Abstract
Personalized medicine aims to individualize care based on a person’s unique genetic, environmental, and clinical profile. Dentists and physicians have long recognized variations between and among patients, and have customized care based on each individual’s health history, environment, and behavior. However, the sequencing of the human genome in 2003 and breakthroughs in regenerative medicine, imaging, and computer science redefined “personalized medicine” as clinical care that takes advantage of new molecular tools to facilitate highly precise health care based on an individual’s unique genomic and molecular characteristics. Major investments in science bring a new urgency toward realizing the promise of personalized medicine; yet, many challenges stand in the way. In this article, we present an overview of the opportunities and challenges that influence the oral health community’s full participation in personalized medicine. We highlight selected research advances that are solidifying the foundation of personalized oral health care, elaborate on their impact on dentistry, and explore obstacles toward their adoption into practice. It is our view that now is the time for oral health professionals, educators, students, researchers, and patients to engage fully in preparations for the arrival of personalized medicine as a means to provide quality, customized, and effective oral health care for all.
Keywords: targeted therapies, genomics, diagnostics, pharmacogenomics, biomarkers, oral diseases
Introduction: A New Era for Biomedicine
In 2000, and referring to the recent completion of the working draft of the Human Genome Project, President Bill Clinton pondered whether one day “our children’s children will know the term ‘cancer’ only as a constellation of stars”. He and others forecast the remarkable biological lessons to be learned from genomic approaches to targeting and treating human diseases (US Department of Energy, 2000). More than 13 years later, although we have not eradicated cancer, it can be said that we have re-defined malignancy as a constellation of different diseases with their own genomic signatures. Many predictions about the profound impact of the Human Genome Project have indeed come true. Among them are an increased use of genetic medicine and genetic tests for diagnosis and treatment, routine use of pre-implantation genetics, and a United States ban on genetic discrimination that opens doors for the safe and equitable application of genomic knowledge to human health (Collins, 2010).
Personalized medicine aims to individualize care based on a person’s unique genetic, environmental, and clinical profile. Dentists and physicians have long recognized variations between and among patients and have provided customized care based on the many biological and behavioral components that shape individual health (Fackler and McGuire, 2009). In this sense, for many years, personalized medicine has been the embodiment of good clinical care. However, the availability of new molecular tools provides researchers—and, increasingly, clinicians—with a means to view health with a higher level of resolution provided by personal genomic and other molecularly based data.
At this juncture, perhaps the area of greatest growth is in the field of targeted oncology diagnostics and therapies based on the molecular classification of underlying pathology. US Food and Drug Administration (FDA)-approved drugs in this arena include signal transduction inhibitors such as imatinib, trastuzumab, gefitinib, and erlotinib, which display greater selectivity for cancer cells, thus reducing adverse side-effects and improving quality of life (Hoelder et al., 2012). In general, molecular diagnoses and targeted therapies are improving survival among patients with melanoma, leukemia, and metastatic lung, breast, and brain cancers (Personalized Medicine Coalition, 2010).
Significant investments in both the public and private sectors have fueled this progress. During the past decade, the number of personalized medicines being used in the United States has steadily increased from only a few in 2001 to several dozen in 2011 (Cohen, 2012). According to a 2010 report from the Tufts Center for the Study of Drug Development, nearly half of the companies surveyed have personalized medicines under development as potential therapies for a range of indications including not only cancer, but also cardiovascular disease and immunological conditions (Milne, 2010). Toward more broad-based clinical adoption, approximately 10% of FDA-approved product labels either directly recommend molecular or genetic testing or note the potential influence of genetic variations on optimal treatment (Frueh et al., 2008; US FDA, 2012).
Expanding the Foundation for Personalized Oral Health Care
The United States Federal Government continues to invest in infrastructure to clarify relationships between genomics and targeted therapies. For example, the National Institutes of Health (NIH) supports the Encyclopedia of DNA Elements (ENCODE) project, which aims to identify all functional elements in the human genome (ENCODE, 2007). To date, ENCODE has mapped regions of transcription, transcription-factor association, chromatin structure, and histone modification. These studies have enabled scientists to assign biochemical functions to 80% of the human genome and have recently highlighted a previously unappreciated but significant role for non-coding RNA (ENCODE, 2012).
The NIH-funded Epigenomics Program focuses on mapping and understanding the potential function of DNA modifications and protein complexes associated with these processes. Epigenomic maps have been overlaid with genetic information associated with various diseases, and the maps are freely available to the scientific community through the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/epige nomics). Finally, the NIH-supported Therapeutics for Rare and Neglected Diseases (TRND) program develops new candidate drugs for rare and neglected diseases, aiming to bridge the gap between discovery science and the testing of new drugs in humans. The TRND program also plays a role in the sub-classification of selected common diseases, toward uncovering new therapies.
