Abstract
Nuclear factor kappa B (NF-κB) signaling plays critical roles in many physiological and pathological processes, including regulating organogenesis. Down-regulation of NF-κB signaling during development results in hypohidrotic ectodermal dysplasia. The roles of NF-κB signaling in tooth development, however, are not fully understood. We examined mice overexpressing IKKβ, an essential component of the NF-κB pathway, under keratin 5 promoter (K5-Ikkβ). K5-Ikkβ mice showed supernumerary incisors whose formation was accompanied by up-regulation of canonical Wnt signaling. Apoptosis that is normally observed in wild-type incisor epithelium was reduced in K5-Ikkβ mice. The supernumerary incisors in K5-Ikkβ mice were found to phenocopy extra incisors in mice with mutations of Wnt inhibitor, Wise. Excess NF-κB activity thus induces an ectopic odontogenesis program that is usually suppressed under physiological conditions.
Keywords: Ikkβ, tooth development, Wnt signaling, enamel, cervical loop, wise
Introduction
Nuclear factor kappa B (NF-κB) signaling plays roles in many physiological and pathological processes including organogenesis, immune and inflammatory responses, apoptosis, cell proliferation, cancer, and stem cell regulations (Oeckinghaus et al. 2011). In mammals, 15 NF-κB homo- or heterodimers are formed among the 5 subunits, NFKB1 (p50, generated from p105), NFKB2 (p52, generated from p100), RelA (p65), RelB, and c-Rel. While in the absence of any stimulus, NF-κB is located in the cytoplasm, and activation of NF-κB signaling by a large variety of NF-κB–specific signals leads to nuclear translocation of NF-κB. Three pathways, classical/canonical, alternative/noncanonical, and a hybrid of both, have been shown to activate NF-κB. Canonical NF-κB activation is usually a rapid and transient response to a wide range of stimuli. In unstimulated cells, the inhibitor of κBα (IκBα) acts to retain canonical NF-κB subunits in the cytoplasm. Exposure to canonical stimuli leads to rapid phosphorylation and subsequently to ubiquitination and degradation of IκBα. IκBα is phosphorylated by a multiprotein kinase complex, the IκB kinase (IKK), composed of 2 catalytic subunits, IKKα and IKKβ, and a regulatory subunit, IKKγ (NEMO). The released NF-κB dimers translocate to the nucleus and regulate target gene transcription (Oeckinghaus et al. 2011). The noncanonical pathway leads slow activation of NF-κB and results in prolonged activation of NF-κB target gene transcription. In noncanonical signaling, NF-κB–inducing kinase (NIK) recruits only IKKα that subsequently phosphorylates p100, which promotes p100 polyubiquitiation and subsequent proteasomal processing to p52. Several types of dimers such as RelB/p52 and p52/p52 with BCL3 are responsible for transcription of noncanonical target genes (Oeckinghaus et al. 2011).
NF-κB signaling is also known to be involved in the development of ectodermal organs, and down-regulation of NF-κB activity leads to hypohydrotic (anhidrotic) ectodermal dysplasia, which is characterized by partial or complete absence of exocrine sweat glands, abnormally sparse hair, and an absence and/or malformation of teeth. The tumor necrosis factor (TNF) superfamily member, ectodysplasin-A1 (EDA-A1), Eda receptor (EDAR), and Eda receptor–associated death-domain (EDARADD) are identified as upstream mediators of NF-κB in the development of the ectodermal organs, which is consistent with the studies of loss of function of NF-κB in the mouse (Mikkola and Thesleff 2003).
Tooth position, number, and shape are consistent in mammals and are under strict genetic control. Teeth develop through a series of reciprocal interactions between the epithelium and underlying mesenchyme. The first morphological sign of tooth development is a localized thickening of the mandibular and maxillary epithelium, which subsequently invaginates into the underlying mesenchyme to form buds. The bud epithelium progressively takes the form of cap and bell configurations. Epithelial cells differentiate into enamel-producing ameloblasts, while mesenchymal cells differentiate into dentin-producing odontoblasts. Mice are the commonly studied mammals for investigating the mechanisms of tooth development; however, mouse incisors are unique teeth as they grow continuously throughout life, and enamel is present on only the labial side. Continuous growth is supported by dental stem cells localized at the apical end of incisor (Harada et al. 1999). Multiple signaling pathways including BMP, FGF, WNT, and SHH are known to play roles in regulating tooth development. The role of NF-κB signaling in rodent incisor tooth development, however, is not fully understood, although loss-of-function studies have identified a role for NF-κB in the specification of cusp formation in molar tooth development (Ohazama et al. 2004).
