Abstract
Periodontitis is one of the most common inflammatory human diseases with a strong genetic component. Due to the limited sample size of available periodontitis cohorts and the underlying trait heterogeneity, genome-wide association studies (GWASs) of chronic periodontitis (CP) have largely been unsuccessful in identifying common susceptibility factors. A combination of quantitative trait loci (QTL) mapping in mice with association studies in humans has the potential to discover novel risk loci. To this end, we assessed alveolar bone loss in response to experimental periodontal infection in 25 lines (286 mice) from the Collaborative Cross (CC) mouse population using micro–computed tomography (µCT) analysis. The orthologous human chromosomal regions of the significant QTL were analyzed for association using imputed genotype data (OmniExpress BeadChip arrays) derived from case-control samples of aggressive periodontitis (AgP; 896 cases, 7,104 controls) and chronic periodontitis (CP; 2,746 cases, 1,864 controls) of northwest European and European American descent, respectively. In the mouse genome, QTL mapping revealed 2 significant loci (–log P = 5.3; false discovery rate = 0.06) on chromosomes 1 (Perio3) and 14 (Perio4). The mapping resolution ranged from ~1.5 to 3 Mb. Perio3 overlaps with a previously reported QTL associated with residual bone volume in F2 cross and includes the murine gene Ccdc121. Its human orthologue showed previously a nominal significant association with CP in humans. Use of variation data from the genomes of the CC founder strains further refined the QTL and suggested 7 candidate genes (CAPN8, DUSP23, PCDH17, SNORA17, PCDH9, LECT1, and LECT2). We found no evidence of association of these candidates with the human orthologues. In conclusion, the CC populations enabled mapping of confined QTL that confer susceptibility to alveolar bone loss in mice and larger human phenotype-genotype samples and additional expression data from gingival tissues are likely required to identify true positive signals.
Keywords: Collaborative Cross, animal model, genetic, alveolar bone loss, QTL mapping, GWAS
Background
Periodontitis is a common multifactorial oral disease caused by a dysbiotic oral microbiota and a deregulated host inflammatory response (Hajishengallis 2014). There is clear evidence that genetic variation contributes to the susceptibility of periodontitis (Corey et al. 1993). However, most suggested susceptibility genes of periodontitis did not meet the significance threshold of association or have not been successfully replicated (Vaithilingam et al. 2014), but some genes are considered true genetic susceptibility factors by giving evidence through independent identification (e.g., NPY [Divaris et al. 2013; Freitag-Wolf et al. 2014], CAMTA1/VAMP3 [Divaris et al. 2012; Bochenek et al. 2013]) or repeated replication in independent case-control populations (e.g., ANRIL [Ernst et al. 2010] and GLT6D1 [Schaefer et al. 2010; Hashim et al. 2015]).
Chromosomal regions responsible for the genetic variance of complex traits in mice can be mapped as quantitative trait loci (QTL) (Iraqi 2000), and orthologous genes can be extended successfully to humans. Recently, 2 QTL associated with periodontitis were reported using F2 (Shusterman, Durrant, et al. 2013) and recombinant inbred lines (RIL) (Sima et al. 2015) mapping approaches. While F2 study reported 3 QTL (Perio1 on chr5, Perio2 on chr3, and Perio3 on chr1) associated with residual bone volume 42 d after oral bacterial infection (Shusterman, Durrant, et al. 2013), the RIL mapping approach suggested 1 QTL (iABLL-on chr2) associated with ligature-induced periodontitis (Sima et al. 2015).
The Collaborative Cross (CC) is a novel RIL mouse population, specifically designed for high-resolution mapping QTL (Churchill et al. 2004; Iraqi et al. 2008). It was created from a full reciprocal mating of 5 classical inbred strains (A/J, C57BL/6J, 129S1/SvImJ, NOD/ShiLtJ, and NZO/HlLtJ) and 3 wild-derived strains (CAST/EiJ, PWK/PhJ, and WSB/EiJ) to capture a much greater level of genetic diversity than existing mouse genetic reference populations (GRPs) (Collaborative Cross Consortium 2012). Recently, we showed that CC lines respond differently to experimental periodontitis 42 d after mixed infection with Porphyromonas gingivalis and Fusobacterium nucleatum (Shusterman, Salyma, et al. 2013).
We hypothesized that a combination of confined QTL from CC mice with association studies in humans has the potential to identify genetic variants associated with periodontal disease in humans. Therefore, we performed an integrated analysis of mouse QTL mapping results, for the first time using the CC population, in conjunction with a genetic analysis of the human orthologous chromosomal regions, using imputed genotype data of 2 large case-control samples of aggressive periodontitis (AgP) and chronic periodontitis (CP).
Methods
Mouse Production and Housing
A total of 286 CC mice (177 males, 109 females), at age 8 to 12 wk from 25 different CC lines (Fig. 1) (8 to 12 mice on average per line, inbreeding generations 10 to 21) of the International Livestock (IL) cohort, were provided by the Small Animal Facility, Faculty of Medicine, Tel Aviv University, Israel, after approval by the Institutional Animal Care and Use Committee (approved number: M-08-044). Mice were divided into 2 groups: infected (138 mice) and control (148 mice). Full details of CC lines development were reported previously (Iraqi et al. 2008). Experiments were conducted with and conformed to the ARRIVE guidelines. Mice were housed on hardwood chip bedding and maintained on standard rodent chow diet for the entire period of the experiment.
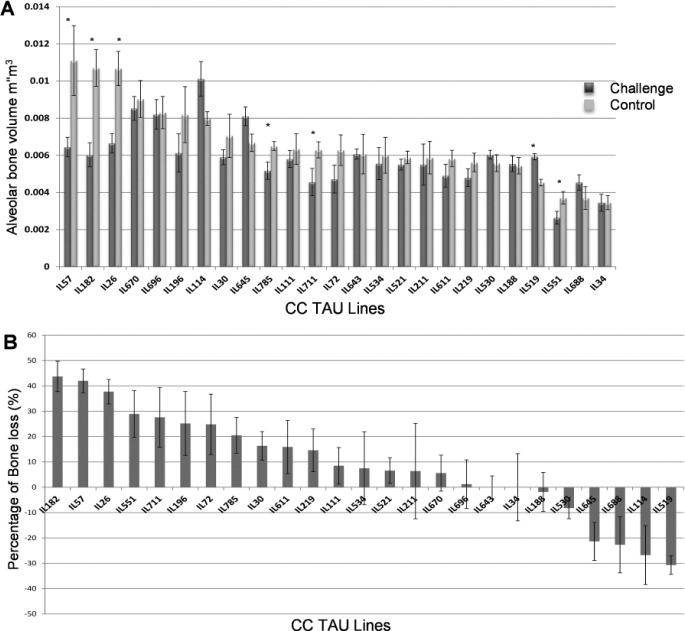
Figure 1.
