Abstract
Nuclear factor of activated T-cells (NFAT5) is a tissue specific, osmoadaptive transcription factor essential for the control of hydration homeostasis in mammalian cells. Nuclear factor of activated T-cells regulates osmolyte transporters aldo-keto reductase family 1 member B1 (AKR1B1) and solute carrier family 5 member 3 (SLC5A3) to maintain fluid equilibrium in cells. The osmotic potential of the extracellular matrix of leiomyomas is attributed to the role of proteoglycans. In leiomyoma cells, NFAT5 is overexpressed compared to myometrial cells. The selective progesterone receptor modulator, ulipristal acetate, has been reported to decrease the size of leiomyomas in clinical trials. When treated with ulipristal acetate, both patient leiomyoma tissue and leiomyoma cells grown in 3-dimensional cultures show a decrease in the expression of NFAT5 protein, solute transporters AKR1B1 and SLC5A3, and results in an associated decline in the expression of proteoglycans, versican, aggrecan, and brevican. In summary, ulipristal acetate induces changes in leiomyoma cell osmoregulation which result in a decrease in proteoglycan expression.
Keywords: NFAT5, ulipristal acetate, leiomyoma, proteoglycans
Introduction
Uterine leiomyomas (fibroids) are the most common tumors of the uterus. Abnormal uterine bleeding (AUB)1,2 and infertility3,4 are often major complaints at presentation. This disease particularly affects African American (AA) females by the end of their reproductive years, but substantially affects Caucasian women as well.5,6 Notably, AA women incur a considerably higher socioeconomic burden, early onset of disease, larger tumor size at presentation, and experience an increased probability of symptomatology.5-9 Further, limited access to health-care services creates an added disadvantage.9 African American women undergo abdominal versus laparoscopic hysterectomies comparatively more often than other non-AA women with the disease.9 As a result, AA women suffer a greater risk of postsurgical complications resulting from a preponderance of abdominal procedures.4,9 In short, uterine fibroids are extremely common, associated with multiple adverse symptoms, and develop in most women before they reach menopause.
Leiomyogenesis is an abnormal fibrotic process of the uterine corpus, resulting in benign tumors of smooth muscle cell origin.10 Increased production of the extracellular matrix (ECM) causes enlargement of the uterus, accompanied by symptoms associated with an increased uterine volume. This aberrant expansion of the matrix is created by excessive and disorganized collagen fibrils11,12 and increased fibronectin and proteoglycan production.13 A number of in vitro studies have reported the ability of various compounds to degrade the ECM. Exposure of cultured leiomyoma cells to curcumin, antiprogestins, GnRH analogs, and inhibitors of retinoic acid metabolism negatively impact ECM protein production.12-16 The advancement of in vitro 3-dimensional (3D) cultured immortalized leiomyoma cells has provided a useful model for investigating leiomyoma formation. However, while studies using this model system have reported ECM degradation by a number of therapeutic compounds,17-19 the search for a treatment that decreases leiomyoma size with minimal patient side effects remains elusive.
Proteoglycans (PGs) are hydrodynamically active constituents of the ECM that owe their diversity of function to the presence of core proteins and attached glycosaminoglycan (GAG) side chains.20 Hyaluronic acid (HA), the backbone structure of PGs, is a nonsulfated protein whose inherent biological properties promote water retention, mediate solute movement through the extracellular space, and regulate signaling pathways involved in inflammation and wound healing.21,22 Regulation of proteoglycan synthesis is through activity of beta 1,3-glucuronosyl transferase 1 (B3GAT1), a member of the glucuronyl transferase gene family that is instrumental in the glucuronyl transfer reaction.22 Glycosaminoglycans are repeating polysaccharide subunits which are attached to HA chains. Proteoglycans versican (VCAN), aggrecan, and brevican (BCAN) interact directly with collagen to maintain ECM structural integrity.21
Ulipristal acetate (UPA) is a selective progesterone receptor modulator (SPRM) that decreases the size of leiomyomas.23 The mechanisms by which this compound exerts its diminutive effects on leiomyoma size are varied. Among these mechanisms, UPA can impact the rate of cell proliferation and initiate apoptosis through an increase in caspase 3 activity.23,24 Treatment with UPA inhibits tissue inhibitors of metalloproteinases and increases matrix metalloproteinase activity.25,26 More recently, UPA was shown to impact cell proliferation and angiogenesis by decreasing Ki-67 activity and reducing expression of vascular endothelial growth factor.27 Ulipristal acetate has been compared to other therapeutic approaches, evaluated for presurgical administration, and has shown considerable effectiveness when used long term in patients with leiomyomas.23,24,28 Ulipristal acetate negatively impacts collagen deposition, and results in a decrease in the expression of both fibronectin and versican proteoglycan in a randomized controlled study.26 Notably, none of these mechanisms provides insight into the relatively rapid impact of UPA on leiomyoma size.
Nuclear factor of activated T-cells (NFAT), formally known as tonicity-responsive enhancer binding protein, belongs to a family of proteins comprising the rel family of transcription factors. It was first implicated in its role in the activation of T-lymphocytes of the immune system.29 Particularly, NFAT5 contains a common DNA binding domain that is shared with other members of the NFAT family of proteins, NFATc1-c4, and nuclear factor-κB.30 Isoforms NFATc1-c4 possess a calcineurin binding domain, absent in NFAT5. Nuclear factor of activated T-cells 5 is found in the cytoplasm as an inactive form and is highly responsive to hypertonic conditions induced by sodium chloride exposure.29,31 Consequently, fluctuations in tissue osmolytes and changes in ECM fluid composition may result in alterations in leiomyoma size.
The objective of this study was to identify an alternate pathway in which UPA might function to impact NFAT5 expression, influence ECM remodeling, and reduce leiomyoma size with short term use of this compound. We hypothesize that ulipristal acetate will regulate NFAT5, and impact the expression of solute transporter proteins controlled by this transcription factor.
Materials and Methods
Tissue Collection
The tissues were collected from a randomized, placebo-controlled double blind clinical trial to evaluate the therapeutic effect of ulipristal acetate on uterine fibroids (Phase I and Phase II). Details of the trial can be found under the National Institutes of Health clinical study 06-CH-0090.26,32,33 Institutional review board approval was obtained from the National Institutes of Health, Bethesda, Maryland, where all surgeries were performed. Institutional review board approval was obtained from the Uniformed Services University, Bethesda, Maryland, where laboratory studies were performed.
For our study, we used the tissues that were collected from patients receiving 10 mg UPA daily for 3 months. Our study included 61 placebo myometrial (MP), 42 UPA treated myometrial, 82 placebo leiomyoma (LP), and 42 UPA treated leiomyoma (LT) patient tissue samples.
Three Dimensional Cell Culture Protocol
Generation of immortalized myometrial and leiomyoma cell cultures and the 3D model system has been described in detail previously.34,35
Quantitative Real-Time Reverse Transcriptase Polymerase Chain Reaction Analysis
We used the SYBR green (Bio-Rad, Hercules, California) for detection of gene expression in real time quantitative polymerase chain reaction (qPCR). Total RNA from both placebo and treated patients was converted to complementary DNA (cDNA) using iScript™ cDNA Synthesis Kit (BioRad). Analysis of NFAT5 gene expression was performed using specific primers for NFAT5.36 Primers specific for B3GAT1 were generated using the National Center for Biotechnology Information/Primer Blast tool. For normalization we used the housekeeping gene 18 S RNA.37
Immunohistochemistry
Tissue immunohistochemistry
Tissues were embedded in paraffin and sectioned for immunohistochemical (IHC) staining. Slideswere deparaffinized in xylene and rehydrated in a series of sequential ethanol baths. Antigen retrieval was performed using 1× sodium citrate buffer (pH = 6.0, Thermo Scientific, Waltham, Massachusetts) heated to 95°C for a 45 minute incubation in a vegetable steamer. Sections were blocked for 1 hour in 1× Tris buffered saline (TBS) containing 3%/bovine serum albumi (BSA)/0.1% Tween-20 along with the appropriate nonimmune serum. Next, tissue sections were incubated with 0.3% H2O2 in phosphate-buffered saline for 1 hour to inactivate tissue peroxidases, followed by a permeabilization step with 0.1% BSA.
For aggrecan detection, the sections were digested with chondroitinase ABC (Thermo Scientific; 50 mU/mL) in buffer containing 100 mM Tris–HCl, pH 8.0 and 30 mM sodium acetate for 1 hour at 37°C. The tissue sections were incubated overnight at 4°C with primary antibody against aggrecan (AB1031; 1:200; EMD Millipore, Burlington, Massachusetts). For NFAT5 detection tissue sections after H2O2 treatment underwent overnight incubation at 4°C with anti-NFAT5 primary antibody (PA1-023; 1:500; Thermo Scientific).
