Abstract
Introduction:
Oxidative stress–mediated fetal membrane cell aging is activated prematurely in preterm premature rupture of membranes (PPROMs). The mechanism of this phenomenon is largely understudied. Progesterone receptor membrane component 1 (PGRMC1) has been recognized as a potential protective component for maintaining fetal membrane integrity and healthy pregnancies. We aimed to investigate the effects of oxidative stress (represented by hydrogen peroxide [H2O2]) on fetal membrane and chorion cell senescence, p38 mitogen-activated protein kinase (MAPK) phosphorylation, and sirtuin 3 (SIRT3) and to examine the roles of PGRMC1 in these effects.
Methods:
Following serum starvation for 24 hours, full-thickness fetal membrane explants and primary chorion cells were treated with H2O2 at 100, 300, and 500 µM for 24 hours. Cells were fixed for cell senescence-associated β-galactosidase assay. Cell lysates were harvested for quantitive reverse transcription polymerase chain reaction to quantify SIRT3 messenger RNA. Cell lysates were harvested for Western blot to semi-quantify SIRT3 protein and p38 MAPK phosphorylation levels, respectively. To examine the role of PGRMC1, primary chorion cells underwent the same treatment mentioned above following PGRMC1 knockdown using validated PGRMC1-specific small-interfering RNA.
Results:
Hydrogen peroxide significantly induced cell senescence and p38 MAPK phosphorylation, and it significantly decreased SIRT3 expression in full-thickness fetal membrane explants and chorion cells. These effects were enhanced by PGRMC1 knockdown.
Discussion:
This study further demonstrated that oxidative stress–induced cell aging is one of the mechanisms of PPROM and PGRMC1 acts as a protective element for maintaining fetal membrane integrity by inhibiting oxidative stress–induced chorion cell aging.
Keywords: fetal membranes and chorion cells, oxidative stress, PGRMC1, PPROM, SIRT3
Introduction
Preterm premature rupture of membrane (PPROM) is poorly understood, yet it is an important contributor to neonatal morbidity and mortality. Premature chorion cell death appears to play a significant role in the mechanisms that lead to PPROM.1–3 In addition, a growing body of literature suggests that PPROM may result from oxidative stress–induced cell death and collagenolysis in fetal membranes.4–6 This hypothesis is supported by epidemiological studies linking clinical conditions known to produce oxidative stress to PPROM,7–9 and it is also supported by in vitro studies in which membrane segments exposed to oxidative stress exhibited tissue alterations consistent with PPROM.4,10–18 Recently, it is reported that oxidative DNA damage and telomere shortening (an aging marker) induced in PPROM and term fetal membrane cells lead to cellular senescence (cell aging).5,18 These studies indicate that oxidative stress–mediated cell senescence is a normal physiological process leading to term delivery, but it is activated prematurely in PPROM.5,18
Oxidative stress reflects an imbalance between the systemic manifestations of reactive oxygen species (ROS), which underlie many pregnancy complications including PPROM.16 Examples of ROS include hydrogen peroxide (H2O2), superoxide, hypochlorous acid, and nitric oxide which are all converted to within cells.19,20 Hydrogen peroxide also has the longest half-life of all ROS in cultured cells. Therefore, H2O2 was chosen to represent oxidative stress generated during pregnancy in this study.
Cells have developed a variety of pathways for responding to oxidative damage. One of the primary effectors of ROS-induced senescence is the p38 mitogen-activated protein kinase (p38 MAPK) pathway.21 The ROS-mediated oxidation of apoptosis signal-regulating kinase (ASK1) activates the p38 MAPK and its downstream effector phosphorylated-p38 MAPK, resulting in cell senescence.22 It has been previously reported that activation of p38 MAPK by oxidative stress is associated with fetal membrane amnion cell senescence.16
In addition to p38 MAPK, recent compelling data demonstrated that sirtuins are intimately linked to the cellular response to oxidative stress and aging.23 Sirtuin proteins bolster stress resistance of mammalian cells by virtue of their ability to remodel metabolism, alter inflammatory response, and enhance the ability to cope with oxidative species. Among the 7 mammalian sirtuins, sirtuin 3 (SIRT3) is the only one that has been associated with human aging.24 Sirtuin 3 deacetylates several proteins that promote resistance to oxidative stress and DNA damage.23,25 Recently, SIRT3 was found to be associated with labor in human myometrium.26 Due to the novelty of SIRT3, its role remains unknown in fetal membranes.
Because of the diversity of oxidative damage phenotypes, the proteins that regulate responses to oxidative damage in different cell types are of great interest. We have previously reported that progesterone receptor membrane component 1 (PGRMC1), a novel mediator of progesterone function in fetal membrane cells, appeared to be regulated by oxidative stress in fetal membrane cells27 and protected fetal membrane cells from pro-inflammation.28,29 Progesterone receptor membrane component 1 is a small protein that may be involved in the ligand-dependent signaling transduction.30–33 It is differentially expressed in gestational tissues and may ultimately contribute to fetal membrane rupture and initiation of parturition.34–37 Other studies have also demonstrated the protective role of PGRMC1 in oxidative damage in human breast cancer cells30 and neuronal cultures.33 More strikingly, it was reported that PGRMC1 might be associated with premature aging of the reproductive tract.38 These functions may translate to a similar role for PGRMC1 in the maintenance of fetal membrane integrity through inhibiting oxidative stress–induced cell aging. To test our postulation that PGRMC1 protects fetal membranes and chorion cells from oxidative stress–induced cell senescence mediated through PGRMC1, we treated primary chorion cells with H2O2 with and without PGRMC1 knockdown with small-interfering RNA (siRNA). Then, we measured cell senescence and cellular senescence signaling intermediators p38 MAPK and SIRT3.
Materials and Methods
This project was approved by the Duke University institutional review board (IRB). The Duke IRB approved the waiver of consent to obtain deidentified tissue that would not be used for clinical purposes.
