Abstract
The pathological changes of subchondral bone during osteoarthritis (OA) development in the temporomandibular joint (TMJ) are poorly understood. In the present study, we investigated the longitudinal alterations of subchondral bone using a rat TMJ-OA model developed in our laboratory. Changes in bone mass were examined by micro-CT, and changes in osteoblast and osteoclast activities were analyzed by real-time PCR, immunohistochemistry, and TRAP staining. Subchondral bone loss was detected from 8 weeks after dental occlusion alteration and reached the maximum at 12 weeks, followed by a repair phase until 32 weeks. Although bone mass increased at late stages, poor mechanical structure and lower bone mineral density (BMD) were found in these rats. The numbers of TRAP-positive cells were increased at 12 weeks, while the numbers of osteocalcin-expressing cells were increased at both 12 and 32 weeks. Levels of mRNA expression of TRAP and cathepsin K were increased at 12 weeks, while levels of ALP and osteocalcin were increased at both 12 and 32 weeks. These findings demonstrated that there is an active bone remodeling in subchondral bone in TMJs in response to alteration in occlusion, although new bone was formed with lower BMD and poor mechanical properties.
Keywords: temporomandibular joint (TMJ), osteoarthritis (OA), bone remodeling, temporomandibular disorders (TMD)
Introduction
It has been reported that up to 10 million Americans suffer from temporomandibular disorders (TMDs) each year (Slavkin, 1996). Temporomandibular joint (TMJ) osteoarthritis (OA), characterized as progressive cartilage degradation and subchondral bone changes (XD Wang et al., 2012), is one of the most serious subgroups of TMD. One percent of the Chinese in Hong Kong was reported to have TMD-related frequent jaw pain with moderate or severe intensity (Pow et al., 2001), and 14.56% of mainland Chinese patients with TMD had radiographic signs of OA (Zhao et al., 2011).
In the early stages of OA of the knee, there is pathological remodeling with low bone mineral density (BMD) (Pelletier et al., 2004; Bouchgua et al., 2009). RANKL and OPG are critical molecules regulating bone resorption (Teitelbaum, 2000). Cathepsin K, a cystein protease mainly expressed by osteoclasts, is involved in osteoclast function and bone resorption via the cleavage of type I collagen (Col1), the major component of bone matrix (Kafienah et al., 1998). During bone formation, osteoblast secretes a range of proteins, including Col1, ALP, osteocalcin, and DMP-1, to form bone matrix. VEGF is also involved in subchondral bone remodeling through the regulation of angiogenesis (Dai and Rabie, 2007).
The role of occlusion in the development of TMD is still under debate (Türp and Schindler, 2012). Based on clinical observations (Wang et al., 2009), we developed a rat TMJ-OA model by creating posterior disordered occlusion. Histological changes within 12 wks were observed in this animal model, including reduced chondrocyte proliferation, increased chondrocyte apoptosis (Jiao et al., 2009), changes in aggrecan and matrix metalloproteases (MMPs) expression, and degradation of mandibular condylar cartilage (Jiao et al., 2010; Wang et al., 2010). Subchondral bone changes were also noted, such as resorption of the subchondral bone (Jiao et al., 2011) and increased osteochondral angiogenesis 24 wks after creation of the disordered occlusion (QY Wang et al., 2012). Long-term longitudinal changes in subchondral bone in response to this disordered occlusion and changes in osteoclast and osteoblast marker gene expression correlated to the morphological changes are worthy of investigation.
In vivo micro-CT scanning is a non-invasive imaging technique and facilitates a continuous and dynamic observation of the three-dimensional subchondral bone morphology of joints (Zhao et al., 2010; Chiba et al., 2011). In this study, a combined approach of in vivo micro-CT technique and histological analysis was used to evaluate the morphological changes in the condylar subchondral bone in the TMJ-OA rat model. Expression of osteoclast and osteoblast marker genes was examined by real-time PCR assay. Changes in mRNA expression of bone resorption-related genes, such as RANKL, OPG, cathepsin K, and TRAP, and bone-formation-related genes, such as Col1a1, Col1a2, ALP, osteocalcin, VEGF, and DMP-1, were examined to evaluate osteoclastic and osteoblastic activities. The goal of this study was to investigate longitudinal changes in the subchondral bone of TMJ-OA rats induced by experimental disordered occlusion.
