Abstract
Regenerative endodontic procedures are stem cell–based treatments for immature teeth with pulp necrosis. The translation of regenerative endodontic procedures into treating mature teeth depends, among other factors, on the availability and delivery of mesenchymal stem cells (MSCs) into the root canal system. The aim of this clinical study was to evaluate whether evoked bleeding from the periapical tissues elicits the influx of MSCs into the root canal system in mature teeth with apical lesions. Participants included in this study (N = 20) were referred for endodontic treatment of mature teeth with apical lesions. Following chemomechanical debridement, intracanal bleeding from the periapical tissues was achieved, and intracanal blood samples were collected. A positive blood aspirate was also collected in the cartridges during local anesthesia. Total RNA was isolated and used as a template in quantitative reverse transcription polymerase chain reactions using MSC-specific arrays. Data were analyzed with the Wilcoxon signed-rank test, and correlation between gene expression and sex or age was tested with Spearman’s rank correlation coefficient test. In addition, MSCs were isolated from an intracanal bleeding sample and subjected to flow cytometry and quantitative osteogenesis assay. Last, the presence and distribution of MSCs within periradicular lesions were evaluated with immunohistochemistry (n = 4). The MSC markers CD73, CD90, CD105, and CD146 were significantly upregulated, with median fold change values of 2.9, 31.7, 4.6, and 6.8, respectively. Conversely, the negative marker for MSCs, CD45, was significantly downregulated (median, –2.7). There was no correlation with age, sex, tooth type, or treatment for any of the evaluated genes. Isolated intracanal cells coexpressed MSC markers and demonstrated robust mineralizing differentiation potential. Finally, immunohistochemical analysis revealed that MSCs were found compartmentalized mainly within vasculature structures located in periapical lesions. Collectively, findings indicate that the evoked-bleeding technique delivers MSCs into the root canal system in mature teeth with apical lesions.
Keywords: periapical periodontitis, endodontics, tissue engineering, cell therapy, bleeding, adult
Introduction
Advances in tissue engineering created an important clinical paradigm shift toward the functional regeneration of damaged or lost tissues. Functional regeneration depends on orchestrating the interplay among stem cells, scaffolds, and growth factors (Langer and Vacanti 1993). Regenerative endodontic procedures (REPs) are biologically based procedures designed to replace damaged structures, including dentin and root structures, as well as cells of the pulp-dentin complex (Murray et al. 2007). The aim of regenerative endodontics is to reproduce the original pulp tissue morphology and function based on tissue engineering principles (Hargreaves et al. 2013). Despite the significant clinical success of these currently used REPs in promoting healing of apical periodontitis, continued root development, and, in certain cases, vitality responses (Diogenes et al. 2013), there is evidence that the tissues formed do not fully mimic the native pulp-dentin complex (Shimizu et al. 2012; Torabinejad and Faras 2012; Lin et al. 2014). Conceptually, the ultimate functional and histologic regeneration of the pulp-dentin complex depends on the identification of autologous sources of mesenchymal stem cells (MSCs), a suitable scaffold, and supportive growth factors.
In immature teeth, the apical papilla represents an enriched pool of MSCs that, among other periradicular stem cell niches, holds promise in endodontic regeneration procedures (Huang et al. 2008; Sonoyama et al. 2008; Huang et al. 2010). Notably, the evoked-bleeding step that follows the manipulation of apical tissues in immature teeth with an apical papilla, as commonly done in REPs, results in a substantial influx of cells expressing MSC markers into the root canal system (Lovelace et al. 2011). This finding implicates a role for MSCs in the regenerative responses seen in immature teeth treated with REPs. However, the successful application of these techniques to the treatment of mature teeth in adult patients with endodontic lesions is uncertain and may be enhanced by the delivery of MSCs into the root canal system of these teeth. Since the apical papilla is no longer present in mature teeth, other sources of MSCs are needed. Although potential sources in mature teeth include bone, periodontal ligament, and inflamed periapical tissues (Liao et al. 2011; Diogenes et al. 2013), studies are lacking that have evaluated the feasibility of the clinical delivery of MSCs into the canal system of mature teeth with endodontic lesions.
Therefore, the aim of this clinical study was to test the hypothesis that intracanal bleeding evoked by the overinstrumentation of periapical tissues elicits the influx of cells with MSC markers into the root canal system of mature, fully formed teeth with apical lesions. The gene expression of MSC markers in blood collected from the canal system following evoked bleeding was compared with systemic blood levels as a primary outcome measure.