After primary antibody incubation, the slides were washed and incubated in biotinylated anti-rabbit/mouse IgG (Vector, Labs, Burlingame, California), and then Elite™ ABC reagent (avidin-biotin peroxidase complex). The peroxidase was then developed with the addition of DAB peroxidase substrate (3, 3′-diaminobenzidine).
Three-dimensional cell culture immunohistochemistry
The IHC protocol for 3D cultures has been describe in detail previously.18 For immunoreactivity in 3D cultures, antiversican primary antibody ( ab19345; 1:500 dilution; Abcam, Cambridge, Massachusetts) was used. Three-dimensional cultures were exposed to a secondary antibody conjugated to alkaline phosphatase reagent (Enzo Life Sciences, Farmingdale, New York). Sections were counterstained with hematoxylin, dehydrated in ethanol, and mounted in Permount for microscopic examination.
Western Blot Analysis
Protein from tissue and 3D cell cultures was isolated using radioimmunoprecipitation assay lysis and extraction buffer (Pierce Biotechnology) containing 1× Halt Protease Inhibitor Cocktail (Pierce Biotechnology, Rockford, IL, USA) as described previously.26,35 Briefly, equal amounts of the proteins extracted from tissue and/or cultured cells were loaded onto Bio-Rad 4% to 15% Tris-glycine gels and underwent electrophoresis under reducing conditions. Trans-Blot Turbo transfer system (Bio-Rad) was used to transfer proteins to nitrocellulose membranes. After incubation in blocking solution (5% nonfat milk in 0.1% Tween 20 in 1× TBS), membranes were washed and exposed to primary antibody against BCAN (sc-166951; 1:400; Santa Cruz Biotechnology), B3GAT1 (1:500; sc-390475), aldo-keto reductase family 1 member B1 (AKR1B1; ab62795; 1:500), solute carrier family 5 member 3 (SLC5A3; ab113245; 1:500; Abcam; ), VCAN (ab28671; 1:500) overnight at 4°C. For detection of proteins, blots were incubated with horseradish peroxidase–conjugated secondary antibody 1:5000 to 1:10 000 (ImmunoPure; Pierce Biotechnology) for 1 hour at room temperature. Clarity Western enhanced chemiluminescence substrate (Bio-Rad) was used for detection of the proteins. Horseradish peroxidase–conjugated antihuman β-actin (sc-1616; 1:10 000) or cytochrome c oxidase IV (4850; 1:2000; Cell Signaling Technology) was used as an internal standard.
Data and Statistical analysis
Immunohistochemistry H-score calculation
We used a modification of H-score interpretation from an analysis used primarily for nuclear staining in breast cancer samples.38 Photographs of stained 2D sections of myometrial and leiomyoma tissues from both placebo and treated patients were taken using a Zeiss Axiocam Imager M2 light microscope. Images were captured, saved, and randomly placed in a PowerPoint presentation for review with the use of Stereo Investigator Bright Field software (64 bit). Each slide was photographed to produce 3 random images at 20× magnification.
Six blinded investigators evaluated the images for histology scoring. Each observer independently reviewed randomized images for each stained slide and were asked to score each slide based on a set of 3 parameters. Each investigator had to determine the absence or presence of staining, stain intensity, and percentage of the slide being stained, based on consensus scoring agreed upon by the lab. These parameters were evaluated on a scale of 0 (no staining) to a score of 4, which represented intense staining for >80% of the slide. The H-score for each slide was assigned using the following formula:
Where “Σ” equals the total of the numbers recorded by each of the observers for each of the slides evaluated. A final score gives a range from 0 to 400 for each respective slide.
Quantitative polymerase chain reaction
For qPCR, relative expression was calculated based on the Pfaffl method.39 The Wilcoxon signed rank test was used for nonparametric statistical evaluation, and P < .05 was considered statistically significant. For the Western blot analysis, calculations were performed with Image Lab software from Bio-Rad Laboratories. Data are presented as the fold difference between relative density units of treated and untreated samples, and are corrected for the internal control β-actin. Data are presented as fold difference ± standard error mean.
Results
Variability of Leiomyoma Tumor Morphology
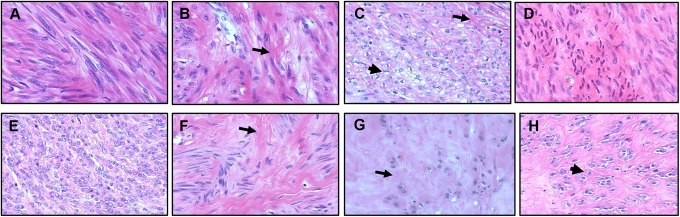
In Figure 1, representative examples of both placebo and LT patient tissue sections stained with hematoxylin and eosin at 40× magnification, illustrate the variable histology of patient leiomyomas. Histologic sections of leiomyoma tissue from both placebo (A-D) and UPA treated patients (E-H) demonstrate considerable microscopic heterogeneity in these tumors. Leiomyoma tissue may be composed of numerous cells with little intervening collagen formation during the early phase of leiomyoma development, as demonstrated in placebo (A) and treated (E) samples.40 As tumors engage in active collagen deposition (arrows; B and C) and (arrows; F and G) cells become fewer in number compared to the degree of hyalinization present. In the final stages of leiomyoma development, tumors are composed of large areas of dense matrix (D and H), accompanied by atrophic cells (D) or cells with increased cytoplasmic vacuolization (arrow heads; C and H).40
Figure 1.
Leiomyomas demonstrate considerable variability of tumor morphology in treated and placebo patient tissue samples. Hematoxylin and eosin stained leiomyoma sections from both placebo (A-D) and treated (E-H) patients are shown above at 40× magnification. Note the absence of smooth muscle fascicles in most of the tissue samples. Leiomyoma tissue may be composed of numerous cells during the initial phase of development (proliferation) as demonstrated in placebo (A) and treated (E) samples. Tumors demonstrate increased collagen deposition (arrows) in placebo (B and C), and ulipristal acetate-treated (F and G) tissues. Note large areas of dense matrix (D and H), atrophic cells (D), or cells with increased cytoplasmic vacuolization (arrow heads) (C and H).
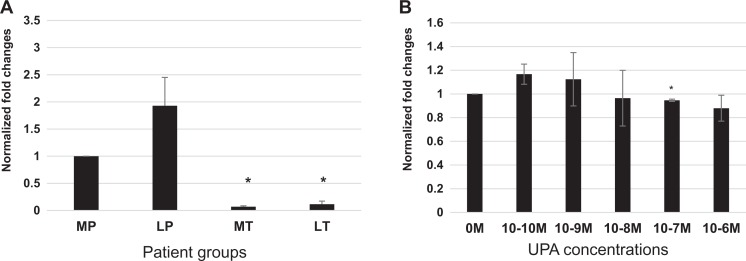
Ulipristal acetate treatment regulates NFAT5 messenger RNA transcripts and protein concentration in LT patient tissues
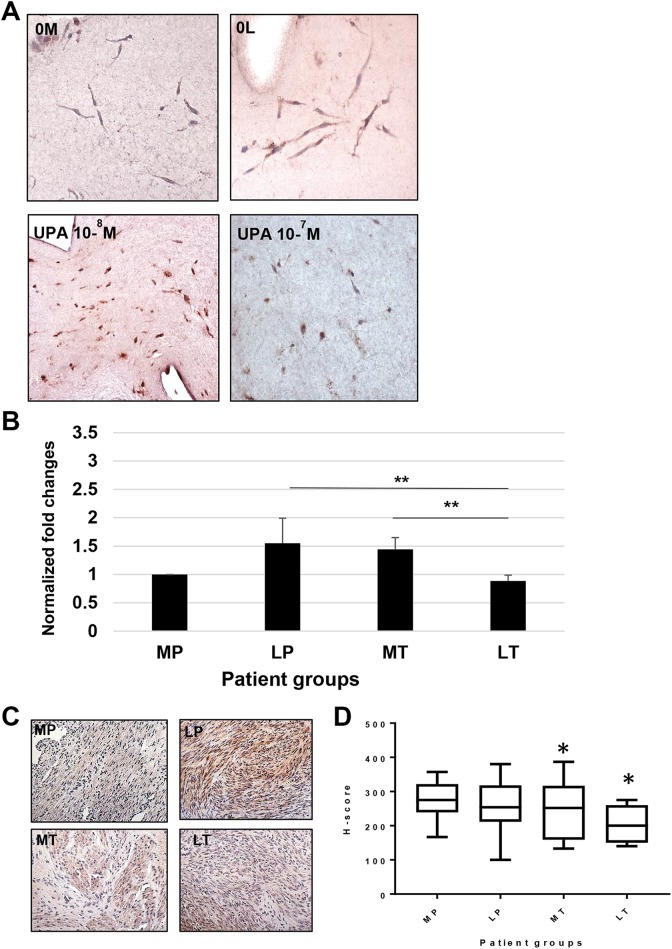
Nuclear factor of activated T-cells 5 transcription factor is responsive to changes in tissue osmolyte concentration. In 3D leiomyoma and myometrial cultures, NFAT5 expression is decreased with UPA treatment (Figure 2A). Compared to myometrium from placebo subjects, an upregulation in messenger RNA (mRNA) transcripts for NFAT5 was observed in leiomyoma from placebo patient tissue samples (1.55 ± 0.44-fold; n = 8) and UPA treated myometrial (1.44 ± 0.20-fold; n = 7) tissue (Figure 2B). When compared to both LP and treated myometrial patient groups, UPA LT patient tissue samples had statistically significant downregulation for NFAT5 mRNA (0.78 ± 0.07-fold; **P < .003).