Estimation of alveolar bone phenotypes. (A) The means of the alveolar bone volumes (± SEM) of 25 different Collaborative Cross (CC) lines. The x-axis represents the different CC lines while the y-axis represents the mean of the alveolar bone volume in mm3 evaluated by micro–computed tomography (CT). The black bars represent the mean of the control alveolar bone volume (CBV) while the gray bars represent the mean of residual bone volume after mixed infection (RBV). Asterisks represent the significant differences between the 2 groups (P < 0.05). (B) Percent alveolar bone volume loss (PBL) due to the mixed infection (± SEM) of 25 different CC lines. The x-axis represents the different CC lines while the y-axis represents the mean percentage of alveolar bone volume loss among the different lines.
Bacterial Cultivation
P. gingivalis strain 381 and F. nucleatum strain PK 1594 were grown in peptone yeast extract containing Hemin (5 mg/L) and vitamin K (0.5 mg/L) (Wilkins Chalgren broth; Oxoid Ltd), in an anaerobic chamber with 85% N2, 5% H2, and 10% CO2, and were taken at the log phase followed by 3 washes in phosphate-buffered saline (PBS). Bacterial concentrations were measured spectrophotometrically standardized to OD650nm = 0.1 for P. gingivalis, corresponding to 1010 bacteria/mL, and OD660nm = 0.26 for F. nucleatum, corresponding to 109 bacteria/mL (Genco et al. 1991; Polak et al. 2009).
Oral Mixed-Infection Model
Mice were treated with sulfamethoxazole (0.8 mg/mL) in drinking water for 10 d, followed by an antibiotic-free period of 3 d, before oral application of mixed culture of P. gingivalis and F. nucleatum (400 µL of 109 bacteria/mL per mouse, P. gingivalis and F. nucleatum with 1:1 ratio) at days 0, 2, and 4 (control groups were treated with PBS and 2% carboxymethycellulose instead) (Polak et al. 2009). Forty-two days postinfection, mice were euthanized after complete anesthesia, using xylisine (Sedaxylan) and ketamine (Clorketam), and maxillary jaws were harvested for micro–computed tomography (µCT) analysis.
Estimation of Percentage of Alveolar Bone Loss Phenotype
A compact fan beam–type computed tomography system (MicroCT40; Scanco Medical) was used for quantitative 3-dimensional analysis (Wilensky et al. 2005). To get high resolution of µCT measurements, we used the jaws for analysis and not the total animal. This requires scarifying the mice for the analysis of its bone, and the control bone volume (CBV) and residual bone volume (RBV) after infection cannot be measured in the same individual mouse. However, since the CC mice are advanced inbred lines, genetic differences would not be present among the mice in a given line, as discussed previously (Shusterman, Salyma, et al. 2013). Therefore, the percentage of alveolar bone loss (PABL) for each infected mouse was calculated relative to a control group from the same line.
Statistical Analysis and Calculation of Heritability
Analysis of variance (ANOVA) was performed (SPSS version 23; SPSS, Inc.) to test the differences of response between and within CC lines, and its output was used to calculate the heritability (H2) (Iraqi et al. 2014).
Genotyping of Mice
CC lines were genotyped with the mouse diversity array (MDA) (Yang et al. 2009), and their genome reconstruction as mosaics of the 8 founders was presented in Durrant et al. (2011). The CC lines were re-genotyped at advanced generations with the new 7500 custom-designed mouse universal genotype array (MUGA), which provided the genome architecture of the CC lines (Collaborative Cross Consortium 2012).
QTL Analysis and Founder Effect
QTL analysis was performed using R-software, including the HAPPY.HBREM R-package (Mott et al. 2000). The probability distribution of descent from the 8 founders at each interval was calculated and used to test for association between founder haplotype at each locus and PABL phenotype. Permutations of the CC lines between the phenotypes were used to set genome-wide significance thresholds levels, and false discovery rate (FDR) was calculated.
Merge Analysis
We used the Sanger mouse genomes database (http://www.sanger.ac.uk/science/data/mouse-genomes-project) in the merge analyses (Yalcin et al. 2005) to test which variants under QTL peak were compatible with a pattern of action at the QTL. This takes advantage of the ancestry of the CC to infer the alleles of each CC line based on its genome mosaic (determined from its single-nucleotide polymorphisms [SNPs]) and sequence variation data in the founder strains. Where a QTL is caused by a single diallelic variant, we expect to have a high chance of testing a very tightly linked tagging SNP with the identical strain distribution pattern and have a higher –log P value than the 8-way haplotype test in the interval containing the variant, due to the reduction in the dimension of the test.
Mouse QTL and Human Genome-Wide Association Study Integration
Orthologous human genes were identified using the Ensembl database (www.ensembl.org). Candidate genes that were nominated by merge-analysis and genes within significant loci were selected for genetic analysis in human case-control samples. In addition, to minimize the likelihood of false negatives, genes at suggestive QTL were analyzed. In addition, genome-wide loci from genome-wide association studies (GWASs) of different human periodontal disease forms were identified from the GWAS-Catalog (Welter et al. 2014).
Human Study Populations
AgP
The AgP patients were recruited throughout Germany, the Netherlands, and Austria. Only patients of German and Dutch ethnical background were included, determined by the location of both parental birthplaces (German and Austrian cases had German family names). Inclusion criteria for the AgP cases were at least 2 affected teeth with >30% alveolar bone loss under the age of 35 y, documented by dental radiographs, and no diabetes. The case sample consisted of 896 AgP patients. The study cases were described previously (Schaefer et al. 2009; Offenbacher et al. 2016).