Material and Methods
The study was conducted in accordance with the ethical principles of the World Medical Association Declaration of Helsinki and received approval from the Institutional Review Board of the University of Texas Health Science Center at San Antonio (UTHSCSA). The subject pool consisted of patients referred to the graduate endodontics clinic at the UTHSCSA for evaluation and root canal treatment. The inclusion criterion for this study was healthy (American Society of Anesthesiologists I or II physical status) adult patients presenting with a mature, fully formed tooth diagnosed with pulp necrosis or previously treated pulp and apical periodontitis that required nonsurgical root canal treatment, nonsurgical root canal retreatment, or periapical surgery. All patients were informed on the scope of this study and signed an informed consent.
Exclusion criteria included American Society of Anesthesio-logists III or IV physical status, systemic health problems that might interfere with healing of apical periodontitis (e.g., immunocompromised, long-term steroid usage), <18 y of age, and unwillingness to participate in the study for any reason.
Root Canal Treatment and Blood Sample Collection
Graduate endodontic residents at the UTHSCSA graduate endodontics clinic performed the root canal treatments. During the first appointment, teeth were anesthetized and accessed under rubber dam isolation. Chemomechanical debridement of the root canal system was achieved with Protaper and Profile systems (Dentsply Maillefer, Ballaigues, Switzerland) for mechanical instrumentation and with 6% sodium hypochlorite irrigation. A final rinse with 17% EDTA was performed, and calcium hydroxide paste (Ultracal XS Paste; Ultradent Products Inc., South Jordan, UT, USA) was placed as intracanal medicament. The access was sealed with Cavit (3M, St. Paul, MN, USA).
One week following the initial appointment, patients returned for a second treatment visit. A positive blood aspirate (systemic blood sample) was collected in the cartridges during local anesthetic injection (2% lidocaine with 1:100,000 epinephrine) and immediately placed into an RNA isolation lysis buffer (RNeasy MiniKit; Qiagen, Valencia, CA, USA) as previously described (Lovelace et al. 2011). Teeth were then isolated, accessed, and irrigated with 6% sodium hypochlorite and 17% EDTA to remove calcium hydroxide from the root canal system. A final rinse with 17% EDTA and saline was performed. The canal was dried with paper points that were collected as control for the study. Intracanal bleeding was evoked with the use of a size 20 K-file at 3 to 5 mm beyond the working length (evoked-bleeding technique). In multirooted teeth, the root canal with the largest apical size was chosen for the evoked-bleeding step. Following verification of intracanal bleeding with the use of microscope at 10× to 16× magnification, a second paper point was placed in the canal for 2 min to collect a blood sample from the canal (intracanal blood sample). The samples were immediately transferred in RNA isolation lysis buffer (Qiagen). Finally, canals were obturated with gutta-percha and resin sealer (AHPlus; Dentsply Maillefer). Both samples from each patient were stored in −80 °C until total RNA isolation was performed for all samples.
RNA Isolation and Real-time Quantitative Reverse Transcription Polymerase Chain Reaction
Total RNA was isolated from systemic control paper points and intracanal blood samples as described previously (Lovelace et al. 2011). cDNA was then synthesized from these RNA samples with the RT2 Nano PreAMP cDNA Synthesis Kit (Qiagen). The preamplified cDNA samples were used as template in real-time quantitative reverse transcription polymerase chain reaction reactions using MSC-specific arrays (RT2 Profiler PCR Array; Qiagen). The reactions were performed on an ABI 7500 ht-fast sequence detector (Applied Biosystems, Palo Alto, CA, USA) with standardized thresholds. The paired raw cycle threshold data from each subject (i.e., systemic and intracanal blood samples from each patient) were uploaded on a web-based software package for the PCR Array System (RT2 Profiler PCR Array Data Analysis; Qiagen). Each target gene mRNA expression was calculated as fold changes of levels found within intracanal blood as compared with systemic blood levels for each patient.
Isolation and Culture of MSCs Delivered into a Root Canal Space
Periapical bleeding was evoked into the canal of mature tooth 8 of a 35-y-old female patient. The intracanal blood sample was aspirated in a 1-cc syringe. Red blood cells were removed with an ammonium chloride treatment (StemCell Technologies, Vancouver, Canada) per the manufacturer’s instructions. Then, cells were centrifuged at 2,500 rpm for 5 min, resuspended, and cultured at 37 °C and 5% CO2 in basal culture media composed of alpha-minimum essential medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine, L-glutamine (Gemini, West Sacramento, CA, USA), penicillin (100 U/mL; Gemini), streptomycin (100mg/mL; Gemini), and amphotericin B (Sigma, St. Louis, MO, USA). Single-cell colonies were transferred to 10-cm culture dishes, maintained, and cultured until ~80% confluency. Cells in third to fourth passage were utilized for flow cytometry and differentiation experiments.
