Abstract
Objective:
To investigate how the mode of conception affects maternal relaxin, creatinine, and electrolyte concentrations.
Background:
Pregnancies achieved by fertility treatment often begin in a nonphysiologic endocrine milieu with no corpus luteum (CL) or with many corpora lutea. The CL produces not only estradiol and progesterone but is also the sole source of relaxin in early pregnancy, a hormone that may contribute to maternal systemic and renal vasodilation. There is limited data about maternal physiology in early pregnancy during fertility treatment, and studies have rarely considered the potential effect of the absence of the CL. To begin to address this gap in knowledge, we sought to investigate how the mode of conception affects maternal relaxin, creatinine, and electrolyte concentrations.
Methods:
One hundred eighty-four women who received care at an academic infertility practice provided serum samples. Levels of relaxin 2, creatinine, and electrolytes were compared between 4 groups defined on the basis of mode of conception which corresponded to categories of CL number: (1) absence of the CL, (2) single CL, (3) multiple CL from ovarian stimulation not including in vitro fertilization (IVF), and (4) multiple CL from IVF with fresh embryo transfer.
Results:
Relaxin-2 levels were undetectable in patients lacking a CL. Creatinine, sodium, and total CO2 levels were significantly higher in the 0 CL group (relaxin absent) compared to all other groups (relaxin present). Compared to clomiphene, use of letrozole was associated with a lower relaxin level.
Conclusion:
Early creatinine and sodium concentrations are increased in the absence of relaxin. Given the increasing utilization of frozen embryo transfer, further studies comparing programmed with natural cycles are warranted.
Keywords: female infertility, assisted reproduction, pregnancy, corpus luteum, relaxin
Introduction
Pregnancy is accompanied by dramatic changes in maternal physiology epitomized by profound vasodilation and changes in osmoregulation. These remarkable transformations actually begin in the luteal phase and then rapidly unfold during the first and early second trimesters, after which they are maintained throughout the remainder of gestation.1–5 In a spontaneous pregnancy achieved without the use of fertility medications, a single corpus luteum (CL) is present, which secretes not only estradiol and progesterone but also relaxin, a 6-kDa peptide hormone. Prior studies suggest that circulating relaxin may contribute to maternal systemic and renal vasodilation and increase glomerular filtration during pregnancy.6–8 In contrast to spontaneous pregnancy, pregnancies achieved via fertility treatment often begin in a nonphysiologic endocrine milieu due to suppression of the pituitary–ovarian axis and the absence of the CL or due to superovulation with the presence of more than 1 CL. Although relaxin will be made by the placenta later in pregnancy, the CL is the sole source of relaxin in early pregnancy.9,10 Studies focusing on the impact of assisted reproductive technology (ART) on maternal physiology in pregnancy are scarce and rarely consider different treatment regimens that assess the potential effect of CL number at the time of conception and in early pregnancy.6,11
What is known is that ART appears to be associated with a higher risk for the development of hypertensive disease in pregnancy such as preeclampsia.12–14 This link was originally demonstrated for in vitro fertilization (IVF) cycles with fresh embryo transfer.12 Recent publications emphasize a higher risk of preeclampsia in frozen embryo transfer cycles compared to fresh transfers.14–16 Frozen embryo transfers are commonly performed in the context of a programmed cycle, with administration of relatively physiologic levels of estradiol and progesterone but in the absence of the CL and thus in the absence of relaxin as the pregnancy begins.14,15 This absence of relaxin in early pregnancy could have physiologic importance given that preeclampsia is thought to have origins early in pregnancy.
In the only human study to carefully examine maternal renal physiology and osmoregulation in the absence of circulating relaxin in early pregnancy, women who conceived with donor eggs and lacked a CL manifested a much subdued increase in 24-hour endogenous creatinine clearance and decrease in plasma osmolality in the first trimester, measures of glomerular filtration rate, and osmoregulation, respectively.6 Thus, although the production of relaxin may not be essential for implantation or gestation in women,17 it may play an important intermediary role in the physiologic adaptations of the late secretory phase and early pregnancy. In turn, deficient or excessive relaxin could have adverse effects on pregnancy outcomes.
In addition to this single study which examined relaxin levels in the absence of the CL, prior studies demonstrated supraphysiologic levels of circulating relaxin in ovarian stimulation throughout pregnancy and in IVF cycles between 11 and 90 days after human chorionic gonadotrophin (HCG) administration18,19 and mostly undetectable levels in pregnancies achieved by oocyte donation.20 However, there are few data addressing renal and osmoregulatory function in early pregnancy achieved with fertility treatment, and there are no recent reports assessing the effect of treatment protocols as currently practiced, including Gonadotropin-releasing hormone (GnRH) antagonist or letrozole, on circulating relaxin levels. To begin to address this gap in knowledge, we examined whether the mode of conception and associated number of CL affects circulating relaxin levels as well as renal and osmoregulatory function during early pregnancy. We also investigated whether commonly used treatment protocols differentially affect maternal serum relaxin concentrations.