The most undeveloped aspect of personalized medicine is the integration of genomic information with clinical and physical examination data. To that end, the NIH’s Electronic Medical Records and Genomics (eMERGE) network seeks to develop and apply approaches to test whether electronic medical record systems can serve as resources for complex genomic analyses of disease and therapeutic outcomes (McCarty et al., 2011). Currently, there are no oral, dental, or craniofacial primary phenotypes under study within the eMERGE network, highlighting a significant future opportunity.
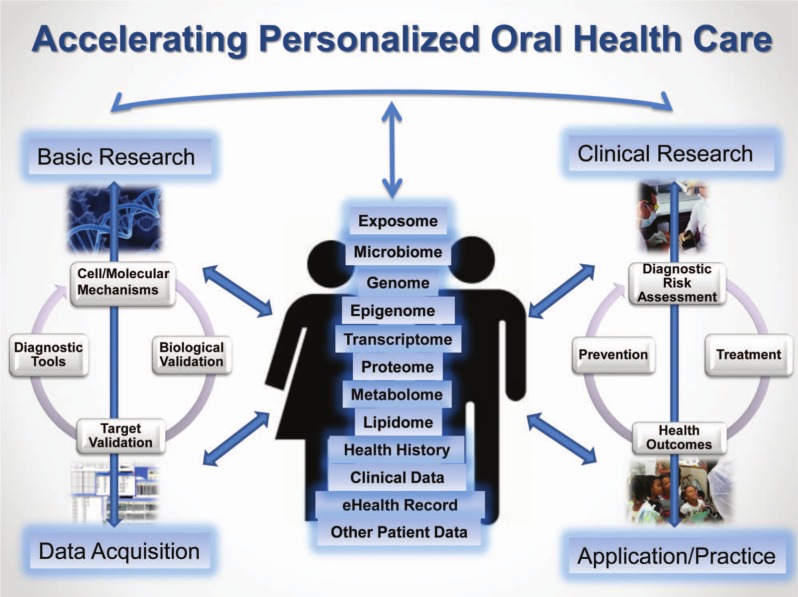
Most oral, dental, and craniofacial diseases and disorders, such as dental caries, periodontal diseases, oral and pharyngeal cancers, chronic orofacial pain, and cleft lip/cleft palate, arise from a complex interaction of genetic, biological, behavioral, and environmental factors. As our understanding of disease pathways, genomic interactions, and novel biomarkers of oral conditions continues to increase, so does the possibility of using high-throughput ‘omics’ approaches to assess risk, prevent disease, and guide treatment (Fig.). In certain areas, we are seeing how research is uncovering molecular targets and guiding new customized therapies. From selected examples, presented below, we can get a glimpse of the extraordinary promise of personalized oral health care.
Figure.
Accelerating personalized oral health care.
Note: Adapted from Research Council (2011). Toward precision medicine: Building a knowledge network for biomedical research and a new taxonomy of disease. Washington DC: The National Academies Press.
Head and Neck Squamous Cell Carcinoma
Head and neck squamous cell carcinoma (HNSCC) is a disease with complex gene alterations that either shut down or amplify regulatory signals within a cell, accelerating cell growth and giving rise to tumors (Elferink and Resto, 2011; Rothenberg and Ellisen, 2012). Current treatment options for head and neck cancer include surgery and cytotoxic therapies, often resulting in drastically reduced quality of life. Better understanding of the biological heterogeneity of head and neck cancer will help customize treatment and optimize outcomes for this malignancy, whose 60% five-year survival rate is the 4th lowest among the 10 leading types of cancer in the United States (Siegel et al., 2012).
Cancer management has long focused on customizing care based on tumor stage, subtype, and histology. Now, knowledge emerging from genomics allows for a more refined tumor classification based on signaling pathways that can be targeted more precisely. Recent findings offer the possibility of reclassifying HNSCC tumors based on their unique molecular features. Molecular techniques are helping to predict which lesions are likely to undergo malignant conversion through a better understanding of a wide range of proteins and transcription factors that participate in the epithelial-to-mesenchymal transition in squamous cell carcinoma (Scanlon et al., 2013). Earlier detection of HNSCC may soon be possible through the identification of specific salivary proteins or DNA abnormalities in pre-cancerous buccal mucosal cells. Nano-sized biochip sensors are currently being tested for their utility to analyze oral cancer biomarkers with high sensitivity and specificity (Weigum et al., 2010).