The AgP control sample consisted of 7,104 controls from Germany and the Netherlands. They were recruited from the Competence Network “FoCus–Food Chain Plus” (Müller et al. 2015), Dortmund Health Study (DHS) (Berger 2012), and the Heinz Nixdorf Recall Studies 1 to 3 (HNR1–3) (Schmermund et al. 2002). The Dutch control sample consisted of 2,891 (1,453 males, 1,438 females), being individuals from the B-Proof Study (Van Wijngaarden et al. 2011).
CP (United States)
The American CP cohort was described in Divaris et al. (2012) and consisted of European American participants of the Dental Atherosclerosis Risk In Communities (ARIC) study (The ARIC Investigators 1989) with moderate CP (n = 1,961 cases; 939 females, 1,022 males; mean age = 63 y) and severe CP (n = 785 cases; 279 females, 506 males; mean age = 64 y). Individuals who were periodontally healthy or had mild periodontitis were used as controls (n = 1,864 controls; 1,197 females, 667 males; mean age = 62 y).
Genotyping and Statistical Tests
All AgP cases and AgP controls were genotyped with OmniExpress arrays on an iScan System (Illumina). SNPs were imputed using 1000G Phase 3 SNPs of Northern Europeans from the HapMap CEPH reference populations (Utah residents with ancestry from northern and western Europe) and the software Impute v2 (Howie et al. 2009). After imputation, the control studies were merged using the genetic analysis software Gtools (http://www.stats.ox.ac.uk/~marchini/software/gwas/gwas.html). Association tests were performed for the AgP case-control sample with SNPTEST v2.5.2 (Marchini et al. 2007), assuming an additive genetic model with sex and a binary variable of smoking status (individuals who never smoked = nonsmokers, current and former smokers = smokers) as covariates.
The association between SNPs and the 2 disease traits (severe and moderate CP) was tested using logistic regression models assuming log-additive allelic effects, adjusting for age, sex, examination center, and ancestry as described in Divaris et al. (2013).
Linkage Disequilibrium Calculation
Linkage disequilibrium (LD) between SNPs was analyzed using the 1000GENOMES: phase_3 sub-population CEU (Utah Residents [centre d’etude du polymorphisme humain; CEPH] with Northern and Western Ancestry) as provided by The Ensembl Project (www.ensembl.org).
Results
Measurement of Susceptibility of Different CC Lines
Our results showed no significant sex effect on bone volume (2-way ANOVA P > 0.05); consequently, both sexes were pooled and treated as same population. The CC strains showed a significant variation in their response to infection (P < 0.01). Six lines (IL-26, IL-182, IL-551, IL-711, IL-785, IL-57) showed a significant decrease (P < 0.05) in bone volume and were considered to be susceptible lines while others were not (Fig. 1A). While some lines showed substantial bone loss, other lines showed negative values of PABL, indicative of bone formation processes (Fig. 1B). One line (IL-519) showed a significant increase of bone volume at 42 d after infection. Heritability estimates of CBV, RBV, and PABL were 0.42, 0.45, and 0.33, respectively.
QTL Analysis and Founder Effect
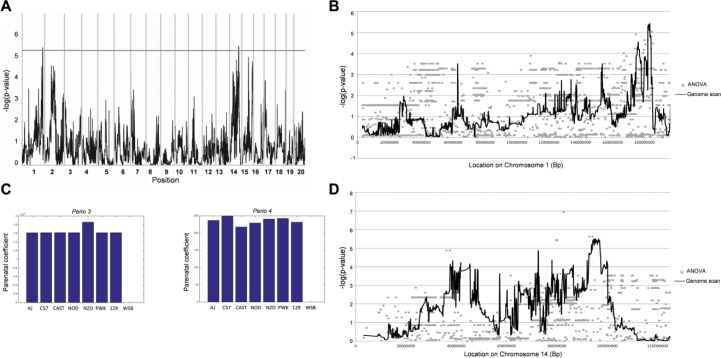
We identified 2 significant QTL (Table 1) associated with PABL on chr1 (180 to 181.5 Mb) and on chr14 (93.5 to 96.5 Mb) at a significance threshold of –log P = 5.3 (FDR = 0.061 in permutation test; Fig. 2A) and designated as Perio3 (periodontitis) and Perio4, respectively. Of note, Perio3, which was mapped in the present study, overlaps with the previous suggested Perio3-QTL in the F2 population (chr1: 178 to 181 Mb) (Shusterman, Durrant, et al. 2013). In total, 80 genes were underlying Perio3 and Perio4 (with flanking region of ~0.5 Mb; listed in Appendix Tables 1 and 2). In addition, 8 suggestive QTL (defined as –log P > 3.52; designated as Perio3 to 10) were mapped on chr1, 14, 15 (2 QTL), 14, 2, 7, and 17, respectively (Table 1). In total, 1,309 mouse genes were underlying all QTL (Appendix Tables 3 to 10). At the 2 most significant QTL (Perio3 and Perio4), we estimated the effect of each founder haplotype on the QTL (Fig. 2B). Both loci on chr1 and chr14 were shown to be less affected by the genetic background of WSB/EiJ (wild-derived strain) than the rest of the parental strains.
Table 1.