Full-Thickness Fetal Membrane Explant and Primary Chorion Cell Culture
Fetal membranes were collected following planned uncomplicated cesarean delivery at term without rupture of membranes or labor. Blood clots were removed by scalpels and full-thickness fetal membranes were washed in warm media. Tissues were cut into 1 × 1 cm pieces and cultured in Dulbecco’s modified Eagle medium–Ham’s F12 media supplemented with 10% fetal bovine serum and antibiotics in 6-well plates. Chorion cells were harvested as we previously described.27 Briefly, fetal membrane tissues were cut into 2 × 2 in squares with forceps and scalpel. The smooth layer of amnion was removed manually. Separation of the decidua and chorion involved blunt dissection with forceps and scalpel. Chorion layer was minced by cross-cutting with scalpel blades. Tissues were processed in digestion buffer [0.125% trypsin and 0.2% collagenase; Sigma-Aldrich, St Louis, Missouri) at 37°C for about 90 minutes with periodic agitation. Cells were filtered through 4 layers of sterile gauze and centrifuged at 2000 rpm for 10 minutes. A cell separation gradient was prepared with an Optiprep column (Sigma-Aldrich) to further purify chorion cells. Purity of primary chorion cells was confirmed using immunofluorescence staining for cytokeratin. Cultures on glass chamber slides were fixed with cold methanol (−20°C) for 5 to 10 minutes. The cells were permeabilized and blocked with 1% bovine serum albumin, 5% normal goat serum, and 0.1% Tween-20 in phosphate buffered saline for 60 minutes at room temperature. After blocking, the cells were incubated with primary antibodies overnight at 4°C in humidified chambers. Primary anticytokeratin mouse monoclonal antibody (Dako, Carpinteria, California) was used at 1:200 dilution. Anti-mouse immunoglobulin G antibody was used as a negative control (R&D system, Minneapolis, Minnesota). Goat anti-mouse 4′,6-diamidino-2-phenylindole secondary antibody Alexa Fluor 488 conjugate (Life Technologies, Carlsbad, California) was used at 1:500 dilution. Slides were mounted using mounting medium for fluorescence with (Vector Laboratories, Burlingame, California) and examined with a Zeiss Axio Imager widefield fluorescence microscope.
Experimental Treatments
Following serum starvation for 24 hours, fetal membrane explants and chorion cells were treated with H2O2 at 100, 300, and 500 µM (H100, H300, and H500, respectively) for 24 hours. Cell viability and proliferation were measured using the CellTiter 96 AQueous One Solution Cell Proliferation Assay (MTS assay) kit following the manufacturer’s instructions (Promega, Madison, Wisconsin). Relative cell viability and proliferation were calculated by normalizing their absorbance to the corresponding control samples. The means and standard error of the means (SEMs) of 5 independent experiments were calculated. Tissue explant and cell lysates were harvested and processed for qRT-PCR and Western blot to determine SIRT3 messenger RNA (mRNA) and protein expression, respectively. Tissue explants and cells were fixed for cell senescence-associated β-galactosidase (SA-β-Gal) assay. To measure p38 MAPK phosphorylation levels by Western blot, tissue explants and chorion cells were treated with H100, H300, and H500 stimulation for 15, 30, and 60 minutes. To examine the role of p38 MAPK phosphorylation in chorion cell senescence, chorion cells were pretreated with p38 MAPK phosphorylation inhibitor, SB203580 at 10 µM (Selleckchem, Houston, Texas), or Dimethyl sulfoxide (vehicle) for 1 hour, and chorion cells were then treated with H300 for 30 minutes for measuring p38 MAPK phosphorylation levels and 24 hours for measuring cell senescence.
qRT-PCR
Total RNA was extracted using RNeasy Mini Kit (Qiagen, Hilden, Germany) and quantified using NanoDrop Spectrophotometer (NanoDrop, Wilmington, Delaware). Total RNA (1 µg) was used to generate complementary DNA using SuperScript III and Oligo dT (Thermo Fisher Scientific, Waltham, Massachusetts) following the manufacturer’s protocols. Complementary DNA (50 ng) was used for qRT-PCR using 2 × IQ SYBR Green supermix cocktail (Bio-Rad, Hercules, California). Primers used for SIRT3 were forward (ACCCAGTGGCATTCCAGAC) and reverse (GGCTTGGGGTTGTGAAAGAAG). Primers used for internal control gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were forward (CATGAGAAGT ATGACAACAGCCT) and reverse (AGTCCTTCCACGATACCAAAGT). The iCycler was programmed for an initial denaturation step of 95°C for 2 minutes, followed by a 2-step amplification phase of 35 cycles of 95°C for 30 seconds and 60°C for 1 minute while sampling for fluorescein emission. Samples were run in duplicates, and the mean cycle threshold (Ct) was normalized to the average GAPDH Ct. Fold changes were calculated using the ΔΔCt method after normalization. Nine replicates were performed using primary cells from 9 subjects.