The NIDCR-funded, highly collaborative Oral Cancer Genome Project (OCGP) is guiding the shift toward individualized oral cancer treatment by defining genetic changes that drive the development of HNSCC at high-resolution levels that were not possible a few years ago. In its discovery phase, using next-generation sequencing, OCGP researchers identified dozens of distinct molecular pathways, each driven by a unique acquired pattern of cancer-causing gene alterations associated with the development of HNSCC. Using high-throughput exome sequencing, they noted alterations in the NOTCH1 gene that deactivate its encoded protein, suggesting that NOTCH1 may function as a tumor suppressor gene rather than as an oncogene in HNSCC (Agrawal et al., 2011). Faulty NOTCH1 expression is involved in tumor formation in many types of cancer, including squamous cell carcinoma of the skin, but it had not been implicated in HNSCC. A better understanding of the role of NOTCH1 may provide insight for the treatment of HNSCC and a range of other malignancies. Many of the results of the discovery-phase research have been corroborated by a companion study (Stransky et al., 2011). This research has also confirmed that HNSCC tumors associated with human papilloma virus infection have a genetic profile different from that of tumors in individuals with a history of tobacco use.
Among the reasons for the modest HNSCC survival rate is local relapse after primary tumor resection, which is estimated to occur in 10% to 30% of cases (Slootweg et al., 2002). Local recurrence even when surgical margins have been designated “clean” is due presumably to the presence of malignant cells that escape routine histopathology or by precancerous lesions near the tumor that are not successfully resected and later become malignant. Several approaches based on protein expression and nucleic acid analysis show promise toward more accurate prediction of patients at risk for local oral cancer recurrence (Braakhuis et al., 2010).
Molecularly targeted therapies have been developed and are being tested for use in HNSCC as well as in other cancers. Some examples include epidermal growth factor receptor (EGFR)-directed drugs, such as monoclonal blocking antibodies (cetuximab) and EGFR-tyrosine kinase inhibitors (gefitinib, erlotinib, lapatinib), which have been tested in clinical trials, with outcomes currently under evaluation (Cassell and Grandis, 2010; Howard et al., 2012). Yet another line of investigation targets the Pl3K/AKT-mTOR signaling pathway, which is frequently dysregulated in HNSCC. A clinical trial is under way to test whether the mTOR inhibitor sirolimus (also known as rapamycin) can decrease pre-surgical tumor size (Czerninski et al., 2009; NIDCR, 2010).
Despite great progress and promise, we issue a cautionary note. Newly developed targeted therapies are not entirely without side-effects, and they are also prone to the development of resistance and toxicity. These realities demand continued studies to refine therapies based on the molecular evolution of disease.
Management of Acute and Chronic Orofacial Pain
Increasing recognition of the potential use of pharmacogenomics toward safer and more effective dosing of drugs for conditions as diverse as depression, anxiety, coronary and peripheral artery disease, inflammatory bowel disease, and cancer points to exciting opportunities for the management of oral health conditions such as chronic orofacial pain. Particularly relevant to dentistry is the well-documented individual genetic variation in the cytochrome P (CYP) 450 superfamily of enzymes involved in the metabolism and bioactivation of about half of all drugs. For instance, people with certain allelic variants in the CYP2D6 gene are unable to convert codeine to morphine. As such, these individuals experience insufficient analgesia, yet they still endure many of the undesirable side-effects associated with opioids (Desmeules et al., 1991). Conversely, individuals with multiple copies of CYP2D6 metabolize codeine extremely rapidly, putting them at risk for morphine intoxication (Gasche et al., 2004).
Variants in the CYP2E1 and OPRM1 genes yield inter-individual differences in response to anesthetics, such as halothane, isoflurane, and synthetic narcotic analgesics such as fentanyl (Eng et al., 2012). Identifying and monitoring such individual genetic variation may enable dentists to customize peri-operative and post-operative pain management such that it is both safer and more effective. A clinical trial is now under way to establish the value of CYP2D6 and OPRM1 testing for opioid treatment in patients with chronic back pain (NIH, 2012).
Genetic variation also plays a role in an individual’s perception of pain, potentially offering clues about susceptibility to chronic orofacial pain conditions such as temporo-mandibular joint disorders (TMD). Ongoing research is helping to identify individuals most susceptible to developing chronic TMD and those who would benefit from early treatment. To this end, the Orofacial Pain: Prospective Evaluation and Risk Assessment (OPPERA) study is the most comprehensive analysis to date of risk factors involved in the onset of orofacial pain conditions (Fillingim et al., 2011). Initial results have revealed that TMD appears to be associated with alterations in autonomic function and pain perception, along with genetic factors (Maixner et al., 2011). The findings confirmed existing associations for 2 genes, HTR2A and COMT, and uncovered new potential risk factors linked to NR3C1, CAMk4, CHRM2, IFRD1, and GRK5, genes known to influence stress response, psychological well-being, and inflammation. Collectively, these gene alterations may represent important markers of TMD risk and, possibly, therapeutic intervention. The OPPERA effort provides a model approach for collaborative, interdisciplinary research. The study integrated genetic, psychosocial, and pain amplification data into cluster analyses that identified a subset of variables that clinicians could incorporate into health-history questionnaires, allowing for sub-diagnosis of patients who show the most abnormal pain sensitivity and psychosocial profiles. While these results are not yet ready for clinical adoption, they provide important clues that dentists and other health care providers might use in combination with imaging and clinical findings to identify individuals most susceptible to TMD and related chronic pain conditions.