Osteogenesis Differentiation
Intracanal MSCs were cultured for 2 wk in basal media or osteogenesis induction media (StemPro Osteogenesis Differentiation Kit; Life Technologies, Grand Island, NY, USA). The quantitative analysis of osteogenesis differentiation was then performed utilizing the Osteogenesis Quantitation Kit (Millipore, Darmstadt, Germany) following the manufacturer’s protocol.
Flow Cytometry
For the multicolor flow cytometry analysis, single-cell suspensions in Stain Buffer (106 cells/mL; BD Biosciences, San Jose, CA, USA) were incubated concomitantly with 5 fluorescent-labeled antibodies for 30 min on ice and protected from light as described previously (Ruparel et al. 2013). The following antibodies were used: PE-CY7 mouse anti-human CD73 (561258; BD Biosciences), APC mouse anti-human CD90 (clone 5E10; BD Biosciences), PerCP-CY5.5 mouse anti-human CD105 (clone 266RUO; BD Biosciences), FITC mouse anti-human CD146 (clone P1H12; BD Biosciences), and APC-H7 mouse anti-human CD45 (clone 2D1BD; Biosciences). Controls consisted of unstained cells and single-color fluorophore controls. Samples were analyzed in a LSR-II cell analyzer using Diva 6.1.3 (BD Biosciences). The gating strategy included doublet discrimination for single cells, scatter gate for gating out small debris, and gating on CD45-negative cells. Positive gates for CD73, CD90, CD105, and CD146 were based on the unstained control. The percentage of cells expressing each marker, as well as the percentage of the cells coexpressing CD73, CD90, CD105, and CD146 simultaneously, was determined.
Immunohistochemistry and Laser Confocal Microscopy
The presence and distribution of MSCs in periapical lesion biopsies were examined as a secondary outcome measure. Surgical biopsies of periradicular lesions in adult patients undergoing apicoectomies were obtained (n = 5). Half of each biopsy was sent to a pathologist for diagnosis, whereas the other half was processed for immunohistochemistry and confocal microscopy as previously described (Henry et al. 2012). One biopsy specimen was diagnosed as normal (i.e., uninflamed tissue) and was used as normal control tissue. Immunohistochemical analysis was performed on tissue slices from each specimen with the MSC markers CD90 (mouse anti-human, 550402, 1:50 dilution; BD Biosciences), CD105 (mouse anti-human, M3527, 1:500 dilution; Dako, Glostrup, Denmark), CD146 (mouse anti-human, 611208, 1:100 dilution; BD Biosciences), or the negative marker CD45 (mouse anti-human, M0701, 1:200 dilution; Dako) in combination with the endothelial cell marker von Willebrand factor antibody (rabbit anti-human, A0082, 1:4,000 dilution; Dako). Secondary antibodies included goat anti-mouse Alexa Fluor 488 and goat anti-rabbit Alexa Fluor 568 (1:100 dilution; Molecular Probes, Eugene, OR, USA). All slides were also incubated with the nuclear stain TO-PRO-3 (1:5,000 dilution; Molecular Probes). Immunoreactivity was visualized with a Nikon C1 si laser confocal imaging system equipped with 90i Nikon microscope (Nikon, Melville, NY, USA). Control preparations consisted of tissue slices that were stained with secondary antibodies but lacked primary antibodies. These control slices showed a lack of immunofluorescence signal.
Statistical Analysis
The calculated fold changes in gene expression for all subjects were combined and tested for normality with the Shapiro-Wilk normality test. Next, differences in median fold change in gene expression were tested with 1-tailed Wilcoxon signed-rank tests. Summary statistics are presented as median (quartile 1, quartile 3; P value). In addition, the Spearman’s rank correlation coefficient test was used to examine the association between each stem cell marker and age in pairwise comparisons, whereas 2-tailed Mann-Whitney U tests were utilized to test for differences in the fold changes in gene expression of the stem cell markers in subgroups stratified by type of treatment (i.e., nonsurgical root canal treatment or retreatment) or sex and for alizarin red quantification. Statistical analysis was carried out with Graph Pad Prism 5.0 (Graph Pad, La Jolla, CA, USA) at α = 0.05 for all tests.
Results
A total of 20 adult patients (12 female, 8 male) with a mean age of 44 y (range, 20-85) participated in the MSC transfer portion of the study: 55% had nonsurgical root canal treatment, and 45% had nonsurgical root canal retreatment. Teeth breakdown was as follows: 45% mandibular molars, 20% maxillary molars, 15% maxillary anteriors, 10% mandibular premolars, and 10% maxillary premolars.