We examined mice overexpressing an essential component of the NF-κB pathway, Ikkβ, under the keratin 5 promoter (K5-Ikkβ), to investigate the role of NF-κB signaling in mouse incisor tooth development. K5-Ikkβ mice showed supernumerary incisors, which initiated from embryonic epithelium. Excess NF-κB is thus able to stimulate extra odontogenic activity in embryonic incisor epithelium.
Materials and Methods
Production and Analysis of Transgenic Mice
The production of mice with mutation of Wise, (Igκ)3xconalacZ, cIκBαΔN, K5-Ikkα, and K5-Ikkβ mice, have previously been described (Schmidt-Ullrich et al. 1996, 2001; Kassai et al. 2005; Lomada et al. 2007; Page et al. 2010). Aly/Aly mice were purchased from CLEA (Tokyo, Japan). Embryonic day 0 (E0) was taken to be midnight prior to finding a vaginal plug.
β-galactosidase Staining
Embryo heads were fixed for 1 h at 4 °C in 1% paraformaldehyde (PFA) and 0.2% glutaraldehyde, and X-gal staining was performed at 36 °C for 48 h.
In Situ Hybridization
Radioactive in situ hybridization with [35S]UTP-labeled riboprobes was carried out as described previously (Ohazama et al. 2008). Decalcification using 0.5 M EDTA (pH 7.6) was performed after fixation of newborn mice.
Micro-CT Analysis
Heads were scanned with Explore Locus SP (GE Healthcare, Little Chalfont, Buckinghamshire, UK) high-resolution micro computed tomography (CT) with a voxel dimension of 8 µm. Three-dimension reconstruction was performed by 3-structure analysis software (MicroView, GE Healthcare).
Contact Microradiography Analysis
Specimens were dehydrated and embedded in polyester resin (Rigolac; Nisshin-EM, Tokyo, Japan). Ground sections were prepared 40 µm in thickness. Contact microradiography was taken with a soft x-ray generator with a 20-µm Ni filter (15 kV, 3 mA, 5 to 6 min; Cu-Kα line; Softex, Austin, TX).
3D Reconstructions of Tooth Epithelium
Contours of dental and adjacent oral epithelium were drawn from histological sections at 7-µm intervals using a Leica DMRB microscope equipped with a drawing chamber at a magnification of 255×. The digitalization of the serial drawings and correlation of successive images have previously been described (Lesot et al. 1996). Three-dimensional images were generated using a volume-rendering program VG Studio Max 2.0 (VG Studio Max, Heidelberg, Baden-Württemberg, Germany).
Immunohistochemistry
Dewaxed sections were treated by proteinase K and then incubated with antibody against phosphorylated p-Smad1/5/8 (Cell Signaling Technology, Beverly, MA). The tyramide signal amplification system (PerkinElmer Life Science, Waltham, MA) was used for detecting the primary antibody.
Results
K5 is expressed in oral/dental epithelium from E11.5 (Appendix Fig. 1; Liu et al. 2010). The K5-Ikkβ mice survived, reproduced normally, and showed minor skin anomalies (Page et al. 2010).
To examine NF-κB activity in K5-Ikkβ mice, we crossed K5-Ikkβ mice with mice carrying a κB-dependent LacZ reporter gene construct ([Igκ]3xconalacZ) that show LacZ expression where the NF-κB pathway is activated. Up-regulation of NF-κB activity was confirmed in incisor regions in (Igκ)3xconalacZ;K5-Ikkβ mice (Fig. 1B).
Figure 1.
