Abstract
Model systems for oral cancer research have progressed from tumor epithelial cell cultures to in vivo systems that mimic oral cancer genetics, pathological characteristics, and tumor-stroma interactions of oral cancer patients. In the era of cancer immunotherapy, it is imperative to use model systems to test oral cancer prevention and therapeutic interventions in the presence of an immune system and to discover mechanisms of stromal contributions to oral cancer carcinogenesis. Here, we review in vivo mouse model systems commonly used for studying oral cancer and discuss the impact these models are having in advancing basic mechanisms, chemoprevention, and therapeutic intervention of oral cancer while highlighting recent discoveries concerning the role of immune cells in oral cancer. Improvements to in vivo model systems that highly recapitulate human oral cancer hold the key to identifying features of oral cancer initiation, progression, and invasion as well as molecular and cellular targets for prevention, therapeutic response, and immunotherapy development.
Keywords: squamous cell carcinoma, genetically engineered mouse models, immunocompetent, cancer biology, immunity, oral carcinogenesis
Introduction
Head and neck cancer, a group of malignancies arising in the epithelial tissue of the paranasal sinus, lip, oral cavity, nasal cavity, pharynx, and larynx, comprises the sixth most common malignancy worldwide, representing 4% of all types of cancers (El-Bayoumy et al. 2017; Siegel et al. 2017). Cancer of the oral cavity (oral cancer) is the most common subtype of this disease. The majority of oral cancer or head and neck cancer is squamous cell carcinoma (oral squamous cell carcinoma [OSCC] or head and neck squamous cell carcinoma [HNSCC]). Oral carcinogenesis is a complex process resulting from accumulation of multiple genetic and epigenetic alterations induced by oral carcinogens (i.e., alcohol, tobacco, betel nut) and/or human papillomavirus (HPV) (Gillison and Restighini 2015; Kumar et al. 2016). Although diagnosis of HPV-associated oral cancer is increasing, this subset of cancer has a distinct biology and generally responds well to therapy. Oral cancer in heavy smokers remains prevalent worldwide and responds more poorly to therapy than HPV-associated HNSCC. Despite advances in understanding, diagnosis, and treatment of head and neck cancers, the 5-y survival rate after diagnosis is around 60% (Pfister et al. 2015), indicating that further improvements in the detection and treatment of HNSCC are crucial to save lives and alleviate complications of basic life functions such as eating, drinking, speaking, and breathing (El-Bayoumy et al. 2017).
Next generation genome-wide sequencing and immunotherapies have revolutionized cancer research, biomarker discovery, and patient care in the past decade. Common genetic and epigenetic alterations in oral cancer have been characterized (Choi and Myers 2008; Stransky et al. 2011; Cancer Genome Atlas 2015), but this knowledge does not positively affect treatment or prognosis without continued research applying the lessons learned from genomics to determine how specific mutations and amplifications can be leveraged to personalize treatment. Investigational and Food and Drug Administration (FDA)–approved immune checkpoint inhibitors that reactivate antitumor immunity are transforming cancer therapy. PD-1 is a coreceptor on T lymphocytes that suppresses T-cell activity when engaged by its ligands, PD-L1 or PD-L2. Consequently, inhibition of PD-L1 or PD-1 increases T-cell activity, and these therapies have been demonstrated to increase T-cell-specific tumor cell killing (Ferris et al. 2016; Seiwert et al. 2016; Bauman et al. 2017; Dogan et al. 2017). The FDA has approved a few PD-1 antibodies for treating recurrent and metastatic HNSCC. However, response rates to anti–PD-1 immunotherapy are ~20% in HNSCC patients (Ferris et al. 2016), and many novel therapies are still in development. Therefore, it is imperative to understand the mechanisms by which cancers evade immune surveillance and define crosstalk between tumor cells and tumor stroma that contributes to metastasis, tumor cell survival, and immune suppression or immune evasion. The use of experimental animal models that possess an immune system to address these mechanistic questions is critical and will complement clinical studies in patients. This article presents an overview of mouse models of oral cancer, reviews studies on oral cancer biology in the presence of a native immune system and immune regulators, and presents current applications of these models for studying novel strategies of chemoprevention and immune-targeted therapies.
Mouse Models of Oral Cancer
Reviews of in vivo models of oral cancer have been published (Supsavhad et al. 2016; Ishida et al. 2017), so we will only briefly introduce mouse models of oral cancer. Broadly speaking, oral cancers can be established in mice by tumor cell transplantation (xenografting and allografting), chemical carcinogenesis, and spontaneous oral cancer initiated by genetic modifications.
Immunocompromised mouse recipients are used to study human cancers in mice because immunocompetent animals reject human cell transplantation. Tumor cells transplanted into the skin of mice are easily monitored for tumor size and response to therapeutic intervention. However, these tumors do not develop in the natural anatomic site of OSCC and do not completely replicate the pathology, microenvironment, or steps of premalignancy and carcinogenesis in human oral cancer (Smith and Thomas 2006). To overcome some of these limitations, tumor cells are orthotopically injected into the mylohyoid muscle in the floor of the oral cavity, tongue muscle, or buccal mucosa, and subsequent tumors are able to develop locoregional metastasis or distant metastasis (Bais et al. 2015; Alsaqer et al. 2017). Patient-derived xenografts (PDX tumors) can be serially passaged in mouse recipients, and their early passages consisting of tumor epithelial and stromal cells retain the genetic and morphological characteristics of the original human oral cancer better than cell line xenograft models (Keysar et al. 2013; Sun and Zhang 2016). To further mimic the tumor microenvironment, oral cancer PDX models are improved upon by transplanting OSCC into the floor of the mouth; these models have better predictive power for therapeutic responses (Keysar et al. 2013). However, immunocompromised mouse recipients exclude the ability to assess contributions of a complete immune system on OSCC. Athymic mice lack functional T lymphocytes but retain B lymphocytes, natural killer (NK) cells, macrophages, and most cells of the innate immune system, but it remains to be determined how these mouse immune cells interact with human tumor cells, due to the species barrier, and whether mouse immune cells have a functional impact on cancer progression similar to human immune cells. NOD-scid IL2Rgammanull (NSG) mice, which are severely immunocompromised (lacking functional T, B, and NK cells as well as severe defects in myeloid lineages), are also used as recipients of PDX OSCCs. Humanized mice are generated by transplantation of human hematopoietic stem and progenitor cells into bone marrow–depleted NSG mice to establish a suite of human immune cells in the mouse host. This model marks a step forward in the evaluation of human immune cells against human tumor cells by partially overcoming the mouse-human species barrier (Morton, Bird, Keysar, et al. 2016; Morton, Bird, Refaeli, et al. 2016; Walsh et al. 2017).