Methods
Study Population
The study was approved by the institutional review board at Stanford University. Patients undergoing treatment at the Stanford Fertility and Reproductive Health Center who achieved a viable pregnancy were recruited at 8 weeks’ gestation between October 2011 and February 2014. At 8 weeks gestation, participants gave written informed consent for use of clinical data and previously stored serum samples for research purposes. The serum samples were collected at 4 weeks of gestation initially for the clinical measurement of quantitative HCG and progesterone. Serum not required for clinical testing was stored at −20°C and after patient consent was aliquoted and stored at −80°C for later analysis.
Electronic medical records were used to obtain demographic and clinical data. Additionally, at the time of consent, participants completed a research questionnaire, which provided information about race, ethnicity, and other demographic data. Participants were sorted into 2 groups: women who delivered singletons and those who delivered twins. The groups were further stratified based on the mode of conception and associated CL number at conception: (1) absence of the CL (programmed frozen embryo transfer or donor egg recipient), (2) single CL (spontaneous pregnancy after infertility, natural cycle intrauterine insemination (IUI) or natural cycle frozen embryo transfer), (3) multiple CL associated with controlled ovarian stimulation not including IVF, and (4) multiple CL associated with controlled ovarian stimulation for IVF and fresh embryo transfer. Primary analysis focused on singleton pregnancies (n = 156).
Depending on the treatment plan (ie, ovulation induction with IUI or IVF), patients followed 1 of 5 treatment protocols. The IVF patients received a long protocol of leuprolide acetate (n = 14), GnRH antagonist (n = 24), or microdose leuprolide acetate (n = 7). Medications used for controlled ovarian stimulation in non-IVF cycles included clomiphene (n = 24), letrozole (n = 11), or gonadotropins (n = 13). Progesterone was administered vaginally in gonadotropin-stimulated IUI cycles, fresh embryo transfer cycles, and programmed autologous frozen embryo transfer cycles. Donor egg recipients used intramuscular and vaginal progesterone. Estradiol was administered via estradiol patch or oral tablet for donor egg recipients and for programmed autologous frozen embryo transfers.
Quantification of Serum Hormones, Creatinine, and Electrolyte Concentrations
Serum levels of relaxin 2 were measured using an updated, validated immunosorbent assay (R&D Quantikine Enzyme-linked Immunosorbent Assay (ELISA), Minneapolis, Minnesota) with a detection range of 7.8 to 500 pg/mL. The validation included the following parameters provided by R&D systems: intra-assay precision 2.7%, interassay precision 7.3%, average % recovery 101%, specificity with no significant cross-reactivity including relaxin-1 or 3, and linearity confirmed with serial dilutions of human relaxin 2. All assay plates included samples from all groups. Serum electrolytes (Na+, K+, Cl−, Ca2+), glucose, total CO2 (tCO2), blood urea nitrogen (BUN), and creatinine were quantified by Piccolo Xpress Analyzer (Abaxis Inc, Union City, California) using Metalyte 8 Chem (Abaxis Inc, Union City, California) panel reagent disks with internal quality controls. Estradiol and progesterone levels were measured on 149 samples by ELISA (EA70 and EA74; Oxford Biomedical, Oxford, Michigan). Estradiol was initially extracted from serum using ethyl ether, and progesterone was extracted using petroleum ether. The interassay coefficient of variation of the estradiol assay was 29.5% for samples with mean value of 213 pg/mL and 14% for samples with a mean value of 88 pg/mL. The interassay coefficient of variation for the progesterone assay was 7%. Samples from an additional 35 patients were run for determination of estradiol and progesterone levels using a Siemens Immulite 1000, Malvern, Pennsylvania; the interassay coefficient of variation of pools ranging from the low to high range of the assay were 6% to 10% for estradiol and 7% to 11% for progesterone. Importantly, all blood samples were collected and processed in a comparable fashion and stored in the freezer over a similar time period for the patient cohorts. Nevertheless, previous studies have confirmed stability of analytes, including electrolytes, proteins, and hormones, under the freeze–thaw and storage conditions used in this study.21–23
Statistical Analysis
Data are presented as median and range for demographic data or 25% and 75% for measured concentrations. Serum analyte concentrations were compared among the 4 CL groups using the Kruskal-Wallis rank sum test. To test for differences among the groups, Kruskal-Wallis pairwise comparisons with Bonferroni correction at an α value of 0.0083 to adjust for multiple comparisons were performed. The coefficient of determination was reported between retrieved egg number and relaxin level. For 2-group comparisons, Mann-Whitney U test was employed (relaxin absent vs present and clomiphene vs letrozole). The type I error was controlled 2 sided at a .05 level. Statistical comparisons mainly focused on singleton pregnancies for which there were sufficient numbers enrolled, although data are presented for twin pregnancies in the Supplemental Tables.