Oral Infectious Diseases
A personalized approach to managing oral infectious diseases is progressing quickly, based on the fruits of research investments. Such knowledge provides a brand new set of tactics for disease prevention. For example, genome sequences of several oral pathogens implicated in caries and periodontal diseases are now available, and researchers are discovering how oral bacterial cells attach to a surface and become established within a biofilm (Kolenbrander et al., 2002). Prevention strategies now include the development of small molecules aimed at blocking or weakening enzymes that enable bacteria associated with caries to form a biofilm or attach to the tooth surface (Liu et al., 2011) and activating lipid mediators to dampen inflammation in periodontal diseases (Recchiuti and Serhan, 2012).
Advances in imaging are providing unique opportunities for better understanding of oral biofilm formation, organization, and composition: The knowledge gained will inform the design of biofilm-manipulating therapies. Researchers have devised a new fluorescent imaging system that successfully distinguished among 28 oral microbes within a single field of view, providing spatial analysis in 3 dimensions (Valm et al., 2011). We can envision that, with advances in these and other technologies, dentists will be able to non-invasively visualize the microbial communities in an individual’s mouth, guiding real-time diagnosis and treatment decisions.
The Human Microbiome Project (HMP), an NIH-funded consortium representing nearly 80 universities and institutions, is laying the foundation for efforts to determine how complex communities of microbes interact in the human body to influence health and disease. The consortium has characterized the microbial communities at 18 body sites, including 9 sites in the oral cavity. More than 200 healthy volunteers have been characterized by 16S rRNA gene sequencing, and the project has generated nearly 1,000 reference genomes from human-associated micro-organisms. In a complementary effort, NIDCR supported the Human Oral Microbiome Database (HOMD) to assemble data on the estimated 1,000 predominant micro-organisms that inhabit the oral cavity and adjacent tissues. An analysis of 35,000 oral clone sequences revealed that roughly 35% originated from unnamed and uncultivable phylotypes (Chen et al., 2010). Efforts such as the HOMD open the door to the development of novel strategies to further improve the prevention, diagnosis, and treatment of polymicrobial oral diseases. In one example, researchers have leveraged the HOMD project to identify a new bacterial species, Scardovia wiggsiae, associated with early childhood caries. This micro-organism represents a potential pathogenic indicator of early childhood caries risk in young children (Tanner et al., 2011a). These and other findings demonstrating the presence of additional pathogens suggest that successful treatment may require a change in microbiota as well as diet (Tanner et al., 2011b).
Genome-wide association studies (GWAS) are a powerful set of approaches made possible by the marriage of human genome sequencing and high-speed computing. GWAS findings from dental caries investigations have identified susceptibility loci, and they have provided evidence that distinct genetic factors may depend on home fluoride exposure levels (Shaffer et al., 2012). In one study, 2 genes, TAS2R38 and TAS1R2, appear to mediate the sensation of taste (Wendell et al., 2010). Although preliminary, the results suggest that some individuals with variations in these genes may have a gustatory predisposition to eat cariogenic foods, offering the potential to introduce genetic factors into risk-assessment algorithms that also consider health history, oral hygiene, dietary practices, and other biologic and environmental risk factors.
Oral Biomarkers
The genomic era has generated a tremendous surge in interest in biomarker research and discovery. Biomarkers are measures of “normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention” (Biomarkers Definitions Working Group, 2001). They are physical, functional, or biochemical indicators with a range of potential applications to clinical practice, including baseline risk assessment, disease prognosis, and treatment guidance. A comprehensive review of biomarker research is beyond the scope of this article and has been presented elsewhere (Castagnola et al., 2011; Al Kawas et al., 2012). We provide a snapshot of particularly relevant developments germane to the interests of oral, dental, and craniofacial research and practice communities.
Saliva has long been recognized to have several advantages over blood as a diagnostic fluid. These include simple, non-invasive collection; potential for lower testing costs; portability; and point-of-care application (Malamud, 1992; Miller et al., 2010). NIDCR is supporting research to establish the foundation of oral-fluid-based diagnostics, toward broadening its technological capabilities. Analysis of existing data supports the presence, in saliva and oral tissues, of disease-related proteins and RNAs for oral cancers (Weigum et al., 2010; Elashoff et al., 2012), cardiovascular disease (Floriano et al., 2009), Sjögren’s syndrome (Alevizos et al., 2011), and other conditions such as connective tissue disorders and periodontal diseases (Miller et al., 2010; Malamud, 2011). In addition to these biomarkers, many other candidate biomarkers have been identified in saliva and oral tissues, including signaling pathways, enzymes, proteins, and cytokines. Further development and validation of oral biomarker approaches could facilitate improved pre-emptive care, more precise therapeutic approaches, and new methods to monitor treatment response.
