and extrahepatic disease (Barcelona Clinic Liver Cancer [BCLC] algorithm), pregnant females, and those with a history of allergy to dyes or iodides (ICG injection).
The diagnosis of HCC was either based on pathological confirmation or on the basis of typical imaging findings, i.e. if the lesion demonstrates characteristic features of hepatocellular carcinoma—that is, arterial phase hyperenhancement and portal venous or delayed phase washout.10 Treatment decision in these patients was made according to the BCLC staging algorithm.
Every patient was evaluated with assessment of CTP and MELD score at baseline and in addition underwent upper gastrointestinal endoscopy, hepatic vein pressure gradient (HVPG) measurement, and ICG retention test. Although the outcome of cirrhotic patients with HCC could be influenced by the severity of PTH and liver function as indicated by HVPG or ICG retention, there was no exclusion based on HVPG measurement and ICG retention test in our study, i.e. patients with any degree of portal hypertension based on HVPG measurements and patients with poor liver function as indicated by ICG clearance test were included, provided Child–Pugh score was less than 9.
ICG clearance test
A solution of commercially available ICG (Aurogreen from Aurolab, Madurai, India) was prepared by diluting the dye in distilled water to a final concentration of 5 mg/ml. A bolus injection of 0.5 mg/kg of ICG solution was injected to the patients through a peripherally placed venous cannula. After injection, venous blood samples were collected at 15 min. The concentration was measured photometrically at 805 nm using a spectrophotometer (Beckmann Coulter DU 640). The dye clearance was measured as a product of volume of distribution and elimination rate. ICG concentration in the plasma was calculated at 15 min after injection to calculate the retention of ICG at 15 min, i.e. ICG-R15.
The study was approved by the scientific review board and institutional review board (IRB). Patient consent was not required for this retrospective study.
Data collection
Baseline demographic information, laboratory measurements, radiological tumor assessment, and clinical information were recorded. The tumor assessments included the number and tumor size. According to the Common Terminology Criteria for Adverse Events V.3.0, it is recommended to assess TACE-related adverse events 4 weeks after the procedure. In this study, we recorded occurrence of PTLF in patients within 1 month after TACE. PTLF was defined as elevation of serum bilirubin three times the baseline or elevation of serum glutamic oxaloacetic transaminase (SGOT)/serum glutamic pyruvic transaminase (SGPT) five times the baseline values occurring within 1 month of TACE.11
Statistical analysis
The statistical results are presented as the mean ± standard deviation or percentages. Continuous variables were compared parametrically using Student's t-test or nonparametrically using the Mann–Whitney U-test.
Univariate and multivariate logistic regression analyses were performed to determine the variables associated with the development of acute hepatic failure after TACE. A two-sided P value < 0.05 was considered statistically significant. Statistical analyses were conducted using the SPSS software.
Result
A total of 42 patients with liver cirrhosis and HCC were included in this study who underwent TACE according to BCLC staging (Table 1). The mean age of the patients was 58.71 ± 11.226 years, with male (83.3%) dominance. The most common etiology for liver cirrhosis was chronic viral hepatitis (40.5%), followed by ethanol-related chronic liver disease (35.7%) and nonalcoholic steatohepatitis (14.3%). The mean Child–Pugh score was 6.60 ± 1.149, and the mean MELD score was 9.57 ± 2.923 in our study population. The mean portal pressure as determined by HVPG was 13.57 ± 4.64 mmHg. Endoscopy revealed the absence of varices in 16 patients, presence of grade I varices in 10 patients, and presence of grade II varices in 16 patients. The mean ICG retention at 15 min in our study patients was 21.571 ± 12.434. ICG-R15 is a ratio between ICG concentration 15 min after injection and initial concentration and hence does not have any units.
Table 1.