Results
Demographics
A total of 184 women were included in the study with 156 deliveries of a singleton pregnancy and 28 deliveries of twins. Demographic data are depicted in Table 1 for singleton deliveries and Supplemental Table 1 for twin deliveries. Participant and partner age were not different for singleton pregnancies among the 4 groups. The majority of patients were either Asian or caucasian. Race is reported to allow the reader to appreciate the population studied and not because a difference was expected across CL groups.
Table 1.
Patient demographics (singleton deliveries).
| Characteristic | 0 CL | 1 CL | Multiple CL non-IVF | Multiple CL IVF | P Value |
|---|---|---|---|---|---|
| n | 36 | 50 | 37 | 33 | |
| Median participant age (range) | 36.7 (27.2-54.0) | 35.7 (27.6-51.8) | 35.0 (27.3-41.3) | 35.9 (30.3-44.5) | 0.24 |
| Participant Race | |||||
| Asian | 18 | 21 | 19 | 20 | |
| African American | 2 | ||||
| White | 15 | 28 | 17 | 11 | |
| Other | 3 | 1 | 3 | 2 | |
| Median Partner Age (range) | 40.1 (27.0-62.7) | 37.1 (23.7-54.9) | 36.7 (29.5-59.9) | 37.7 (30.0-49.0) | 0.37 |
| Partner race | |||||
| American Indian/Alaska | 1 | ||||
| Native | |||||
| Asian | 12 | 16 | 16 | 18 | |
| Native Hawaiian/Pacific Islander | 1 | ||||
| White | 22 | 33 | 19 | 11 | |
| Other | 1 | 2 | 1 | 2 | |
| Unknown | 1 | 1 | 1 | ||
Abbreviations: CL, corpus luteum; IVF, in vitro fertilization.
Hormone Concentrations
Serum hormone concentrations are presented in Table 2 (singleton deliveries) and Supplemental Table 2 (twin deliveries). Serum relaxin was undetectable in the absence of a CL (<7.8 pg/mL). Relaxin and progesterone concentrations were lower in women without a CL compared to all other groups (both P < .001). Progesterone concentrations were also lower in women without a CL compared to women with multiple CL after IVF (P < .001). We did not observe a linear relationship between number of retrieved oocytes in fresh IVF cycles and relaxin concentrations (R 2 = 0.12). Among women with delivery of twins, relaxin and progesterone levels were also significantly lower when pregnancy was achieved in the absence of a CL compared to all other CL groups (P < .001).
Table 2.
Hormone Levels of Singleton Pregnancies (Median, 25th and 75th Percentile, P Value Comparing Across 4 CL Number Groups).
| Hormones | 0 CL | 1 CL | Multiple CL non-IVF | Multiple CL IVF | P Value |
|---|---|---|---|---|---|
| n | 36 | 50 | 37 | 33 | |
| Relaxin (pg/mL) | n.d. | 182.4a (109.6, 330.5) | 284.0a (110.0, 515.5) | 173.0a (18.3, 396) | <.001 |
| Progesterone (ng/mL) | 7.5 (4.7, 14.3) | 23.9b (17.4, 29.7) | 29.1b (23.0, 41.8) | 53.8b,c (26.8, 112.4) | <.001 |
| Estradiol (pg/mL) | 201.5 (156.0, 392.9) | 191.0 (124.0, 288.0) | 253.0 (122.0, 408.0) | 354.5 (156.2, 696.8) | .07 |
Abbreviation: n.d., not detectable.
aRelaxin: comparing 1 CL, multiple CL non-IVF, or IVF to 7.8 pg/mL (the detection threshold for 0 CL): P < .001.
bProgesterone: comparing 0 CL vs. 1 CL, multiple CL non-IVF or IVF: P < .001.
cProgesterone: comparing 1 CL versus multiple CL IVF: P < .001.
Creatinine, BUN, Electrolyte, Glucose, and tCO2 Concentrations
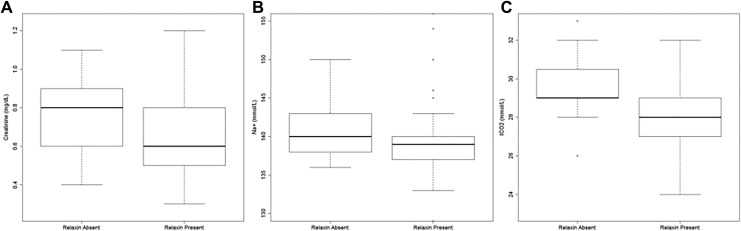
Serum creatinine and sodium concentrations were significantly higher in women without a CL compared to women with 1 CL in singleton pregnancies (Table 3). Despite similar median, women without a CL had a statistically higher tCO2 concentration (due to higher 25th and 75th percentiles) compared to women with 1 CL (P = .049). As further illustrated in Figure 1A to C, creatinine (P < .001), Na+ (P = .011), and tCO2 (P = .001) concentrations were all significantly higher in the absence of relaxin compared to the presence of relaxin. Glucose, BUN, Ca2+, K+, and Cl− did not differ among CL groups. Except for tCO2, which was higher in the absence of a CL, all other serum parameters were not different among CL groups in twin pregnancies (Supplemental Table 3). There was no linear relationship between relaxin concentrations and serum creatinine (singleton: R 2 = .006; twins: R 2 = .102), Na+ (singleton: R 2 = .02; twins: R 2 = .01), or tCO2 (singleton: R 2 = .12; twins: R 2 = .173). Progesterone levels were not related to serum creatinine (singleton: R 2 = 0; twins: R 2 = .079), Na+ (singleton: R 2 = .001; twins: R 2 = .018), or tCO2 (singleton: R 2 = .057; twins: R 2 = .017) concentrations in singleton or in twin pregnancies.