Materials & Methods
Animals
Thirty-six 8-week-old female Sprague-Dawley rats (weight, 180-200 g) were used. All orthodontic and animal care procedures were approved by the university ethics committee and performed according to institutional guidelines. The rats were randomly assigned to 2 control and 2 experimental groups. Each group had 9 animals (n = 9). Sixteen of the 36 rats used for in vivo micro-CT scanning were randomly assigned to control and experimental groups (n = 8) (Appendix Table 1). Rats in experimental groups were randomly paired with those in control groups. In experimental groups, disordered occlusion was created as described previously (QY Wang et al., 2012). Briefly, an elastic rubber band (1/8#; 3M Unitek, Saint Paul, MN, USA), about 1 mm in diameter, was inserted between the first and second molars of the left side of the maxillary dentition and the right side of the mandibular dentition. In this way, the first molars were gradually moved medially by the elastic force of the rubber bands. One wk later, the rubber bands were replaced with self-curing resin (Zhangjiang Biomaterial, Shanghai, China) to maintain the gaps (about 0.8 mm) between the first and second molars until the end of the treatment. Ten days after the beginning of the experiment, the same method was used to push the left maxillary and right mandibular third molars distally. In this way, 2 first and 2 third molars were moved away from their original positions and no longer intercuspated their opposite molars. In control groups, rats underwent the same procedure, but the rubber bands were immediately pulled out so that the molars did not receive elastic force from the bands, and no resin remained between molars. Rats in experimental groups were monitored every other day. If a rubber band or self-curing resin was lost, the procedure was repeated. At the same time, a repeated sham operation was performed to the matched control rat(s). In this way, the numbers of operating times between experimental and control groups were similar, and the effects of repeated operations were balanced between the experimental and control groups. The photos showing the disordered occlusion post mortem were presented in previous reports (Wang et al., 2010; QY Wang et al., 2012).
Longitudinal Micro-CT Analysis
The rats were scanned with an in vivo micro-CT system (Inveon, Siemens, MUC, Bavaria, Germany) before and 8, 12, 16, 24, and 32 wks after surgery. Scanning was performed at 80 kV and 500 mAs. The x-ray beam was collimated to irradiate only the head of the rat and reconstructed with an isotropic voxel size of 30 µm. The micro-CT volume was calibrated in the conventional linear scale of CT numbers, otherwise known as Hounsfield units (HU), wherein the values for water and air are 0 and -1,000, respectively. The three-dimensional images acquired from microtomographic slices were utilized for quantitative evaluation. The width and length of the condylar heads were measured directly with software provided by the manufacturer (Fig. 1B). In previous studies, we observed obvious cartilage degradation and subchondral bone resorption at the middle and posterior parts of the mandibular condyle using the same animal model (Jiao et al., 2009, 2011). In the present studies, 2 cubic regions of interest (0.5 x 0.5 x 0.5 mm) at the middle points of the center and posterior condyle were selected for examination of the longitudinal changes in subchondral bone (Fig. 1C). The following parameters—BV/TV (the ratio of bone volume to tissue volume), BS/BV (the ratio of bone surface area to bone volume), Tb.Th (trabecular thickness), Tb.Sp (trabecular separation), Tb.N (trabecular number), and BMD (bone mineral density) (Appendix Table 2)—were measured and compared between experimental and control groups at different time points.