Baseline Characteristics of Study Subjects (n = 42).
| Variable | Baseline (mean ± SD) |
|---|---|
| Age (years) | 58.71 ± 11.226 |
| Sex | |
| M | 35 (83.3%) |
| F | 7 (16.7%) |
| Etiology of cirrhosis (n, %) | |
| Chronic viral hepatitis | 17 (40.5%) |
| Ethanol | 15 (35.7%) |
| NASH | 06 (14.3%) |
| Cryptogenic | 04 (9.5%) |
| Mean Child score | 6.60 ± 1.149 |
| Mean MELD Score | 9.57 ± 2.923 |
| Hemoglobin (g/dl) | 11.633 ± 2.173 |
| Serum bilirubin (mg/dl) | 1.783 ± 1.128 |
| SGOT (IU/L) | 70.05 ± 49.568 |
| SGPT (IU/L) | 44.40 ± 22.157 |
| Serum albumin (g/dl) | 3.231 ± 0.508 |
| INR | 1.095 ± 0.215 |
| AFP (ng/ml) | 53.277 ± 45.803 |
| Serum creatinine (mg/dl) | 0.765 ± 0.147 |
| Mean maximum tumor size (cm) | 5.893 ± 4.410 |
| Number Of HCC | 1.12 ± 0.328 |
| BCLC stage (n, %) | |
| A | 1 (2.39%) |
| B | 34 (80.95%) |
| C | 7 (16.67%) |
| HVPG (mmHg) | 13.57 ± 4.644 |
| ICG (R15) | 21.571 ± 12.434 |
| Esophageal varices (n, %) | |
| Absent | 16 (38.09%) |
| Grade I | 10 (23.80%) |
| Grade II | 16 (38.09%) |
AFP, Alpha-fetoprotein; MELD, Model for End-Stage Liver Disease; HCC, hepatocellular carcinoma; ICG, indocyanine green; BCLC, Barcelona Clinic Liver Cancer.
PTLF (within 1 month after TACE) developed in 10 (23.80%) patients (group 1). Thirty-two (76.19%) patients did not have PTLF (group 2).
Univariate analysis
Univariate analysis was performed to compare the two groups (patients with and without hepatic failure) for the different preprocedure variables that might predict acute hepatic failure after TACE. There were 10 patients in the PTLF group (group 1) and 32, in the group without hepatic failure (group 2). Mean Child score was higher in group 1 (7.7 ± 1.252) than in group 2 (6.25 ± 0.880), which was found to be statistically significant, P = 0.004. We found that the mean MELD score was significantly higher in group 1 (13.40 ± 2.757) than in group 2 (8.38 ± 1.699), P = 0.006. In group 1, mean serum albumin levels were significantly lower (2.640 ± 0.395) than in group 2 (3.416 ± 0.386), P = 0.001. Similarly, as shown in Table 2, mean tumor size, serum bilirubin, serum creatinine, and alfa-fetoprotein levels in group 1 were found to be significantly higher than in group 2 (table 2). Mean ICG retention in the group of patients developing acute hepatic failure was found to be higher (38.950 ± 6.698) than in the group of patients not developing acute hepatic failure after TACE (18.48 ± 8.37), and this was statistically significant, P = 0.002. Portal pressure as determined by HVPG in group 1 was significantly higher than that in group 2 (16.50 ± 4.275 vs 12.66 ± 4.426, P = 0.035). There was no significant difference (P > 0.05) between group 1 and group 2 with regard to age, mean hemoglobin level, serum bilirubin, SGOT, SGPT, international normalized ratio (INR), and the number of tumors (Table 2).
Table 2.