Comparison of the levels of MSC markers observed in the systemic blood with those identified in blood obtained from the root canal system by the evoked-bleeding step revealed a median 4.4-fold (0.4, 50.6; P = 0.048) intracanal increase in CD73, a median 32.6-fold (2.3, 210; P < 0.001) intracanal increase in CD90, a median 4.3-fold (2.3, 34.8; P = 0.006) increase of CD105, and a median 6.9-fold (1.3, 34.9; P = 0.012) increase in CD146 transcripts (Fig. 1). In addition, there was a significant downregulation in the intracanal expression of the negative marker CD45 (median, −2.7 [−10, −1.3]; P = 0.036; Fig. 1). Results showed that 40% of subjects had a significant upregulation of all 4 positive markers and a significant downregulation of CD45 in intracanal blood samples. In addition to the MSC markers, polymerase chain reaction array genes related to stem cell stemness, stem cell survival, homing, proliferation, and differentiation were significantly upregulated (Table). There were no detectable transcripts in the saline sample collected from canals prior to evoked bleeding.
Figure 1.

Real-time quantitative reverse transcription polymerase chain reaction analysis; fold change in gene expression for the mesenchymal stem cell (MSC) markers CD73, CD90, CD105, CD146, and CD45. Data are presented as median fold change in gene expression in the intracanal blood samples normalized to the systemic blood levels for each marker. The positive MSC markers CD73, CD90, CD105, and CD146 were significantly upregulated. The negative MSC marker CD45 was significantly downregulated. *P < 0.05. **P < 0.01. ***P < 0.001.
Table.
Expression Genes Included in the MSC Polymerase Chain Reaction Array That Had a Change >2-fold.
| Gene Name | Gene Symbol | Median Fold Change (P Value) | Function |
|---|---|---|---|
| Vascular cell adhesion molecule 1 | VCAM1 (CD106) | 20.35 (0.014) | Endothelial marker, also expressed in perivascular stem cells (Gronthos et al. 2000; Carter and Wicks 2001) |
| Osteocalcin | OCN | 56.2 (0.013) | Mainly expressed by osteoblasts; regulates bone formation and mineralization (Lee et al. 2007) |
| Collagen, type I, alpha 1 | COL1A1 | 117.6 (<0.001) | Main collagen type in dental pulp and dentin; regulates dentin formation (Mizuno et al. 2003) |
| Fibroblast growth factor 10 | FGF10 | 5.9 (0.043) | Maintains stem cell survival, stemness (Harada et al. 2002) |
| KIT ligand | SCF | 6.1 (0.022) | Promotes stem cell homing and participates in angiogenesis (Pan et al. 2013) |
| Granulocyte colony-stimulating factor | GCSF | 4.7 (0.036) | Chemotactic agent for MSCs; also induces maturation of hematopoietic cells among other functions (Fukuda and Fujita 2005; Horibe et al. 2014) |
| Matrix metalloproteinase 2 | MMP2 | 42.9 (<0.001) | Key role in differentiation of odontoblast-like cells and reparative dentinogenesis (Chaussain et al. 2009) |
| Insulinlike growth factor 1 | IGF1 | 33.4 (0.01) | Odontoblastic differentiation (Onishi et al. 1999) |
| Nestin | NES | 8.27 (0.04) | Odontoblastic and neuronal differentiation (About et al. 2000; Abe et al. 2012) |
These genes were significantly upregulated in the root canal system after the evoked bleeding step as compared with systemic blood.
MSC, mesenchymal stem cell.
Correlation analysis revealed no significant association between the fold changes in MSC marker gene expressions and age (all P > 0.08). Similarly, there was no effect of the sex or treatment type with the median fold change in gene expression of the MSC markers (all P > 0.05).
Isolated MSCs transferred into root canals following evoked bleeding coexpressed CD73 and CD90 in >95% of all cells. Of these, 67% of the cells also expressed CD105 and CD146 while being negative for CD45 (Fig. 2). Importantly, these cells demonstrated robust differentiation into a mineralizing phenotype in culture (Fig. 3).
Figure 2.