Table 3.
Concentrations of Serum Electrolytes and Other Parameters in Singleton Pregnancies (Median, 25th and 75th Percentile, P Value Comparing Across 4 CL Number Groups).
| Serum Parameters | 0 CL | 1 CL | Multiple CL Non-IVF | Multiple CL IVF | P Value |
|---|---|---|---|---|---|
| n | 35 | 35 | 23 | 23 | |
| Glucose, mg/dL | 93.0 (85.0, 107.5) | 86.0 (81.5, 98.5) | 88.0 (78.0, 92.0) | 91.0 (82.0, 97.0) | .14 |
| BUN, mg/dL | 11.0 (10.0, 13.0) | 11.0 (10.0, 12.0) | 12.0 (10.0, 13.0) | 11.0 (9.0, 13.0) | .46 |
| Ca2+, mg/dL | 9.4 (9.2, 9.8) | 9.5 (9.4, 9.7) | 9.6 (9.4, 9.7) | 9.6 (9.5, 9.8) | .29 |
| Creatinine, mg/dL | 0.8 (0.7, 0.9) | 0.7 (0.6, 0.8)a | 0.6 (0.5, 0.8)b | 0.6 (0.6, 0.8)c | .01 |
| Na+, mmol/L | 140.0 (138.0, 144.0) | 139.0 (137.0, 141.0)d | 137.0 (136.0, 139.5)e | 140.0 (138.5, 140.0)f | .004 |
| K+, mmol/dL | 4.3 (4.1, 5.0) | 4.4 (4.2, 4.6) | 4.5 (4.2, 4.9) | 4.6 (4.4, 4.9) | .26 |
| Cl−, mmol/dL | 104.0 (102.0, 106.0) | 104.0 (102.0, 105.0) | 103.0 (102.0, 105.0) | 105.0 (103.0, 105.0) | .52 |
| tCO2, mmol/L | 29.0 (29.0, 31.0) | 29.0 (28.0, 30.0)g | 28.0 (27.0, 28.0)h,i | 28.0 (27.5, 30.0)j | <.001 |
Abbreviations: BUN, blood urea nitrogen; CL, corpus luteum; IVF, in vitro fertilization.
aCreatinine: comparing 0 CL versus 1 CL: P = .07.
bCreatinine: comparing 0 CL versus multiple CL non-IVF: P = .008.
cCreatinine: comparing 0 CL versus multiple CL IVF: P = .004.
dNa+: comparing 0 CL versus 1 CL: P = .04.
eNa+: comparing 0 CL versus multiple CL non-IVF: P = .001.
fNa+: comparing multiple CL non-IVF versus multiple CL IVF: P = .014.
gtCO2: comparing 0 CL versus 1 CL: P = .049.
htCO2: comparing 0 CL versus multiple CL non-IVF: P < .001.
itCO2: comparing 1 CL versus multiple CL non-IVF: P = .009.
jtCO2: comparing multiple CL non-IVF versus multiple CL IVF: P = .017.
Figure 1.
Creatinine (A), Na+ (B), and tCO2 (C) levels depending on relaxin status (absent vs present) in singleton pregnancies. Box plots represent median and 25th and 75th percentile. Creatinine (P < .001), sodium (P = .011), and tCO2 (P = .001) concentrations are significantly higher in the absence of relaxin compared to the presence of relaxin.
Impact of Treatment Protocols on Serum Hormone Levels
Women who received letrozole for ovulation induction in non-IVF cycles had significantly lower serum relaxin concentrations compared to women who were prescribed clomiphene (Table 4). There were no differences in estradiol or progesterone concentration between clomiphene and letrozole. Hormone levels did not differ among treatment regimens used for pituitary downregulation in IVF cycles (leuprolide acetate long, leuprolide acetate microdose/flare, or GnRH antagonist; Table 4).
Table 4.