Univariate Analysis of Risk Factors for PTLF After TACE.
| Variable | Patients with hepatic failure (n = 10, mean ± SD | Patients without hepatic failure (n = 32, mean ± SD | P value | OR | 95% CI |
|---|---|---|---|---|---|
| Age (years) | 63.20 ± 11.429 | 57.31 ± 10.965 | 0.155 | 1.057 | 0.979–1.140 |
| Mean Child score | 7.70 ± 1.252 | 6.25 ± 0.880 | 0.004 | 4.350 | 1.595–11.862 |
| Mean MELD score | 13.40 ± 2.757 | 8.38 ± 1.699 | 0.006 | 3.017 | 1.362–6.684 |
| Hb (g/dl) | 11.960 ± 2.453 | 11.960 ± 2.453 | 0.583 | 1.100 | 0.782–1.547 |
| Serum bilirubin (mg/dl) | 3.240 ± 1.070 | 1.328 ± 0.672 | 0.001 | 8.340 | 2.325–29.917 |
| SGOT (IU/L) | 68.90 ± 22.268 | 70.41 ± 55.723 | 0.932 | 0.999 | 0.985–1.014 |
| SGPT (IU/L) | 52.40 ± 23.448 | 41.91 ± 21.508 | 0.196 | 1.021 | 0.989–1.055 |
| Serum albumin (g/dl) | 2.640 ± 0.395 | 3.416 ± 0.386 | 0.005 | 0.002 | 0.000–0.150 |
| INR | 1.014 ± 0.082 | 1.120 ± 0.238 | 0.075 | 0.000 | 0.000–2.842 |
| AFP (ng/ml) | 110.023 ± 39.033 | 35.544 ± 31.128 | 0.003 | 1.062 | 1.021–1.104 |
| Serum creatinine (mg/dl) | 0.853 ± 0.098 | 0.738 ± 0.150 | 0.040 | 1.314 | −0.218–0.0119 |
| Mean tumor size (cm) | 11.790 ± 4.420 | 4.050 ± 2.309 | 0.001 | 1.754 | 1.248–2.464 |
| Number Of HCC lesions | 1.20 ± 0.422 | 1.09 ± 0.296 | 0.376 | 2.417 | 0.343–17.036 |
| HVPG (mm Hg) | 16.50 ± 4.275 | 12.66 ± 4.426 | 0.035 | 1.251 | 1.016–1.541 |
| ICG (R15) | 38.950 ± 6.698 | 18.48 ± 8.37 | 0.002 | 1.448 | 1.117–1.006 |
CI, confidence interval; MELD, Model for End-Stage Liver Disease; HCC, hepatocellular carcinoma; HVPG, hepatic venous pressure gradient; ICG, indocyanine green; OR, odds ratio; PTLF, post-TACE liver failure.
Multivariate analysis
From the univariate analysis, Child score, MELD score, serum bilirubin, serum albumin, alfa-fetoprotein, serum creatinine, mean tumor size, HVPG, and ICG retention were statistically significant factors predictive of acute hepatic failure. Of these, MELD score, serum albumin, HVPG, and ICG were analyzed by stepwise binary logistic regression multivariate analysis. Other variables were not included in multivariate analysis as they reduced the statistical significance of other significant variables due to indirect interdependence. On multivariate analysis, ICG (P value = 0.002 at 95% confidence interval [CI]) was the only statistically significant independent factor predictive of PTLF with an odds ratio (OR) of 1.339 (Table 3).
Table 3.
Stepwise Logistic Regression Multivariate Analysis of the Statistically Significant Variable of the Univariate Analysis.
| Variable | P value | OR | 95% CI |
|---|---|---|---|
| ICG | 0.002 | 1.339 | 1.117–1.606 |
CI, confidence interval; ICG, indocyanine green; OR, odds ratio.