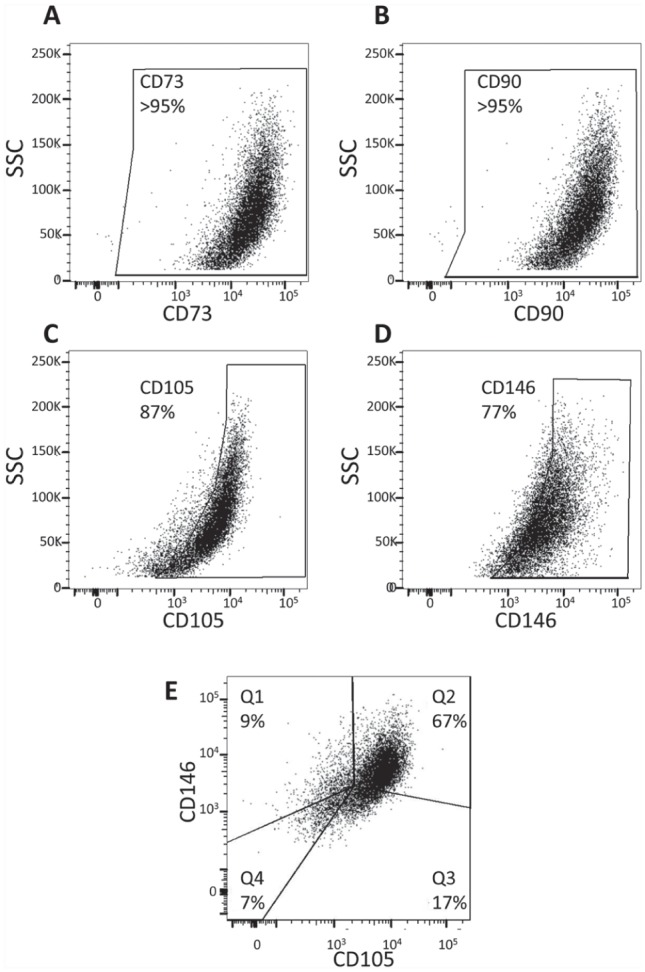
Scatter plots showing the expression of mesenchymal stem cell markers by multicolor flow cytometry analysis. The majority of cells isolated from intracanal blood following overinstrumentation of the periapical tissues coexpress mesenchymal stem cell markers. After doublet discrimination, CD45-negative cells were analyzed for CD73, CD90, CD105, and CD146 using gates based on the unstained control. CD73 (A) and CD90 (B) were found to be expressed in >95% of cells, whereas CD105 (C) and CD146 (D) were expressed in 87% and 77% of cells, respectively. (E) Of the CD73 and CD90 coexpressing cells, 9% also expressed CD146 but not CD105 (Q1); 67% expressed both CD105 and CD146 (Q2); 17% expressed only CD105 but not CD146 (Q3); and 7% did not express either CD105 or CD146. SSC, side scatter.
Figure 3.

Mesenchymal stem cells isolated from an intracanal blood following overinstrumentation of the periapical tissues demonstrated robust mineralizing differentiation potential. Cells were cultured for 2 wk in basal or osteogenic media. Alizarin red staining was performed (A) and quantified (B). Cells cultured in osteogenic media (osteogenesis group) expressed significantly higher mineralization activity as compared with cells cultured in standard culture media (control group). The mineralization activity was measured by absorbance at 405 nm. Data are presented as mean absorbance (n = 6). ***P < 0.001, Student’s t test.
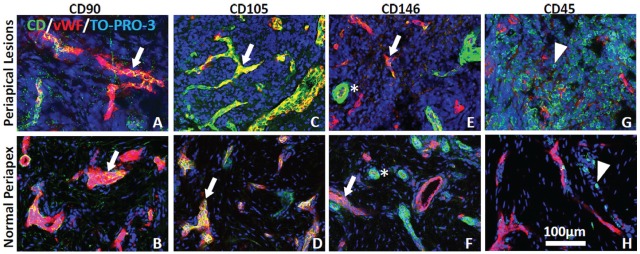
Confocal microscopic evaluation of tissue sections from control and periapical lesion biopsy specimens revealed that cells expressing the positive MSC markers CD90, CD105, and CD146 were colocalized with von Willebrand factor–identified endothelial cells in vascular structures (Fig. 4). In contrast, specific immunofluorescence staining with the negative MSC marker, CD45, was identified within immune cells but was lacking in the same cells that showed staining with the MSC markers (Fig. 4).
Figure 4.
Immunohistochemistry and confocal microscopy. Cells expressing mesenchymal stem cell markers CD90, CD105, and CD146 (green) are mostly colocalized with von Willebrand factor–identified (red) endothelial cells (arrows) in periapical lesions (A, C, E) and normal periapex (B, D, F), while CD146 is also expressed in vascular-associated smooth muscle (E, F; asterisks). In contrast, CD45 (green) is expressed in immune cells (arrowheads) in periapical lesion (G) and normal periapex (H). Nuclei are identified by TO-PRO-3 staining (blue), and scale bar in panel H applies to all images.