Western Blot
Cells in culture were washed with cold PBS. The Radioimmunoprecipitation assay buffer (Sigma-Aldrich) with the complete mini-protease inhibitor cocktail (Roche, Mannheim, Germany) was added and cells were incubated on ice for 5 minutes. Cells were scraped off the plate and kept on ice for 30 minutes in centrifuge tubes and spun at 12 000g for 20 minutes at 4°C. The supernatant was collected. Frozen tissue explants (∼500 mg) were first pulverized and then homogenized in 1.0 mL of RIPA buffer that contained protease inhibitor. Protein concentration was determined with the Bradford assay (Bio-Rad). Total protein (25 μg) was loaded onto 10% sodium dodecyl sulfate polyacrylamide gels, separated, and then transferred onto a polyvinylidene difluoride membrane. The membranes were blocked with 5% milk in Tris-buffered saline and Tween-20 buffer and probed with primary antibody in blocking buffer overnight at 4°C. All antibodies were purchased from Cell Signaling Technology (Beverly, Massachusetts): rabbit anti-human GAPDH antibody (1:20,000), rabbit anti-human SIRT3 antibody (1:1000), rabbit anti-human phosphorylated-p38 MAPK antibody (1:1000), rabbit anti-human total p38 MAPK antibody (1:1000), and secondary antibody (1:2000). SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific) was used for film developing. Film developing was optimized to keep bands within the linear range. Band intensity was measured using ImageJ analysis software (National Institutes of Health, Bethesda, Maryland), and SIRT3 data are presented as ratios after being normalized to GAPDH. Phosphorylated p38 MAPK data are presented as ratios after being normalized to total p38 MAPK. Normalization was performed as such band intensity of target protein/band intensity of internal control (GAPDH or total p38 MAPK). Target protein is either SIRT3 or phosphorylated p38 MAPK.
Small-Interfering RNA Transfection and Experimental Treatments
Chorion cells were plated in 6-well plates and grown to 40% to 50% confluence. Cells were then transfected with scramble siRNA (Thermo Fisher Scientific, catalog# AM4611) or predesigned PGRMC1 siRNA (catalog# s21310) using Lipofectamine RNAiMAX (Thermo Fisher Scientific) to yield a final concentration of 10 nM. The siRNA transfection was performed as outlined in protocols provided by Thermo Fisher Scientific. After 24 hours of transfection, cells were harvested and total RNA was extracted for qRT-PCR to determine knockdown of PGRMC1 mRNA expression. After 72 hours of transfection, cell lysates were harvested and processed for Western blot to determine knockdown of PGRMC1 expression. The primary antibody used was rabbit anti-human PGRMC1 antibody (1:2000; Sigma-Aldrich). In parallel, cells were treated with H300 for 30 minutes (for p38 MAPK phosphorylation) or 24 hours (for SIRT3). Cells were also fixed for cell senescence assay.
Cell Senescence by SA-β-Gal Assay
Senescence cells were identified using a histochemical staining kit to measure SA-β-Gal activity, according to the manufacturer’s instruction (Sigma-Aldrich). The proportion of senescence cells in the total cell population was counted manually and reported as a percentage. The senescence cells in tissue explants were detected using senescence detection kit purchased from BioVision (Milpitas, California). Manufacturer’s instruction was followed accordingly.
Statistical Analysis
Experiments performed using tissue explant or primary chorion cells culture from 1 subject was counted as 1 repeat. All experiments were repeated more than 5 times (N > 5). Data are presented as means ± SEMs. The 2-tailed Student t test was used for 2 comparisons. The 1-way analysis of variance with the post hoc Dunnett test was used for multiple comparisons. A P < .05 was considered statistically significant. Statistical analyses were performed using GraphPad Prism 6.0 (GraphPad Software, Inc, La Jolla, California).
Results
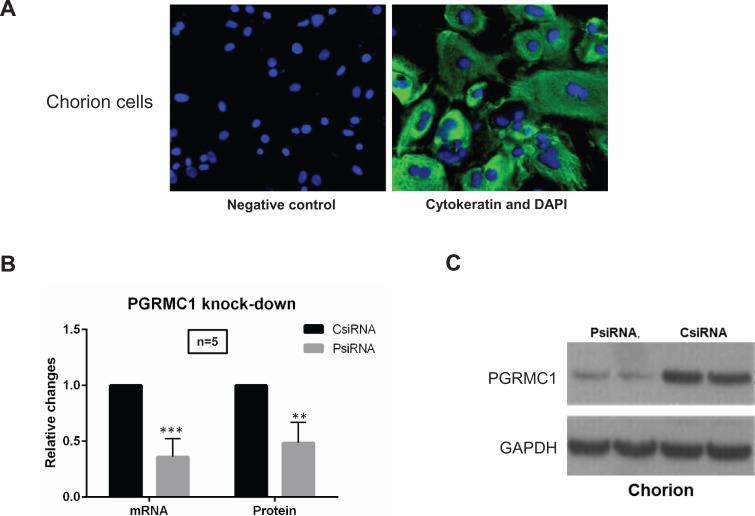
Primary chorion cell cultures were verified by immunofluorescence staining with cytokeratin (epithelial cell marker, Figure 1A) as well as observation of cell morphology. Chorion cells in our culture are epithelial-like cells manifested in a polygonal shape. Western blot analysis confirmed that PGRMC1-specific siRNA transfection significantly reduced PGRMC1 mRNA and protein expression (>50%) compared with scramble siRNA-transfected controls (Figure 1B and C).
Figure 1.
A, Immunofluorescence staining of chorion cells. Anti-human cytokeratin antibody was used for staining to distinguish chorion cell cultures (green) from mesenchymal type of cells along with observation of cell morphology. DAPI counterstaining was performed to visualize nuclei (blue). Images of cells were captured using digital camera interfaced with a fluorescence microscope. Magnification is ×40. B, C, Progesterone receptor membrane component 1 (PGRMC1) messenger RNA (mRNA) and protein knockdown in chorion cells. PsiRNA is for PGRMC1 small-interfering RNA (siRNA)-transfected group; CsiRNA is for control siRNA-transfected group. qPCR (B) and Western blot (C) were performed to confirm the knockdown of PGRMC1 mRNA (in 24 hours) and protein expression (in 72 hours), respectively. Representative Western blot and densitometry data are presented in (C).