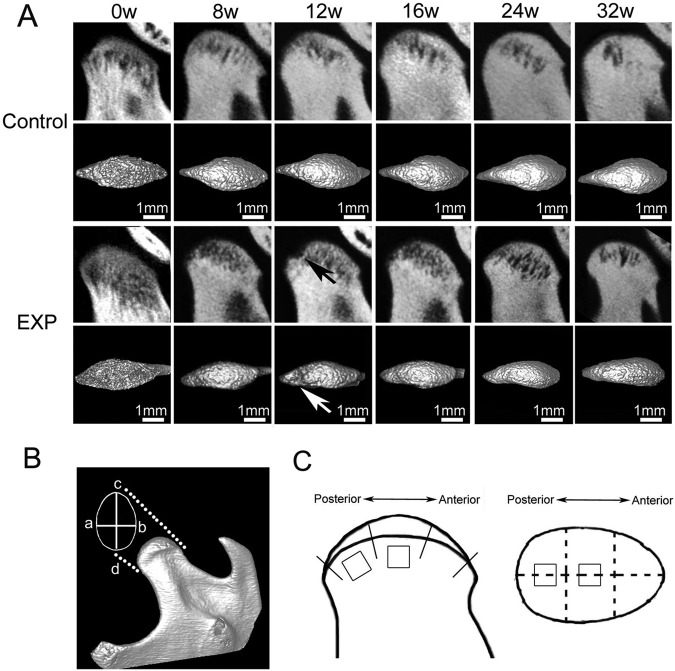
Figure 1.
In vivo micro-CT images of the TMJ condylar head from the control and experimental (EXP) groups. (A) The images were arranged from left to right according to the time sequence. Local subchondral bone lesions were observed in the experimental group. In general, the lesion regions (indicated by arrows) were larger at 12 wks after surgery than those at the other time-points. (B) Illustration of the methods for measuring the width (a–b) and length (c–d) of the condylar head. (a) The outermost point of the ventral contour of the condyle, (b) the outermost point of the dorsal contour of the condyle, (c) the most anterior point of the condyle, and (d) the most posterior point of the condyle. (C) Illustration of the method for locating the region of interest (ROI) for micro-CT analysis. The surface of the condylar head was equally divided into 3 parts, and the ROIs were located at the center of the middle and posterior parts.
Sample Preparation
Animals were sacrificed at 12 and 32 wks after surgery. Because no side differences were found in our previous work (Jiao et al., 2010), TMJ blocks on the right side of the 9 rats in each group were removed and embedded in paraffin. Five-µm-thick sections were used for HE, TRAP, and IHC staining. The subchondral bones of the condyle on the left side of the rats in each group were preserved at -70oC for mRNA extraction.
Histomorphometric Measurements
The central sagittal sections of each joint stained with HE were imaged with a Leica DFC490 system (Leica, Solms, Hessen, Germany). Two square frames (0.5 x 0.5 mm) under the interface of cartilage and subchondral bone were located at the middle of the center and posterior third of the mandibular condylar (Fig. 2B, black frames). Within the selected area, BV/TV, Tb.Th, Tb.Sp, and Tb.N were measured as described previously (Jiao et al., 2011).
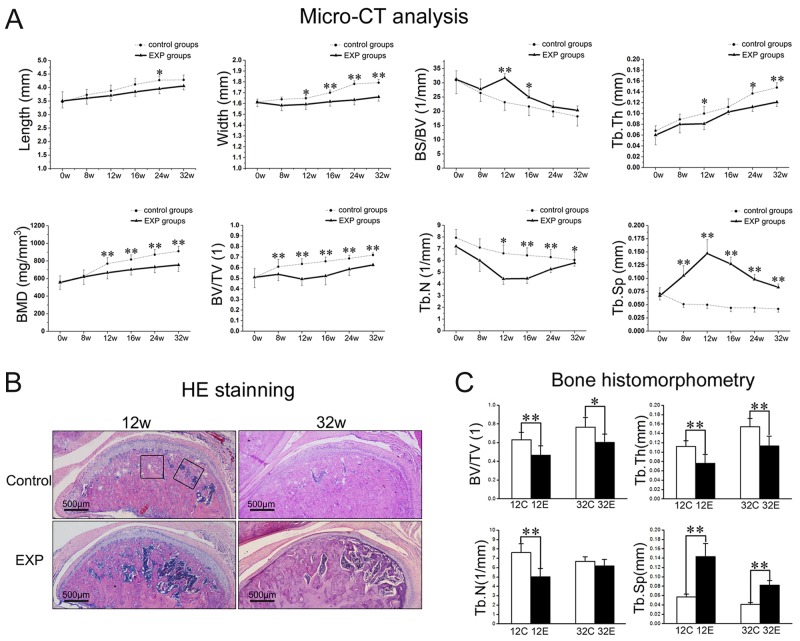
Figure 2.