Considering the close relationship between tobacco use and oral carcinogenesis, tobacco-mimicking carcinogen application to the oral cavity is an ideal model to study carcinogen-related oral cancer. 4-Nitroquinoline-1 oxide (4-NQO), a synthetic water-soluble carcinogen (Wallenius and Lekholm 1973), exerts potent intracellular oxidative stress and metabolic by-products that bind to DNA predominantly at guanine residues, causing damage similar to carcinogens in tobacco and inducing molecular and pathological changes similar to human OSCC (Turesky 1994; Tang et al. 2004). Histologically, 4-NQO–induced lesions have sequential stages of epithelial carcinogenesis, including hyperplasia, various degrees of dysplasia, carcinoma in situ, and invasive squamous cell carcinoma (SCC) occurring in the endogenous epithelia and microenvironment, resembling multistage carcinogenesis of human oral cancer (Tang et al. 2004; Li et al. 2013). Therefore, this model provides a valuable tool to study OSCC or precursor lesions in the native stroma and immune system.
Genetically engineered mouse models (GEMMs) and carcinogen-induced OSCC animal models provide the research community with immunocompetent cancer models that mimic multistage oral carcinogenesis observed in patients (Fig. 1). Oral cancer GEMMs are now mainly produced by inducible systems, allowing modification of a gene of interest in oral epithelia at specific times. In addition to tetracycline (Tet)–inducible systems summarized by Baron and Bujard (2000) and estrogen receptor (ER)–inducible systems described by Feil et al. (1997), RU486-inducible, oral epithelium–specific GEMMs have been developed in our laboratory. The “gene-switch” system allows RU486-inducible activation of a gene of interest in the oral epithelium (Fig. 2A). Targeting RU486-inducible cre recombinase (CrePR1) (Kellendonk et al. 1996) to stratified epithelium with K5 or K14 promoters allows for inducible control of target gene expression or gene deletion in basal keratinocytes (including stem cells and non–stem cells) of oral epithelia (Lu et al. 2006; Bornstein et al. 2009), and the K15 promoter restricts expression to stem cells of hair follicles or oral epithelia (White et al. 2013) (Fig. 2B).
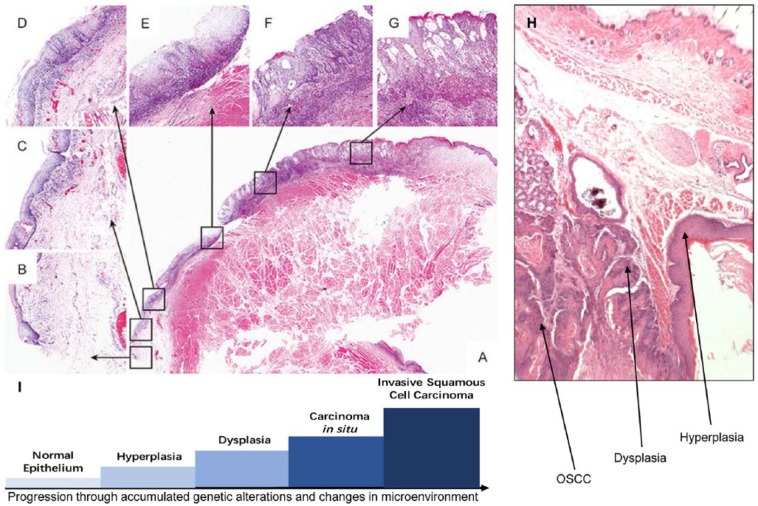
Figure 1.
Multistage carcinogenesis in oral squamous cell carcinoma (OSCC). (A–G) From a case report illustrating sequential stages of oral carcinogenesis in a human patient. A low-power view of an oral lesion with multiple histologies is presented in panel A, and high-power magnification of different locations of this lesion is presented in panels B–G as indicated by the arrows. (A) Pathological whole lesion; (B) normal epithelium; (C) hyperplasia; (D) mild dysplasia; (E) moderate dysplasia; (F) severe dysplasia/carcinoma in situ; (G) local invasive squamous cell carcinomas. Figure adapted from Wei et al. (2016). (H) An example of sequential stages of oral carcinogenesis induced by Smad4 deletion in the oral mucosa of mice (Bornstein et al. 2009). (I) Graphical representation of normal epithelium transformation by accumulated genetic lesions and microenvironmental changes.
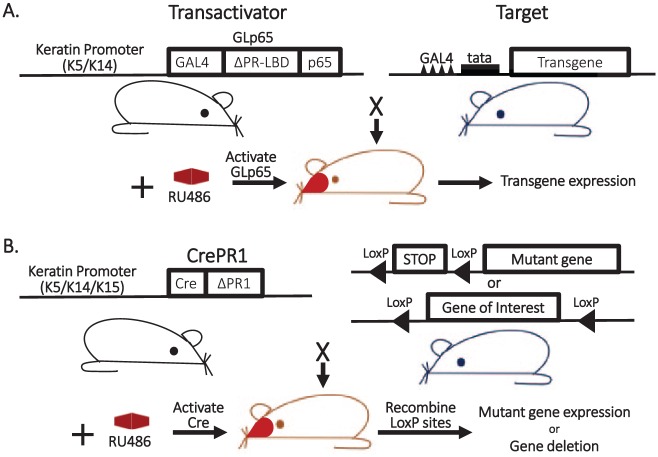
Figure 2.
RU486-inducible, oral-specific genetically engineered mouse models. (A) Inducible “gene-switch” keratinocyte-specific transgenic system: the GLp65 transactivator is inserted downstream of a keratin 5 (K5) or keratin 14 (K14) promoter. The GLp65 transactivator is composed of the DNA binding domain of the yeast transcription factor (GAL4), the ligand binding domain of the truncated progesterone receptor (ΔPR-LBD) that selectively binds progesterone antagonists (e.g., RU486), and the NF-κB p65 transactivation domain. The target consists of a transgene under the control of a minimal tata promoter containing 4 copies of the GAL4 DNA binding sequence upstream of the promoter. A transactivator mouse line is bred with a target mouse line to generate bitransgenic mice containing both transgenes. Expression of the target gene is induced by topical application of RU486 to mouse oral epithelia. (B) Inducible keratinocyte-specific knock-in or knockout system: mice expressing a Cre recombinase transgene fused to a truncated progesterone receptor (CreΔPR1) under transcriptional control of a K5, K14, or K15 promoter are bred with mice containing genes engineered to contain LoxP sites: (top) a mutant gene preceded by a lox-stop-lox cassette or (bottom) a gene of interest flanked by LoxP sites. RU486 application to the oral cavity of bigenic mice activates CrePR1 activity that recombines LoxP sites, resulting in excision of the stop codon (inducing mutant gene activation) or excision of the gene of interest (inducing loss of gene activity).