QTL and Genes.
| QTL | Chr. | Position (Interval) | No. of Genes | Human Chr. | No. of Human Genes | P Value of QTL | Total No. of Human Genes | |
|---|---|---|---|---|---|---|---|---|
| Significant QTL | Perio3 | 1 | 180 to 181.5 Mbp (1.5 Mb) | 70 | 1 | 28 | –log P = 5.3 | 31 |
| 2 | 1 | |||||||
| Perio4 | 14 | 93.5 to 96.5 Mbp (3 Mb) | 10 | 13 | 2 | |||
| Suggestive QTL | Perio3′ | 1 | 172.6 to 182.2 (9.6 Mb) | 226 | 1 | 83 | –log P = 3.52 | 685 |
| 2 | ||||||||
| Perio4′ | 14 | 71.6 to 73.03 (1.43 Mb) | 43 | 13 | 13 | |||
| 81.83 to 86.2 (4.37 Mb) | ||||||||
| 91.29 to 99.88 (8.59 Mb) | ||||||||
| Perio5 | 15 | 63.08 to 65.68 (2.6 Mb) | 9 | 8 | 7 | |||
| Perio6 | 15 | 93.5 to 99.2 (5.7 Mb) | 105 | 12 | 68 | |||
| Perio7 | 14 | 36.4 to 45.1 (8.7 Mb) | 91 | 14 | 26 | |||
| 10 | ||||||||
| Perl8 | 2 | 66.01 to 70.19 (4.18 Mb) 78.61 to 102.58 (23.97 Mb) |
648 | 1 | 373 | |||
| 2 | ||||||||
| 11 | ||||||||
| Perio9 | 7 | 3.1 to 12.8 (9.7 Mb) | 184 | 19 | 112 | |||
| Perio10 | 17 | 9.8 to 11.9 (2.1 Mb) | 3 | 6 | 3 |
The locations of the mouse QTL, their genomic intervals, and their corresponding human orthologous are listed in the table, according to different significance threshold of QTL.
Chr., chromosome; QTL, quantitative trait loci.
Figure 2.
QTL, founder effect and merge analysis. (A) Genome scans of susceptibility to alveolar bone volume loss percentage in 25 different Collaborative Cross (CC) lines. The x-axis represents genome location; the y-axis represents the –log P of the test of association between locus and percentage of bone loss. Two quantitative trait loci (QTL) associated with percentage of bone loss after infection mapped on chromosome 1 with genome coordinate (181.5 to 182.5 Mbp) and chromosome 14 with genome coordinate (93.5 to 96.5 Mbp). (B) Founder effect-estimated haplotype effects at QTL for alveolar bone loss after mixed infection with Porphyromonas gingivalis and Fusobacterium nucleatum. Effects are shown as deviations relative to WSB/EiJ, which is arbitrarily assigned the trait effect of 0. The x-axis of each plot shows the founder strains; the y-axis shows the estimated haplotype effects of the CC founders. A, Perio3; B, Perio4. Merge analysis of sequence variants at (C) Perio3 and (D) Perio4. The x-axis is genome location; y-axis is the –log P value of the test of association between locus and alveolar bone loss phenotype. The continuous black lines are sections of the genome scans in A while gray dots are the results of analysis of variance tests of sequence.
Merge Analysis (Association Analysis of Sequence Variants)
The merge analyses for QTL, Perio3, and Perio4 are shown in Figure 2C, D. Appendix Table 11 lists the 7 candidate genes (CAPN8, DUSP23, PCDH17, SNORA17, PCDH9, LECT1, LECT2) with the significant merge adjacent SNPs. Two genes, protocadherin 17 (PCDH17) and protocadherin 9 (PCDH9), were shown to have a high influence on Perio4. While the PCDH17 reached the highest significance in the merge analysis (–log P = 6.9), PCDH9 was found to be the closest gene, with 2 SNPs with –log P = 5.3 and 5.6. Two genes, leukocyte cell-derived chemotaxin 1 and 2 (LECT1 and LECT2) had a –log P of ~5.4 and were nominated as suggestive candidate genes. However, the 2 genes on chr1, calpain 8 (CAPN8) and dual-specificity phosphatase 23 (DUSP23), had lower association P values than in the haplotype mapping (–log P = 5.04 and 4.91, respectively).
Integration of Mouse QTL Analysis and Human GWAS
None of 7 candidate genes (CAPN8, DUSP23, PCDH17, SNORA17, PCDH9, LECT1, LECT2) that selected from merge analysis (Table 2) showed significant single SNP marker associations with human periodontitis in either AgP or CP. However, 3 genes showed gene-centric associations of periodontal subphenotypes: LECT1 (“orange complex,” P = 3.72 × 10–4), DUSP23 (P. gingivalis colonization, P = 0.037), and PCDH17 (P. gingivalis colonization, P = 0.049) (Rhodin et al. 2014).
Table 2.
Candidate Genes Revealed through Integration of Mouse QTL and Human GWAS.
| QTL Analysis in Mice |
Human GWAS |
|||||
|---|---|---|---|---|---|---|
| QTL | Candidate Gene | P Value of QTL | AgP, Best Associated SNP (P Value, OR/β) | CP Moderate, Best Associated SNP (P Value, OR/β) | CP Severe, Best Associated SNP (P Value, OR/β) | Reported Trait Association; Reference (SNP, P Value, OR/β) |
| Perio3 | Coiled-coil domain containing 121 (CCDC121) | −log P = 5.3 | rs35605899 (0.027, 1.16/0.144) | rs3749147 (0.207, 0.93/–0.0699) | rs7382 (0.313, 1.070/0.068) | CP; Teumer et al. 2013 (rs111571364, 7.98 × 10–6, 3.46) |
| Perio7 | Neuregulin 3 (NRG3) | –log P = 3.52 | rs2250828 (7.5 × 10–5, 1.41/0.344) | rs9971278 (7.2 × 10–5, 0.74/–0.303) | rs10509436 (8.3 × 10–5, 1.39/0.33) | — |
| Perio9 | Zinc finger ZNF579 | rs310432 (0.134, 1.09/0.088) | rs2902925 (0.34, 1.11/0.106) | rs10421563 (0.11, 0.899/–0.107) | CP; Teumer et al. 2013 (rs149546760, 5.12 × 10–6, 0.34) | |
| Zinc finger (FIZ1) | rs3803890 (0.078, 1.14/0.13) | rs520125 (0.168, 0.87/–0.140) | rs310469 (0.155, 0.885/–0.122) | CP; Teumer et al. 2013 (rs140900046, 4.44 × 10–6, 0.34) | ||
| Zinc finger (ZNF524) | ||||||
| Perio10 | Parkin RBR E3 ubiquitin protein ligase (PARK2) | rs4709556 (0.0013, 1.27/0.242) | rs9458343 (0.0003, 0.80/–0.222) | rs2803101 (0.0076, 1.199/0.182) | — | |
| PARK2 coregulated (PACRG) | ||||||
Gene symbols, their mouse QTL significance value, and the significance of the best associated SNP marker in the human CP/AgP data are listed in the table.