Subchondral bone loss in the TMJ condyle. (A) Micro-CT analysis showed that significant bone loss started from the end of 8 wks and reached the peak at around 12 wks. (B) Hematoxylin-eosin (HE) staining of sections showed larger trabecular spacing in both the 12- and 32-week experimental groups. (C) Histomorphological parameters confirmed the significant subchondral bone loss in both the 12- and 32-week experimental groups. The black areas show the selected regions used for analysis of the histomorphological parameters (*p < 0.05, **p < 0.01).
Histochemical and Immunohistochemical Staining
We performed TRAP staining (387-A, Sigma, St. Louis, MO, USA) to examine changes in osteoclast numbers. A standard, three-step, avidin-biotin complex staining procedure was performed with anti-osteocalcin primary antibody (1:100; sc-30044; Santa Cruz Biotechnology, Santa Cruz, CA, USA) for detection of changes in osteoblast numbers. The numbers of TRAP-positive osteoclasts (Oc.N) and osteocalcin-positive (osteocalcin+) cells were counted in 5 randomly selected high-power (400x) fields under light microscopy.
RNA Extraction and Real-time PCR Assay
In each subgroup, every 3 out of 9 subchondral bone tissues were pooled to create a single sample, and 3 independent pooled samples in both experimental and control groups were prepared for analysis. RNA was isolated by Trizol (Invitrogen, Carlsbad, CA, USA), and purified with an RNeasy Mini Kit (Qiagen, Hilden, USA). Gene expression was analyzed with the Applied Biosystems 7500 Real-time PCR machine (Applied Biosystems, Foster City, CA, USA; Appendix Table 3). Data were collected from a minimum of 3 independent experiments.
Statistical Analysis
Procedures for quantification of parameters from micro-CT images were performed twice in a blinded fashion, over an interval of 1 wk, by two independent observers with no knowledge of the experimental design. There was a high level of agreement for all the above parameters (r > 0.9) between the two observers. The mean of each of the 2 measurements was used for statistical analysis. Two factors, treatment and time, were taken into consideration when 2-way analysis of variance (ANOVA) was applied for statistical analysis. When significant differences were found, a Student-Newman-Keuls post-test was performed to compare changes between groups. The p values < 0.05 were considered statistically significant.
Results
During the experiment, no difference in body weight was found in rats between experimental and age-matched control groups (data not shown).
Subchondral Trabecular Bone Loss in TMJ Condyle
Local and gradual subchondral bone lesions were observed in the experimental groups. The bone lesion regions reached the maximum at the end of 12 wks but gradually recovered thereafter (Fig. 1A). The sizes, especially the widths, of the condyles in the experimental groups were significantly smaller than those of their age-matched controls (Fig. 2A, p < 0.05). Micro-CT analysis revealed that the significantly lower values of BV/TV, but higher values of Tb.Sp, were initially observed in the experimental group at 8 wks after surgery, while lower values of BMD, Tb.N, and Tb.Th became apparent at 12 wks. These changes persisted to the end of the experiment (p < 0.05) (Fig. 2A). The BS/BV increased only at 12 and 16 wks compared with their controls (all p < 0.05) (Fig. 2A). It seems that the maximal bone loss was reached at 12 wks after surgery.