Despite the fact that biomarkers hold great promise for oral health care, implementation into clinical practice remains a lengthy and expensive enterprise requiring strong and creative collaborations among scientists, clinicians, and industry. The Biomarkers Consortium, a public-private biomedical research partnership, was established to develop, validate, and qualify promising biomarkers (http://www.biomarkersconsortium.org). Toward furthering the development, testing, and implementation of new biomarkers, other diagnostics, and novel drug development strategies, the NIH created the National Center for Advancing Translational Sciences (NCATS), which serves to facilitate translational research across the NIH and complement other public and private efforts (Collins, 2011).
Integrating Personalized Medicine into Dental Practice
The pursuit of personalized medicine has been likened to the construction of the national highway system in the United States after World War II, which, while providing necessary infrastructure for road travel, did not tell drivers where to go. Rather, the US highway system “built the roads and set the standards for safety” (Hamburg and Collins, 2010). The same holds true for personalized medicine: With required research infrastructure now largely in place, clinicians, patients, and researchers will all need to “travel the roads” before the promise of personalized medicine is fully realized.
Where will this scientific infrastructure take practicing dentists? Just as for evidence-based dentistry, or the emergence of the Internet in the 1990s, some dentists will be early adopters, and others will be more cautious to embrace a new approach. Early advances could benefit dentistry in the near term, and timely identification of barriers to implementation will be important to accelerate progress. The NIDCR-supported National Practice-based Research Network (PBRN), an effort to link practicing dentists and scientists, presents an extraordinary opportunity to catalyze the adoption of new tools and technologies brought about by personalized health care.
More broadly speaking, multiple genomic medicine projects are being implemented in the United States, demonstrating that personalized health care is achievable (Manolio et al., 2013). One of the barriers that impedes the clinical integration of genomics applies equally to medicine and dentistry: the skepticism by providers and payers of the added value of genomics to improve patient care. This reluctance is driven in part by the somewhat sparse evidence of clinical usefulness (Scheuner et al., 2008). Certainly, public and private insurers will require robust evidence of the clinical and cost-effectiveness of prognostic tests and personalized treatment approaches before endorsing these approaches in health care. Another challenge is the need to integrate genomic and proteomic profiles with clinical and health-history data, toward the development of decision-supported clinical tools. Still a work in progress in medicine, this area is even less mature in dentistry. Progress awaits considerable improvement of information technology systems that can enable dental patient care and decision-making to be supported (Mendonça, 2004; Schleyer et al., 2012).
Challenges for the Future
In many ways, personalized medicine is indeed here, but many improvements are needed to validate its routine and effective use in oral health care. Current scientific and technological gaps include: definitive linkages between both biomarkers and genotypes and clinical outcomes; cost-effective and non-invasive imaging technologies for the detection of pathologies of the oral-craniofacial complex; clarification of the association between and among the environment, microbiome, and genetics; more accurate disease risk and drug response prediction; improved drug design and delivery; as well as an enhanced system for facile yet appropriate use and access to electronic health records and databases (Collins, 2010; Hamburg and Collins, 2011).
The time is now for electronic oral health records to overcome the challenges of interoperability and accessibility, as well as to seek common standards. Given that 50% of the US population in 2010 had medical information available in electronic health records (Hsiao et al., 2010), addressing this opportunity has achieved a new sense of urgency. A survey conducted among members of the Dental PBRN found that 73.8% of solo dental practitioners use a computer to manage patient information, with 14.3% indicating that they have gone completely paperless (Schleyer et al., 2013). Yet, the lack of integration between electronic medical and dental data in most US health care settings, where medical and dental systems were developed separately, remains a critical hurdle to be overcome (Rudman et al., 2010).
On the educational front, future health professionals will need to be well-versed in the scientific underpinnings of personalized care. As genetic testing becomes more common, it is unclear how well-prepared health care providers, including dentists, will be to interpret them. Dental schools will need to incorporate genetics and genomics into their professional curricula, and dentists will need to keep up with rapidly changing technologies—including but not limited to ‘omics’—to keep abreast of the modern clinical care patients expect.