AgP, aggressive periodontitis; CP, chronic periodontitis; GWAS, genome-wide association study; OR, odds ratio; QTL, quantitative trait loci; SNP, single-nucleotide polymorphism.
The human orthologous genes (31 human orthologous genes) of both significant QTL Perio3 and Perio4 were analyzed for their association to periodontal disease in the AgP and CP GWAS data. We found that 1 orthologous gene, coiled-coil domain containing 121 genes (CCDC121), which was underlying the significant QTL-Perio3 (–log P = 5.3 at FDR = 0.06), is located 5 kb upstream of a variant (rs111571364) that was reported as a suggestive risk variant of CP (Teumer et al. 2013) (P = 8.0 × 10–6, OR = 3.46). However, we could not replicate this association in our AgP and CP samples. In addition, 14 genes, out of 31 human orthologous genes, showed gene-centric associations (P < 0.05) with periodontal subphenotypes: severe CP, ‘orange complex,” “red complex,” Aggregatibacter actinomycetemcomitans and P. gingivalis colonization (Rhodin et al. 2014) (Appendix Table 12).
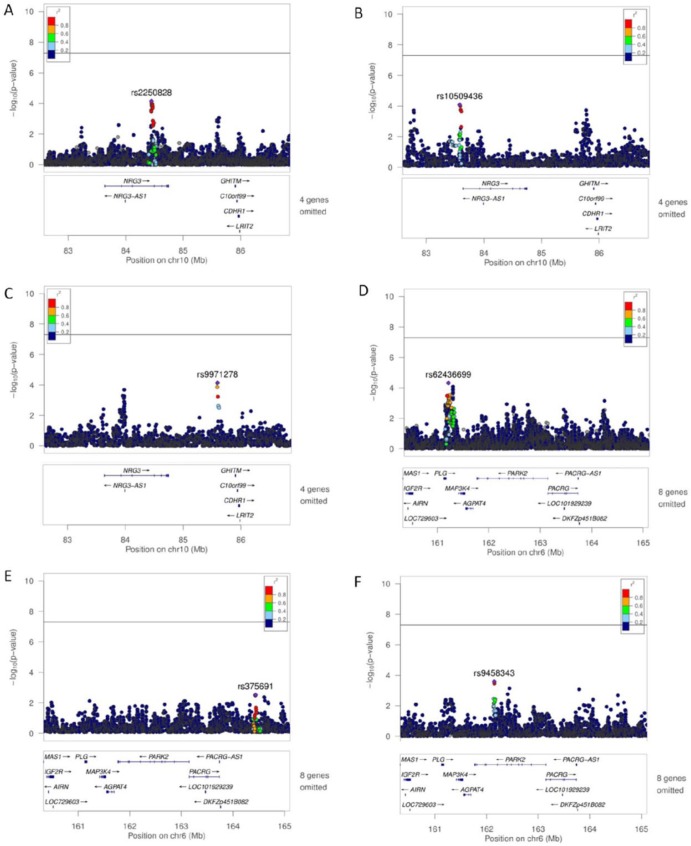
We analyzed the human corresponding genes for additional 1,229 mouse genes underlying suggestive QTL (defined as –log P > 3.52). We suggest 6 genes (NRG3, ZNF579, FIZ1, ZNF524, PARK2, and PACRG) as candidate genes, because they showed nominal significant associations with either AgP or CP at P < 3 × 10–4. While 3 genes—ZNF579 (rs149546760, P = 5.1 × 10–6), FIZ1 (rs140900046, P = 4.4 × 10–6), and ZNF524—showed a nominal significant association with CP (Teumer et al. 2013), the other three genes (NRG3, PARK2, and PACRG) showed an association with both AgP and CP (either moderate or severe) in our data. Regional association plots of these genes are shown in Figure 3.
Figure 3.
Association plots of imputed genotypes at the NRG3, PARK2, and PACRG loci. Regional association plots of imputed genotypes for aggressive periodontitis (A), severe chronic periodontitis (B), and moderate chronic periodontitis (C) for the chromosomal region spanning NRG3. Regional association plots of imputed genotypes for aggressive periodontitis (D), severe chronic periodontitis (E), and moderate chronic periodontitis (F) for the chromosomal region spanning PARK2 and PACRG. The –log P values of the analyzed single-nucleotide polymorphisms (SNPs) were plotted as a function of the genomic SNP position. SNP annotation provided by LocusZoom databases.
In total, 7 candidate genes were nominated and are summarized in Table 2, based on the integration of mouse QTL and human GWAS.
Discussion
Because of study limitations of investigations of complex traits in humans, genetic components underlying susceptibility to periodontitis remain largely unknown. Here, we performed a combined analysis of high-resolution mapping QTL in CC mice and analysis of the orthologous human chromosomal regions using imputed genotype sets of human periodontitis. Our analyses suggest a set of genes to be associated with periodontal disease susceptibility through the combined QTL-GWAS analysis.
While most of the CC showed bone loss 42 d after infection, 1 line showed significant higher bone volume 42 d after infection. We speculate that this observation may occur due to bone production rather than bone destruction caused by transient infection in this specific line, similarly to condensing osteitis. Further studies at the histological level should been done on this line to understand the nature of this observation. A recent QTL study that used the F2 cross to map loci that contribute to increased resorption of the alveolar bone after periodontal infection reported 2 significant QTL with a genomic interval of 33 Mb on chr5 and chr3. These QTL were designated Perio1 and Perio2, respectively. One additional suggestive QTL on chr1 with a genomic interval of ~3 Mb was named Perio3 (Shusterman, Durrant, et al. 2013). In addition, a suggested genomic region on mouse chr2 (iBALL-QTL chr2: 20 to 76.8 Mb) was recently reported as associated with ligature-mediated periodontal inflammation (Sima et al. 2015). By using the CC model, we mapped 2 significant QTL with ~1.5 to 3 Mb resolution, validating previous reports on the power of the CC model for high-resolution QTL mapping (Durrant et al. 2011). While the previous reported QTL Perio1, Perio2, and iABLL were not replicated in our study, interestingly, Perio3 was replicated with higher association. We believe that the discrepancies between the previously reported QTL and the current QTL are due to the different phenotype definition, disease induction, phenotype quantification techniques, and genetic characteristics of the CC lines compared to the F2 and classical RIL.