By combining these mouse models of oral cancer, several important discoveries have been made that will affect our approach to treating patients. While not discussed here, feline, hamster, and rat models have contributed to advancing our knowledge of oral cancer, and readers are pointed to a relevant review (Supsavhad et al. 2016).
Models to Study Cancer Stem Cells and Metastasis of Oral Cancer
Cancer stem cells (CSCs) are tumor cells capable of self-renewal and reestablishing a tumor after therapy, contributing to relapse (Beck and Blanpain 2013; Dionne et al. 2015). As such, defining mechanisms that drive CSC function and their therapeutic liabilities is important to eradicate these major players in OSCC relapse. 4-NQO carcinogenesis was used to generate a spectrum of oral lesions and perform lineage tracing of BMI1+ cells. This study identified BMI1 as a bona fide marker of CSCs responsible for tumor initiation, reinitiation after chemotherapy, and driver of metastasis (Chen et al. 2017). While chemotherapy decreased tumor cell proliferation, it increased CSC abundance. Targeting BMI1 (or its effector AP-1) decreased CSCs, and when combined with chemotherapy, the most pronounced effect on both primary tumor size and lymph node metastases was observed. This study highlights milestones in lineage tracing of functional CSCs, mechanisms for OSCC chemoresistance, and therapeutic intervention to combat CSC-mediated metastasis. Model systems such as this provide a valuable tool to further study the immune evasion niche of CSCs and use these models to track how immune-targeting agents eradicate CSCs and metastatic cells. In our own study, we targeted KrasG12D.Smad4−/− into keratin 15 (K15)–positive stem cells, which reside in the hair follicle and tongue epithelium. These mutated cells rapidly developed into aggressive SCCs that metastasized to the lung (White et al. 2013). In addition to our finding that miR-9 is critical for CSC expansion and metastasis, several other nonepithelial signature microRNAs, particularly microRNAs of hematopoietic origin, are overexpressed in these tumors (White et al. 2013). This raises the question of whether CSC reprograming by miRNAs causes them to be more immune evasive than non-CSCs.
Other examples of CSC regulators are from studies using PDX models of HNSCC to determine the efficacy of targeting interleukin (IL)–6 or 5T4 oncofetal glycoprotein (a fetal protein reexpressed in cancer) using experimental therapeutics. 5T4 was found to be a biomarker of reduced patient survival and a critical regulator of HNSCC CSC maintenance; targeted killing of 5T4-expressing cells with a single dose of MEDI0641 (anti-5T4 antibody conjugated to a cytotoxic drug) resulted in long-term tumor growth inhibition, reduction of CSCs, and prevention of recurrence (Kerk et al. 2017). Inhibition of IL-6 reduced CSC abundance and prevented recurrence in PDX models of HNSCC (Finkel et al. 2016). Elimination of CSCs is a critical barrier to prevent relapse, so translating the use of 5T4- or IL-6 targeted therapies into immune-competent models that can evaluate the effect of therapeutic antibodies in the context of native stroma and immune system is necessary to move these agents forward. Because IL-6 is a potent inflammatory cytokine, it would be particularly important to understand how IL-6 inhibition influences CSCs and immune response in immunocompetent models with traceable CSCs.
Phosphatidylinositol-3 kinase (PI3K) is another critical regulator of HNSCC CSCs. Studies in HNSCC PDX cohorts identified consistently high tumor initiating capacity of ALDH+/CD44high cells and demonstrated the reliance of these HNSCC CSCs on PI3K and SOX2 activity (Keysar et al. 2017). When combined with the 4-NQO carcinogenesis model, inducible overexpression of PI3K resulted in invasive tumor formation enriched for CSCs. Tumors with overexpressed PI3K had increased inflammation in the tumor stroma, including tumor-associated macrophages (TAMs), infiltrated leukocytes, and cancer-associated inflammatory chemokines and their receptors (CCL3, CCR7, CXCL12, CXCR4, and TGFβ1) (Du et al. 2016), suggesting that PI3K overexpression has effects on both tumor and stromal cells. The tumor suppressor gene NDRG2 (N-myc downregulated gene 2) is a recently identified regulator of metastasis and PI3K in OSCC: 4-NQO carcinogenesis in Ndrg2-deficient mice resulted in larger, more numerous oral lesions that frequently metastasized to cervical lymph nodes compared to wild-type mice subjected to 4-NQO carcinogenesis. Low levels of NDRG2 were observed in human metastatic OSCC and reexpression of NDRG2-suppressed PI3K signaling, while reduced NDRG2 correlated with increased PI3K signaling and invasion (Tamura et al. 2017). Because PI3K is a critical regulator of immune cell migration, maturation, and function, and the PI3K pathway is activated by amplification or mutation of core PI3K genes or upstream receptors in >40% to 50% of HNSCCs (Cancer Genome Atlas 2015; Fruman et al. 2017; Soulieres et al. 2017), it will be critical to evaluate the influence of PI3K inhibitors in immune-competent models of OSCC to demonstrate the feasibility of these inhibitors to target CSCs and their effect on inflammatory cytokines, as well as identify consequences on antitumor immunity. Induction of oral cancer CSCs and metastasis by epidermal growth factor required PI3K-dependent glycolytic glucose metabolism (Xu et al. 2017), implying that PI3K regulation of glucose metabolism also requires attention and is discussed further below.
Models Revealing Immune Regulatory Mechanisms in Oral Cancer
Mouse models of OSCC have identified a number of immune cells, cytokines, and chemokines critical to oral carcinogenesis. 4-NQO carcinogenesis increased the chemokine CCL3 and carcinogenesis was decreased in CCL3−/− mice (da Silva et al. 2017), suggesting that CCL3 recruitment of inflammatory cells is necessary for carcinogenesis and may be a critical factor of 4-NQO + PI3K carcinogenesis described above. Similarly, 4-NQO carcinogenesis was blunted in mice lacking macrophage migration inhibitory factor (MIF), and loss of MIF decreased CCL3 and myeloid cell infiltration (presumably myeloid-derived suppressor cells) (Oghumu et al. 2016). Conversely, depletion of B lymphocytes sensitized SCCs to chemotherapy by upregulating antitumor macrophages that in turn recruited cytotoxic T lymphocytes (Affara et al. 2014). Together, these studies demonstrate that myeloid-derived cells and their recruitment by CCL3 can have both pro- and antitumor activity in SCC models, and systematic, controlled evaluation of specific chemokines and subsets of myeloid-derived cells is imperative to identify pro- and antitumor biomarkers, mechanisms of tumor progression, and immune regulation.