Impact of Treatment Protocols on Serum Hormone Concentrations in non-IVF (Clomiphene, Letrozole) and IVF (Leuprolide Long, Leuprolide Microdose Flare, GnRH Antagonist) Cycles.a
| Treatment Protocols | Relaxin, pg/mL | Progesterone, ng/mL | Estradiol, pg/mL |
|---|---|---|---|
| Non-IVF cycles | |||
| Clomiphene (n = 24) | 328.2b (120.8, 558.5) | 29.8 (23.3, 44.8) | 253.0 (129.5, 343.5) |
| Letrozole (n = 11) | 122.0 (81.5, 193.2) | 33.4 (24.4, 38.5) | 217.0 (104.0, 337.5) |
| IVF cycles | |||
| leuprolide long (n = 14) | 108 (12.0, 542.2) | 31.7 (16.7, 66.9) | 431.5 (240.5, 759.8) |
| leuprolide microdose flare (n = 7) | 121.0 (41.3, 734.2) | 55.6 (18.4, 70.4) | 266.5 (133.0, 668.5) |
| GnRH antagonist (n = 24) | 288.2 (36.2, 652.2) | 67.1 (36.5, 128.4) | 368.0 (164.0, 664.5) |
aData are shown as median and 25th and 75th percentiles.
b P = .03 comparing clomiphene and letrozole.
Discussion
The major finding of this work is that women lacking a CL with undetectable circulating serum relaxin have significantly higher serum creatinine, sodium, and tCO2 concentrations compared to those values for patients in which relaxin was present. This is the first study to report serum sodium and creatinine concentrations for autologous programmed frozen embryo transfers. These findings suggest potential compromise of the normal renal and osmoregulatory changes of pregnancy in the absence of the CL that might contribute to the higher risk of adverse pregnancy outcomes. In addition, this study is the first study to demonstrate that use of letrozole in non-IVF protocols is associated with lower serum relaxin levels compared to clomiphene administration.
As outlined in the introduction, fertility treatments may perturb maternal pregnancy physiology with some treatments occurring in the absence of the CL. Circulating relaxin derives solely from the CL in early human pregnancy,10,24–27 peaks at the end of first trimester, and maintains intermediate levels thereafter.28–30 In this regard, our data show a clear effect of the absence of a CL on relaxin levels in early pregnancy, essentially confirming 1 prior study.20
Luteinized granulosa cells of the ovary produce relaxin,31,32 and thus in an IVF cycle, one may expect to find an increase in serum relaxin level as the number of oocytes retrieved increases. However, similar to the findings of 1 prior group of investigators,33 we too did not find a linear relationship between the number of retrieved oocytes in fresh IVF cycles with multiple CL and the relaxin concentration. In contrast to other investigators,18,19 we did not find higher relaxin concentrations with IVF cycles compared to superovulation cycles that did not employ IVF, possibly attributable to our oocyte retrieval technique with aspiration and frequent flushing of follicles leading to removal of many granulosa cells34 or due to a difference in the protocols used for ovarian stimulation and pituitary suppression compared to the older studies.
Normal human pregnancy is characterized by profound changes in the maternal cardiovascular system with a marked decline in renal and systemic vascular resistance reaching a nadir by the end of the first or beginning of the second trimester (reviewed in35). Consequently, glomerular filtration rate and renal plasma flow increase by as much as 50% and 80%, respectively, while cardiac output rises by ∼40%. Plasma sodium and osmolality also fall during the first trimester.36 These cardiovascular and renal changes in pregnancy parallel the rise of relaxin concentrations in early gestation, providing one clue that relaxin may be important in the normal maternal renal and cardiovascular adaptations to pregnancy.37
A number of other observations support the hypothesis that relaxin plays an important role in the maternal renal and cardiovascular adaptation to pregnancy. In nonpregnant rats and humans, relaxin administration augmented cardiac output, glomerular filtration rate, renal blood flow, and reduced plasma sodium concentration and osmolality.38–40 Elimination of circulating relaxin in rats by ovariectomy or specific neutralization antibodies inhibited these hemodynamic and osmoregulatory changes at midterm pregnancy in conscious rats.7,8 Moreover, the rise in glomerular filtration rate and the fall in plasma osmolality during the first 12 weeks of pregnancy in women who conceived through egg donation, thus lacked a functioning CL and detectable relaxin levels, were significantly subdued compared to women with normal ovarian function.6
The present results provide a critically needed, independent data set, which corroborates the small study by Smith et al,40 insofar as serum creatinine, a reflection of glomerular filtration rate was significantly higher in women who lacked a CL and circulating relaxin. Although the difference in serum creatinine concentration was numerically small (∼0.8 vs 0.6 mg/dL), this reflects a 25% difference in glomerular filtration rate. In addition, in the current study, the serum sodium concentration was significantly elevated in pregnancies which began in the absence of the CL, also potentially reflecting compromise of the normal osmoregulatory adaptation to pregnancy. Although the difference in plasma sodium concentration was small (∼140 vs 138 mmol/L), this reflects a 4 mOsm/kg H2O difference in plasma osmolality, consistent with the attenuated decline in plasma osmolality observed by Smith et al.6 These findings may have true clinical importance, given the observation that subdued renal and cardiovascular changes in the first trimester have been associated with severe early onset preeclampsia or normotensive fetal growth restriction41,42 and conditions are more often observed in women who conceived with ART.12,13,16
Although it is widely held that relaxin is a key hormone involved in exerting osmoregulatory changes in pregnancy,7,43,44 it has also been suggested that sex steroids may play a role.45 In our study, serum progesterone did not correlate with plasma creatinine or sodium concentrations suggesting that absent relaxin and not low progesterone concentrations mediated the differences observed between pregnant women with and without a CL.