Merge analysis revealed 6 SNPs at Perio4 with higher P values than the haplotype mapping data; thus, these polymorphisms were considered candidate causal variants, responsible for the QTL rise. On the other hand, 2 SNPs on Perio3 showed lower values than the haplotype mapping; one possibility other than a false-positive observation is that Perio3 is raised by a combination of linked variants effect. While 3 of 7 genes showed gene-centric association with periodontal subphenotypes, none of human orthologous regions showed significant single SNP marker association with either CP or AgP. This could be due to differences in the frequency of these variants between CC mice compared to human and/or different regulatory elements that affect periodontitis susceptibility in mouse and human.
Of 31 orthologous genes underlying the significant QTL, 1 genetic locus at Perio3 (CCDC121-GPN1) showed a nominal significant association with chronic periodontitis (rs111571364, MAF EUR = 0.02%) (Teumer et al. 2013). However, this association did not replicate in our CP and AgP samples. This could indicate a false-positive association of the previous study or be an expression of the trait heterogeneity of periodontitis, as the 3 GWASs applied different diagnostic criteria. The precise function of CCDC121 is unknown, and it is not clear if the yet-unknown causative variant has a cis effect on CCDC121 or on other genes. Interestingly, 3.4 kb upstream of the CP-associated SNP at CCDC121 lies an SNP (rs6547741, MAF EUR = 0.50%) that is associated with oral cavity cancer at a genome-wide significance level (Lesseur et al. 2016), which adds to the hypothesis that this genomic region has a role in oral health. Interestingly, both SNPs are in linkage disequilibrium (D′ = 1), and for the common variant rs6547741, expression QTL studies reported several effects on gene expression on a chromosomal region 200 to 100 kb upstream (from SNX17, P = 8.6 × 10–15; tissue: skeletal muscle, to GKCR, P = 2.3 × 10–8; tissue: spleen), a region that carries numerous GWAS lead SNPs of lipid and glycemic traits but also Crohn’s disease. Taking into account that the mouse QTL encompassed CCDC121 but not the genes from SNPX17 to GKCR, it is possible, although speculative, that the associated SNPs may exert their trans-regulatory effects by influencing CCDC121 function.
Although significant QTL with high resolution were mapped in the CC mice, we did not find evidence of association of the orthologous regions in AgP and CP samples. It is possible that the molecular mechanisms that regulate gene expression are different in humans compared to mice and that the orthologous regions do not carry risk variants in both mice and humans. Accordingly, we may have missed the regions that are relevant for human periodontitis by focusing on the confined mouse QTL. In addition, the lack of validated associations in the human samples may be explained by the instance that the effects of potential causative variants within the orthologues regions are too small for identification in limited size of our case-control samples. Of note, GWAS can employ sample sizes of up to hundreds of thousands of individuals.
Analyzing the suggestive QTL reveals 5 genetic suggestive regions to be associated with periodontitis. Two regions (NRG3, PARK2-PACRG) showed a nominal significant association in the AgP and CP samples, but signals were located at different regions of these genes. Interestingly, PARK2-PACRG are located near the gene plasminogen (PLG), which is significantly associated with AgP (Schaefer et al. 2015) and CP (Munz et al. 2017). It is possible that these associations are false positives or that they point to different regulatory elements of the analyzed subphenotypes of periodontitis. Two more loci at ZNF579 and FIZ1-ZNF524 were not associated with our AgP and CP samples, but they showed previously a nominal significant association with CP (Teumer et al. 2013). The nonvalidation of these 2 reported associations may again indicate false positives or the heterogeneity of the different periodontitis phenotypes.
In conclusion, the CC populations enabled mapping confined QTL that confer susceptibility to alveolar bone loss in mice. However, larger human phenotype-genotype samples and additional genome-wide expression data from gingival tissues are likely required to identify true-positive associations.
Author Contributions
A. Nashef, A. Schaefer, F.A. Iraqi, Y.H. Haddad, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; R. Qabaja, P. Hoffmann, A. Franke, K. Divaris, contributed to data acquisition and analysis, critically revised the manuscript; Y. Salaymeh, contributed to data acquisition, analysis, and interpretation, drafted the manuscript; M. Botzman, contributed to design and data analysis, critically revised the manuscript; M. Munz, contributed to data analysis and interpretation, critically revised the manuscript; H. Dommisch, M. Laudes, K. Berger, T. Kocher, B. Loos, A.G. Uitterlinden, L.C.P.G.M. de Groot, S. Offenbacher, W. Lieb, contributed to data acquisition, critically revised the manuscript; B. Krone, J. Wellmann, N. van der Velde, R. Mott, I. Gat-Viks, contributed to data analysis, critically revised the manuscript; E. Wiess, contributed to conception, design, and data interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Acknowledgments
We further thank Corinna Bruckmann, Christof Dörfer, Peter Eickholz, Yvonne Jockel-Schneider, Jörg Meyle, Barbara Noack, and Ingmar Staufenbiel for the recruitment of cases of aggressive periodontitis.
Footnotes
A supplemental appendix to this article is available online.