Discussion
Although the evoked-bleeding step in revascularization procedures leads to a substantial influx of MSCs into the root canal system in immature teeth, similar studies in mature, fully formed teeth are lacking (Lovelace et al. 2011). Therefore, the aim of the present study was to evaluate whether the same procedure would also result in the influx of cells with MSC markers in mature teeth. MSCs can be identified by the expression of a specific cluster of differentiation markers, including CD105, CD90, CD73, and CD146, while being negative for CD45 among other markers (Dominici et al. 2006; Russell et al. 2010). The present study used polymerase chain reaction array technology to evaluate the expression of these CD markers in combination with the differential expression of other genes associated with MSCs. Importantly, the positive markers of MSCs were found to be significantly increased following intracanal influx of apical blood. Conversely, the expression of CD45 was found to be significantly decreased, suggesting that the laceration of the apical tissues promoted the influx of cells that meet the minimal criteria for identification as MSCs.
Besides specific MSC markers, defining criteria for MSCs also include adherence to plastic when in culture and differentiation potential (Dominici et al. 2006). To evaluate these cell properties, a periapical MSC population was isolated from an intracanal blood sample and cultured. These cells adhered to plastic, formed single colonies, and differentiated in a mineralizing phenotype when induced in vitro. Importantly, they also showed ability to coexpress all the required MSC markers while lacking CD45. These results indicate that cells transferred into root canals by periapical overinstrumentation and bleeding possess MSC properties.
Immunohistochemical characterization of the expression pattern of MSC markers was performed in surgical biopsies from apical lesions and compared with staining seen in normal control apical tissue. The expression of the positive markers CD90, CD105, and CD146 was mainly compartmentalized within perivascular niches in periapical lesions. Findings from this study agree with previous results that inflamed periapical tissues harbor precursor cells (Liao et al. 2011). The enthothelial marker CD106 was also significantly increased in the intracanal bleeding samples, which further indicates a possible association of the MSCs found in the root canal system with the periapical vasculature (Gronthos et al. 2000). Nonetheless, the current study does not directly address the source of the cells delivered into root canals following the evoked-bleeding step that include several MSC niches (Diogenes et al. 2013). For example, the high expression of osteocalcin and collagen type 1 suggests that cells with a mineralizing phenotype, such as osteoblast-like cells, were also likely delivered intracanally. However, the relative high abundance of MSCs in the richly vascularized apical inflamed tissues supports the hypothesis that inflamed periradicular tissues represent one of the primary sources of the cells delivered into root canals in this study.
Several genes related to MSCs were signicantly upregulated in addition to MSC markers. Fibroblast growth factor 10 is an important factor that maintains stem cell survival and stemness and regulates mitogenesis and fate decision of adult stem cells (Harada et al. 2002). The stem cell factor is a potent chemotactic factor that has been identified as a potential therapeutic factor to promote cell homing of dental pulp stem cells (Pan et al. 2013). Clinically, transplantation of dental pulp stem cells mobilized by granulocyte colony-stimulating factor in canine pulpectomzed teeth has been shown to lead to significantly greater odontogenic differentiation when compared with transplantation with pulp stem cells alone (Iohara et al. 2013). Importantly, granulocyte colony-stimulating factor mobilized dental pulp stem cells are currently being used in a pulpal regeneration clinical trial (Nakashima and Iohara 2014). Matrix metalloproteinase 2, found increased in this study, is known to be expressed in dental stem cells, where it has been shown to be able to cleave dentin matrix protein 1, releasing bioactive peptides that participate in the differentiation of odontoblast-like cells and reparative dentinogenesis (Chaussain et al. 2009). The differentiation of stem cells into odontoblast-like cells has been shown to be increased by insulin-like growth factor 1, another gene found increased in this study (Onishi et al. 1999). Furthermore, increased expression of nestin has been shown to be associated with odontoblastic and neuronal differentiation (Terling et al. 1995; About et al. 2000; Abe et al. 2012). Last, nestin expression is indicative of a neural crestal origin, potentially being derived from glial cells surrounding the periapical innervation, as demonstrated recently (Kaukua et al. 2014). Collectively, these findings reveal that the evoked-bleeding step in mature teeth delivers cells expressing numerous gene transcripts important for regulating the proliferation, migration, and differentiation of dental stem cells.