Finally, a critical element of the success of personalized dentistry is public awareness and acceptance of the benefits and risks of personal genome sequencing. Socio-ethical and legal barriers continue to face the fields of genomic medicine, stem cell therapy, and other molecular approaches to health care, and these issues need to be addressed thoughtfully and with public involvement. The 2008 passage of the Genetic Information Nondiscrimination Act (P.L. 110-233; US Government, 2008) was a significant step toward safeguarding individuals against potential discrimination from health insurers based on genetic risk factors. Still, the possibility for over- and misinterpretation of genomics-based results—with the potential to cause undue alarm, lead to unnecessary interventions, and/or contribute to psychological harm—continues to be a significant issue confronting modern biomedicine (Drmanac, 2011). This reality underscores the need for a deeper understanding of the public’s unique educational requirements, as well as for genomic education efforts including various communication channels and approaches such as social media and comprehensive educational campaigns (Manolio et al., 2013).
Concluding Thoughts
Major investments in science have leveled the ground for the foundation of precise and personalized oral health care. Tremendous progress in many areas of dental research will undoubtedly accelerate personalized approaches and fill critical voids in our knowledge. These include advances in microbiology, immunology, tissue engineering, imaging, neuropharmacology, stem cell biology, and nanotechnology. Finally, and deserving separate focus, is vigorous and careful investigation using tools of the behavioral and social sciences. The dental health profession must engage in dialogue focused on preparing the current and next generation of clinicians, educators, researchers, and the public for the transformation in health care that is already in progress and will continue to evolve rapidly.
Acknowledgments
The authors are grateful to Drs. Amy Adams, Alison Davis, Khara Ramos, and Sundar Venkatachalam for their manuscript review and helpful comments.
Footnotes
The authors received no financial support and declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Agrawal N, Frederick MJ, Pickering CR, Bettegowda C, Chang K, Li RJ, et al. (2011). Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science 333:1154-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Kawas S, Rahim ZH, Ferguson DB. (2012). Potential uses of human salivary protein and peptide analysis in the diagnosis of disease. Arch Oral Biol 57:1-9. [DOI] [PubMed] [Google Scholar]
- Alevizos I, Alexander S, Turner J, Illei GG. (2011). MicroRNA expression profiles as biomarkers of minor salivary gland inflammation and dysfunction in Sjögren’s syndrome. Arthritis Rheum 63:535-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biomarkers Definitions Working Group (2001). Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 69:89-95. [DOI] [PubMed] [Google Scholar]
- Braakhuis BJ, Bloemena E, Leemans CR, Brakenhoff RH. (2010). Molecular analysis of surgical margins in head and neck cancer: More than a marginal issue. Oral Oncol 46:535-544. [DOI] [PubMed] [Google Scholar]
- Cassell A, Grandis JR. (2010). Investigational EGFR-targeted therapies in HNSCC. Expert Opin Investig Drugs 6:709-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagnola M, Cabras T, Vitali A, Sanna MT, Messana I. (2011). Biotechnological implications of the salivary proteome. Trends Biotechnol 29:409-418. [DOI] [PubMed] [Google Scholar]
- Chen T, Yu H, Izard J, Baranova O, Kakshmanan A, Dewhirst FE. (2010). The Human Oral Microbiome Database: a Web accessible resource for investigating oral microbe taxonomic and genomic information. Database Article ID baq-013, doi: 10.1093/database/baq013 Accessed on 4/1/2013 at: http://www.ncbi.nlm.nih.gov/pubmed/20624719. [DOI] [PMC free article] [PubMed]
- Cohen JP. (2012). Overcoming regulatory and economic challenges facing pharmacogenomics. N Biotechnol 29:751-756. [DOI] [PubMed] [Google Scholar]
- Collins FS. (2010). Has the revolution arrived? Nature 464:674-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins FS. (2011). Reengineering translational science: the time is right. Sci Transl Med 3:90cm17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerninski R, Amornphimoltham P, Patel V, Molinolo AA, Gutkind JS. (2009). Targeting mammalian target of rapamycin by rapamycin prevents tumor progression in an oral-specific chemical carcinogenesis model. Cancer Prev Res 2:27-36. [DOI] [PubMed] [Google Scholar]
- Desmeules J, Gascon MP, Dayer P, Magistris M. (1991). Impact of environmental and genetic factors on codeine analgesia. Eur J Clin Phamarmacol 41:23-26. [DOI] [PubMed] [Google Scholar]
- Drmanac R. (2011). The advent of personal genome sequencing. Genet Med 13:188-190. [DOI] [PubMed] [Google Scholar]