Larger marrow cavities within subchondral bone were observed in both the 12- and 32-week experimental groups than in the age-matched control groups (Fig. 2B). Histomorphometric analysis showed that BV/TV and Tb.Th significantly decreased and Tb.Sp increased in the experimental groups compared with the control groups (p < 0.05) (Fig. 2C). The Tb.N decreased in the 12-week experimental group (p < 0.05) but showed no difference in the 32-week experimental group compared with the control groups (Fig. 2C).
Osteoclast Activity
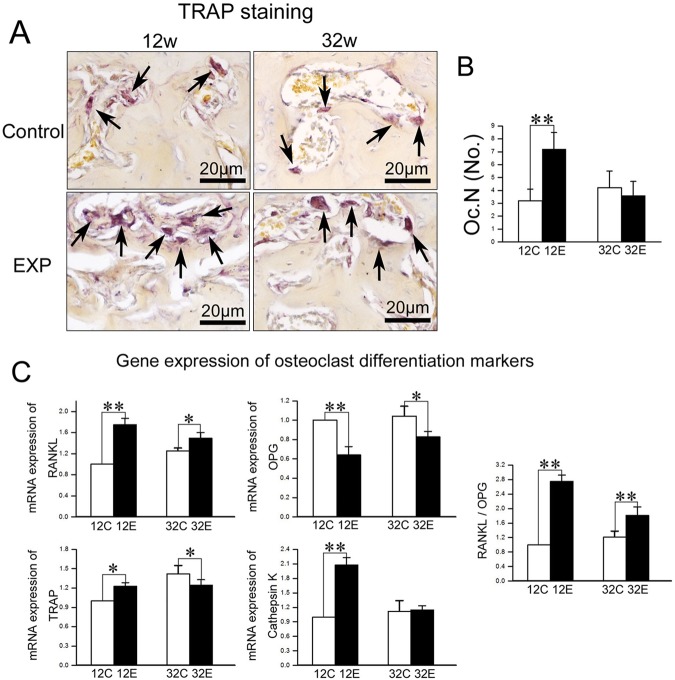
In control groups, few TRAP-positive cells were found in the subchondral bone area, while the numbers of TRAP-positive cells were much larger in the 12-week experimental group than in the age-matched control group (p < 0.01), but returned to control levels in the 32-week experimental group (Fig. 3B).
Figure 3.
Analysis of osteoclast activity in TMJ condylar subchondral bone. (A) TRAP staining of TMJ subchondral bone from 12- and 32-week control and experimental groups. (B) TRAP-positive cells with more than 3 nuclei were counted as the number of osteoclasts (Oc.N). (C) Real-time PCR analysis of expression of RANKL, OPG, TRAP, and cathepsin K genes from the subchondral bone of mandibular condylar heads in 12- and 32-week control and experimental groups (*p < 0.05, **p < 0.01).
The RANKL mRNA expression was increased while OPG expression was decreased, resulting in an increased RANKL/OPG ratio in both 12- and 32-week experimental groups compared with the age-matched control groups (p < 0.05) (Fig. 3C). The mRNA expression of TRAP and cathepsin K was increased in the 12-week experimental group, but returned to control or even lower levels in the 32-week experimental group (p < 0.05) (Fig. 3C).