TGFβ is a critical inhibitor of epithelial growth but also a well-known immune suppressor. TGFβ1 overexpression occurs in over 60% of tobacco-associated HNSCCs (Lu et al. 2004). Oral epithelial expression of TGFβ1 in mice surprisingly induced massive inflammation in oral mucosa that indirectly induced epithelial hyperproliferation (Lu et al. 2004). This is contradictory to the role of TGFβ in other organs, where it is primarily immune suppressive and anti-inflammatory, highlighting the context-specific role of TGFβ in oral cancer. Interestingly, disrupting TGFβ signaling components often results in compensatory TGFβ overexpression. For example, mice expressing KrasG12D with TGFβRII deletion in the oral cavity develop HNSCC with full penetrance characterized by increased levels of TGFβ1 that is secreted into the tumor stroma, inducing macrophage recruitment and induction of IL-1β, MIP2, and SDF1 cytokines (Lu et al. 2006). This finding sheds light on human HNSCCs that have a 69% rate of TGFβRII reduction (Lu et al. 2006). Similarly, genetic SMAD4 loss, primarily through large 18q chromosomal deletion (1- or 2-allele loss), occurs in 53% of HNSCCs (149/279 patients in the Cancer Genome Atlas [TCGA]), predominately in tobacco-associated oral cancer patients (Cancer Genome Atlas 2015). Smad4 deletion is sufficient to induce spontaneous oral cancer, with a small population of tumor-bearing mice harboring regional lymph node metastases. Again, TGFβ1 is overexpressed in these tumors, causing massive leukocyte infiltration that includes macrophages, granulocytes, lymphocytes, and proinflammatory Th17 cells (Bornstein et al. 2009). We are currently using these models to investigate the role that immune cells and TGFβ signaling play in mediating tumor progression and antitumor immunity.
Oral Cancer Chemoprevention Testing in Mouse Models
The reproducible nature of mouse models of OSCC is particularly useful to test chemoprevention agents. Although PD-1 inhibitors are being used to induce antitumor T-cell activity, PD-1 blockade also prevented 4-NQO malignant progression by increasing T-cell accumulation and activity (Wang et al. 2017), suggesting that PD-1 inhibitors may be useful for chemoprevention. However, the use of a chemoprevention agent requires the agent be safe to use in healthy individuals. While the safety and cost of PD-1 inhibitors as a preventative agent for the general public are dubious, their use in high-risk individuals with preneoplastic lesions is intriguing. Dietary agents and supplements with a long history of safe consumption are attractive for chemoprevention. Bitter melon extract blunted 4-NQO carcinogenesis, preventing dysplasia and SCC progression while also suppressing proinflammatory IL-1β, IL-23, and immune checkpoint PD-1 expression (Sur et al. 2018), suggesting that this extract may exert its anticancer activity in an immune-dependent manner. Grapeseed extract and resveratrol, natural dietary phytochemicals with anticancer activity, prevented 4-NQO–induced oral carcinogenesis, including decreased severity of hyperplasia, dysplasia, and SCC, by decreasing proliferation and increasing apoptosis of tumor cells through adenosine monophosphate–activated protein kinase (AMPK) activation, a critical metabolic checkpoint (Shrotriya et al. 2015). Indeed, modulation between catabolic activities of AMPK versus anabolic activities of mTOR is a central theme in transformation and therefore chemoprevention (Inoki et al. 2012; Saxton and Sabatini 2017).
Metformin and rapamycin activation of AMPK and inhibition of mTOR are being scrutinized for HNSCC chemoprevention. Metformin has long been used to treat type II diabetes, and decades of data suggest it is relatively safe (Pernicova and Korbonits 2014). Rapamycin and its derivatives (mTOR inhibitors) are immunosuppressants used to reduce organ transplant rejection that have been used safely for decades. Metformin activates AMPK, thereby inactivating mTOR, creating a metabolic state of catabolism and opposing anabolic mTOR activities of growth and proliferation that support tumorigenesis (Inoki et al. 2012; Pernicova and Korbonits 2014). Administration of metformin prevented 4-NQO oral carcinogenesis by inhibiting mTOR, decreasing premalignant lesion size/number, and inhibiting conversion to invasive SCC (Vitale-Cross et al. 2012). In fact, metformin is being evaluated in a clinical trial to determine whether it prevents OSCC in patients with premalignant oral lesions (ClinicalTrials.gov identifier: NCT02581137). Heavy tobacco use and HPV infection are 2 risk factors for preneoplastic oral lesions and progression to OSCC. Expression of HPV E6/E7 proteins in the basal squamous epithelia induces malignant transformation when exposed to a single dose of the carcinogen 7,12-dimethylbenz[a]anthracene (DMBA). Rapamycin prevented oral tumor development driven by E6/E7 + DMBA (Callejas-Valera et al. 2016), suggesting that mTOR inhibition may also be a chemoprevention agent in HPV+ patients at risk for HNSCC. Continued research using these mouse models of tobacco- and HPV-induced premalignancy and HNSCC will identify immune cell and mTOR biomarkers that predict when a patient may benefit from immune- or mTOR-targeted prevention strategies before the onset of cancer, potentially sparing at-risk patients from developing cancer and the trauma of surgery, radiation, and complex treatment regimens.
Immune-Targeted Therapies in Oral Cancer Using Mouse Models
The low response rate of HNSCCs to FDA-approved PD-1 inhibitors highlights the importance of studying basic mechanisms of immune evasion and resistance to immunotherapy as well as developing new therapeutic strategies to combine immunotherapy with other therapeutic approaches. In an experimental therapeutics study, combination of a PD-L1 inhibitor with radiation, the current “standard of care” for oral cancer treatment, to treat mice transplanted with syngeneic oral cancer cells in the buccal mucosa decreased tumor growth and increased animal survival in a T lymphocyte–dependent manner (which did not occur with radiation or PD-L1 inhibitor alone), suggesting that radiotherapy may increase immune checkpoint therapy efficacy (Oweida et al. 2017). Similarly, combining cisplatin and PD-1 blockade delayed HNSCC syngeneic flank tumor growth and prolonged mouse survival (Tran et al. 2017). In both of these studies, radiation or cisplatin increased PD-L1 expression, suggesting that PD-L1/PD-1 inhibition is necessary to overcome immune suppression mediated by these therapies and also highlights that genotoxic therapies do not necessarily impede T-cell function. However, further studies are needed to determine how treatment dosing schedules influence T-cell survival and function to optimize tumor cell killing and maintain T-cell function. Toll-like receptor (TLR) agonists, which stimulate innate immunity, are also being explored as experimental therapeutics in mouse models and human clinical trials in combination with PD-1 blockade (ClinicalTrials.gov Identifier: NCT02521870). TLR7/9 agonists in combination with PD-1 blockade suppressed syngeneic flank HPV– and HPV+ HNSCC primary tumor growth and metastasis by stimulating antitumor macrophage activity and sustained tumor-specific adaptive T-cell immune response that suppressed secondary tumor challenge (Sato-Kaneko et al. 2017). These studies serve as a foundation of successful therapeutic combinations. Future studies may ask questions regarding specific innate and adaptive immune cell activity, cytokine/chemokine influence on treatment, new agonists and antagonists, biomarkers that may predict response to therapies, or mechanisms of resistance to these therapies.