Figure 2.
Ulipristal acetate results in a decrease in NFAT5 mRNA transcripts and protein in treated leiomyoma tissue. A, Anti-NFAT5 antibody demonstrates a predominately cytoplasmic location, and is increased in untreated 3D leiomyoma cells (0 L) compared to 3D myometrial cells (0 M). Leiomyoma cells treated with 10−8 M UPA show little change from 0 L. However, 3D leiomyoma cultures show a marked decrease in NFAT5 expression at 10−7 M UPA treatment. B, Messenger RNA transcriptions for NFAT5 are increased in both placebo leiomyoma (1.55 ± 0.44-fold) and treated myometrial (1.44 ± 0.15-fold) patient samples. A comparison of NFAT5 transcripts between placebo leiomyoma, LT (0.78 ± 0.07-fold), and treated myometrial tissues is significant (**P < .003). C, Anti-NFAT5 primary antibody demonstrates detection of this protein in the cytoplasm of strongly positive placebo myometrial (MP) and leiomyoma (LP) cells, and significant staining in treated myometrial tissue (MT) in the representative sections shown above. Treated leiomyoma (LT) tissue indicates that few cells are staining for this protein, and at a lesser intensity. D, Analysis of NFAT5 protein H-scores show that when compared to MP tissue (276 ± 9.8), placebo leiomyoma tissue (267.6 ± 10.3) appear similar in protein concentrations. Ulipristal acetate treatment resulted in a decrease in treated myometrium (248 ± 15.8 *P < .01), and a marked decrease in the expression of NFAT5 in treated leiomyoma patient tissues (205 ± 16.6 *P < .02). UPA indicates ulipristal acetate.
Histologic sections of represented patient myometrial and leiomyoma tissues evaluated for NFAT5 demonstrate predominately cytoplasmic staining (Figure 2C). As observed in Figure 2D, analysis of H-score comparisons between MP (276 ± 9.8; n = 6) and patient matched LP (267.6 ± 10.3; n = 10) did not demonstrate any significant change. Compared to MP, treated myometrium (MT; 248 ± 15.8 *P < .01; n = 10) and LT (205 ± 16.6 *P < .02; n = 11) samples demonstrated a statistically significant decrease in NFAT5 protein. Nuclear factor of activated T-cells 5 protein was also significantly (*P < .05) decreased in LT compared to LP (Figure 2D).
Ulipristal acetate treatment decreases the expression of solute transporter proteins in 3D myometrial and leiomyoma cells
Transporter protein AKR1B1 facilitates the movement of sorbitol across cell membranes in response to changes in cellular fluid equilibrium. As such, activity of AKR1B1 responds to alterations in tissue osmolarity. A comparison between LP (n = 7) and UPA LT tissue samples (n = 5) demonstrated that 80% of treated patient samples resulted in a 1.25-fold to 5-fold decrease in AKR1B1 protein (data not shown). Similarly, SLC5A3 protein demonstrated considerable variability in UPA treated as compared to placebo patient tissues (data not shown). Due to this observed variation in tissue samples, we examined AKR1B1and SLC5A3 protein expression in 3D cultures of leiomyoma and patient matched myometrial cell cultures in response to UPA treatment.
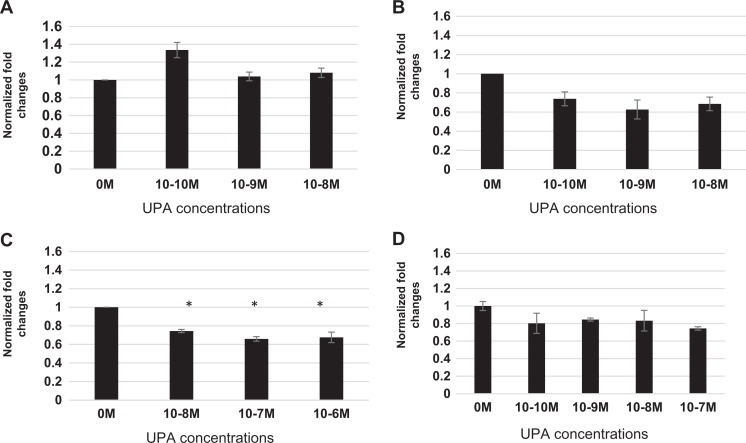
In Figure 3A, no significant effect was observed for AKR1B1 protein in myometrial cells, on treatment with different concentrations of UPA. Three dimensional leiomyoma cultures (Figure 3B) demonstrate a significant (*P < .05) decrease of AKR1B1 protein; however, the change was not concentration dependent. The decline in AKR1B1 protein was observed at concentrations as low as 10−9 M (0.63 ± 0.09-fold) and 10−8 M (0.69 ± 0.07-fold). A lower concentration of 10−10 M resulted in a slightly lower fold expression for AKR1B1 (0.74 ± 0.07-fold), suggesting that this concentration may have reached a maximal effect in these cells.
Figure 3.
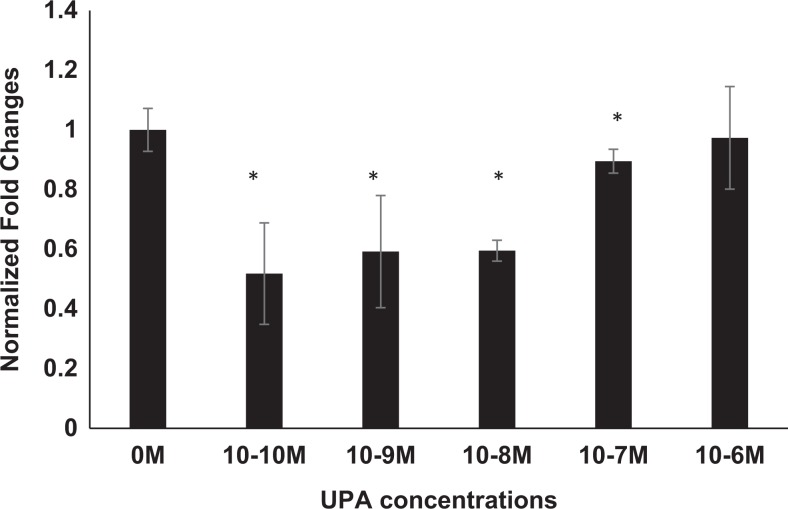
Ulipristal acetate regulates solute transporter proteins in 3D myometrial and leiomyoma cells. A, Western blot analysis of myometrial cells exposed to UPA show only a slight increase in AKR1B1 protein at the lowest concentration of 10−10 M (1.34 ± 0.08-fold) when compared to placebo myometrial cells. Higher concentrations of UPA show no change in AKR1B1 expression in these cells from placebo controls. B, Western blot analysis of leiomyoma cells treated with UPA show a decrease in the expression of AKR1B1 at all concentrations: 10−10 M (0.72 ± 0.07-fold), 10−9 M (0.63 ± 0.09-fold), and 10−8 M (0.69 ± 0.07-fold) evaluated. C, Western blot analysis of 3D leiomyoma cells demonstrate a decrease in the expression of SLC5A3 protein at 10−8 M (0.74 ± 0.01), 10−7 M (0.66 ± 0.02), and 10−6 M (0.67 ± 0.05) compared to placebo myometrium, when exposed to UPA treatment (*P < .05). D, Western blot analysis of leiomyoma cells treated with UPA demonstrate a maximally 1.34-fold decrease in the expression of SLC5A3 protein for all concentrations of UPA: 10−10 M (0.80 ± 0.12), 10−9 M (0.84 ± 0.01), 10−8 M (0.83 ± 0.12), and 10−7 M (0.74 ± 0.02) evaluated, that was not concentration dependent. AKR1B1 indicates aldo-keto reductase family 1 member B1; SLC5A3, solute carrier family 5 member 3; UPA, ulipristal acetate.