Osteoblast Activity
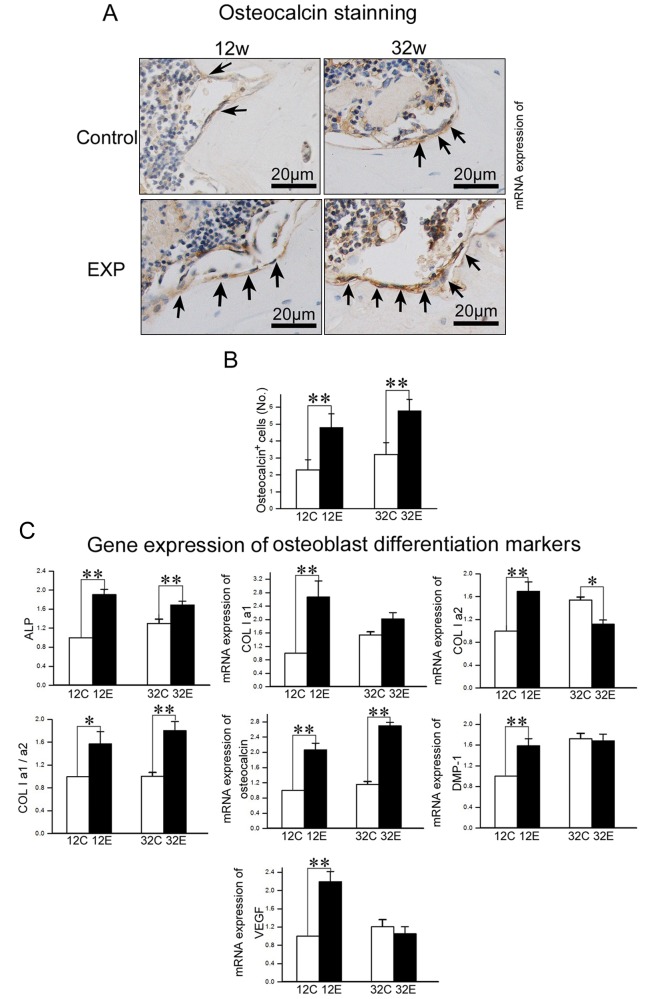
Osteocalcin-positive cells were mainly located on the trabecular bone surface, and the numbers of osteocalcin-positive cells in 12- and 32-week experimental groups were larger than those in the age-matched control groups (p < 0.01) (Figs. 4A, 4B). Compared with the controls, the mRNA expression of ALP and osteocalcin and the ratio of Col1a1/Col1a2 were significantly higher in 12- and 32-week experimental groups (p < 0.05), while expression of Col1a1, DMP-1, and VEGF was significantly increased in the 12-week (p < 0.01), but not the 32-week, experimental group (p > 0.05) (Fig. 4C).
Figure 4.
Analysis of osteoblast activity in TMJ condylar subchondral bone. (A) Osteocalcin immunohistochemical (IHC) staining of TMJ subchondral bone from 12- and 32-week control and experimental groups. (B) The number of osteocalcin-positive cells within the selected area was counted. (C) Real-time PCR analysis of expression of ALP, Col1a1, Col1a2, the ratio of Col1a1/Col1a2, osteocalcin, DMP-1, and VEGF genes from the subchondral bone of the mandibular condylar head in 12- and 32-week control and experimental groups (*p < 0.05, **p < 0.01).
Discussion
In this study, we performed a longitudinal observation on condylar subchondral bone changes using an in vivo micro-CT method. The current findings demonstrated a predominantly resorptive activity in the subchondral bone of TMJ condyles in the initial phases, followed by increased bone formation in late stages in response to changes in dental occlusion. Consistent with the higher resorptive activity in the 12-week experimental group, expression of RANKL and TRAP and the RANKL/OPG ratio were significantly increased in the experimental group. These changes returned to control levels in the 32-week experimental group. Meanwhile, the osteoblast activity and expression of ALP and osteocalcin were significantly increased in both the 12- and 32-week experimental groups. The increased ratio of mRNA expression of Col1a1/Col1a2 suggests a poor quality of newly formed bone (Bailey et al., 2002). These results are consistent with the increased subchondral bone remodeling observed in primary knee osteoarthritis (Ding et al., 2006) and a surgically induced knee OA mouse model (Pelletier et al., 2004) and also agree with our previous reports, in which we demonstrated the OA-like changes in this rat disordered occlusion model (Jiao et al., 2009; Wang et al., 2010).