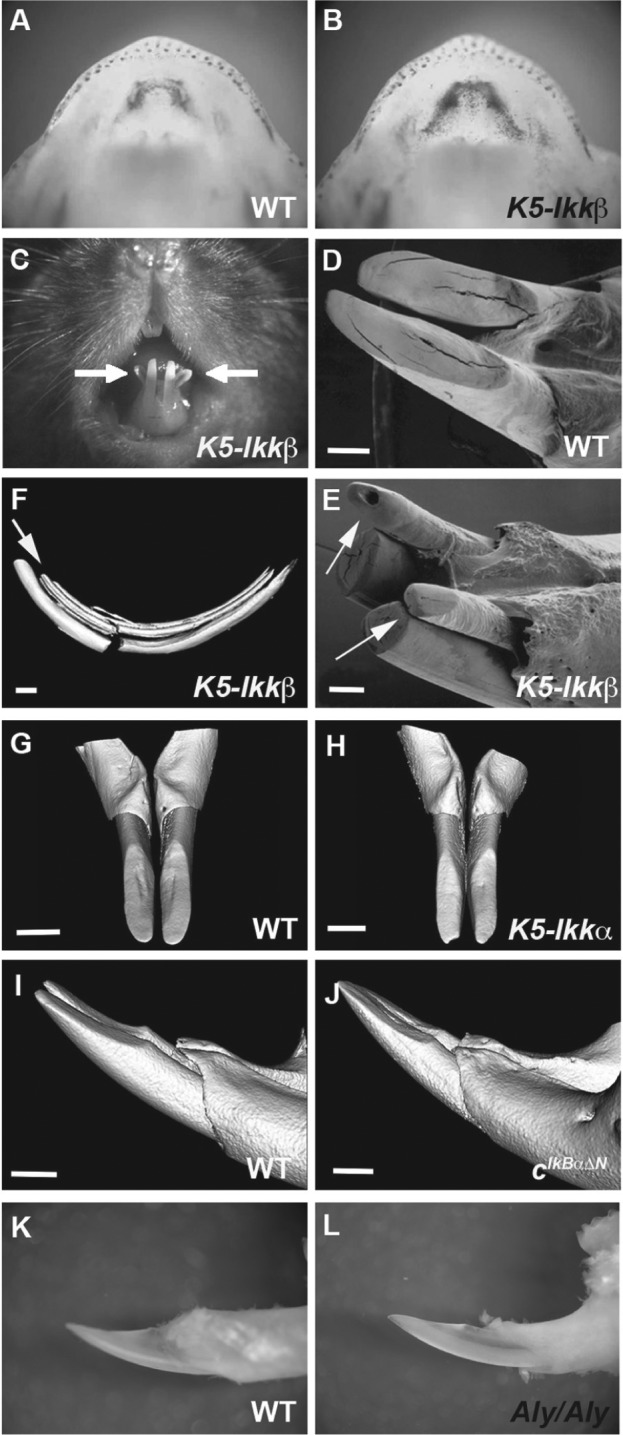
Lower incisor phenotypes in transgenic mice. (A, B) LacZ staining in (Igκ)3xconalacZ (A) and (Igκ)3xconalacZ;K5-Ikkβ (B) mice at E14.5. (C–L) Lower incisors in wild-type (D, G, I, K), K5-Ikkβ (C, E, F), K5-Ikka (H), cIκBαΔN (J), and Aly/Aly (L) mice at 6 wk of age. Arrows indicate the lingual incisors (C, E, F). (D, E) Scanning electron microscopy. (F-J) Three-dimensional reconstructions based on micro–computed tomography scans. Scale bars: 1.0 mm (D-J).
Six-week-old K5-Ikkβ mice revealed an additional pair of incisors on the mandible, lingual to the endogenous incisors (Fig. 1C, 1E; Appendix Table; these will hereafter be referred to as “lingual incisors”). Micro-CT analysis showed the lingual incisors to be separate entities from the endogenous incisors (Fig. 1F). The lingual incisors, however, shared the same bone socket with the endogenous incisor, suggesting that the lingual incisors develop dependent on endogenous incisor tooth germs. Upper incisors of K5-Ikkβ mice showed longitudinal grooves on their labial surfaces but no extra incisors (data not shown).