Solute carrier family 5 member 3 induces myo-inositol transport under conditions of hypotonic stress. Adjustments in tissue concentrations of this protein might be expected to aid in the correction of tissue osmolytes. Three dimensional cultures of myometrial cells treated with UPA demonstrated a statistically significant (*P < .05) decrease in SLC5A3 protein. As observed in Figure 3C, a maximum of 1.35-fold decrease at the highest UPA concentration of 10−7 M (0.66 ± 0.02-fold) in SLC5A3 expression was observed. Statistically significant changes at lower concentrations were also observed at 10−8 M (0.74 ± 0.01-fold) and 10−6 M (0.66 ± 0.05-fold; Figure 3C). As observed in Figure 3D, when compared to untreated leiomyoma cells (0 M), UPA LT cells demonstrate a reduction in SLC5A3 expression at 10−10 M (0.80 ± 0.12-fold), 10−9 M (0.84 ± 0.01-fold), 10−8 M (0.83 ± 0.12-fold), and a statistically significant reduction at 10−7 M (0.74 ± 0.02-fold).
Ulipristal acetate decreases proteoglycan expression in patient tissues and cells
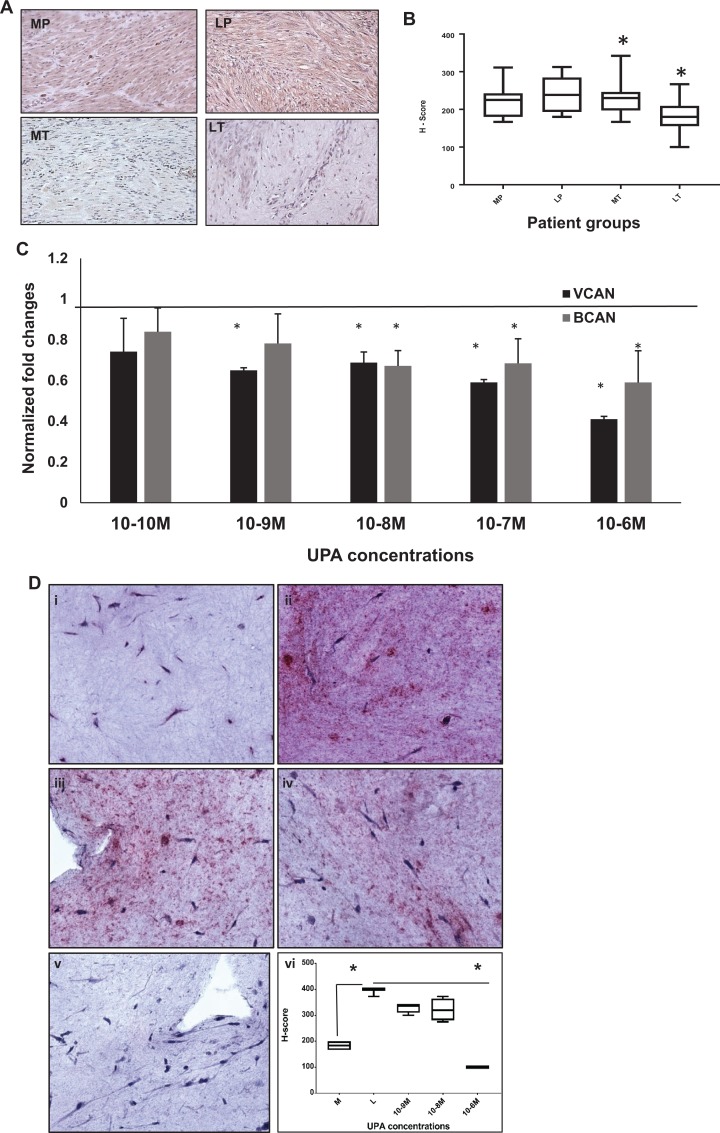
Proteoglycans represent a major portion of the ECM components in leiomyomas. These molecules contain hydrophilic side chains that aid in the regulation of water homeostasis. Figure 4A shows representative stained tissue sections of patient samples for aggrecan protein. Histologic sections for aggrecan demonstrated a prevalence for cytoplasmic staining. Both the representative MP and patient matched LP tissue samples demonstrate strong cytoplasmic immunoreactivity for aggrecan protein. Treated myometrium shows less stain intensity for > 90% of cells depicted in this slide. The cytoplasm of MT and patient matched leiomyoma indicates little to no immunoreactivity for aggrecan protein in this matched patient tissue sample.
Figure 4.
Ulipristal acetate treatment decreases the expression of proteoglycans in treated leiomyoma (LT) tissue and 3D cultures. A, Primary antibody directed against aggrecan protein is demonstrated in the cytoplasm of positively stained myometrial and leiomyoma patient tissue samples. Both treated myometrium (MT) and LT tissues resulted in a decrease in the expression of aggrecan PG, indicated by less stain intensity for this protein. B, Analysis of the expression of aggrecan (H-scores) in myometrial and leiomyoma patient tissue. Both placebo leiomyoma tissue (255 ± 8.5) and treated myometrial tissue (223.8 ± 8.4) was similar to placebo myometrium (241.9 ± 10.8). Treated leiomyoma patient samples demonstrated a decrease in the expression of aggrecan (172.3 ± 6.7) that was statistically significant (**P < .003). C, Western blot analysis of 3D cultures treated with UPA demonstrate a decrease in both versican and brevican proteoglycans at all UPA concentrations. D, Immunohistochemical analysis of VCAN production in UPA-treated 3D leiomyoma cells stained for versican (i-vi) are shown. (i) Myometrial 3D culture; 0 M, (ii) Leiomyoma culture; 0 M, (iii-v) Leiomyoma treated with UPA at 10−9 M, 10−8 M, and 10−6 M. Note that at UPA concentration of 10−6 M (v) the stain intensity for VCAN is markedly diminished, resembling that of myometrial 3D cultures. (vi) H-score analysis of VCAN images: Myometrium (i; 137.6 ± 29.6), Leiomyoma (ii; 315.4 ± 75.6), 10−9 M (iii; 237.5 ± 58.8), 10−8 M (iv; 321.9 ± 21.5), and 10−6 M (v; 100.0 ± 0) (*P < .03). PG indicates proteoglycans; UPA, ulipristal acetate; VCAN, versican.
As observed in Figure 4B, evaluation of H-score comparisons between MP (241.9 ± 10.8; n = 6), with LP samples (255 ± 8.5; n = 10) and MT (223.8 ± 8.4; n = 9) were not statistically relevant. When compared to MP, LP, and MT, the expression of aggrecan in LT tissue was significantly decreased (172.3 ± 6.7 **P < .003; n = 9).
We have previously demonstrated that VCAN is significantly reduced in UPA treated patient samples as compared to placebo patients.26 As observed in Figure 4C, compared to untreated leiomyoma cells (0 M; represented as a straight line as 1-fold change), UPA LT cells (black bars) demonstrated a significant reduction in the expression for VCAN proteins. This decline in VCAN expression was noted in all concentrations of UPA ranging from 10−10 M (0.74 ± 0.16-fold), 10−9 M (0.65 ± 0.012-fold), 10−8 M (0.68 ± 0.05-fold), 10−7 M (0.59 ± 0.013-fold), and 10−6 M (0.41 ± 0.01-fold) in leiomyoma cells. Similarly, expression of BCAN in UPA LT cells demonstrated a concentration dependent reduction in protein expression (Figure 4C; grey bars). Concentrations as low as 10−10 M (0.84 ± 0.12-fold) and 10−9 M (0.78 ± 0.14-fold) demonstrated an effect on BCAN. At higher concentrations, the decrease in BCAN became statistically significant; 10−8 M (0.67 ± 0.07-fold) and 10−7 M (0.68 ± 0.12-fold), and the lowest decrease was observed (0.59 ± 0.16-fold) at 10−6 M concentration.
We further examined the spatial expression of VCAN in 3D leiomyoma cultures using IHC analysis. As observed in Figure 4D, a concentration dependent decrease in VCAN protein staining was observed with increasing concentration of UPA. Increased intensity of staining in untreated leiomyoma (0 M, 4D-ii) as compared to untreated myometrial cultures (0 M, 4D-i) indicated higher VCAN production by leiomyoma cells. At the highest concentration of UPA, 10−6 M (4D-v), there was very little staining observed in the leiomyoma 3D culture. The final Figure (4D-vi) represents the H-score for these images. On treatment with UPA, myometrial cells demonstrate little or no significant decrease in BCAN protein concentration of any of the 3 proteins compared to MP cells (data not shown).
Ulipristal acetate decreases B3GAT1 in leiomyoma tissues and cells
Beta 1,3-glucuronosyl transferase 1 is the enzyme that controls proteoglycan formation by regulating synthesis of the sulfated GAG, chondroitin sulfate. As observed in Figure 5A, B3GAT1 mRNA transcripts were significantly upregulated (1.93 ± 0.52-fold) in LP tissue samples (n = 5) compared to normal patient matched myometrial tissue. There was a marked downregulation of mRNA transcripts for B3GAT1 in LT samples (n = 5; 0.12 ± 0.06-fold *P < .05) of greater than 5 fold, and a greater than 10-fold downregulation in treated myometrial samples (MT; 0.07 ± 0.02-fold) when compared to MP.
Figure 5.