While PD-1 inhibition may have therapeutic effects in HNSCC, especially when combined with other inhibitors as discussed above, upregulation of other inhibitory T-cell receptors can mitigate antitumor activity of PD-1 inhibition. Smad4−/− tumor cells transplanted into syngeneic mouse recipients escaped immune surveillance due to coexpression of inhibitory receptors PD-1 and LAG-3 on most CD8+ T cells (Mishra et al. 2016). Dual blockade of PD-1 and LAG-3 inhibited growth of these tumors, demonstrating the feasibility of dual-checkpoint immunotherapy to treat aggressive SCCs (Mishra et al. 2016), and this treatment combination is in clinical trials for advanced solid tumors, including HNSCC (ClinicalTrials.gov Identifiers: NCT01968109, NCT02966548, NCT03005782). Similarly, LAG-3 was shown to be correlated with larger, metastatic, and higher-grade HNSCCs, and inhibition of LAG-3 induced CD8+ T-cell antitumor response in an immunocompetent mouse model of HNSCC driven by loss of Pten and TGFβR1 (Deng et al. 2016). The early administration of LAG-3 inhibitor in this model was intended as a preventative agent, but the authors’ discovery of potential myeloid-derived suppressor cells, regulatory T cells, and chemokines that regulate myeloid cell recruitment that were suppressed by LAG-3 inhibition has incited the need for informative functional follow-up studies. Another immune suppressive molecule, B7-H4, in the same B7 family as PD-L1 and PD-L2, is reported to be elevated in human OSCC samples and correlates with poor overall survival (Wu et al. 2016). The authors show that B7-H4 is overexpressed in a genetic mouse HNSCC model induced by loss of TGFβR1 and Pten, suggesting that this model may be excellent for determining whether anti–B7-H4 induces antitumor activity or whether other checkpoint receptors may override B7-H4 therapy. To study HPV oncogene-associated OSCC in the mouse, C57BL/6 mouse tonsil epithelial cells transformed with HPV oncoproteins E6/E7 and oncogenic H-RasG12V, “MEER cells,” are transplanted into syngeneic C57BL/6 hosts (Hoover et al. 2007). Shayan et al. (2017) subcutaneously transplanted MEER cells into syngeneic hosts and found that another immune checkpoint receptor, TIM-3, is upregulated in tumors that acquire resistance to PD-1 inhibition, and significant antitumor activity could be achieved by adding a TIM-3 blocking antibody (Shayan et al. 2017). Importantly, combination of anti–PD-1 and anti–TIM-3 therapies is being tested clinically (ClinicalTrials.gov Identifier: NCT02817633), suggesting mouse models could be a surrogate to help answer questions emerging from the clinic. Because upregulation of TIM-3 required PI3K signaling (Shayan et al. 2017), the impact of PI3K/mTOR inhibition on tumor immunity is a tantalizing line of investigation.
With PI3K/mTOR playing critical roles in metabolism, tumor progression, and immune cell activity, PI3K/mTOR inhibitors as antitumor agents are being evaluated in several laboratories using multiple model systems. Because high glucose metabolism correlates with decreased T-cell infiltration in SCCs (Ottensmeier et al. 2016), the use of PI3K/mTOR inhibitors to combat glucose metabolism and increase immune cell infiltration may be as critical as the direct effect of the inhibitor against tumor cells. Additional benefit may be derived because inhibition of glycolytic metabolism in T cells increases their memory function and antitumor activity (Sukumar et al. 2013). Rapamycin plus cisplatin/radiation therapy (CRT) effectively increased mouse survival in a subcutaneous syngeneic MEER cell tumor model while decreasing primary tumor burden, lymph node metastasis, and lung metastasis with rapamycin resensitizing CRT-resistant tumors to CRT therapy (Coppock et al. 2016). Interestingly, the glycolytic metabolism of tumors generates lactate that suppresses T-cell function; mTOR inhibition reduced lactate production and increased antitumor immune response in the same tumor model (Coppock et al. 2013). Inhibition of mTOR with metformin had similar results with the OCT3 transporter, highly expressed in both HPV– and HPV+ HNSCC, serving as a biomarker of metformin responsiveness (Madera et al. 2015). Finally, a study using multiple tumor models including syngeneic MEER and SCCVII cell transplant models of HNSCC demonstrated that PI3Kγ activity in tumor macrophages suppressed T-cell activation and that inhibition of PI3Kγ promoted T-cell-dependent antitumor activity, which was further increased by addition of PD-1 blockade (Kaneda et al. 2016). The observation that PI3Kδ inhibition suppressed T-cell activity and promoted tumor growth demonstrates that PI3K isoform specificity is a critical factor in antitumor activity. These studies suggest that inhibition of mTOR/PI3K has multifunctional beneficial effects against HNSCC, and further studies will clarify how to leverage these effects for maximum efficacy.
Perspective
No single in vivo system is sufficient to investigate oral carcinogenesis and treatment. However, GEMMs and chemical carcinogenesis models have proven to be valuable tools for the study of oral cancer arising from native epithelia in immunocompetent animals. GEMMs are powerful for assessing which commonly detected genetic alterations in human oral cancer are “driver mutations” and which mutations cooperate with other events in tumorigenesis while chemical carcinogenesis models mimic numerous genetic insults from environmental carcinogens in oral cancer. Studies combining both model systems overcome single-system limitations and shorten experimental courses. Because immune-targeted therapies are entering into OSCC treatment and new immune-targeting therapies such TLR agonists, multiple immune checkpoint inhibitors, vaccines, and adoptive T-cell transfer are entering human trials (reviewed in Economopoulou et al. 2016), it is important that researchers evaluate the influence of immune cells and cytokines/chemokines in the context of their model, treatments, and experimental questions. For example, HNSCC models in immune-compromised mice have generated a wealth of knowledge regarding the genes and signaling pathways that drive tumor growth, metastasis, and CSCs, but translating these models into immune competent models is a critical next step. Such research can assess how immune cells contribute to the process in question and how therapeutic interventions may have positive or negative effects on antitumor immunity. Similarly, the evaluation of immune cell contributions to early, preneoplastic transitions in oral epithelia is critical to develop novel chemoprevention strategies. Given the fundamental differences between the immune systems of humans and mice, humanized PDX models (Morton, Bird, Refaeli, et al. 2016) can contribute to understanding human oral cancer biology. Combining mouse models and humanized PDX models will provide complementary model systems to study oral cancer immune evasion, develop strategies for prevention, and optimize therapies targeting the immune system and tumor microenvironment that will complement human clinical trials and provide models for testing hypotheses borne out of human trials.