This work was supported by the German Research Foundation DFG (Deutsche Forschungsgemeinschaft; GZ: SCHA 1582/3-1 and SCHA 1582/4-1) and by the Israel Science Foundation (grant number 429/09). The data for the European American CP cases were generated by the following research grants of the US National Institutes of Health (NIH): RO1-DE022527, UL1-TR001111, and RO1-DE021418. The Federal Ministry of Education and Research (01GR0468) supported collection of the popgen control sample. The Dortmund Health Study (DHS) is supported by the German Migraine & Headache Society (DMKG) and by unrestricted grants of equal share from Almirall, AstraZeneca, Berlin Chemie, Boehringer, Boots Health Care, Glaxo-Smith-Kline, Janssen Cilag, McNeil Pharma, MSD Sharp & Dohme, and Pfizer to the University of Münster (collection of sociodemographic and clinical data). The Institute of Epidemiology and Social Medicine, University of Münster funded blood collection in the Dortmund Health Study, and the Federal Ministry of Research and Education (BMBF, grant 01ER0816) supported genotyping. FOCUS was supported by the Federal Ministry of Education and Research BMBF (FKZ 0315540A). The Heinz Nixdorf Foundation (Germany) supported the HNR study. In addition, the Federal Ministry of Education and Science and the German Research Council (DFG; Project SI 236/8-1, SI 236/9-1, ER 155/6-1) funded it. The German Centre financed Genotyping of the Illumina HumanOmni-1 Quad BeadChips of the HNR subjects for Neurodegenerative Diseases (DZNE), Bonn.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
ORCID iD: K. Divaris  https://orcid.org/0000-0003-1290-7251
https://orcid.org/0000-0003-1290-7251
References
- The ARIC Investigators. 1989. The atherosclerosis risk in communities (ARIC) study: design and objectives. Am J Epidemiol. 129(4):687–702. [PubMed] [Google Scholar]
- Berger K. 2012. The Dortmund health study [in German]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 55(6–7):816–821. [DOI] [PubMed] [Google Scholar]
- Bochenek G, Häsler R, Nour Eddine M, König IR, Loos BG, Jepsen S, Philip R, Schreiber S, Schaefer AS. 2013. The large non-coding RNA ANRIL, which is associated with atherosclerosis, periodontitis and several forms of cancer, regulates ADIPOR1, VAMP3 and C11ORF10. Hum Mol Genet. 22(22):4516–4527. [DOI] [PubMed] [Google Scholar]
- Churchill G, Airey D, Allayee H, Angel JM, Attie A, Beatty J, Beavis W, Belknap J, Bennett B, Berrettini W, et al. 2004. The Collaborative Cross, a community resource for the genetic analysis of complex traits. Nat Genet. 36(11):1133–1137. [DOI] [PubMed] [Google Scholar]
- Collaborative Cross Consortium. 2012. The genome architecture of the Collaborative Cross mouse genetic reference population. Genetics. 190(2):389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey LA, Nance WE, Hofstede P, Schenkein HA. 1993. Self-reported periodontal disease in a Virginia twin population. J Periodontol. 64(12):1205–1208. [DOI] [PubMed] [Google Scholar]
- Divaris K, Monda K, North K, Olshan A, Lange E, Moss K, Barros SP, Beck JD, Offenbacher S, et al. 2012. Genome-wide association study of periodontal pathogen colonization. J Dent Res 91 Suppl 7:21S–28S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divaris K, Monda K, North K, Olshan A, Reynolds L, Hsueh W, Lange E, Moss K, Barros S, Weyant R, et al. 2013. Exploring the genetic basis of chronic periodontitis: a genome-wide association study. Hum Mol Genet. 22(11):2312–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant C, Tayem H, Yalcin B, Cleak J, Goodstadt L, De Villena FP-M, Mott R, Iraqi F. 2011. Collaborative Cross mice and their power to map host susceptibility to Aspergillus fumigatus infection. Genome Res. 21(8):1239–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst FD, Linden GJ, Homuth G, Kocher T. 2010. Replication of the association of chromosomal region 9p21.3 with generalized aggressive periodontitis (gAgP) using an independent case-control cohort. BMC Med Genet. 11:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag-Wolf S, Dommisch H, Graetz C, Jockel-Schneider Y, Harks I, Staufenbiel I, Meyle J, Eickholz P, Noack B, Bruckmann C, et al. 2014. Genome-wide exploration identifies sex-specific genetic effects of alleles upstream NPY to increase the risk of severe periodontitis in men. J Clin Periodontol. 41(12):1115–1121. [DOI] [PubMed] [Google Scholar]
- Genco CA, Cutler CW, Kapczynski D, Maloney K, Arnold RR. 1991. A novel mouse model to study the virulence of and host response to Porphyromonas (Bacteroides) gingivalis. Infect Immun. 59(4):1255–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GTEx Consortium. 2015. Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science. 348(6235):648–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G. 2014. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 15(1):30–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashim N, Linden G, Ibrahim M, Gismalla B, Lundy F, Hughes FJ, El Karim I. 2015. Replication of the association of GLT6D1 with aggressive periodontitis in a Sudanese population. J Clin Periodontol. 42(4):319–324. [DOI] [PubMed] [Google Scholar]
- Howie BN, Donnelly P, Marchini J. 2009. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 5(6):e1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iraqi F. 2000. Fine mapping of quantitative trait loci using advanced intercross lines of mice and positional cloning of the corresponding genes. Exp Lung Res. 26(8):641–649. [DOI] [PubMed] [Google Scholar]
- Iraqi F, Athamni H, Dorman A, Salymah Y, Tomlinson I, Nashif A, Shusterman A, Weiss E, Houri-Haddad Y, Mott R, et al. 2014. Heritability and coefficient of genetic variation analyses of phenotypic traits provide strong basis for high-resolution QTL mapping in the Collaborative Cross mouse genetic reference population. Mamm Genome. 25(3–4):109–119. [DOI] [PubMed] [Google Scholar]
- Iraqi FA, Churchill G, Mott R. 2008. The Collaborative Cross, developing a resource for mammalian systems genetics: a status report of the Wellcome Trust cohort. Mamm Genome. 19(6):379–381. [DOI] [PubMed] [Google Scholar]