Ulipristal acetate decreased B3GAT1 mRNA in treated myometrial and leiomyoma tissue. A, B3GAT1 mRNA transcripts are increased in placebo leiomyoma (1.93 ± 0.52-fold) samples compared to placebo myometrium. Ulipristal acetate treatment results in a greater than 10-fold downregulation in B3GAT1 transcription in both treated myometrial (0.07 ± 0.02-fold *P < .05) and leiomyoma (0.12 ± 0.05-fold *P < .05) patient tissue samples. B, UPA decreases B3GAT1 protein expression in 3D leiomyoma cells. The expression of B3GAT1protein shows a slight decrease in leiomyoma cells grown in 3D at higher concentrations of 10−7 M (0.95 ± 0.01-fold *P < .05) and 10−6 M (0.88 ± 0.11) UPA concentrations. B3GAT1 indicates beta 1,3-glucuronosyl transferase 1; mRNA, messenger RNA; UPA, ulipristal acetate.
The expression of B3GAT1 in 3D leiomyoma cell cultures was decreased at higher concentrations of 10−7 M (*P < .05) and 10−6 M of UPA by Western blot analysis. Lower concentrations have minimal effect on B3GAT1 expression compared to MP controls (Figure 5B).
Ulipristal acetate decreases the expression of transforming growth factor β3 in 3D leiomyoma tissue and cells
The transforming growth factor β (TGFβ) has been established as a profibrotic mediator in leiomyoma pathogenesis.41 The process of leiomyogenesis increases ECM components that include the PGs. Transforming growth factor β expression has also been associated with the regulation of B3GAT1 in nucleus pulposus cells in interventricular disc disease.42
We have already demonstrated that patient tissue from UPA treated samples show a significant decline in TGFβ3 protein when compared to placebo patient tissues.43 As observed in Figure 6, 3D leiomyoma cultures at lower concentrations of UPA significantly reduced the total amount of TGFβ3 protein in LT cells (∼2-fold) when compared to untreated leiomyoma cells (0 M), though the effect was not concentration dependent. At suprapharmacologic concentrations of UPA (10−6 M), leiomyoma cells demonstrated little or no response to UPA. This finding may be the result of nonspecific effects at high concentrations, or rare contaminants that only impact expression when treatment concentrations are in the micromolar range.
Figure 6.
Ulipristal acetate decreases the expression of TGFβ3 in 3D leiomyoma cells. Treated leiomyoma cells grown in 3D cultures show a statistically significant decrease in the expression of TGFβ3 when treated with UPA at low concentrations (10−10 M to 10−8 M), independent of concentration. TGFβ3 indicates transforming growth factor β3; UPA, ulipristal acetate.
Discussion
Earlier studies in fibroid research demonstrated that cultured leiomyoma cells responded to 100 ng/mL of UPA (also known as CDB-2914) by regulating progesterone receptor (PR) isoforms.44 Notably, the effect of UPA on the expression of PR-A was observed to be a dose-dependent increase in leiomyoma cells45; a finding not appreciated in normal myometrial cells.46,47 A randomized, double-blind, placebo-controlled study documented that administration of UPA for 3 to 6 months results in a significant impact on the quality of life due to a decrease in the size of leiomyomas in women involved in the study.33 We assessed the effect of UPA on patient matched myometrial and leiomyoma tissue samples taken from a randomized controlled study of women who underwent treatment for 3 months. Our study demonstrates differences in the responses of placebo and treated patient tissues to UPA. For example, results were highly dependent upon which placebo-treated pairs were correlated. Microscopic examination of LP tissues revealed that many of the samples showed areas of a clear distinction in the number of cells relative to the amount of ECM present (Figure 1). Of note, a considerable degree of diversity was also observed within the same patient when different leiomyomas were examined microscopically. We propose that the observed dissimilarity between patient samples might best be explained by the presence of contrasting morphology in these tumors. Conceivably, differences in tissue architecture could result in a variation in tissue responses to compounds that impact gene expression. In fact, a characterization of the natural evolution of uterine leiomyomas has been reported. Over time, leiomyoma cytoarchitectural features deviate from that of clearly delineated fascicles found in the myometrium. The collection of collagen fibrils as fascicles define the functionality of smooth muscle cell contraction. Leiomyomas develop clearly recognizable histologic characteristics that distinguish them from normal myometrium. In our study, we observed significant differences in leiomyoma patient tissue samples microscopically (Figure 1). Leiomyoma cytoarchitecture can appear as tumors with high cellularity (A and E) and little collagen deposition in the early phase of development. Tumors that are actively producing matrix are shown in B and C in placebo patients and F and G in treated patient samples. As leiomyoma development progresses, tumors with predominately hyaline matrix (H) having an abundance of “atrophic myocytes” (D) can be identified.40 To address the limitation of broad variability of responses generated by patient tissue samples, we used a previously validated 3D model system to explore pathways that might be regulated by UPA treatment. The use of 3D cultures for the in vitro study of leiomyomas offers several distinct advantages. Exposure of cultured leiomyoma and myometrial cells grown 3-dimensionally to ECM proteins and cytokines creates an environment that closely mimics actual in vivo conditions. This unique feature contrasts significantly with cells grown 2-dimensionally. Another advantage of the 3D system is its ability to preserve intercellular communication, supporting those processes that contribute to cell–cell and cell–matrix interactions affected by mechanical stimulation.17
A chief manifestation of patients presenting with uterine leiomyomas is AUB. Ulipristal acetate provides women with symptomatic leiomyomas, relief from excessive bleeding that frequently accompanies the syndrome of uterine leiomyomas.24 Additionally, intermittent oral dosages of 10 mg of UPA for 12 weeks significantly impacts the quality of life of affected patients by controlling pain, bleeding, and reducing tumor volume.23 Another evaluation of patient matched myometrial and leiomyoma tissue samples from a randomized controlled study involving women treated with UPA for 3 months, illustrated a mechanism by which these patients might experience a rapid decline in leiomyoma volume.26 In addition to changes in ECM proteins, an impact on pathways linked to matrix synthesis and degradation, and possibly changes in leiomyoma hydration were observed. This study indicated that UPA plays a vital role in the remodeling and dissolution of leiomyoma matrix components that may also contribute to changes in water content.26
Many in vitro studies designed to investigate leiomyoma ECM production have described varying responses of myometrium and leiomyoma tissue to therapy. Differential ECM expression of leiomyoma and myometrial tissue to GnRH analogs,13,18,38 and selectivity for steroid hormone receptors exposed to SPRMs45 are prime examples. In a like manner, revelation of an abnormality in leiomyoma regulation of cell volume homeostasis could be of some clinical value. Essential to this approach is the identification of pathways related to the ability of leiomyoma cells to maintain fluid equilibrium.
Increasing evidence supports that NFAT signaling and regulation of transcription functions in cells other than those comprising the immune system. When cultured leiomyoma cells are exposed to hyperosmolar conditions, there is an elevation in the expression of NFAT5.48 Nuclear factor of activated T-cells 5 is known to behave as an “osmoadaptive” transcription factor, active in a number of divergent tissue types. Osmoresponsive proteins known to be regulated by NFAT5, are AKR1B1 and SLC5A3. These glycoproteins regulate sorbitol and inositol transport across cell membranes. Counterbalancing responses like tight control of solute transport are critical to cell survival.30,31 Consequently, NFAT5 directs the correction of electrolyte imbalances and restores cell volume in affected tissues.
The role of NFAT5 appears to be primarily tissue specific. Distinct roles for NFAT5 transcription point to its purpose in maintaining hydration homeostasis, while other studies support the capacity of NFAT5 to control cellular processes unrelated to osmoregulation.49 Studies in mammalian cells show that the expression of NFAT5 contributes to various cellular functions including the control of solute transport, responses to low oxygen tension, promotion of inflammatory processes, and matrix remodeling.50-53
We evaluated NFAT5 expression in 3D myometrial and leiomyoma cells and found a decreased expression in leiomyoma cells treated with UPA at 10−7 M (Figure 2). Additionally, an assessment of tissue samples from patients treated with UPA in a prospective, randomized, placebo-controlled trial revealed similar results. Unpaired analysis of patient samples demonstrated an elevation in NFAT5 transcripts in leiomyoma tissue from subjects treated with placebo compared to placebo-MT, but the increase was not statistically significant. This was confirmed by quantitative IHC analysis based on H-score that revealed comparable levels of NFAT5 protein in MP and leiomyoma samples. When compared to placebo-treated myometrial tissue, UPA-treated myometrial tissue showed an adjusted increase in NFAT5 transcripts, whereas leiomyoma tissue demonstrated a statistically significant decrease in NFAT5 transcripts. Conversely, NFAT5 protein in LT and myometrial specimens was significantly lower compared to MP samples (Figure 2). These findings suggest that myometrial tissue responds to UPA treatment with an elevation in NFAT5 expression to compensate for the decrease in protein, a predictable response not evident in leiomyoma tissue. Leiomyoma tissue appears incapable of compensating for a changing tissue environment induced by UPA to the same degree as the myometrium. This event may also be explained by the presence of a substantially higher number of PRs in leiomyoma tissue compared to myometrial cells.43 Clearly, UPA treatment influences the expression of NFAT5 in these samples, and this aberrant response requires future investigation of proteins that are directly regulated by this transcription factor.