This study is exploring a new area of research and has limitations. The assessment is limited to the very early pregnancy and immediate effects of treatment protocols. Our data do not address the sustainability of the observed effects throughout pregnancy. The number of participants is limited particularly for comparison of medications used for treatment protocols. While clomiphene citrate, a nonsteroidal selective estrogen receptor modulator, causes a drop in circulating estrogen and a rise in gonadotropin levels, letrozole is used as an alternative drug to induce ovulation. The aromatase inhibitor reduces the conversion of androgens to estrogens and also increases the release of follicle-stimulating hormone due to estrogenic negative feedback. At this point, it remains unclear why relaxin levels are lower with letrozole treatment compared to clomiphene. Patients were not randomized to particular treatment protocols, and the medications were chosen by the treating physician.
It is interesting to speculate about how the practice of IVF could change whether the findings from this study are confirmed. Assuming that it is relaxin and not some other, as of yet unidentified, vasodilator from the CL that is responsible, then inclusion of relaxin in the luteal support regimen in women without a CL might ultimately be considered. Furthermore, if the findings are confirmed, it may ultimately be determined to be preferable to perform frozen embryo transfers in the context of a natural cycle rather than using a programmed cycle.
Conclusion
This study further supports the concept that circulating relaxin contributes to the early gestational changes in renal function and osmoregulation, insofar as in those women without circulating relaxin, serum creatinine, and sodium concentrations were significantly higher than in women with circulating relaxin. Given the increasing utilization of frozen embryo transfer within a programmed cycle which lacks a CL but involves a higher risk of preeclampsia, further studies that examine the differences in maternal renal and cardiovascular adaptation to pregnancy between programmed cycles and natural cycle frozen embryo transfers are warranted.
Supplemental Material
Supplementary_Tables for Effect of Mode of Conception on Maternal Serum Relaxin, Creatinine, and Sodium Concentrations in an Infertile Population by Frauke von Versen-Höynck, Nairi K. Strauch, Jing Liu, Yueh-Yun Chi, Maureen Keller-Woods, Kirk P. Conrad, and Valerie L. Baker in Reproductive Sciences
Acknowledgments
The authors would like to thank all participants and health-care providers who enabled the collection of these data, Raquel R. Fleischmann, DVM, CCRP, for recruiting participants and collecting data, Jessica L. Cline for performing the Piccolo Clinical Chemistry Analysis and Elaine Sumners for performing the relaxin-2 ELISA.
Authors’ Note: Frauke von Versen-Höynck and Nairi K. Strauch consider that the first two authors should be regarded as joint First Authors. The work reported was done at Stanford University and University of Florida.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: KPC discloses use patents for relaxin. All other authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Award Number P01 HD 065647-01A1 from the National Institute of Child Health and Human Development. Frauke von Versen-Höynck was funded by the German Research Foundation Heisenberg Fellowship Award (VE490/8-1).
Supplemental Material: Supplemental material is available for this article online.
References
- 1. Chapman AB, Abraham WT, Zamudio S, et al. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int. 1998;54(6):2056–2063. [DOI] [PubMed] [Google Scholar]
- 2. Petersen JW, Liu J, Chi YY, et al. Comparison of multiple non-invasive methods of measuring cardiac output during pregnancy reveals marked heterogeneity in the magnitude of cardiac output change between women. Physiol Rep. 2017;5(8):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Capeless EL, Clapp JF. Cardiovascular changes in early phase of pregnancy. Am J Obstet Gynecol. 1989;161(6 pt 1):1449–1453. [DOI] [PubMed] [Google Scholar]
- 4. Robson SC, Hunter S, Boys RJ, Dunlop W. Serial study of factors influencing changes in cardiac output during human pregnancy. Am J Physiol. 1989;256(4 pt 2):H1060–H1065. [DOI] [PubMed] [Google Scholar]