During normal bone remodeling, osteoclast activity is coupled with osteoblast function. This coupling seems to be interrupted during OA development, characterized by a predominant bone resorption in the early stage and enhanced bone formation with poor quality in the late stage (Petersson et al., 1998; Bailey et al., 2001; Hunter et al., 2003). This enhanced osteoblast activity seems unable to repair the bone loss caused by increased osteoclast activity occurring at the early stage of the present rat model. The newly formed bone, which has a lower BMD value in the experimental group, is more liable to deformation when loading is applied (Day et al., 2001). The increased ratio of Col1a1/Col1a2 also suggests a poor quality of the newly formed bone (Bailey et al., 2002). In addition, the increased BS/BV in the experimental group suggests a higher remodeling activity of subchondral bone (Cowin, 2004).
The possible wear of teeth in the orthodontically placed position may be a potential contributing factor for bone formation in the late stage of the experiment. We did not evaluate the occlusal contact because it is beyond our current technical ability. It should be pointed out that human TMJ OA is a complicated disease, caused by different pathological mechanisms. The current animal model may explain 1 of these reasons, so the current findings should be interpreted with caution. The contribution of dental occlusion to TMJ OA development in patients requires further investigation. The current findings may provide significant insight into the role of dental occlusion in TMJ OA development, and thus will help us develop better treatment strategies for patients with TMD problems.
In summary, the present longitudinal in vivo micro-CT studies demonstrated a predominantly resorptive activity in the subchondral bone of TMJ condyles in the initial phases and a reparative capability in later stages in response to changes in dental occlusion. The newly formed subchondral bone is of poor quality.
Supplementary Material
Footnotes
This work was supported by the National Natural Science Foundation of China (Nos. 30872870, 30901699, and 30801303).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Bailey AJ, Buckland-Wright C, Metz D. (2001). The role of bone in osteoarthritis. Age Ageing 30:374-378. [DOI] [PubMed] [Google Scholar]
- Bailey AJ, Sims TJ, Knott L. (2002). Phenotypic expression of osteoblast collagen in osteoarthritic bone: production of type I homotrimer. Int J Biochem Cell Biol 34:176-182. [DOI] [PubMed] [Google Scholar]
- Bouchgua M, Alexander K, Carmel EN, d’Anjou MA, Beauchamp G, Richard H, et al. (2009). Use of routine clinical multimodality imaging in a rabbit model of osteoarthritis—Part II: bone mineral density assessment. Osteoarthritis Cartilage 17:197-204. [DOI] [PubMed] [Google Scholar]
- Chiba K, Ito M, Osaki M, Uetani M, Shindo H. (2011). In vivo structural analysis of subchondral trabecular bone in osteoarthritis of the hip using multi-detector row CT. Osteoarthritis Cartilage 19:180-185. [DOI] [PubMed] [Google Scholar]
- Cowin SC. (2004). Tissue growth and remodeling. Annu Rev Biomed Eng 6:77-107. [DOI] [PubMed] [Google Scholar]
- Dai J, Rabie AB. (2007). VEGF: an essential mediator of both angiogenesis and endochondral ossification. J Dent Res 86:937-950. [DOI] [PubMed] [Google Scholar]
- Day JS, Ding M, van der Linden JC, Hvid I, Sumner DR, Weinans H. (2001). A decreased subchondral trabecular bone tissue elastic modulus is associated with pre-arthritic cartilage damage. J Orthop Res 19:914-918. [DOI] [PubMed] [Google Scholar]
- Ding M, Danielsen CC, Hvid I. (2006). Age-related three-dimensional microarchitectural adaptations of subchondral bone tissues in guinea pig primary osteoarthrosis. Calcif Tissue Int 78:113-22. [DOI] [PubMed] [Google Scholar]