A significant observation from the present study was that there was a high variability in the concentration of these MSC markers among patients. The variability noted in this sample population implies that factors other than age, sex, and tooth type modulates the relative abundance of these cells. These factors could be related to unknown host factors or the nature and duration of the etiology (e.g., duration and virulence of an endodontic infection). The ability of multipotent stem cells to replace damaged tissues has been shown to decline with age (Jones and Rando 2011). However, in the present study, the relative abundance of MSC markers was not correlated with age. The differentiation potential of these cells is paramount for tissue-engineering approaches, in addition to their presence and relative abundance. Dental pulp from aged dogs has been shown to contain MSCs that display high proliferation and migration potential but lower differentiation potential than that of MSCs derived from younger dogs (Iohara et al. 2014). Therefore, age might be a factor that may not significantly determine the amount of inflow of MSCs into the root canal system in adults but may influence their differentiation capacity.
In conclusion, this study is the first to demonstrate that the evoked-bleeding technique delivers undifferentiated MSCs into the root canal systems of adult patients with mature teeth. Knowledge gained related to the availability, identity, and influx of stem cells from the apical region in mature teeth could provide the basis for future clinical procedures incorporating the use of suitable scaffolds and chemotactic factors to promote cell homing and the regeneration of the pulp-dentin complex in mature, fully formed teeth.
Author Contributions
V. Chrepa, contributed to design, data acquisition, analysis and interpretation, drafted the manuscript; M.A. Henry, B.J. Daniel, contributed to data acquisition and analysis, critically revised the manuscript; A. Diogenes, contributed to conception, design, and data analysis, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Footnotes
This study was supported by start-up funds (A.D.) from the University of Texas Health Science Center at San Antonio (UTHSCSA). The Flow Cytometry Shared Resource Facility is supported by the UTHSCSA and the National Institutes of Health–National Cancer Institute (grant P30 CA054174-20, Cancer Therapy and Research Center at the UTHSCSA; grant UL1 TR001120, Clinical and Translational Science Award).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Abe S, Hamada K, Miura M, Yamaguchi S. 2012. Neural crest stem cell property of apical pulp cells derived from human developing tooth. Cell Biol Int. 36(10):927–936. [DOI] [PubMed] [Google Scholar]
- About I, Bottero MJ, de Denato P, Camps J, Franquin JC, Mitsiadis TA. 2000. Human dentin production in vitro. Exp Cell Res. 258(1):33–41. [DOI] [PubMed] [Google Scholar]
- Carter RA, Wicks IP. 2001. Vascular cell adhesion molecule 1 (CD106): a multifaceted regulator of joint inflammation. Arthritis Rheum. 44(5):985–994. [DOI] [PubMed] [Google Scholar]
- Chaussain C, Eapen AS, Huet E, Floris C, Ravindran S, Hao J, Menashi S, George A. 2009. MMP2-cleavage of DMP1 generates a bioactive peptide promoting differentiation of dental pulp stem/progenitor cell. Eur Cell Mater. 18:84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diogenes A, Henry MA, Teixeira FB, Hargreaves KM. 2013. An update on clinical regenerative endodontics. Endod Topics. 28(1):2–23. [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E. 2006. Minimal criteria for defining multipotent mesenchymal stromal cells: the International Society for Cellular Therapy position statement. Cytotherapy. 8(4):315–317. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Fujita J. 2005. Mesenchymal, but not hematopoietic, stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction in mice. Kidney Int. 68(5):1940–1943. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. 2000. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 97(25):13625–13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada H, Toyono T, Toyoshima K, Yamasaki M, Itoh N, Kato S, Sekine K, Ohuchi H. 2002. FGF10 maintains stem cell compartment in developing mouse incisors. Development. 129(6):1533–1541. [DOI] [PubMed] [Google Scholar]
- Hargreaves KM, Diogenes A, Teixeira FB. 2013. Treatment options: biological basis of regenerative endodontic procedures. J Endod. 39(3):S30–S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry MA, Luo S, Levinson SR. 2012. Unmyelinated nerve fibers in the human dental pulp express markers for myelinated fibers and show sodium channel accumulations. BMC Neurosci. 13:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horibe H, Murakami M, Iohara K, Hayashi Y, Takeuchi N, Takei Y, Kurita K, Nakashima M. 2014. Isolation of a stable subpopulation of mobilized dental pulp stem cells (MDPSCs) with high proliferation, migration, and regeneration potential is independent of age. PLoS One. 9(5):e98553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GT, Sonoyama W, Liu Y, Liu H, Wang S, Shi S. 2008. The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. J Endod. 34(6):645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GT, Yamaza T, Shea LD, Djouad F, Kuhn NZ, Tuan RS, Shi S. 2010. Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng Part A. 16(2):605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iohara K, Murakami M, Nakata K, Nakashima M. 2014. Age-dependent decline in dental pulp regeneration after pulpectomy in dogs. Exp Gerontol. 52:39–45. [DOI] [PubMed] [Google Scholar]