- Elashoff D, Zhou H, Reiss J, Wang J, Xiao H, Henson B, et al. (2012). Prevalidation of salivary biomarkers for oral cancer detection. Cancer Epidemiol Biomarkers Prev 21:664-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elferink LA, Resto VA. (2011). Receptor-tyrosine-kinase-targeted therapies for head and neck cancer. J Sig Transduct 2011:982870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENCODE Project Consortium (2007). Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature 447:799-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENCODE Project Consortium (2012). An integrated encyclopedia of DNA elements in the human genome. Nature 489:57-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng G, Chen A, Vess T, Ginsburg GS. (2012). Genome technologies and personalized dental medicine. Oral Dis 18:223-235. [DOI] [PubMed] [Google Scholar]
- Fackler JL, McGuire AL. (2009). Paving the way to personalized medicine: steps to successful implementation. Curr Pharmacogenomics Person Med 7:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingim RB, Slade GD, Diatchenko L, Dubner R, Greenspan JD, Knott C, et al. (2011). Summary of findings from the OPPERA baseline case-control study: implications and future directions. J Pain 12(11 Suppl):102S-107S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floriano PN, Christodoulides N, Miller CS, Ebersole JL, Spertus J, Rose BG, et al. (2009). Use of saliva-based nano-biochip tests for acute myocardial infarction at the point of care: a feasibility study. Clin Chem 55:1530-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frueh FW, Amur S, Mummaneni P, Epstein RS, Aubert RE, DeLuca TM, et al. (2008). Pharmacogenomic biomarker information in drug labels approved by the United States Food and Drug Administration: prevalence of related drug use. Pharmacotherapy 28:992-998. [DOI] [PubMed] [Google Scholar]
- Gasche Y, Daali Y, Fathi M, Chiappe A, Cottini S, Dayer P, et al. (2004). Codeine intoxication associated with ultrarapid CYP2D6 metabolism. N Engl J Med 351:2827-2731; erratum in N Engl J Med 352:638, 2005. [DOI] [PubMed] [Google Scholar]
- Hamburg MA, Collins FS. (2010). The path to personalized medicine. N Engl J Med 363:301-304; erratum in N Engl J Med 363:1912, 2010. [DOI] [PubMed] [Google Scholar]
- Hoelder S, Clarke PA, Workman P. (2012). Discovery of small molecule cancer drugs: successes, challenges and opportunities Mol Oncol 6:155-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard JD, Lu B, Chung CH. (2012). Therapeutic targets in head and neck squamous cell carcinoma: identification, evaluation, and clinical translation. Oral Oncol 48:10-17. [DOI] [PubMed] [Google Scholar]
- Hsiao CJ, Hing E, Socey TC, Cai B. (2010). Centers for Disease Control and Prevention, National Center for Health Statistics, 2010. Division of Health Care Statistics Electronic Medical Record/Electronic Health Record Systems of Office-based Physicians: United States, 2009 and Preliminary 2010 Estimates. Accessed on 4/1/2013 at: http://www.cdc.gov/nchs/data/hestat/emr_ehr_09/emr_ehr_09.pdf
- Kolenbrander PE, Andersen RN, Blehert DS, Egland PG, Foster JS, Palmer RJ., Jr (2002). Communication among oral bacteria. Microbiol Mol Biol Rev 66:486-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Worthington RJ, Melander C, Wu H. (2011). A new small molecule specifically inhibits the cariogenic bacterium Streptococcus mutans in multispecies niofilms. Antimicrob Agents Chemother 55:2679-2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolio TA, Chisholm RL, Ozenberger B, Roden DM, Williams MS, Wilson R, et al. (2013). Implementing genomic medicine in the clinic: the future is here. Genet Med 15:258-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maixner W, Greenspan JD, Dubner R, Bair E, Mulkey F, Miller V, et al. (2011). Potential autonomic risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case-control study. J Pain 12(11 Suppl):75S-91S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamud D. (1992). Saliva as a diagnostic fluid. BMJ 305:207-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamud D. (2011). Saliva as a diagnostic fluid. Dent Clin North Am 55:159-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty CA, Chisholm RL, Chute CG, Kullo IJ, Jarvik GP, Larson EB, eMERGE Team et al. (2011). The eMERGE Network: a consortium of biorepositories linked to electronic medical records data for conducting genomic studies. BMC Med Genomics 4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonça EA. (2004). Clinical decision support systems: perspectives in dentistry. J Dent Educ 6:589-597. [PubMed] [Google Scholar]
- Miller CS, Foley JD, Bailey AL, Campell CL, Humphries RL, Christodoulides N, et al. (2010). Current developments in salivary diagnostics. Biomark Med 4:171-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne CP. (2010). Personalized medicine is playing a growing role in developmental pipelines. Tufts Center for the Study of Drug Development Impact Report 12:6. [Google Scholar]