- Hunter DJ, Hart D, Snieder H, Bettica P, Swaminathan R, Spector TD. (2003). Evidence of altered bone turnover, vitamin D and calcium regulation with knee osteoarthritis in female twins. Rheumatology (Oxford) 42:1311-1316. [DOI] [PubMed] [Google Scholar]
- Jiao K, Wang MQ, Niu LN, Dai J, Yu SB, Liu XD, et al. (2009). Death and proliferation of chondrocytes in the degraded mandibular condylar cartilage of rats induced by experimentally created disordered occlusion. Apoptosis 14:22-30. [DOI] [PubMed] [Google Scholar]
- Jiao K, Wang MQ, Niu LN, Dai J, Yu SB, Liu XD. (2010). Mandibular condylar cartilage response to moving 2 molars in rats. Am J Orthod Dentofacial Orthop 137:460, e1-e8. [DOI] [PubMed] [Google Scholar]
- Jiao K, Niu LN, Wang MQ, Dai J, Yu SB, Liu XD, et al. (2011). Subchondral bone loss following orthodontically induced cartilage degradation in the mandibular condyles of rats. Bone 48:362-371. [DOI] [PubMed] [Google Scholar]
- Kafienah W, Bromme D, Buttle DJ, Croucher LJ, Hollander AP. (1998). Human cathepsin K cleaves native type I and II collagens at the N-terminal end of the triple helix. Biochem J 331(Pt 3):727-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier JP, Boileau C, Brunet J, Boily M, Lajeunesse D, Reboul P, et al. (2004). The inhibition of subchondral bone resorption in the early phase of experimental dog osteoarthritis by licofelone is associated with a reduction in the synthesis of MMP-13 and cathepsin K. Bone 34:527-538. [DOI] [PubMed] [Google Scholar]
- Petersson IF, Boegard T, Svensson B, Heinegard D, Saxne T. (1998). Changes in cartilage and bone metabolism identified by serum markers in early osteoarthritis of the knee joint. Br J Rheumatol 37:46-50. [DOI] [PubMed] [Google Scholar]
- Pow EH, Leung KC, McMillan AS. (2001). Prevalence of symptoms associated with temporomandibular disorders in Hong Kong Chinese. J Orofac Pain 15:228-234. [PubMed] [Google Scholar]
- Slavkin HC. (1996). A lifetime of motion: temporomandibular joints. J Am Dent Assoc 127:1093-1098. [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL. (2000). Bone resorption by osteoclasts. Science 289:1504-1508. [DOI] [PubMed] [Google Scholar]
- Türp JC, Schindler H. (2012). The dental occlusion as a suspected cause for TMDs: epidemiological and etiological considerations. J Oral Rehabil 39:502-512. [DOI] [PubMed] [Google Scholar]
- Wang GW, Wang MQ, Wang XJ, Yu SB, Liu XD, Jiao K. (2010). Changes in the expression of MMP-3, MMP-9, TIMP-1 and aggrecan in the condylar cartilage of rats induced by experimentally created disordered occlusion. Arch Oral Biol 55:887-895. [DOI] [PubMed] [Google Scholar]
- Wang MQ, Xue F, He JJ, Chen JH, Chen CS, Raustia A. (2009). Missing posterior teeth and risk of temporomandibular disorders. J Dent Res 88:942-945. [DOI] [PubMed] [Google Scholar]
- Wang QY, Dai J, Kuang B, Zhang J, Yu SB, Duan YZ, et al. (2012b). Osteochondral angiogenesis in rat mandibular condyles with osteoarthritis-like changes. Arch Oral Biol 57:620-629. [DOI] [PubMed] [Google Scholar]
- Wang XD, Kou XX, He DQ, Zeng MM, Meng Z, Bi RY, et al. (2012a). Progression of cartilage degradation, bone resorption and pain in rat temporomandibular joint osteoarthritis induced by injection of iodoacetate. PLoS One 7:e45036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Kurita H, Kurashina K, Hosoya A, Arai Y, Nakamura H. (2010). Temporomandibular joint response to mandibular deviation in rabbits detected by 3D micro-CT imaging. Arch Oral Biol 55:929-937. [DOI] [PubMed] [Google Scholar]
- Zhao YP, Zhang ZY, Wu YT, Zhang WL, Ma XC. (2011). Investigation of the clinical and radiographic features of osteoarthrosis of the temporomandibular joints in adolescents and young adults. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 111:e27-e34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.