Hydrogen Peroxide–Induced Cell Senescence, p38 MAPK Phosphorylation, and Decreased SIRT3 Expression in Primary Chorion Cells
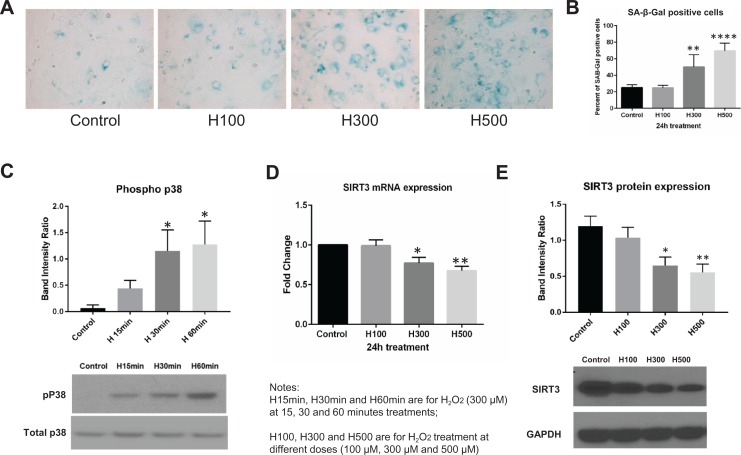
Compared with untreated controls, the proportion of SA-β-Gal-positive chorion cells was higher after exposure to H300 (25% vs 50.2%, P < .01, N = 5) and H500 (25% vs 69.6%, P < .001, N = 5), but not H100 (Figure 2A and B). These results were consistent with Western blot data demonstrating increased p38 MAPK phosphorylation after H300 treatments (Figure 2C). The increase in p38 MAPK phosphorylation reached statistical significance at 30 and 60 minutes compared with the untreated control (usually nondetectable; P < .05, N = 8). Conversely, both SIRT3 mRNA and protein expressions were significantly downregulated by treatments of H300 (P < .05 for SIRT3 mRNA, N = 9; P < .01 for SIRT3 protein, N = 14) and H500 (P < .01 for SIRT3 mRNA; P < .001 for SIRT3 protein), but not H100 (Figure 2D and E).
Figure 2.
Hydrogen peroxide (H2O2)-induced cell senescence, p38 mitogen-activated protein kinase (MAPK) phosphorylation, and downregulated sirtuin 3 (SIRT3) expression in chorion cells. A, Senescence cell histochemical staining of chorion cells treated with 0, 100, 300, and 500 μM H2O2 for 24 hours. SA-β-Gal-16 positive chorion cells are blue in representative images. B, The proportion of SA-β-Gal-positive chorion cells were summarized in a bar graph. C, Representative Western blot image to demonstrate the p38 MAPK phosphorylation under 300 μM H2O2 treatment at 15, 30, and 60 minutes. Densitometry analysis was summarized in a bar graph (N = 8). D, SIRT3 mRNA expression level in fold change calculated using the ΔΔCt method after normalization (24-hour treatments, N = 9). E, Representative Western blot image to demonstrate SIRT3 protein expression and densitometry analysis was summarized in a bar graph (24-hour treatments, N = 9).
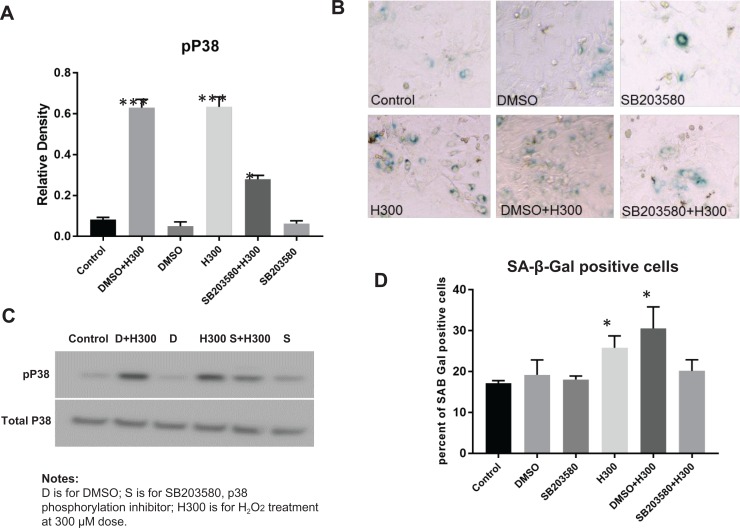
p38 MAPK Phosphorylation Inhibitor, SB203580, Inhibited H2O2-Induced p38 MAPK Phosphorylation and Attenuated H2O2-Induced Senescence in Primary Chorion Cells
Pretreatment with 10 µM SB203580 for 1 hour effectively inhibited 300 µM H2O2-induced p38 MAPK phosphorylation in chorion cells (P = .002, N = 3; Figure 3A and B). Vehicle (DMSO) did not affect p38 MAPK phosphorylation. In addition, the specificity of the p38 MAPK phosphorylation inhibitor was confirmed by the observation that no apparent change in total p38 MAPK by the inhibitor was observed. Pretreatment with SB203580 partially attenuated the 300 µM H2O2-induced chorion cell senescence manifested by reduced SA-β-Gal-positive cells in SB203580 + H300 group compared with DMSO + H300 group (20.1% ± 2.7% vs 30.5% ± 5.3%, P = .05, N = 3; Figure 3C and D). The percentage of SA-β-Gal-positive cells showed no significant differences among control and DMSO treatment groups (17.2% ± 0.6% vs 19.17% ± 3.7%, P = .6). SB203580 pretreatment did not reduce the baseline level of chorion cell senescence (SB203580 vs control: 18.0% ± 0.9% vs 17.2% ± 0.6%, P = .5). It seems that DMSO and H2O2 had synergistic effects toward inducing chorion cell senescence, but it was not statistically significant (H300 vs DMSO + H300: 25.8% ± 2.9% vs 30.5% ± 5.3%, P = .3).
Figure 3.
p38 mitogen-activated protein kinase (MAPK) phosphorylation inhibitor, SB203580, inhibited p38 MAPK phosphorylation and attenuated H2O2-induced cell senescence in chorion cells.