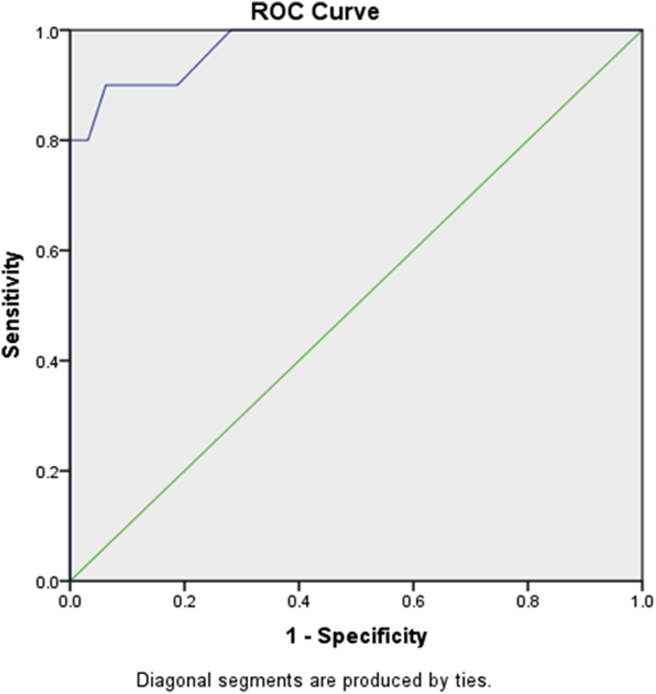
Receiver operating characteristic (ROC) curve for ICG-R15 for the prediction of post-TACE hepatic failure: ROC was performed for ICG-R15 normalized to the group of patients with PTLF (group 1, n = 10) and the group of patients without hepatic failure (group 2, n = 32). The area under the curve (AUC) for ICG-R15 is 0.972, with a P value 0.002 (Figure 1).
Figure 1.
Receiver operating characteristic (ROC) curve for ICG-R15 for the prediction of post-TACE hepatic failure. Area under the curve (AUC) for ICG-R15 is 0.972 with a P value 0.002.
Discussion
A total of 42 patients with liver cirrhosis and inoperable nonmetastatic hepatocellular carcinoma were included in this study who received TACE according to their BCLC stage. We tried to predict risk factors for postprocedure acute hepatic failure based on routine laboratory tests, ICG retention at 15 min (ICG-R15), and portal pressure (as determined by HVPG) available at baseline. In this study, post-TACE acute hepatic failure was defined as an elevation of serum bilirubin three times the baseline or elevation of SGOT/SGPT five times the baseline values observed within one month after TACE.
Significantly higher proportion of patients developing acute hepatic failure after TACE in our study population had lower mean serum albumin level (2.640 g/dl ± 0.395 in group 1 vs 3.416 g/dl ± 0.385 in group 2, P = 0.005), higher Child–Pugh score (7.70 ± 1.252 in group 1 vs 6.25 ± 0.880 in group 2, P = 0.004), and higher MELD score (13.40 ± 2.757 in group 1 vs 8.38 ± 1.699 in group 2, P = 0.006). This is consistent with most of the previously published literature including two recent prospective studies showing the role of lower serum albumin in predicting acute decompensation/failure after TACE (3.416 g/dl ± 0.386 vs 2.640 g/dl ± 0.395, P = 0.033 at 95% CI, OR = 0.31, Mohamed A S Kohla et al and 3.6 g/dl Vs 3.35 g/dl, P = 0.028 and OR = 0.547, Yang Won Min et al).12, 13 The MELD score is a composite index that is dependent on serum creatinine, serum bilirubin, and INR. This is reflected in our study as patients in group 1 had higher mean serum bilirubin and serum creatinine.
In a study, larger tumors (mean tumor size) and multiple tumors (higher tumor burden) were associated with poorer outcome after TACE14 (P = 0.004 at 95% CI and OR = 4.45). Another study found no association of postprocedure hepatic decompensation with tumor size12 (P < 0.687); however, in our study, we found larger tumor size in the group of patients developing post-TACE acute hepatic failure (11.790 cm ± 4.420 vs 4.050 cm ± 2.309, P = 0.001). Although the dose of embolizing agents was not measured in our study, it is likely that higher dosages were used in larger tumors, and therefore, greater ischemia occurred resulting in higher incidence of hepatic failure in these patients.