Nuclear factor of activated T-cells 5 transcription factor is an essential element required for the response of mammalian cells to osmotic stimuli.29,30 Aldo-keto reductase family 1 member B1 is under the control of NFAT5 transcription. Aldo-keto reductase family 1 member B1 aids the cell in the accumulation of organic osmolytes that prevent damage to cellular components. It is expressed in normal endometrium, myometrium, and the cervix, and may also be involved in carcinogenesis and cancer drug resistance.54 Reportedly, the role of AKR1B1in the pathogenesis of uterine leiomyomas involves mediators of inflammation, and AKR1B1 transcription controls changes in cell tonicity which act as a kind of “switch” to control NFAT binding to the promoter of AKR1B1.54-56
Ulipristal acetate treatment of myometrial and leiomyoma 3D cultures exhibited a decrease in NFAT5 gene expression (data not shown) that was accompanied by a decrease in AKR1B1 expression. Treated myometrial cells demonstrated little to no change in AKR1B1 expression. Examination of leiomyoma cells grown in 3D exhibit a concentration dependent decrease in AKR1B1 which plateaued at 10−8 M concentration, illustrating an impaired reaction of imposed osmotic stress on these cells, caused by UPA treatment. Leiomyoma cells respond to UPA treatment by lowering AKR1B1 expression, a protein that has a direct impact on intracellular osmolyte concentration. This response of leiomyoma cells suggests an impairment of cell volume autoregulation. Ulipristal acetate treatment appears to modify the ECM in a manner that results in selective expression of the AKR1B1 transporter in the myometrium.
The second solute transporter protein that is impacted by NFAT5 transcriptional activity is SLC5A3. Various human cell types exposed to hypotonic conditions demonstrate an upregulation of SLC5A3 gene expression implying that these cells respond to changes in osmolarity by increasing myo-inositol transport.57,58 Myometrial cells grown in 3D show a decline in expression of SLC5A3 when treated with UPA. The maximum fold change of 1.5-fold was noted at 10−7 M. The decrease in this protein was independent of all concentrations of UPA evaluated. In 3D leiomyoma cells exposed to UPA, the expression of SLC5A3 resulted in a maximal decrease at the highest concentration (10−7 M), resulting in a 1.35-fold decrease. Logically, a decrease in the expression of NFAT5 transcription factor might be expected to be accompanied by a decrease in promoter activity for proteins under NFAT regulation. If UPA modifies intracellular electrolytes, then an upregulation in SLC5A3 would be consistent with an appropriate response by uterine tissue. This unexpected result might be explained by the fact that proteins from 3D cultures treated with UPA were collected after 72 hours. Remarkably, UPA continues to impact cells after 72 hours of exposure. This time point had been previously determined to be optimal for witnessing changes in ECM production by other compounds. In the case of SLC5A3, tissue osmolyte equilibrium may have become reestablished to normal levels, sometime before protein samples were collected. Our results suggest the possibility that the window for UPA impact on SLC5A3 expression might have been missed, thus limiting accurate assessment of the kinetics of UPA regulation for this protein. An assessment of treated 3D leiomyoma and myometrial samples taken at earlier time points after UPA treatment may be more illustrative of the influence of UPA on this protein.
In addition to collagen, proteoglycan molecules comprise a major portion of uterine leiomyoma ECM constituents. In a previous study, patients treated with UPA exhibited a marked decrease in versican proteoglycan in 80% of patients evaluated.26 Similar structurally to versican, aggrecan is a high molecular weight, chondroitin sulfate containing proteoglycan molecule. Specifically, aggrecan complexes with HA to create hydrated gel-like complexes that help to stabilize collagen fibrils and other matrix components.20,21 The hydrophilic properties of these dynamic molecules may also contribute to the osmotic potential of the ECM.21 We evaluated the expression of both aggrecan and BCAN PGs in patients treated with UPA for 3 months. Consequently, we also investigated the influence that UPA treatment might have on PG production in 3D cultures.
Aggrecan proteoglycan was expressed both in myometrial and patient matched leiomyoma patient samples as observed by IHC. Expression of aggrecan has been reported in endometrium,59 but this is a first report on the presence of this PG in uterine leiomyomas. On treatment with UPA, leiomyoma tissue demonstrated a loss of aggrecan protein as compared to patient matched myometrial tissue. Interestingly, examining the H scores, taking in consideration unpaired analysis, UPA MT and leiomyoma tissues demonstrated statistically significant reduction in aggrecan protein compared to MP tissue. Yasuo and coworkers demonstrated progesterone induction of aggrecan in the endometrium60; which may explain the effect of UPA, an antiprogestin, on the expression of aggrecan in uterine tissues.
Brevican PG is abundant in the central nervous system.59,61 Although brevican was not reported in the endometrium,60 we were able to quantitate the protein both in patient tissue and our cell lines. An initial comparison between patient matched myometrial and leiomyoma tissue in placebo and treated patients showed little change between these groups for BCAN (data not shown). Multivariate comparisons between placebo and UPA LT tissue samples illustrated that the production of BCAN was decreased by as much as 2-fold. However, 50% of comparisons made between placebo and LT tissue showed an almost 3-fold increase in this same protein depending on patient comparisons (data not shown). Again, these disparities in BCAN expression noted between different placebo and treated patient samples highlight relative differences in the cellularity of leiomyomas. In 3D leiomyoma cultures, BCAN expression revealed a concentration dependent decrease in protein amount when compared to untreated cells. Though supra pharmacological concentrations of UPA (10−6 M) showed an over 1.6-fold decrease in BCAN expression in LT cells, a decreased amount of protein was also observed at lower concentrations.
Analogous to the response of tissue samples for versican protein,26 leiomyoma cell cultures demonstrate a marked concentration dependent decrease in VCAN protein when treated with UPA, which was clearly highlighted in the matrix of the 3D cultures. Undoubtedly, UPA treatment contributes to ECM remodeling by decreasing the production of PG proteins like versican, aggrecan, and brevican.
As mentioned earlier, inherent to proteoglycan structure is the presence of GAGs composed of sulfated disaccharide subunits like chondroitin sulfate. Our next task was to investigate whether UPA treatment would also regulate expression of an enzyme that might result in measurable changes in ECM proteoglycan production. In other model systems, NFAT5 transcription mediates aggrecan synthesis and increases transcriptional activity at the promoter region of B3GAT1 in response to TGFβ stimulation.42,62 Beta 1,3-glucuronosyl transferase 1 enzyme is involved in the synthesis of chondroitin sulfate, promoting the production of PGs when stimulated with the TGFβ growth factor.42
Beta 1,3-glucuronosyl transferase 1 transcripts in placebo and LT samples were increased as compared to patient matched myometrial samples, though due to the appreciable variability in leiomyoma morphology, statistical significance was lost. In an unpaired analysis of our clinical patient samples, we observed that UPA treatment resulted in a marked decline in the expression of B3GAT1 in both myometrial and leiomyoma patient tissue samples when compared to the placebo paired samples. Similarly, the protein expression from patient samples was inconclusive as the decrease in transcripts on treatment was observed in less than 10% of the patients (data not shown). We concluded that this may be due to the longer period of exposure (3 months) in clinical patient samples. To evaluate whether there was any early effect on treatment, we used cell lines. We observed that it was at higher concentrations of UPA that decreased B3GAT1 protein in 3D leiomyoma cells. Overall, a decrease in both the mRNA transcripts and protein levels for B3GAT1 supports our findings for PG depletion with UPA treatment.
Our next step was to identify a pathway involved in leiomyogenesis that might be impacted by UPA treatment and also affects proteoglycan production. Transforming growth factor β induces elevated PGs like BCAN and aggrecan in the brain and spinal cord.61,63 Interestingly, a recent report presents evidence that a 3 month exposure to UPA causes a decline in serum levels of TGFβ3 in treated patients compared to controls.64 Transforming growth factor β3 regulation of ECM production in leiomyomas has been well documented.41 Studies have shown that the expression of ECM genes are increased in myometrial cells grown in the presence of TGFβ3, to levels equivalent to that of leiomyoma cells. This finding demonstrates how growth factor stimulation impacts myometrial cell transcriptional activity and alters the expression profile of these cells to one that mimics leiomyoma cells.37 When myometrial and leiomyoma cells are stimulated with TGFβ3, the expression of versican proteoglycan variants rich in GAG increased by several fold.65 Inhibition of TGFβ3 culminated in a reversal of the stimulatory effects on versican production.37 Our evaluation of the effect of UPA on TGFβ3 expression in leiomyoma cells grown in 3D culture demonstrated a statistically significant 2-fold decrease in expression at lower concentrations of UPA, whereas at higher concentrations, the levels of TGFβ3 expression in these cells approaches that of untreated cells. Considering our results, as well as the abovementioned published findings, the association between the TGFβ3 pathway and decreased PG synthesis is apparent. We hypothesize that a decrease in serum levels of TGFβ3 in patients treated with UPA might be expected to result in alterations in B3GAT1 expression. Consequently, the production of chondroitin sulfate containing compounds like PGs are affected, resulting in a decrease in the ECM of leiomyoma tumors exposed to UPA treatment.