Hydrogen Peroxide–Induced cell senescence, p38 MAPK Phosphorylation, and Decreased SIRT3 Expression in Full-Thickness Fetal Membrane Explants
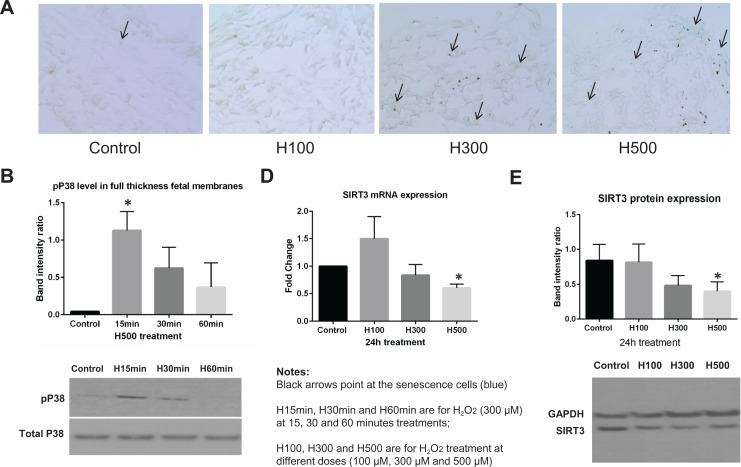
Due to the limitations of using immunohistochemistry staining for quantification, we only presented representative immunohistochemical staining images from each group. We observed a trend suggesting that the proportion of SA-β-Gal-positive cells was higher after exposure to H500 when compared with untreated controls (Figure 4A). This observation was consistent with the Western blot data showing increased p38 MAPK phosphorylation after H500 treatments (Figure 4B). The increase in p38 MAPK phosphorylation reached statistical significance at 15 minutes compared with untreated controls (P < .05, N = 6). Conversely, both SIRT3 mRNA and protein expressions were significantly downregulated by treatments of H500 (P < .05, N = 6), but not H100 and H300 (Figure 4D and E).
Figure 4.
Hydrogen peroxide (H2O2)-induced cell senescence, p38 mitogen-activated protein kinase (MAPK) phosphorylation, and downregulated sirtuin 3 (SIRT3) expression in full-thickness fetal membrane explants. A, Senescence cell histochemical staining of full-thickness fetal membrane explants treated with 0, 100, 300, and 500 μM H2O2 for 24 hours. SA-β-Gal-positive cells are blue in representative images. B, The proportion of SA-β-Gal-positive cells was summarized in a bar graph. C, Representative Western blot image to demonstrate the p38 MAPK phosphorylation under 500 μM H2O2 treatment at 15, 30, and 60 minutes. Densitometry analysis was summarized in a bar graph (N = 6). D, SIRT3 mRNA expression level in fold change calculated using the ΔΔCt method after normalization (24-hour treatments, N = 6). E, Representative Western blot image to demonstrate SIRT3 protein expression and densitometry analysis was summarized in a bar graph (24-hour treatments, N = 6).
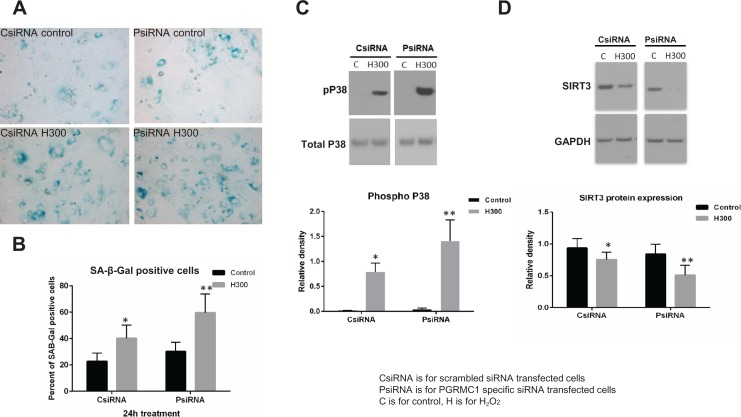
Hydrogen Peroxide–Induced Cell Senescence, p38 MAPK Phosphorylation, and Downregulation of SIRT3 Expression Were Enhanced in PGRMC1 Knockdown Cells
In PGRMC1 knockdown cells (PsiRNA), H300-induced cell senescence (N = 5) and p38 MAPK phosphorylation (P < .05, N = 8) were enhanced compared with scrambled siRNA-transfected chorion cells (CsiRNA; Figure 5A–C). The proportion of SA-β-Gal-positive cells in control and H300-treated chorion cells was 22.6% and 40.2%, respectively, in CsiRNA cells and 30.2% and 59.6%, respectively, in PsiRNA cells (P < .05). Decreased SIRT3 protein expression by H300 was also enhanced in PsiRNA chorion cells compared with CsiRNA cells (P < .05, N = 10; Figure 5D).
Figure 5.
The increased cell senescence, p38 mitogen-activated protein kinase (MAPK) phosphorylation, and downregulated sirtuin 3 (SIRT3) expression induced by hydrogen peroxide (H2O2) were enhanced in PGRMC1 knockdown chorion cells. A, Senescence cell histochemical staining of chorion cells treated with 0 and 300 μM H2O2 for 24 hours in scrambled small-interfering RNA (siRNA)-transfected chorion cells (CsiRNA) and in PGRMC1 knockdown cells (PsiRNA). SA-β-Gal-positive chorion cells are blue in representative images. B, The proportion of SA-β-Gal-positive chorion cells is summarized in a bar graph. C, Representative Western blot image to demonstrate the p38 MAPK phosphorylation under 300 μM H2O2 treatment at 30 minutes in CsiRNA and PsiRNA groups. Densitometry analysis was summarized in a bar graph (N = 8). D, Representative Western blot image to demonstrate SIRT3 protein expression in CsiRNA and PsiRNA groups and densitometry analysis was summarized in a bar graph (N = 10). MAPK indicates mitogen-activated protein kinases; H2O2, hydrogen peroxide; PGRMC1, progesterone receptor membrane component 1; siRNA, small-interfering RNA; SIRT3, sirtuin 3.