Author Contributions
J.J. Luo, C.D. Young, H.M. Zhou, X.J. Wang, contributed to conception, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Acknowledgments
The authors thank Pamela Garl for her critical review and proofreading of this manuscript. Due to space constraints, we apologize for being unable to present and discuss many important studies pertaining to this review.
Footnotes
The original work in the Wang lab is supported by National Institutes of Health grants to X.J. Wang. J.J. Luo is supported by the State Scholarship Fund of China Scholarship Council (File No. 201506240122).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Affara NI, Ruffell B, Medler TR, Gunderson AJ, Johansson M, Bornstein S, Bergsland E, Steinhoff M, Li Y, Gong Q, et al. 2014. B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas. Cancer Cell. 25(6):809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsaqer SF, Tashkandi MM, Kartha VK, Yang YT, Alkheriji Y, Salama A, Varelas X, Kukuruzinska M, Monti S, Bais MV. 2017. Inhibition of LSD1 epigenetically attenuates oral cancer growth and metastasis. Oncotarget. 8(43):73372–73386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bais MV, Kukuruzinska M, Trackman PC. 2015. Orthotopic non-metastatic and metastatic oral cancer mouse models. Oral Oncol. 51(5):476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron U, Bujard H. 2000. Tet repressor-based system for regulated gene expression in eukaryotic cells: principles and advances. Methods Enzymol. 327:401–421. [DOI] [PubMed] [Google Scholar]
- Bauman JE, Cohen E, Ferris RL, Adelstein DJ, Brizel DM, Ridge JA, O’Sullivan B, Burtness BA, Butterfield LH, Carson WE, et al. 2017. Immunotherapy of head and neck cancer: emerging clinical trials from a National Cancer Institute head and neck cancer steering committee planning meeting. Cancer. 123(7):1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck B, Blanpain C. 2013. Unravelling cancer stem cell potential. Nat Rev Cancer. 13(10):727–738. [DOI] [PubMed] [Google Scholar]
- Bornstein S, White R, Malkoski S, Oka M, Han G, Cleaver T, Reh D, Andersen P, Gross N, Olson S, et al. 2009. Smad4 loss in mice causes spontaneous head and neck cancer with increased genomic instability and inflammation. J Clin Invest. 119(11):3408–3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callejas-Valera JL, Iglesias-Bartolome R, Amornphimoltham P, Palacios-Garcia J, Martin D, Califano JA, Molinolo AA, Gutkind JS. 2016. mTOR inhibition prevents rapid-onset of carcinogen-induced malignancies in a novel inducible HPV-16 E6/E7 mouse model. Carcinogenesis. 37(10):1014–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Network. 2015. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 517(7536):576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Wu M, Li Y, Chang I, Yuan Q, Ekimyan-Salvo M, Deng P, Yu B, Yu Y, Dong J, et al. 2017. Targeting BMI1(+) cancer stem cells overcomes chemoresistance and inhibits metastases in squamous cell carcinoma. Cell Stem Cell. 20(5):621–634.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Myers JN. 2008. Molecular pathogenesis of oral squamous cell carcinoma: implications for therapy. J Dent Res. 87(1):14–32. [DOI] [PubMed] [Google Scholar]
- Coppock JD, Vermeer PD, Vermeer DW, Lee KM, Miskimins WK, Spanos WC, Lee JH. 2016. mTOR inhibition as an adjuvant therapy in a metastatic model of HPV+ HNSCC. Oncotarget. 7(17):24228–24241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppock JD, Wieking BG, Molinolo AA, Gutkind JS, Miskimins WK, Lee JH. 2013. Improved clearance during treatment of HPV-positive head and neck cancer through mTOR inhibition. Neoplasia. 15(6):620–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva JM Moreira Dos Santos TP Sobral LM Queiroz-Junior CM Rachid MA Proudfoot AEI Garlet GP Batista AC Teixeira MM Leopoldino AM et al. . 2017. Relevance of CCL3/CCR5 axis in oral carcinogenesis. Oncotarget. 8(31):51024–51036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng WW, Mao L, Yu GT, Bu LL, Ma SR, Liu B, Gutkind JS, Kulkarni AB, Zhang WF, Sun ZJ. 2016. LAG-3 confers poor prognosis and its blockade reshapes antitumor response in head and neck squamous cell carcinoma. Oncoimmunology. 5(11):e1239005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne LK, Driver ER, Wang XJ. 2015. Head and neck cancer stem cells: from identification to tumor immune network. J Dent Res. 94(11):1524–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan V, Rieckmann T, Munscher A, Busch CJ. 2017. Current studies of immunotherapy in head and neck cancer. Clin Otolaryngol. 43(1):13–21. [DOI] [PubMed] [Google Scholar]
- Du L, Chen X, Cao Y, Lu L, Zhang F, Bornstein S, Li Y, Owens P, Malkoski S, Said S, et al. 2016. Overexpression of PIK3CA in murine head and neck epithelium drives tumor invasion and metastasis through PDK1 and enhanced TGFβ signaling. Oncogene. 35(35):4641–4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economopoulou P, Perisanidis C, Giotakis EI, Psyrri A. 2016. The emerging role of immunotherapy in head and neck squamous cell carcinoma (HNSCC): anti-tumor immunity and clinical applications. Ann Transl Med. 4(9):173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Bayoumy K, Chen KM, Zhang SM, Sun YW, Amin S, Stoner G, Guttenplan JB. 2017. Carcinogenesis of the oral cavity: environmental causes and potential prevention by black raspberry. Chem Res Toxicol. 30(1):126–144. [DOI] [PubMed] [Google Scholar]
- Feil R, Wagner J, Metzger D, Chambon P. 1997. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun. 237(3):752–757. [DOI] [PubMed] [Google Scholar]
- Ferris RL, Blumenschein G, Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, et al. 2016. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 375(19):1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel KA, Warner KA, Kerk S, Bradford CR, McLean SA, Prince ME, Zhong H, Hurt EM, Hollingsworth RE, Wicha MS, et al. 2016. IL-6 inhibition with MEDI5117 decreases the fraction of head and neck cancer stem cells and prevents tumor recurrence. Neoplasia. 18(5):273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT. 2017. The PI3K pathway in human disease. Cell. 170(4):605–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillison ML, Restighini C. 2015. Anticipation of the impact of human papillomavirus on clinical decision making for the head and neck cancer patient. Hematol Oncol Clin North Am. 29(6):1045–1060. [DOI] [PubMed] [Google Scholar]