In conclusion, UPA acts to impact global alterations in uterine proteoglycan expression, defining a role for this compound in osmoregulation. However, the study does not address what effect other compounds regarded as SPRMs may contribute to this phenomenon. Our study uniquely demonstrates the impact of ulipristal acetate on the transcriptional activity of the osmotic stress inducible transcription factor, NFAT5. We have established the effect of UPA on the regulation of NFAT5 expression as well as those osmoresponsive proteins, AKR1B1 and SLC5A3, under NFAT5 control. We also demonstrate that UPA treatment decreases TGFβ3 expression in cultured 3D leiomyoma cells, which directly correlated with B3GAT1 activity. A decrease in B3GAT1, corresponded to decreases in PGs versican, aggrecan, and brevican in patient samples and the 3D model system. Attached GAG enhance the affinity of PG molecules to retain water within the tissue. Ulipristal acetate induces a loss in PGs, which corresponds to fluctuations in the osmotic potential of the ECM. Regulation of tissue hydration via changes in PG concentrations may explain alterations in leiomyoma volume associated with the clinical application of this compound.
Acknowledgments
The authors would like to thank Dr Lynnette K. Neiman, Diabetes, Endocrine and Obesity Branch, The National Institutes of Health (NIDDK) for the use of all clinical specimens used in this study.
Authors’ Note: The opinions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the Department of Defense.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: W.H.C. received a research grant from Allergan, and serves as a consultant for Allergan.
Funding: The author(s) disclosed receipt of the following support for the research, authorship and/or publication of this article: W.H.C. received a research grant from Allergan. This research was supported by The Uniformed Services University of the Health Sciences, Military Women’s Health Award; grant number 309325.2.00.64622; and was also supported by Allergan. It was also supported in part by the intramural research program of the National Institutes of Health, Program in Reproductive and Adult Endocrinology, NIH.
References
- 1. Munro MG. Uterine leiomyomas, current concepts: pathogenesis, impact on reproductive health, and medical, procedural, and surgical management. Obstet Gynecol Clin N Am. 2011;38(4):703–731. [DOI] [PubMed] [Google Scholar]
- 2. Whitaker L., Critchley HO. Abnormal uterine bleeding. Best Pract Res Clin Obstet Gynaecol. 2016;34:54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carranza-Mamane B, Havelock J, Hemmings R; Reproductive Endocrinology and Infertility Committee; Special Contributor. The management of uterine fibroids in women with otherwise unexplained infertility. J Obstet Gynaecol Can. 2015;37(3):277–285. [DOI] [PubMed] [Google Scholar]
- 4. Owen C, Armstrong AY. Clinical management of leiomyoma. Obstet Gynecol Clin North Am. 2015;42(1):67–85. [DOI] [PubMed] [Google Scholar]
- 5. Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100–107. [DOI] [PubMed] [Google Scholar]
- 6. Catherino WH, Eltoukhi HM, Al-Hendy A. Racial and ethnic differences in the pathogenesis and clinical manifestations of uterine leiomyoma. Semin Reprod Med. 2013;31(5):370–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bulun SE. Uterine fibroids. N Engl J Med. 2013;369(14):1344–1313. [DOI] [PubMed] [Google Scholar]
- 8. Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science. 2005;308(5728):1589–1592. [DOI] [PubMed] [Google Scholar]
- 9. Eltoukhi HM, Modi MN, Weston M, Armstrong AY, Stewart EA. The health disparities of uterine fibroid tumors for African American women: a public health issue. Am J Obstet Gynecol. 2014;210(3):194–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holdsworth-Carson SJ, Zaitseva M, Vollenhoven BJ, Rogers PA. Clonality of smooth muscle and fibroblast cell populations isolated from human fibroid and myometrial tissues. Mol Hum Reprod. 2014;20(3):250–259. [DOI] [PubMed] [Google Scholar]
- 11. Leppert PC, Catherino WH, Segars JH. A new hypothesis about the origin of uterine fibroids based on gene expression profiling with microarrays. Am J Obstet Gynecol. 2006;195(2):415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Malik M, Mendoza M, Payson M, Catherino WH. Curcumin, a nutritional supplement with antineoplastic activity, enhances leiomyoma cell apoptosis and decreases fibronectin expression. Fertil Steril. 2009;91(5 suppl):2177–2184. [DOI] [PubMed] [Google Scholar]
- 13. Britten JL, Malik M, Levy G, Mendoza M, Catherino WH. Gonadotropin-releasing hormone (GnRH) agonist leuprolide acetate and GnRH antagonist cetrorelix acetate directly inhibit leiomyoma extracellular matrix production. Fertil Steril. 2012;98(5):1299–1307. [DOI] [PubMed] [Google Scholar]
- 14. Patel A, Malik M, Britten J, Cox J, Catherino WH. Mifepristone inhibits extracellular matrix formation in uterine leiomyoma. Fertil Steril. 2016;105(4):1102–1110. [DOI] [PubMed] [Google Scholar]
- 15. Gilden M, Malik M, Britten J, Delgado T, Levy G, Catherino WH. Leiomyoma fibrosis inhibited by liarozole, a retinoic acid metabolic blocking agent. Fertil Steril. 2012;98(6):1557–1562. [DOI] [PubMed] [Google Scholar]
- 16. Levy G, Malik M, Britten J, Gilden M, Segars J, Catherino WH. Liarozole inhibits transforming growth factor-β3-mediated extracellular matrix formation in human three-dimensional leiomyoma cultures. Fertil Steril. 2014;102(1):272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Malik M, Catherino WH. Development and validation of a three-dimensional in vitro model for uterine leiomyoma and patient-matched myometrium. Fertil Steril. 2012;97(6):1287–1293. [DOI] [PubMed] [Google Scholar]
- 18. Malik M, Britten J, Cox J, Patel A, Catherino WH. Gonadotropin-releasing hormone analogues inhibit leiomyoma extracellular matrix despite presence of gonadal hormones. Fertil Steril. 2016;105(1):214–224. [DOI] [PubMed] [Google Scholar]
- 19. Malik M, Britten J, Segars J, Catherino WH. Leiomyoma cells in 3-dimensional cultures demonstrate an attenuated response to fasudil, a rho-kinase inhibitor, when compared to 2-dimensional cultures. Reprod Sci. 2014;21(9):1126–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Perrimon N, Bernfield M. Cellular functions of proteoglycans—an overview. Semin Cell Dev Biol. 2001;12(2):65–67. [DOI] [PubMed] [Google Scholar]
- 21. Iozzo RV, Schaefer L. Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol. 2015;42:11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dicker KT, Gurski LA, Pradhan-Bhatt S, Witt RL, Farach-Carson MC, Jia X. Hyaluronan: a simple polysaccharide with diverse biological functions. Acta Biomater. 2014;10(4):1558-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Courtoy GE, Donnez J, Marbaix E, Dolmans MM. In vivo mechanisms of uterine myoma volume reduction with ulipristal acetate treatment. Fertil Steril. 2015;104(2):426–434. [DOI] [PubMed] [Google Scholar]
- 24. Donnez J, Tatarchuk TF, Bouchard P, et al. PEARL I Study Group. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med. 2012;366(5):409–420. [DOI] [PubMed] [Google Scholar]
- 25. Donnez J, Donnez O, Matule D, et al. Long-term medical management of uterine fibroids with ulipristal acetate. Fertil Steril. 2016;105(1):165–173. [DOI] [PubMed] [Google Scholar]
- 26. Cox J, Malik M, Britten J, Lewis T, Catherino WH. Ulipristal acetate and extracellular matrix production in human leiomyomas in vivo: a laboratory analysis of a randomized placebo controlled trial. Reprod Sci. 2018;25(2):198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Demura TA, Revazova ZV, Kogan EA, Adamyan LV. The molecular mechanisms and morphological manifestations of leiomyoma reduction induced by selective progesterone receptor modulators. Arkh Patol. 2017;79(3):19–26. [DOI] [PubMed] [Google Scholar]
- 28. Donnez J, Hudecek R, Donnez O, et al. Efficacy and safety of repeated use of ulipristal acetate in uterine fibroids. Fertil Steril. 2015;103(2):519–527. [DOI] [PubMed] [Google Scholar]
- 29. Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5(6):472–484. [DOI] [PubMed] [Google Scholar]
- 30. Halterman JA, Kwon HM, Wamhoff BR. Tonicity-independent regulation of the osmosensitive transcription factor TonEBP (NFAT5). Am J Physiol Cell Physiol. 2012;302(1):C1–C8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. López-Rodríguez C, Aramburu J, Jin L, Rakeman AS, Michino M, Rao A. Bridging the NFAT and NF-kappaB families: NFAT5 dimerization regulates cytokine gene transcription in response to osmotic stress. Immunity. 2001;15(1):47–58. [DOI] [PubMed] [Google Scholar]