ICG-R15 is a quantitative liver function test reflecting both liver function impairment and hemodynamic alterations. Higher ICG retention (ICG-R15) has been associated with increase in incidence of clinical decompensation in previously compensated cirrhotics13 (Child class A). Similar findings have also been found in a previous study that showed pre-TACE ICG-R15 as a risk factor for deterioration of liver function after TACE + RFA15 (ICG-R15, >30% Vs <30%, OR - = 0.92, 95% CI = 0.12–7.59). This correlated with the finding in our study showing higher rate of acute hepatic failure in patients with higher ICG retention at 15 min (38.950 ± 6.698 in group 1 vs 18.48 ± 8.37 in group 2, P = 0.002). On subsequent multivariate analysis, ICG retention was found to be an independent risk factor for predicting post-TACE acute hepatic failure. Its accuracy to predict acute hepatic failure after TACE was found to be 92.9%, while a cutoff of 25 shows a sensitivity of 90% and a specificity of 87.5%.
According to Shalimar et al, ICG-PDR (plasma disappearance rate) performs similar to MELD, MELD-Na, and CTP score for predicting development of PTLF. In their study, ICG-PDR values were lower in those patients who developed PTLF (7.4%/min vs 10.6%/min; P = 0.008).16
We found that baseline portal pressure was higher in the group of patients developing post-TACE acute hepatic failure than in those without hepatic failure (16.50 ± 4.275 mm Hg vs 12.66 ± 4.426 mm Hg). It is likely that patients with higher portal pressure have more advanced liver cirrhosis and hence are at higher risk of deterioration of hepatic function after TACE.
In most of the previous studies, age was not found to be a risk factor for post-TACE hepatic failure (P > 0.05)9, 12. Higher rate of acute hepatic failure after treatment of HCC with TACE can be anticipated and is likely due to age-related decrease in liver volume and liver blood flow; hepatocyte changes including increased oxidative stress, increased inflammatory response, accelerated cellular senescence, and progressive organ dysfunction significantly affect cellular responses to injury.17 However, we did not observe any significantly higher rate of hepatic failure related to age.
Our study has few limitations such as small cohort, retrospective nature of study, and lack of pathologic confirmation for HCC diagnosis.
In summary, pretreatment evaluation of routine liver function is of fundamental importance before TACE. Patients with higher Child–Pugh score, MELD sore, serum bilirubin, serum creatinine, alfa-fetoprotein, larger tumor, portal pressure, and lower serum albumin at baseline are likely to have higher chance of acute hepatic failure after TACE. Baseline ICG retention test (ICG-R15) is a marker indicating the state of liver function in patients undergoing TACE and is an independent predictor for acute hepatic failure with high accuracy, sensitivity, and specificity.
Conflicts of interest
The authors have none to declare.
Footnotes
Study performed at: Department of Interventional Radiology, Institute of Liver & Biliary Sciences, D-1, Vasant Kunj, New Delhi, India. Ph: +91-11-46300000.