(Igκ)3xconalacZ shows activity of both canonical and noncanonical NF-κB pathway. Although Ikkβ is known to be nvolved in only canonical NF-κB signaling, noncanonical signaling always requires precedent canonical signaling. We therefore next confirmed whether lingual incisor formation was caused by the up-regulation of only canonical NF-κB signaling. To address this question, we examined mice with overexpression of Ikkα from the K5 promoter (K5-Ikkα) to see if increased noncanonical NF-κB signaling can also induce lingual incisors, since Ikkα is known to play a central role in regulating noncanonical NF-κB (Oeckinghaus et al. 2011). K5-Ikkα mice, however, showed no supernumerary incisors, suggesting that lingual incisor formation in K5-Ikkβ mice is caused solely by up-regulation of canonical NF-κB signaling (Fig. 1H; Blackburn et al. 2012). Mutation of Eda (upstream ligand of the NF-κB pathway in ectodermal organ development) has also been shown to result in extra tooth formation in the diastema (Peterková et al. 2002; Mustonen et al. 2003). To investigate whether reduced NF-κB signal activity also affects lingual incisor formation, we examined mice expressing the IκBα superrepressor IκBαΔN (cIκBαΔN), which ubiquitously suppresses NF-κB activity, and mice with a mutation in Nik (Aly/Aly) in which noncanonical signaling is inhibited. Both lines did not show any lingual incisors, suggesting that lingual incisor formation is entirely dependent on increased canonical NF-κB signaling (Fig. 1J, 1L; Ohazama et al. 2004).
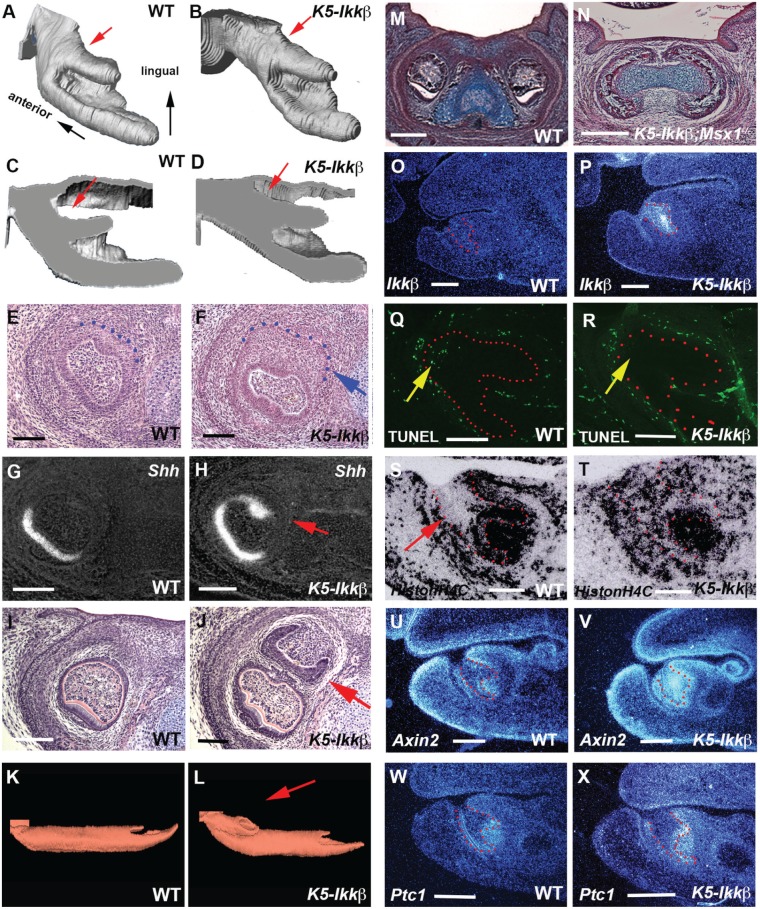
To establish the timing of the first appearance of the lingual incisor teeth, K5-Ikkβ embryos were examined at different time points during embryonic development. The obvious sign of lingual incisor formation could be visualized from E14.5 as a lingual enlargement of the endogenous incisor epithelium, which was confirmed by ectopic Shh expression at E15.5 (Fig. 2A-2H; Appendix Figs. 2 and 3; Appendix Table; n = 43; E13.5, n = 40; E14.5, n = 46; E15.5). Lingual incisor tooth germs were more obvious at E17.5 (Fig. 2J; Appendix Fig. 3). Analysis of 3D reconstructions of the dental epithelium and histological sections clearly demonstrated that lingual incisor tooth germs developed from the epithelium of the endogenous incisors at the most anterior/lingual extent (Fig. 2L; Appendix Fig. 3). To confirm whether lingual incisor tooth development requires endogenous incisor tooth germ, we crossed K5-Ikkβ mice with Msx1 mutants (K5-Ikkβ; Msx1-/-). Msx1 mutation is known to result in the arrest of tooth development at the bud stage (Satokata and Maas 1994), whereas the K5-Ikkβ lingual incisor initiates when the endogenous incisor tooth reaches the cap stage. K5-Ikkβ;Msx1-/- mice showed no incisor formation, suggesting that the ectopic lingual incisor development depends on endogenous incisor tooth development (Fig. 2N).