- 32. Levens ED, Potlog-Nahari C, Armstrong AY, et al. CDB-2914 for uterine leiomyomata treatment: a randomized controlled trial. Obstet Gynecol. 2008;111(5):1129–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nieman LK, Blocker W, Nansel T, et al. Efficacy and tolerability of CDB-2914 treatment for symptomatic uterine fibroids: a randomized, double-blind, placebo-controlled, phase IIb study. Fertil Steril. 2011;95(2):767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Malik M, Webb J, Catherino WH. Retinoic acid treatment of human leiomyoma cells transformed the cell phenotype to one strongly resembling myometrial cells. Clin Endocrinol. 2008;69(3):462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Malik M, Catherino WH. Development and validation of a three-dimensional in-vitro model for uterine leiomyoma and patient-matched myometrium. Fertil Steril. 2012;97(6):1287–1293. [DOI] [PubMed] [Google Scholar]
- 36. Pont JN, McArdle CA, López Bernal A. Oxytocin-stimulated NFAT transcriptional activation in human myometrial cells. Mol Endocrinol. 2012;26(10):1743–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Joseph DS, Malik M, Nurudeen S, Catherino WH. Myometrial cells undergo fibrotic transformation under the influence of transforming growth factor beta-3. Fertil Steril. 2010;93(5):1500–1508. [DOI] [PubMed] [Google Scholar]
- 38. van Diest PJ, van Dam P, Henzen-Logmans SC, et al. A scoring system for immunohistochemical staining: consensus report of the task force for basic research of the EORTC-GCCG. European Organization for Research and Treatment of Cancer-Gynaecological Cancer Cooperative Group. J Clin Pathol. 1997;50(10):801–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Flake GP, Moore AB, Sutton D, et al. The natural history of uterine leiomyomas: light and electron microscopic studies of fibroid phases, interstitial ischemia, inanosis and reclamation. Obstet Gynecol Int. 2013;2013:528376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chegini N, Zhao Y, Williams RS, Flanders KC. Human uterine tissue throughout the menstrual cycle expresses transforming growth factor-beta 1 (TGF beta 1), TGF beta 2, TGF beta 3, and TGF beta type II receptor messenger ribonucleic acid and protein and contains [125I]TGF beta 1-binding sites. Endocrinology. 1994;135(1):439–449. [DOI] [PubMed] [Google Scholar]
- 42. Hiyama A, Gogate SS, Gajghate S, Mochida J, Shapiro IM, Risbud MV. BMP-2 and TGF-beta stimulate expression of beta1,3-glucuronosyl transferase 1 (GlcAT-1) in nucleus pulposus cells through AP1, TonEBP, and Sp1: role of MAPKs. J Bone Miner Res. 2010;25(5):1179–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lewis TD, Malik M, Britten J, San Pablo AM, Catherino WH. A comprehensive review of the pharmacologic management of uterine leiomyoma. Biomed Res Int. 2018;2018:2414609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xu Q, Ohara N, Chen W, et al. Progesterone receptor modulator CDB-2914 down-regulates vascular endothelial growth factor, adrenomedullin and their receptors and modulates progesterone receptor content in cultured human uterine leiomyoma cells. Hum Reprod. 2006;21(9):2408–2416. [DOI] [PubMed] [Google Scholar]
- 45. Catherino WH, Malik M, Driggers P, Chappel S, Segars J, Davis J. Novel, orally active selective progesterone receptor modulator CP8947 inhibits leiomyoma cell proliferation without adversely affecting endometrium or myometrium. J Steroid Biochem Mol Biol. 2010;122(4):279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Horak P, Mara M, Dundr P, et al. Effect of a selective progesterone receptor modulator on induction of apoptosis in uterine fibroids in vivo. Int J Endocrinol. 2012;2012:436174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bouchard P, Chabbert-Buffet N. The history and use of the progesterone receptor modulator ulipristal acetate for heavy menstrual bleeding with uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2017;40:105–110. [DOI] [PubMed] [Google Scholar]
- 48. McCarthy-Keith DM, Malik M, Britten J, Segars J, Catherino WH. Gonadotropin-releasing hormone agonist increases expression of osmotic response genes in leiomyoma cells. Fertil Steril. 2011;95(7):2383–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Park JK, Kang TG, Kang MY, et al. Increased NFAT5 expression stimulates transcription of Hsp70 in preeclamptic placentas. Placenta. 2014;35(2):109–116. [DOI] [PubMed] [Google Scholar]
- 50. Arroyo JA, Garcia-Jones P, Graham A, Teng CC, Battaglia FC, Galan HL. Placental TonEBP/NFAT5 osmolyte regulation in an ovine model of intrauterine growth restriction. Biol Reprod. 2012;86(3):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lee GT, Price MD, Mejia CA, Galan HL, Arroyo JA. Increased trophoblast expression of NFAT5/TonEBP in pre-eclamptic placentas and hyperosmolar-treated BeWo cells. Eur J Obstet Gynecol Reprod Biol. 2014;183:37–43. [DOI] [PubMed] [Google Scholar]
- 52. Dobierzewska A, Palominos M, Irarrazabal CE, et al. NFAT5 is up-regulated by hypoxia: possible implications in preeclampsia and intrauterine growth restriction. Biol Reprod. 2015;93(1):1–11. [DOI] [PubMed] [Google Scholar]
- 53. Johnson ZI, Doolittle AC, Snuggs JW, Shapiro IM, Le Maitre CL, Risbud MV. TNF-α promotes nuclear enrichment of the transcription factor TonEBP/NFAT5 to selectively control inflammatory but not osmoregulatory responses in nucleus pulposus cells. J Biol Chem. 2017;292(42):17561–17575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rizner TL. Enzymes of the AKR1B and AKR1C subfamilies and uterine diseases. Front Pharmacol. 2012;3:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bresson E, Lacroix-Pépin N, Boucher-Kovalik S, Chapdelaine P, Fortier MA. The prostaglandin F synthase activity of the human aldose reductase AKR1B1 brings new lenses to look at pathologic conditions. Front Pharmacol. 2012;3:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tong EH, Guo JJ, Xu SX, et al. Inducible nucleosome depletion at OREBP-binding-sites by hypertonic stress. PLoS One. 2009;4(12): e8435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Andronic J, Shirakashi R, Pickel SU, et al. Hypotonic activation of the myo-inositol transporter SLC5A3 in HEK293 cells probed by cell volumetry, confocal and super-resolution microscopy. PLoS One. 2015;10(3):e0119990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chauvin TR, Griswold MD. Characterization of the expression and regulation of genes necessary for myo-inositol biosynthesis and transport in the seminiferous epithelium. Biol Reprod. 2004;70(3):744–751. [DOI] [PubMed] [Google Scholar]
- 59. Valenzuela JC, Heise C, Franken G, et al. Hyaluronan-based extracellular matrix under conditions of homeostatic plasticity. Philos Trans R Soc Lond B Biol Sci. 2014;369(1654):20130606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yasuo T, Yamaguchi T, Kitaya K. Progesterone induction of chondroitin sulfate proteoglycan aggrecan expression in human endometrial epithelial cells. J Steroid Biochem Mol Biol. 2010; 122(4):159–163. [DOI] [PubMed] [Google Scholar]
- 61. Dauth S, Grevesse T, Pantazopoulos H, et al. Extracellular matrix protein expression is brain region dependent. J Comp Neurol. 2016;524(7):1309–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Johnson ZI, Shapiro IM, Risbud MV. Extracellular osmolarity regulates matrix homeostasis in the intervertebral disc and articular cartilage: evolving role of TonEBP. Matrix Biol. 2014;40:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jahan N, Hannila SS. Transforming growth factor β-induced expression of chondroitin sulfate proteoglycans is mediated through non-Smad signaling pathways. Exp Neurol. 2015;263:372–384. [DOI] [PubMed] [Google Scholar]
- 64. Ciebiera M, Włodarczyk M, Wrzosek M, et al. Ulipristal acetate decreases transforming growth factor β3 serum and tumor tissue concentrations in patients with uterine fibroids. Fertil Steril. 2018;109(3):501–507. [DOI] [PubMed] [Google Scholar]
- 65. Norian JM, Malik M, Parker CY, et al. Transforming growth factor beta3 regulates the versican variants in the extracellular matrix-rich uterine leiomyomas. Reprod Sci. 2009;16(12):1153–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]