- Hoover AC, Spanos WC, Harris GF, Anderson ME, Klingelhutz AJ, Lee JH. 2007. The role of human papillomavirus 16 E6 in anchorage-independent and invasive growth of mouse tonsil epithelium. Arch Otolaryngol Head Neck Surg. 133(5):495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Kim J, Guan KL. 2012. AMPK and mTOR in cellular energy homeostasis and drug targets. Annu Rev Pharmacol Toxicol. 52:381–400. [DOI] [PubMed] [Google Scholar]
- Ishida K, Tomita H, Nakashima T, Hirata A, Tanaka T, Shibata T, Hara A. 2017. Current mouse models of oral squamous cell carcinoma: genetic and chemically induced models. Oral Oncol. 73:16–20. [DOI] [PubMed] [Google Scholar]
- Kaneda MM, Messer KS, Ralainirina N, Li H, Leem CJ, Gorjestani S, Woo G, Nguyen AV, Figueiredo CC, Foubert P, et al. 2016. PI3Kγ is a molecular switch that controls immune suppression. Nature. 539(7629):437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellendonk C, Tronche F, Monaghan AP, Angrand PO, Stewart F, Schutz G. 1996. Regulation of Cre recombinase activity by the synthetic steroid RU 486. Nucleic Acids Res. 24(8):1404–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerk SA, Finkel KA, Pearson AT, Warner KA, Zhang Z, Nor F, Wagner VP, Vargas PA, Wicha MS, Hurt EM, et al. 2017. 5T4-targeted therapy ablates cancer stem cells and prevents recurrence of head and neck squamous cell carcinoma. Clin Cancer Res. 23(10):2516–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keysar SB, Astling DP, Anderson RT, Vogler BW, Bowles DW, Morton JJ, Paylor JJ, Glogowska MJ, Le PN, Eagles-Soukup JR, et al. 2013. A patient tumor transplant model of squamous cell cancer identifies PI3K inhibitors as candidate therapeutics in defined molecular bins. Mol Oncol. 7(4):776–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keysar SB, Le PN, Miller B, Jackson BC, Eagles JR, Nieto C, Kim J, Tang B, Glogowska MJ, Morton JJ, et al. 2017. Regulation of head and neck squamous cancer stem cells by PI3K and SOX2. J Natl Cancer Inst. 109(1): djw189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Nanavati R, Modi TG, Dobariya C. 2016. Oral cancer: etiology and risk factors: a review. J Cancer Res Ther. 12(2):458–463. [DOI] [PubMed] [Google Scholar]
- Li J, Liang F, Yu D, Qing H, Yang Y. 2013. Development of a 4-nitroquinoline-1-oxide model of lymph node metastasis in oral squamous cell carcinoma. Oral Oncol. 49(4):299–305. [DOI] [PubMed] [Google Scholar]
- Lu SL, Herrington H, Reh D, Weber S, Bornstein S, Wang D, Li AG, Tang CF, Siddiqui Y, Nord J, et al. 2006. Loss of transforming growth factor-beta type II receptor promotes metastatic head-and-neck squamous cell carcinoma. Genes Dev. 20(10):1331–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SL, Reh D, Li AG, Woods J, Corless CL, Kulesz-Martin M, Wang XJ. 2004. Overexpression of transforming growth factor beta1 in head and neck epithelia results in inflammation, angiogenesis, and epithelial hyperproliferation. Cancer Res. 64(13):4405–4410. [DOI] [PubMed] [Google Scholar]
- Madera D, Vitale-Cross L, Martin D, Schneider A, Molinolo AA, Gangane N, Carey TE, McHugh JB, Komarck CM, Walline HM, et al. 2015. Prevention of tumor growth driven by PIK3CA and HPV oncogenes by targeting mTOR signaling with metformin in oral squamous carcinomas expressing OCT3. Cancer Prev Res (Phila). 8(3):197-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra AK, Kadoishi T, Wang X, Driver E, Chen Z, Wang XJ, Wang JH. 2016. Squamous cell carcinomas escape immune surveillance via inducing chronic activation and exhaustion of CD8+ T cells co-expressing PD-1 and LAG-3 inhibitory receptors. Oncotarget. 7(49):81341–81356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton JJ, Bird G, Keysar SB, Astling DP, Lyons TR, Anderson RT, Glogowska MJ, Estes P, Eagles JR, Le PN, et al. 2016. Xactmice: humanizing mouse bone marrow enables microenvironment reconstitution in a patient-derived xenograft model of head and neck cancer. Oncogene. 35(3):290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton JJ, Bird G, Refaeli Y, Jimeno A. 2016. Humanized mouse xenograft models: narrowing the tumor-microenvironment gap. Cancer Res. 76(21):6153–6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oghumu S, Knobloch TJ, Terrazas C, Varikuti S, Ahn-Jarvis J, Bollinger CE, Iwenofu H, Weghorst CM, Satoskar AR. 2016. Deletion of macrophage migration inhibitory factor inhibits murine oral carcinogenesis: potential role for chronic pro-inflammatory immune mediators. Int J Cancer. 139(6):1379–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottensmeier CH, Perry KL, Harden EL, Stasakova J, Jenei V, Fleming J, Wood O, Woo J, Woelk CH, Thomas GJ, et al. 2016. Upregulated glucose metabolism correlates inversely with CD8+ T-cell infiltration and survival in squamous cell carcinoma. Cancer Res. 76(14):4136–4148. [DOI] [PubMed] [Google Scholar]
- Oweida A, Lennon S, Calame D, Korpela S, Bhatia S, Sharma J, Graham C, Binder D, Serkova N, Raben D, et al. 2017. Ionizing radiation sensitizes tumors to PD-L1 immune checkpoint blockade in orthotopic murine head and neck squamous cell carcinoma. Oncoimmunology. 6(10):e1356153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernicova I, Korbonits M. 2014. Metformin—mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol. 10(3):143–156. [DOI] [PubMed] [Google Scholar]