- 5. Chapman AB, Zamudio S, Woodmansee W, et al. Systemic and renal hemodynamic changes in the luteal phase of the menstrual cycle mimic early pregnancy. Am J Physiol. 1997;273(5 pt 2):F777–F782. [DOI] [PubMed] [Google Scholar]
- 6. Smith MC, Murdoch AP, Danielson LA, Conrad KP, Davison JM. Relaxin has a role in establishing a renal response in pregnancy. Fertil Steril. 2006;86(1):253–255. [DOI] [PubMed] [Google Scholar]
- 7. Novak J, Danielson LA, Kerchner LJ, et al. Relaxin is essential for renal vasodilation during pregnancy in conscious rats. J Clin Invest. 2001;107(11):1469–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Debrah DO, Novak J, Matthews JE, Ramirez RJ, Shroff SG, Conrad KP. Relaxin is essential for systemic vasodilation and increased global arterial compliance during early pregnancy in conscious rats. Endocrinology. 2006;147(11):5126–5131. [DOI] [PubMed] [Google Scholar]
- 9. Conrad KP. Maternal vasodilation in pregnancy: the emerging role of relaxin. Am J Physiol Regul Integr Comp Physiol. 2011;301(2):R267–R275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Emmi AM, Skurnick J, Goldsmith LT, et al. Ovarian control of pituitary hormone secretion in early human pregnancy. J Clin Endocrinol Metab. 1991;72(6):1359–1369. [DOI] [PubMed] [Google Scholar]
- 11. Conrad KP, Baker VL. Corpus luteal contribution to maternal pregnancy physiology and outcomes in assisted reproductive technologies. Am J Physiol Regul Integr Comp Physiol. 2013;304(2):R69–R72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhu L, Zhang Y, Liu Y, et al. Maternal and live-birth outcomes of pregnancies following assisted reproductive technology: a retrospective cohort study. Sci Rep. 2016;6:35141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Storgaard M, Loft A, Bergh C, et al. Obstetric and neonatal complications in pregnancies conceived after oocyte donation: a systematic review and meta-analysis. BJOG. 2017;124(4):561–572. [DOI] [PubMed] [Google Scholar]
- 14. Sites CK, Wilson D, Barsky M, et al. Embryo cryopreservation and preeclampsia risk. Fertil Steril. 2017;108(5):784–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen ZJ, Shi Y, Sun Y, et al. Fresh versus frozen embryos for infertility in the polycystic ovary syndrome. N Engl J Med. 2016;375(6):523–533. [DOI] [PubMed] [Google Scholar]
- 16. Wong KM, van Wely M, Mol F, Repping S, Mastenbroek S. Fresh versus frozen embryo transfers in assisted reproduction. Cochrane Database Syst Rev. 2017;3:CD011184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Johnson MR, Okokon E, Collins WP, Sharma V, Lightman SL. The effect of human chorionic gonadotropin and pregnancy on the circulating level of relaxin. J Clin Endocrinol Metab. 1991;72(5):1042–1047. [DOI] [PubMed] [Google Scholar]
- 18. Haning RV, Jr, Goldsmith LT, Seifer DB, et al. Relaxin secretion in in vitro fertilization pregnancies. Am J Obstet Gynecol. 1996;174(1 pt 1):233–240. [DOI] [PubMed] [Google Scholar]
- 19. Mushayandebvu TI, Goldsmith LT, Von Hagen S, Santoro N, Thurston D, Weiss G. Elevated maternal serum relaxin concentrations throughout pregnancy in singleton gestations after superovulation. Obstet Gynecol. 1998;92(1):17–20. [DOI] [PubMed] [Google Scholar]
- 20. Johnson MR, Abdalla H, Allman AC, Wren ME, Kirkland A, Lightman SL. Relaxin levels in ovum donation pregnancies. Fertil Steril. 1991;56(1):59–61. [PubMed] [Google Scholar]
- 21. DiMagno EP, Corle D, O’Brien JF, Masnyk IJ, Go VL, Aamodt R. Effect of long-term freezer storage, thawing, and refreezing on selected constituents of serum. Mayo Clin Proc. 1989;64(10):1226–1234. [DOI] [PubMed] [Google Scholar]
- 22. Gislefoss RE, Grimsrud TK, Morkrid L. Long-term stability of serum components in the Janus Serum Bank. Scand J Clin Lab Invest. 2008;68(5):402–409. [DOI] [PubMed] [Google Scholar]
- 23. Kale VP, Patel SG, Gunjal PS, et al. Effect of repeated freezing and thawing on 18 clinical chemistry analytes in rat serum. J Am Assoc Lab Anim Sci. 2012;51(4):475–478. [PMC free article] [PubMed] [Google Scholar]
- 24. Stoelk E, Chegini N, Lei ZM, Rao CV, Bryant-Greenwood G, Sanfilippo J. Immunocytochemical localization of relaxin in human corpora lutea: cellular and subcellular distribution and dependence on reproductive state. Biol Reprod. 1991;44(6):1140–1147. [DOI] [PubMed] [Google Scholar]
- 25. Sherwood OD. Relaxin’s physiological roles and other diverse actions. Endocr Rev. 2004;25(2):205–234. [DOI] [PubMed] [Google Scholar]