- Lesseur C, Diergaarde B, Olshan AF, Wünsch-Filho V, Ness AR, Liu G, Lacko M, Eluf-Neto J, Franceschi S, Lagiou P, et al. 2016. Genome-wide association analyses identify new susceptibility loci for oral cavity and pharyngeal cancer. Nat Genet. 48(12):1544–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchini J, Howie BN, Myers S, McVean G, Donnelly P. 2007. A new multipoint method for genome-wide association studies by imputation of genotypes. Nat Genet. 39(7):906–913. [DOI] [PubMed] [Google Scholar]
- Mott R, Talbot CJ, Turri MG, Collins AC, Flint J. 2000. A method for fine mapping quantitative trait loci in outbred animal stocks. Proc Natl Acad Sci USA. 97(23):12649–12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller N, Schulte DM, Türk K, Freitag-Wolf S, Hampe J, Zeuner R, Schröder J, Gouni-Berthold I, Berthold H, Krone W, et al. 2015. IL-6 blockade by monoclonal antibodies inhibits apolipoprotein (a) expression and lipoprotein (a) synthesis in humans. J Lipid Res. 56(5):1034–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munz M, Chen H, Jockel-Schneider Y, Adam K, Hoffman P, Berger K, Kocher T, Meyle J, Eickholz P, Doerfer C, et al. 2017. A haplotype block downstream of plasminogen is associated with chronic and aggressive periodontitis. J Clin Periodontol. 44(10):962–970. [DOI] [PubMed] [Google Scholar]
- Offenbacher S, Divaris K, Barros SP, Moss KL, Marchesan JT, Morelli T, Zhang S, Kim S, Sun L, Beck JD, et al. 2016. Genome-wide association study of biologically-informed periodontal complex traits offers novel insights into the genetic basis of periodontal disease. Hum Mol Genet. 25(10):2113–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak D, Wilensky A, Shapira L, Halabi A, Goldstein D, Weiss EI, Houri-Haddad Y. 2009. Mouse model of experimental periodontitis induced by Porphyromonas gingivalis/Fusobacterium nucleatum infection: bone loss and host response. J Clin Periodontol. 36(5):406–410. [DOI] [PubMed] [Google Scholar]
- Rhodin K, Divaris K, North KE, Barros SP, Moss K, Beck JD, Offenbacher S. 2014. Chronic periodontitis genome-wide association studies: gene-centric and gene set enrichment analyses. J Dent Res. 93(9):882–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer AS, Bochenek G, Jochens A, Ellinghaus D, Dommisch H, Zeldemir A, Akanat E, Graetz C, Harks I, Jockel-Schneider Y, et al. 2015. Genetic evidence for PLASMINOGEN as a shared genetic risk factor of coronary artery disease and periodontitis. Circ Cardiovasc Genet. 8(1):159–167. [DOI] [PubMed] [Google Scholar]
- Schaefer AS, Richter GM, Groessner-Schreiber B, Noack B, Nothnagel M, El Mokhtari NE, Loos BG, Jepsen S, Schreiber S. 2009. Identification of a shared genetic susceptibility locus for coronary heart disease and periodontitis. PLoS Genet. 5(2):e1000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer AS, Richter GM, Nothnagel M, Manke T, Dommisch H, Jacobs G, Arlt A, Rosenstiel P, Noack B, Groessner-Schreiber B, et al. 2010. A genome-wide association study identifies GLT6D1 as a susceptibility locus for periodontitis. Hum Mol Genet. 19(3):553–562. [DOI] [PubMed] [Google Scholar]
- Schmermund A, Möhlenkamp S, Stang A, Grönemeyer D, Seibel R, Hirche H, Mann K, Siffert W, Lauterbach K, Siegrist J, et al. 2002. Assessment of clinically silent atherosclerotic disease and established and novel risk factors for predicting myocardial infarction and cardiac death in healthy middle-aged subjects: rationale and design of the Heinz Nixdorf RECALL study. Am Heart J. 144(2):212–218. [DOI] [PubMed] [Google Scholar]
- Shusterman A, Durrant C, Mott R, Polak D, Schaefer A, Weiss EI, Iraqi F, Houri-Haddad Y. 2013. Host susceptibility to periodontitis: mapping murine genomic regions. J Dent Res. 92(5):438–443. [DOI] [PubMed] [Google Scholar]
- Shusterman A, Salyma Y, Nashef A, Soller M, Wilensky A, Mott R, Weiss EI, Houri-Haddad Y, Iraqi F. 2013. Genotype is an important determinant factor of host susceptibility to periodontitis in the Collaborative Cross and inbred mouse populations. BMC Genet. 14:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sima C, Cheng Q, Rautava J, Levesque C, Sherman P, Glogauer M. 2015. Identification of quantitative trait loci influencing inflammation-mediated alveolar bone loss: insights into polygenic inheritance of host-biofilm disequilibria in periodontitis. J Periodontal Res. 51(2):237–249. [DOI] [PubMed] [Google Scholar]
- Teumer A, Holtfreter B, Völker U, Petersmann A, Nauck M, Biffar R, Völzke H, Kroemer H, Meisel P, Homuth G, et al. 2013. Genome-wide association study of chronic periodontitis in a general German population. J Clin Periodontol. 40(11):977–985. [DOI] [PubMed] [Google Scholar]
- Vaithilingam RD, Safii SH, Baharuddin NA, Ng CC, Cheong SC, Bartold PM, Schaefer S, Loos BG. 2014. Moving into a new era of periodontal genetic studies: relevance of large case-control samples using severe phenotypes for genome-wide association studies. J Periodontal Res. 49(6):683–695. [DOI] [PubMed] [Google Scholar]
- Van Wijngaarden JP, Dhonukshe-Rutten RAM, van Schoor NM, van der Velde N, Swart KMA, Enneman AW, Van Dijk S, Brouwer-Brolsma E, Zillikens M, Van-Meurs J, et al. 2011. Rationale and design of the B-PROOF study, a randomized controlled trial on the effect of supplemental intake of vitamin B12 and folic acid on fracture incidence. BMC Geriatr. 11:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welter D, MacArthur J, Morales J, Burdett T, Hall P, Junkins H, Klemm A, Flicek P, Manolio T, Hindorff T, et al. 2014. The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res. 42 (Database issue):D1001–D1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky A, Gabet Y, Yumoto H, Houri-Haddad Y, Shapira L. 2005. Three-dimensional quantification of alveolar bone loss in Porphyromonas gingivalis–infected mice using micro-computed tomography. J Periodontol. 76(8):1282–1286. [DOI] [PubMed] [Google Scholar]
- Yalcin B, Flint J, Mott R. 2005. Using progenitor strain information to identify quantitative trait nucleotides in outbred mice. Genetics. 171(2):673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Ding Y, Hutchins LN, Szatkiewicz J, Bell TA, Paigen BJ, Graber J H, De Villena FP-M, Churchill G. 2009. A customized and versatile high-density genotyping array for the mouse. Nat Methods. 6(9):663–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.