Discussion
This study provides further molecular insight into the pathophysiology of PPROM by oxidative stress. Our principal findings are 2-fold: first, the oxidative stress–induced cell senescence and related signaling pathways in full-thickness fetal membrane explants and primary chorion cell cultures, and second, the oxidative stress–induced cell senescence and related signaling pathways enhanced after depletion of PGRMC1, indicating a critical role for PGRMC1 in protecting feta membranes and chorion cells from oxidative stress–induced aging.
The chorioamnion is a biologically active membrane that is vulnerable to oxidative stress stimulation.13 Although the amnion layer provides the greatest tensile strength of the fetal membrane layers,39 the chorion layer serves an important role in maintaining pregnancy by providing a defense against infection and regulating cell death and inflammation.1,40 Previous research demonstrates evidence of accelerated chorion thinning in both term and PPROM subjects.2,3,41 In recent years, there has been growing evidence supporting the implication of chorion senescence in preterm birth.9,42–44 Here, we further demonstrated that chorion aging can be intensified by exposure to oxidative stress.
The doses of H2O2 used in this study were decided by referencing a previous study45 and our preliminary optimization study. The previous study demonstrated that only high doses of H2O2 (1 mM = 1000 µM) can induce apoptosis in amnion-derived WISH cells. We found that H2O2 did not induce cell death until the doses were above 500 µM in chorion cells. Our preliminary study also suggested that lower doses of H2O2 (under 100 µM) might represent the normal physiological level of oxidative stress in fetal membranes. Therefore, doses between 100 and 500 µM were chosen for this study. Chorion cell death was not observed under H2O2 treatments in this study. However, chorion cell proliferation was reduced by ∼20% with 500 µM H2O2 treatment compared with untreated cells (nonsignificance). While this finding was not statistically significant, we were concerned that reduced cell proliferation may influence the effects of 500 µM H2O2 on chorion cells. Therefore, all definitive conclusions were made based on the results of the 300 µM H2O2 treatments in primary chorion cells. We also previously demonstrated that H2O2 treatments do not change chorion PGRMC1 expression.27
Among the reported outcomes, the downregulation of SIRT3 by H2O2 is intriguing. Sirtuin 3 is a member of the silent information regulator 2 (Sir2) family, which is a group of NAD(+)-dependent deacetylases/ADP-ribosyltransferases (type III histone deacetylase). Sirtuin 3 has been shown to decrease ROS production in brown adipocytes.46 Embryonic fibroblasts from SIRT3-deficient mice exhibit a malignancy-prone phenotype with increased stress-induced superoxide levels and genomic instability.47 Sirtuin 3 appears to be a critical protein in the protection of early embryos against stress conditions during in vitro fertilization and culture.48 Sirtuin 3 protects neurons from oxidative stress–induced cell death.49 The SIRT3 activity may play a role in the increased oxidative stress associated with pregnancies complicated by obesity.50 Among all Sir2 family, SIRT3 was the only one which might play a role in regulating inflammation-induced labor in human myometrium.26 Inflammatory reactions induce the production of ROS: The reverse sequence of these events is also true. Given the reports described above and the findings in this study, loss or downregulation of SIRT3 could be interpreted to contribute to detrimental effects of oxidative stress to fetal membrane cells. Alternatively, downregulation of SIRT3 could increase the susceptibility of chorion cells to stress during pregnancy.
Both SIRT3 and p38 MAPK signaling pathways appear to be important in the physiological process of oxidative stress–induced fetal membrane cell aging. Through measuring the regulation of these pathways, we further discovered the critical roles of PGRMC1 in oxidative stress–induced chorion cell aging. Progesterone receptor membrane component 1 is highly expressed and actively regulated in amnion and chorion cells.27,37 Clinical and molecular studies from our group suggest potential roles of PGRMC1 in mediating progestin function in fetal membranes and maintaining fetal membrane integrity.28,37 Progesterone receptor membrane component 1 shares key structural motifs with cytochrome b5. Progesterone receptor membrane component 1 binds and activates P450 proteins to synthesize sterols and protect cells from DNA damage in unicellular eukaryotes.30 Sterols reduce the permeability of the plasma membrane to protons (positive hydrogen ions: oxidative stress). Here, we demonstrated that knockdown of PGRMC1 promoted H2O2-induced cell senescence, p38 MAPK phosphorylation, and downregulation of SIRT3 in chorion cells. This suggests that PGRMC1 provides protection against oxidative stress–induced chorion cell aging. Importantly, we previously reported that PGRMC1 expression appears to be diminished in PPROM subjects compared with term and preterm subjects without labor.37 This current finding indicates that fetal membranes with diminished PGRMC1 might be more susceptible to oxidative stress–induced cell aging. The PGRMC1 signaling pathways in this process and the potential clinical implications warrant further investigation.
Footnotes
Authors’ Note: Liping Feng designed the study, performed experiments, analyzed and interpreted the data, and prepared the manuscript. Terrence Allen participated in the statistical analysis and contributed to the manuscript preparation. William Marinello performed some of the experiments. Amy Murtha contributed to the study design and participated in manuscript preparation.