- 26. Khan-Dawood FS, Goldsmith LT, Weiss G, Dawood MY. Human corpus luteum secretion of relaxin, oxytocin, and progesterone. J Clin Endocrinol Metab. 1989;68(3):627–631. [DOI] [PubMed] [Google Scholar]
- 27. Johnson MR, Abdalla H, Allman AC, Wren ME, Kirkland A, Lightman SL. Relaxin levels in ovum donation pregnancies. Fertil Steril. 1991;56(1):59–61. [PubMed] [Google Scholar]
- 28. Sherwood O. Relaxin In: Knobil E, Neill J, Greenwald GS, et al., eds. The Physiology of Reproduction. New York, NY: Raven; 1994:861–1009. [Google Scholar]
- 29. Stewart DR, Celniker AC, Taylor CA, Jr, Cragun JR, Overstreet JW, Lasley BL. Relaxin in the peri-implantation period. J Clin Endocrinol Metab. 1990;70(6):1771–1773. [DOI] [PubMed] [Google Scholar]
- 30. Quagliarello J, Szlachter N, Steinetz BG, Goldsmith LT, Weiss G. Serial relaxin concentrations in human pregnancy. Am J Obstet Gynecol. 1979;135(1):43–44. [PubMed] [Google Scholar]
- 31. Quagliarello J, Goldsmith L, Steinetz B, Lustig DS, Weiss G. Induction of relaxin secretion in nonpregnant women by human chorionic gonadotropin. J Clin Endocrinol Metab. 1980;51(1):74–77. [DOI] [PubMed] [Google Scholar]
- 32. Gagliardi CL, Goldsmith LT, Saketos M, Weiss G, Schmidt CL. Human chorionic gonadotropin stimulation of relaxin secretion by luteinized human granulosa cells. Fertil Steril. 1992;58(2):314–320. [PubMed] [Google Scholar]
- 33. Arthur ID, Anthony FW, Adams S, Thomas EJ. Serum relaxin and the major endometrial secretory proteins in in-vitro fertilization and down-regulated hormone-supported and natural cycle frozen embryo transfer. Hum Reprod. 1996;11(1):88–91. [DOI] [PubMed] [Google Scholar]
- 34. Mok-Lin E, Brauer AA, Schattman G, Zaninovic N, Rosenwaks Z, Spandorfer S. Follicular flushing and in vitro fertilization outcomes in the poorest responders: a randomized controlled trial. Hum Reprod. 2013;28(11):2990–2995. [DOI] [PubMed] [Google Scholar]
- 35. Jeyabalan A, Conrad KP. Renal physiology and pathophysiology in pregnancy. Renal and Electrolyte Disorders. 7th ed Philadelphia, PA: Lippincott Williams & Wilkins; 2010:462–518. [Google Scholar]
- 36. Davison JM, Gilmore EA, Durr J, Robertson GL, Lindheimer MD. Altered osmotic thresholds for vasopressin secretion and thirst in human pregnancy. Am J Physiol. 1984;246(1 pt 2):F105–F109. [DOI] [PubMed] [Google Scholar]
- 37. Haning RV, Jr, Canick JA, Goldsmith LT, Shahinian KA, Erinakes NJ, Weiss G. The effect of ovulation induction on the concentration of maternal serum relaxin in twin pregnancies. Am J Obstet Gynecol. 1996;174(1 pt 1):227–232. [DOI] [PubMed] [Google Scholar]
- 38. Danielson LA, Sherwood OD, Conrad KP. Relaxin is a potent renal vasodilator in conscious rats. J Clin Invest. 1999;103(4):525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Conrad KP, Debrah DO, Novak J, Danielson LA, Shroff SG. Relaxin modifies systemic arterial resistance and compliance in conscious, nonpregnant rats. Endocrinology. 2004;145(7):3289–3296. [DOI] [PubMed] [Google Scholar]
- 40. Smith MC, Danielson LA, Conrad KP, Davison JM. Influence of recombinant human relaxin on renal hemodynamics in healthy volunteers. J Am Soc Nephrol. 2006;17(11):3192–3197. [DOI] [PubMed] [Google Scholar]
- 41. Easterling TR, Benedetti TJ, Schmucker BC, Millard SP. Maternal hemodynamics in normal and preeclamptic pregnancies: a longitudinal study. Obstet Gynecol. 1990;76(6):1061–1069. [PubMed] [Google Scholar]
- 42. Bosio PM, McKenna PJ, Conroy R, O’Herlihy C. Maternal central hemodynamics in hypertensive disorders of pregnancy. Obstet Gynecol. 1999;94(6):978–984. [DOI] [PubMed] [Google Scholar]
- 43. Weisinger RS, Burns P, Eddie LW, Wintour EM. Relaxin alters the plasma osmolality-arginine vasopressin relationship in the rat. J Endocrinol. 1993;137(3):505–510. [DOI] [PubMed] [Google Scholar]
- 44. Brunton PJ, Russell JA. Endocrine induced changes in brain function during pregnancy. Brain Res. 2010;1364:198–215. [DOI] [PubMed] [Google Scholar]
- 45. Wenner MM, Stachenfeld NS. Blood pressure and water regulation: understanding sex hormone effects within and between men and women. J Physiol. 2012;590(23):5949–5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary_Tables for Effect of Mode of Conception on Maternal Serum Relaxin, Creatinine, and Sodium Concentrations in an Infertile Population by Frauke von Versen-Höynck, Nairi K. Strauch, Jing Liu, Yueh-Yun Chi, Maureen Keller-Woods, Kirk P. Conrad, and Valerie L. Baker in Reproductive Sciences