- National Institute of Dental and Craniofacial Research (NIDCR). (2010). Rapamycin therapy in head and neck squamous carcinoma. In: ClinicalTrials.gov [Internet]. Bethesda, MD: National Library of Medicine (US) [cited 2013 Feb 14]. Accessed on 4/1/2013 at: http://www.clinicaltrials.gov/ct2/show/NCT01195922 NLM Identifier NCT01195922 [Google Scholar]
- National Institutes of Health (2012). A study of genetic variation influencing pain and response to opioid medications in patients with chronic pain. In: ClinicalTrials.gov [Internet]. Bethesda, MD: National Library of Medicine (US): Stanford University; Accessed on 4/1/2013 at: http://www.clinicaltrials.gov/ct2/show/record/NCT01293994. NLM Identifier NCT01293994. [Google Scholar]
- Personalized Medicine Coalition (2010). The case for personalized medicine. 3rd ed. Accessed on 4/1/2013 at: http://www.personalizedmedicinecoalition.org/sites/default/files/files/Case_for_PM_3rd_edition.pdf [Google Scholar]
- Recchiuti A, Serhan CN. (2012). Pro-resolving lipid mediators (SPMs) and their actions in regulating miRNA in novel resolution circuits in inflammation. Front Immunol 3:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg SM, Ellisen LW. (2012). The molecular pathogenesis of head and neck squamous cell carcinoma. J Clin Invest 122:1951-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudman W, Hart-Hester S, Jones W, Caputo N, Madison M. (2010). Integrating medical and dental records: a new frontier in health information management. J AHIMA [American Health Information Management Association] 81:36-39. [PubMed] [Google Scholar]
- Scanlon CS, Van Tubergen EA, Inglehart RC, D’Silva NJ. (2013). Biomarkers of epithelial-mesenchymal transition in squamous cell carcinoma. J Dent Res 92:114-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuner MT, Sieverding P, Shekelle PG. (2008). Delivery of genomic medicine for common chronic adult diseases: a systematic review. J Am Med Assoc 299:1320-1334. [DOI] [PubMed] [Google Scholar]
- Schleyer T, Thyvalikakath TP, Spallek H, Dziabiak MP, Johnson LA. (2012). From information technology to informatics: the information revolution in dental education. J Dent Educ 76:143-153. [PMC free article] [PubMed] [Google Scholar]
- Schleyer T, Song M, Gilbert GH, Rindal DB, Fellows JL, Gordan VV, et al. (2013). Electronic dental record use and clinical information management patterns among practitioner-investigators in the Dental Practice-based Research Network. J Am Dent Assoc 144:49-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer JR, Feingold E, Wang X, Tcuenco KT, Weeks DE, DeSensi RS, et al. (2012). Heritable patterns of tooth decay in the permanent dentition: principal components and factor analyses. BMC Oral Health 12:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. (2012). Cancer statistics, 2012. CA Cancer J Clin 62:10-29. [DOI] [PubMed] [Google Scholar]
- Slootweg PJ, Hordijk GJ, Schade Y, van Es RJ, Koole R. (2002). Treatment failure and margin status in head and neck cancer. A critical view on the potential value of molecular pathology. Oral Oncol 38: 500-503. [DOI] [PubMed] [Google Scholar]
- Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, et al. (2011). The mutational landscape of head and neck squamous cell carcinoma. Science 333:1157-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner AC, Mathney JM, Kent RL, Chalmers NI, Hughes CV, Loo CY, et al. (2011a). Cultivable anaerobic microbiota of severe early childhood caries. J Clin Microbiol 49:1464-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner AC, Kent RL, Jr, Holgerson PL, Hughes CV, Loo DY, Pradhan N, et al. (2011b) Microbiota of severe early childhood caries before and after therapy. J Dent Res 90:1298-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Energy, Office of Science. White House press release, June 26, 2000. Accessed on 4/1/2013 at: http://www.ornl.gov/sci/techresources/Human_Genome/project/clinton2.shtml.
- US Food and Drug Administration (2012). FDA Table of Pharmacogenomic Biomarkers in Drug Labels. Accessed on 4/1/2013 at: http://www.fda.gov/Drugs/ScienceResearch/ResearchAreas/Pharmacogenetics/ucm083378.htm.
- US Government Printing Office (2008). P.L. 100-233 122 Stat. 881. May 21, 2008. Accessed on 4/1/2013 at: http://www.gpo.gov/fdsys/pkg/PLAW-110publ233/pdf/PLAW-110publ233.pdf
- Valm AM, Mark Welch JL, Rieken CW, Hasegawa Y, Sogin ML, Oldenbourg R, et al. (2011). Systems level analysis of microbial community organization through combinatorial labeling and spectral imaging. Proc Natl Acad Sci USA 108:4152-4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigum SE, Floriano PN, Redding SW, Yeh CK, Westbrook SD, McGuff HS, et al. (2010). Nano-Bio-Chip sensor platform for examination of oral exfoliative cytology. Cancer Prev Res 3:518-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendell S, Wang X, Brown M, Cooper ME, DeSensi RS, Weyant RJ, et al. (2010). Taste genes associated with dental caries. J Dent Res 89:1198-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]