Declaration of Conflicting Interests: The author(s) declared no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Charles Hammond Fund of the Department of Obstetrics and Gynecology, Duke University (PI: Liping Feng) and National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number KL2TR001115 (PI: Terrence K. Allen). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Fortner KB, Grotegut CA, Ransom CE, et al. Bacteria localization and chorion thinning among preterm premature rupture of membranes. PLoS One. 2014;9(1):e83338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Canzoneri BJ, Feng L, Grotegut CA, Bentley RC, Heine RP, Murtha AP. The chorion layer of fetal membranes is prematurely destroyed in women with preterm premature rupture of the membranes. Reprod Sci. 2013;20(10):1246–1254. [DOI] [PubMed] [Google Scholar]
- 3. George RB, Kalich J, Yonish B, Murtha AP. Apoptosis in the chorion of fetal membranes in preterm premature rupture of membranes. Am J Perinatol. 2008;25(1):29–32. [DOI] [PubMed] [Google Scholar]
- 4. Menon R, Boldogh I, Hawkins HK, et al. Histological evidence of oxidative stress and premature senescence in preterm premature rupture of the human fetal membranes recapitulated in vitro. Am J Pathol. 2014;184(6):1740–1751. [DOI] [PubMed] [Google Scholar]
- 5. Menon R, Yu J, Basanta-Henry P, et al. Short fetal leukocyte telomere length and preterm prelabor rupture of the membranes. PLoS One. 2012;7(2):e31136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Woods JR., Jr Reactive oxygen species and preterm premature rupture of membranes—a review. Placenta. 2001;22(suppl A):S38–S44. [DOI] [PubMed] [Google Scholar]
- 7. Kacerovsky M, Tothova L, Menon R, et al. Amniotic fluid markers of oxidative stress in pregnancies complicated by preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med, 2014:1–10. [DOI] [PubMed] [Google Scholar]
- 8. Ilhan N, Celik E, Kumbak B. Maternal plasma levels of interleukin-6, C-reactive protein, vitamins C, E and A, 8-isoprostane and oxidative status in women with preterm premature rupture of membranes. J Matern Fetal Neonatal Med. 2015;28(3):316–319. [DOI] [PubMed] [Google Scholar]
- 9. Menon R, Behnia F, Polettini J, Saade GR, Campisi J, Velarde M. Placental membrane aging and HMGB1 signaling associated with human parturition. Aging (Albany NY). 2016;8(2):216–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Menon R, Fortunato SJ, Yu J, et al. Cigarette smoke induces oxidative stress and apoptosis in normal term fetal membranes. Placenta. 2011;32(4):317–322. [DOI] [PubMed] [Google Scholar]
- 11. Plessinger MA, Woods JR, Jr, Miller RK. Pretreatment of human amnion-chorion with vitamins C and E prevents hypochlorous acid-induced damage. Am J Obstet Gynecol. 2000;183(4):979–985. [DOI] [PubMed] [Google Scholar]
- 12. Michaelis J, Vissers MC, Winterbourn CC. Different effects of hypochlorous acid on human neutrophil metalloproteinases: activation of collagenase and inactivation of collagenase and gelatinase. Arch Biochem Biophys. 1992;292(2):555–562. [DOI] [PubMed] [Google Scholar]
- 13. Buhimschi IA, Kramer WB, Buhimschi CS, Thompson LP, Weiner CP. Reduction-oxidation (redox) state regulation of matrix metalloproteinase activity in human fetal membranes. Am J Obstet Gynecol. 2000;182(2):458–464. [DOI] [PubMed] [Google Scholar]
- 14. Masumoto N, Tasaka K, Mizuki J, Miyake A, Tanizawa O. Regulation of intracellular Mg2+ by superoxide in amnion cells. Biochem Biophys Res Commun. 1992;182(2):906–912. [DOI] [PubMed] [Google Scholar]
- 15. Ikebuchi Y, Masumoto N, Tasaka K, et al. Superoxide anion increases intracellular pH, intracellular free calcium, and arachidonate release in human amnion cells. J Biol Chem. 1991;266(20):13233–13237. [PubMed] [Google Scholar]
- 16. Menon R, Boldogh I, Urrabaz-Garza R, et al. Senescence of primary amniotic cells via oxidative DNA damage. PLoS One. 2013;8(12):e83416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Menon R, Polettini J, Syed TA, Saade GR, Boldogh I. Expression of 8-oxoguanine glycosylase in human fetal membranes. Am J Reprod Immunol. 2014;72(1):75–84. [DOI] [PubMed] [Google Scholar]
- 18. Polettini J, Behnia F, Taylor BD, Saade GR, Taylor RN, Menon R. Telomere fragment induced amnion cell senescence: a contributor to parturition? PLoS One. 2015;10(9):e0137188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alfadda AA, Sallam RM. Reactive oxygen species in health and disease. J Biomed Biotechnol. 2012;2012:936486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Newsholme P, Rebelato E, Abdulkader F, Krause M, Carpinelli A, Curi R. Reactive oxygen and nitrogen species generation, antioxidant defenses, and beta-cell function: a critical role for amino acids. J Endocrinol. 2012;214(1):11–20. [DOI] [PubMed] [Google Scholar]
- 21. Freund A, Patil CK, Campisi J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011;30(8):1536–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hsieh CC, Kuro-o M, Rosenblatt KP, Brobey R, Papaconstantinou J. The ASK1-signalosome regulates p38 MAPK activity in response to levels of endogenous oxidative stress in the Klotho mouse models of aging. Aging (Albany NY). 2010;2(9):597–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Merksamer PI, Liu Y, He W, Hirschey MD, Chen D, Verdin E. The sirtuins, oxidative stress and aging: an emerging link. Aging (Albany NY). 2013;5(3):144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hall JA, Dominy JE, Lee Y, Puigserver P. The sirtuin family’s role in aging and age-associated pathologies. J Clin Invest. 2013;123(3):973–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang YY, Zhou LM. Sirt3 inhibits hepatocellular carcinoma cell growth through reducing Mdm2- mediated p53 degradation. Biochem Biophys Res Commun. 2012;423(1):26–31. [DOI] [PubMed] [Google Scholar]