- Pfister DG, Spencer S, Brizel DM, Burtness B, Busse PM, Caudell JJ, Cmelak AJ, Colevas AD, Dunphy F, Eisele DW, et al. 2015. Head and neck cancers, version 1.2015. J Natl Compr Canc Netw. 13(7):847–855; quiz 856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato-Kaneko F, Yao S, Ahmadi A, Zhang SS, Hosoya T, Kaneda MM, Varner JA, Pu M, Messer KS, Guiducci C, et al. 2017. Combination immunotherapy with TLR agonists and checkpoint inhibitors suppresses head and neck cancer. JCI Insight. 2(18): 93397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton RA, Sabatini DM. 2017. mTOR signaling in growth, metabolism, and disease. Cell. 168(6):960–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP, Heath K, McClanahan T, Lunceford J, Gause C, et al. 2016. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 17(7):956–965. [DOI] [PubMed] [Google Scholar]
- Shayan G, Srivastava R, Li J, Schmitt N, Kane LP, Ferris RL. 2017. Adaptive resistance to anti-PD1 therapy by Tim-3 upregulation is mediated by the PI3K-Akt pathway in head and neck cancer. Oncoimmunology. 6(1):e1261779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrotriya S, Tyagi A, Deep G, Orlicky DJ, Wisell J, Wang XJ, Sclafani RA, Agarwal R, Agarwal C. 2015. Grape seed extract and resveratrol prevent 4-nitroquinoline 1-oxide induced oral tumorigenesis in mice by modulating AMPK activation and associated biological responses. Mol Carcinog. 54(4):291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. 2017. Cancer statistics, 2017. CA Cancer J Clin. 67(1):7–30. [DOI] [PubMed] [Google Scholar]
- Smith LP, Thomas GR. 2006. Animal models for the study of squamous cell carcinoma of the upper aerodigestive tract: a historical perspective with review of their utility and limitations. Part a. Chemically-induced de novo cancer, syngeneic animal models of HNSCC, animal models of transplanted xenogeneic human tumors. Int J Cancer. 118(9):2111–2122. [DOI] [PubMed] [Google Scholar]
- Soulieres D, Faivre S, Mesia R, Remenar E, Li SH, Karpenko A, Dechaphunkul A, Ochsenreither S, Kiss LA, Lin JC, et al. 2017. Buparlisib and paclitaxel in patients with platinum-pretreated recurrent or metastatic squamous cell carcinoma of the head and neck (BERIL-1): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Oncol. 18(3):323–335. [DOI] [PubMed] [Google Scholar]
- Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, Kryukov GV, Lawrence MS, Sougnez C, McKenna A, et al. 2011. The mutational landscape of head and neck squamous cell carcinoma. Science. 333(6046):1157–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumar M, Liu J, Ji Y, Subramanian M, Crompton JG, Yu Z, Roychoudhuri R, Palmer DC, Muranski P, Karoly ED, et al. 2013. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J Clin Invest. 123(10):4479–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Zhang Z. 2016. Patient-derived xenograft platform of OSCC: a renewable human bio-bank for preclinical cancer research and a new co-clinical model for treatment optimization. Front Med. 10(1):104–110. [DOI] [PubMed] [Google Scholar]
- Supsavhad W, Dirksen WP, Martin CK, Rosol TJ. 2016. Animal models of head and neck squamous cell carcinoma. Vet J. 210:7–16. [DOI] [PubMed] [Google Scholar]
- Sur S, Steele R, Aurora R, Varvares M, Schwetye KE, Ray RB. 2018. Bitter melon prevents the development of 4-NQO-induced oral squamous cell carcinoma in an immunocompetent mouse model by modulating immune signaling. Cancer Prev Res (Phila). 11(4):191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T, Ichikawa T, Nakahata S, Kondo Y, Tagawa Y, Yamamoto K, Nagai K, Baba T, Yamaguchi R, Futakuchi M, et al. 2017. Loss of NDRG2 expression confers oral squamous cell carcinoma with enhanced metastatic potential. Cancer Res. 77(9):2363–2374. [DOI] [PubMed] [Google Scholar]
- Tang XH, Knudsen B, Bemis D, Tickoo S, Gudas LJ. 2004. Oral cavity and esophageal carcinogenesis modeled in carcinogen-treated mice. Clin Cancer Res. 10(1 Pt 1):301–313. [DOI] [PubMed] [Google Scholar]
- Tran L, Allen CT, Xiao R, Moore E, Davis R, Park SJ, Spielbauer K, Van Waes C, Schmitt NC. 2017. Cisplatin alters antitumor immunity and synergizes with PD-1/PD-l1 inhibition in head and neck squamous cell carcinoma. Cancer Immunol Res. 5(12):1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turesky RJ. 1994. DNA adducts of heterocyclic aromatic amines, arylazides and 4-nitroquinoline 1-oxide. IARC Sci Publ. (125):217–228. [PubMed] [Google Scholar]
- Vitale-Cross L, Molinolo AA, Martin D, Younis RH, Maruyama T, Patel V, Chen W, Schneider A, Gutkind JS. 2012. Metformin prevents the development of oral squamous cell carcinomas from carcinogen-induced premalignant lesions. Cancer Prev Res (Phila). 5(4):562–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenius K, Lekholm U. 1973. Oral cancer in rats induced by the water-soluble carcinogen 4-nitrochinoline N-oxide. Odontol Revy. 24(1):39-48. [PubMed] [Google Scholar]
- Walsh NC, Kenney LL, Jangalwe S, Aryee KE, Greiner DL, Brehm MA, Shultz LD. 2017. Humanized mouse models of clinical disease. Annu Rev Pathol. 12:187–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Xie T, Wang B, William WN, Jr, Heymach JV, El-Naggar AK, Myers JN, Caulin C. 2017. PD-1 blockade prevents the development and progression of carcinogen-induced oral premalignant lesions. Cancer Prev Res (Phila). 10(12):684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei CL, Gao QH, Wu LY, Guo YQ, Wang XJ, Liu CX, Lin M, Zhou HM. 2016. Non/micro-invasive clinicopathologic methods in the assessment of oral leukoplakia multistep carcinogenesis: a case report. Int J Clin Exp Pathol. 9(9):9687–9693. [Google Scholar]
- White RA, Neiman JM, Reddi A, Han G, Birlea S, Mitra D, Dionne L, Fernandez P, Murao K, Bian L, et al. 2013. Epithelial stem cell mutations that promote squamous cell carcinoma metastasis. J Clin Invest. 123(10):4390–4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Deng WW, Yu GT, Mao L, Bu LL, Ma SR, Liu B, Zhang WF, Sun ZJ. 2016. B7-H4 expression indicates poor prognosis of oral squamous cell carcinoma. Cancer Immunol Immunother. 65(9):1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Zhang Q, Ishida Y, Hajjar S, Tang X, Shi H, Dang CV, Le AD. 2017. EGF induces epithelial-mesenchymal transition and cancer stem-like cell properties in human oral cancer cells via promoting Warburg effect. Oncotarget. 8(6):9557–9571. [DOI] [PMC free article] [PubMed] [Google Scholar]