- Iohara K, Murakami M, Takeuchi N, Osako Y, Ito M, Ishizaka R, Utunomiya S, Nakamura H, Matsushita K, Nakashima M. 2013. A novel combinatorial therapy with pulp stem cells and granulocyte colony-stimulating factor for total pulp regeneration. Stem Cells Transl Med. 2(7):521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DL, Rando TA. 2011. Emerging models and paradigms for stem cell ageing. Nat Cell Biol. 13(5):506–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaukua N, Shahidi MK, Konstantinidou C, Dyachuk V, Kaucka M, Furlan A, An Z, Wang L, Hultman I, Ahrlund-Richter L, et al. 2014. Glial origin of mesenchymal stem cells in a tooth model system. Nature. 513(7519):551–554. [DOI] [PubMed] [Google Scholar]
- Langer R, Vacanti JP. 1993. Tissue engineering. Science. 260(5110):920–926. [DOI] [PubMed] [Google Scholar]
- Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKeee MD, Jung DY, et al. 2007. Endocrine regulation of energy metabolism by the skeleton. Cell. 130(3):456–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J, Al Shahrani M, Al-Habib M, Tanaka T, Huang GT. 2011. Cells isolated from inflamed periapical tissue express mesenchymal stem cell markers and are highly osteogenic. J Endod. 37(9):1217–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LM, Shimizu E, Gibbs JL, Loghin S, Ricucci D. 2014. Histologic and histobacteriologic observations of failed revascularization/revitalization therapy: a case report. J Endod. 40(2):291–295. [DOI] [PubMed] [Google Scholar]
- Lovelace TW, Henry MA, Hargreaves KM, Diogenes A. 2011. Evaluation of the delivery of mesenchymal stem cells into the root canal space of necrotic immature teeth after clinical regenerative endodontic procedure. J Endod. 37(2):133–138. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Miyamoto T, Wada K, Watatani S, Zhang GX. 2003. Type I collagen regulated dentin matrix protein-1 (Dmp-1) and osteocalcin (OCN) gene expression of rat dental pulp cells. J Cell Biochem. 88(6):1112–1119. [DOI] [PubMed] [Google Scholar]
- Murray PE, Garcia-Godoy F, Hargreaves KM. 2007. Regenerative endodontics: a review of current status and a call for action. J Endod. 33(4):377–390. [DOI] [PubMed] [Google Scholar]
- Nakashima M, Iohara K. 2014. Mobilized dental pulp stem cells for pulp regeneration: initiation of clinical trial. J Endod. 40(4):S26–S32. [DOI] [PubMed] [Google Scholar]
- Onishi T, Kinoshita S, Shintani S, Sobue S, Ooshima T. 1999. Stimulation of proliferation and differentiation of dog dental pulp cells in serum-free culture medium by insulin-like growth factor. Arch Oral Biol 44(4):361–371. [DOI] [PubMed] [Google Scholar]
- Pan S, Dangaria S, Gopinathan G, Yan X, Lu X, Kolokythas A, Niu Y, Luan X. 2013. SCF promotes dental pulp progenitor migration, neovascularization, and collagen remodeling: potential applications as a homing factor in dental pulp regeneration. Stem Cell Rev. 9(5):655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruparel NB, de Almeida JF, Henry MA, Diogenes A. 2013. Characterization of a stem cell of apical papilla cell line: effect of passage on cellular phenotype. J Endod. 39(3):357–363. [DOI] [PubMed] [Google Scholar]
- Russell KC, Phinney DG, Lacey MR, Barrilleaux BL, Meyertholen KE, O’Connor KC. 2010. In vitro high-capacity assay to quantify the clonal heterogeneity in trilineage potential of mesenchymal stem cells reveals a complex hierarchy of lineage commitment. Stem Cells. 28(4):788–798. [DOI] [PubMed] [Google Scholar]
- Shimizu E, Jong G, Partridge N, Rosenberg PA, Lin LM. 2012. Histologic observation of a human immature permanent tooth with irreversible pulpitis after revascularization/regeneration procedure. J Endod. 38(9):1293–1297. [DOI] [PubMed] [Google Scholar]
- Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, Huang GT. 2008. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 34(2):166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terling C, Rass A, Mitsiadis TA, Fried K, Lendahl U, Wroblewski J. 1995. Expression of the intermediate filament nestin during rodent tooth development. Int J Dev Biol. 39(6):947–956. [PubMed] [Google Scholar]
- Torabinejad M, Faras H. 2012. A clinical and histological report of a tooth with an open apex treated with regenerative endodontics using platelet-rich plasma. J Endod. 38(6):864–868. [DOI] [PubMed] [Google Scholar]