- 26. Lim R, Barker G, Menon R, Lappas M. A novel role for SIRT3 in regulating mediators involved in the terminal pathways of human labor and delivery. Biol Reprod. 2016;95(5):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meng Y, Murtha AP, Feng L. Progesterone, inflammatory cytokine (TNF-alpha), and oxidative stress (H2O2) regulate progesterone receptor membrane component 1 expression in fetal membrane cells. Reprod Sci. 2016;23(9):1168–1178. [DOI] [PubMed] [Google Scholar]
- 28. Allen TK, Feng L, Grotegut CA, Murtha AP. Progesterone receptor membrane component 1 as the mediator of the inhibitory effect of progestins on cytokine-induced matrix metalloproteinase 9 activity in vitro. Reprod Sci. 2014;21(2):260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Allen TK, Feng L, Nazzal M, Grotegut CA, Buhimschi IA, Murtha AP. The effect of progestins on tumor necrosis factor alpha-induced matrix metalloproteinase-9 activity and gene expression in human primary amnion and chorion cells in vitro. Anesth Analg. 2015;120(5):1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hand RA, Craven RJ. Hpr6.6 protein mediates cell death from oxidative damage in MCF-7 human breast cancer cells. J Cell Biochem. 2003;90(3):534–547. [DOI] [PubMed] [Google Scholar]
- 31. Peluso JJ. Multiplicity of progesterone’s actions and receptors in the mammalian ovary. Biol Reprod. 2006;75(1):2–8. [DOI] [PubMed] [Google Scholar]
- 32. Hughes AL, Powell DW, Bard M, et al. Dap1/PGRMC1 binds and regulates cytochrome P450 enzymes. Cell Metab. 2007;5(2):143–149. [DOI] [PubMed] [Google Scholar]
- 33. Sun F, Nguyen T, Jin X, et al. Pgrmc1/BDNF signaling plays a critical role in mediating glia-neuron cross talk. Endocrinology. 2016;157(5):2067–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zachariades E, Mparmpakas D, Pang Y, Rand-Weaver M, Thomas P, Karteris E. Changes in placental progesterone receptors in term and preterm labour. Placenta. 2012;33(5):367–372. [DOI] [PubMed] [Google Scholar]
- 35. Wang R, Sheehan PM, Brennecke SP. Changes in myometrial expression of progesterone receptor membrane components 1 and 2 are associated with human parturition at term. Reprod Fertil Dev. 2016;28(5):618–627. [DOI] [PubMed] [Google Scholar]
- 36. Wu W, Shi SQ, Huang HJ, Balducci J, Garfield RE. Changes in PGRMC1, a potential progesterone receptor, in human myometrium during pregnancy and labour at term and preterm. Mol Hum Reprod. 2011;17(4):233–242. [DOI] [PubMed] [Google Scholar]
- 37. Feng L, Antczak BC, Lan L, et al. Progesterone receptor membrane component 1 (PGRMC1) expression in fetal membranes among women with preterm premature rupture of the membranes (PPROM). Placenta. 2014;35(5):331–333. [DOI] [PubMed] [Google Scholar]
- 38. Clark NC, Pru CA, Yee SP, Lydon JP, Peluso JJ, Pru JK. Conditional ablation of progesterone receptor membrane component 2 causes female premature reproductive senescence. Endocrinology. 2017;158(3):640–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oyen ML, Calvin SE, Landers DV. Premature rupture of the fetal membranes: is the amnion the major determinant? Am J Obstet Gynecol. 2006;195(2):510–515. [DOI] [PubMed] [Google Scholar]
- 40. Uchide N, Ohyama K, Bessho T, Toyoda H. Induction of pro-inflammatory cytokine gene expression and apoptosis in human chorion cells of fetal membranes by influenza virus infection: possible implications for maintenance and interruption of pregnancy during infection. Med Sci Monit. 2005;11(1):RA7–A16. [PubMed] [Google Scholar]
- 41. Murtha AP, Auten R, Herbert WN. Apoptosis in the chorion laeve of term patients with histologic chorioamnionitis. Infect Dis Obstet Gynecol. 2002;10(2):93–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jones CW, Gambala C, Esteves KC, et al. Differences in placental telomere length suggest a link between racial disparities in birth outcomes and cellular aging. Am J Obstet Gynecol. 2017;216(3):294.e291–e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gomez-Lopez N, Romero R, Plazyo O, et al. Preterm labor in the absence of acute histologic chorioamnionitis is characterized by cellular senescence of the chorioamniotic membranes. Am J Obstet Gynecol. 2017;217(5):592.e1–e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Behnia F, Taylor BD, Woodson M, et al. Chorioamniotic membrane senescence: a signal for parturition? Am J Obstet Gynecol. 2015;213(3):359.e351–e316. [DOI] [PubMed] [Google Scholar]
- 45. Kumar D, Lundgren DW, Moore RM, Silver RJ, Moore JJ. Hydrogen peroxide induced apoptosis in amnion-derived WISH cells is not inhibited by vitamin C. Placenta. 2004;25(4):266–272. [DOI] [PubMed] [Google Scholar]
- 46. Shi T, Wang F, Stieren E, Tong Q. SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J Biol Chem. 2005;280(14):13560–13567. [DOI] [PubMed] [Google Scholar]
- 47. Kim HS, Patel K, Muldoon-Jacobs K, et al. SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell. 2010;17(1):41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tatone C, Di Emidio G, Vitti M, et al. Sirtuin functions in female fertility: possible role in oxidative stress and aging. Oxid Med Cell Longev. 2015;2015: 659687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Someya S, Yu W, Hallows WC, et al. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143(5):802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Boyle KE, Newsom SA, Janssen RC, Lappas M, Friedman JE. Skeletal muscle MnSOD, mitochondrial complex II, and SIRT3 enzyme activities are decreased in maternal obesity during human pregnancy and gestational diabetes mellitus. J Clin Endocrinol Metab. 2013;98(10):E1601–E1609. [DOI] [PMC free article] [PubMed] [Google Scholar]