References
- 1.El Sherbiny W., AbdelRahman A., Diasty M. Changes in Doppler parameters of portal pressure after interventional management of hepatocellular carcinoma. AbdomRadiol (NY) 2016 Aug;41(8):15328. doi: 10.1007/s00261-016-0704-0. [DOI] [PubMed] [Google Scholar]
- 2.Cho Y.K., Kim J.K., Kim W.T. Hepatic resection versus radiofrequency ablation for very early stage hepatocellular carcinoma: a Markov model analysis. Hepatology. 2010;51:1284–1290. doi: 10.1002/hep.23466. [DOI] [PubMed] [Google Scholar]
- 3.Murotani K., Nakai M., Sato M. Change in portal vein hemodynamics after chemoembolization for hepatocellular carcinoma: evaluation through multilevel dynamic multidetector computed tomography during arterial portography. J Comput Assist Tomogr. 2015;39:396–400. doi: 10.1097/RCT.0000000000000229. [DOI] [PubMed] [Google Scholar]
- 4.Elia C., Venon W.D., Stradella D. Transcatheter arterial chemoembolization for hepatocellular carcinoma in cirrhosis: influence on portal hypertension. Eur J Gastroenterol Hepatol. 2011;23:573–577. doi: 10.1097/MEG.0b013e32834701f5. [DOI] [PubMed] [Google Scholar]
- 5.Spahr L., Becker C., Pugin J. Acute portal hemodynamics and cytokine changes following selective transarterial chemoembolization in patients with cirrhosis and hepatocellular carcinoma. Med SciMonit. 2003;9(9):CR383–CR388. [PubMed] [Google Scholar]
- 6.Mullin E.J., Metcalfe M.S., Maddern G.J. How much liver resection is too much? Am J Surg. 2005;190:87–97. doi: 10.1016/j.amjsurg.2005.01.043. [DOI] [PubMed] [Google Scholar]
- 7.Kamei H., Fujimoto Y., Nagai S., Suda R., Yamamoto H., Kiuchi T. Impact of non-congestive graft size in living donor liver transplantation: new indicator for additional vein reconstruction in right liver graft. Liver Transplant. 2007;13:1295–1301. doi: 10.1002/lt.21231. [DOI] [PubMed] [Google Scholar]
- 8.Hsieh C.B., Chen C.J., Chen T.W. Accuracy of indocyanine green pulse spectrophotometry clearance test for liver function prediction in transplanted patients. World J Gastroenterol. 2004;10:2394–2396. doi: 10.3748/wjg.v10.i16.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lisotti A., Azzaroli F., Cucchetti A. Relationship between indocyanine green retention test, decompensation and survival in patients with Child-Pugh A cirrhosis and portal hypertension. Liver Int. 2016 Sep;36(9):1313–1321. doi: 10.1111/liv.13070. [DOI] [PubMed] [Google Scholar]
- 10.McEvoy Sinead H., McCarthy Colin J., Lavelle Lisa P. Hepatocellular carcinoma: illustrated guide to systematic radiologic diagnosis and staging according to guidelines of the american association for the study of liver diseases. Radiographics. 2013;33(6):1653–1668. doi: 10.1148/rg.336125104. [DOI] [PubMed] [Google Scholar]
- 11.Trotti A., Colevas A.D., Setser A. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13(3):176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 12.Min Y.W., Kim J., Kim S. Risk factors and a predictive model for acute hepatic failure after transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. Liver Int. 2013 Feb;33(2):197–202. doi: 10.1111/liv.12023. [DOI] [PubMed] [Google Scholar]
- 13.Kohla M.A.S., Abu Zeid M.I., Al-Warraky M. Predictors of hepatic decompensation after TACE for hepatocellular carcinoma. BMJ Open Gastroenterol. 2015 Jun 23;2(1) doi: 10.1136/bmjgast-2015-000032. e000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kima Hee, Kisseleva Tatiana, David A. Brenner. Aging and liver disease. Curr Opin Gastroenterol. 2015 May;31(3):184–191. doi: 10.1097/MOG.0000000000000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J.X., Wu H., Huang J.W., Zeng Y. The influence on liver function after transcatheter arterial chemoembolization combined with percutaneous radiofrequency ablation in patients with hepatocellular carcinoma. J Formos Med Assoc. 2012 Sep;111(9):510–515. doi: 10.1016/j.jfma.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 16.Shalimar Jain S., Gamanagatti S.R., Kedia S. Role of indocyanine green in predicting post- transarterial chemoembolization liver failure in hepatocellular carcinoma. J Clin Exp Hepatol. 2018 Mar;8(1):28–34. doi: 10.1016/j.jceh.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tasneem A.A., Abbas Z., Luck N.H., Hassan S.M., Faiq S.M. Adverse events following transarterial chemoembolization for hepatocellular carcinoma and factors predicting such events. J Pakistan Med Assoc. 2013 Feb;63(2):239–244. [PubMed] [Google Scholar]