Figure 2.
Molecular changes in lower incisors of K5-Ikkβ mice. (A–D, K, L) Three-dimensional (3D) reconstructions of the epithelium in the incisor tooth germs of wild type (A, C, K) and K5-Ikkβ (B, D, L) embryos at E14.5 (A–D) and E17.5 (K, L). Dental epithelium is viewed as the mediolingual axis (A, B). Sagittal sections through the 3D model (C, D). Arrows indicating the lingual aspect of endogenous incisors (A–D) and lingual incisor tooth germ (L). (E–J, M, N) Frontal sections showing lower incisors in wild-type (E, G, I, M), K5-Ikkβ (F, H, J) and K5-Ikkβ;Msx1-/- (N) embryos at E15.5 (E-H) and E17.5 (I, J, M, N). (O–X) Sagittal section showing lower incisors in wild-type (O, Q, S, U, W) and K5-Ikkβ mice (P, R, T, V, X) at E13.5 (O, P) and E14.5 (Q-X). Expression of Shh (G, H), Ikkβ (O, P), HistonH4C (S, T), Axin2 (U, V), and Ptc1 (W, X) by radioactive in situ hybridization. (Q, R) TUNEL assay. Arrows indicating the lingual enlargement of endogenous incisor epithelium (F), colocalized ectopic Shh expression (H), lingual incisor tooth germ (J), apoptotic activity (Q, R), and the region showing the low level expression of HistonH4C (S). Tooth epithelium is outlined by red or blue dots. Scale bars: 100 µm (E-H), 200 µm (O–T), 400 µm (U–X), 500 µm (I, J, M, N).
Increased Ikkβ expression was confirmed in the epithelium where lingual incisors initiate (Fig. 2P). Increased Ikkβ expression and NF-κB activity was also observed in the upper incisor tooth germs, although the extra incisor was not found in the upper incisors (Appendix Fig. 4). Apoptotic activity has been shown in the mouse lower incisor epithelium at E14.5 (Munne et al. 2009). K5-Ikkβ embryos exhibited reduced apoptotic cells (Fig. 2R). Cell proliferation was low in wild-type epithelium, whereas there was extensive proliferation in K5-Ikkβ epithelium (Fig. 2S, 2T).
Increased Wnt signaling during tooth development has been shown to result in supernumerary tooth formation (Järvinen et al. 2006). The canonical Wnt signaling pathway (examined by expression of Axin2; a transcriptional target of the canonical Wnt signaling pathway) was up-regulated in K5-Ikkβ incisor epithelium where the lingual incisors initiate (Fig. 2V). K5-Ikkβ embryos also showed increased expression of Ptch1 (transcriptional target of the Shh signaling pathway) at the lingual aspect of incisor tooth germs, which was consistent with ectopic Shh expression (Fig. 2H, 2X). Although supernumerary incisors have also been shown following change of Fgf or Bmp signaling, K5-Ikkβ embryos exhibited no significant differences in Bmp or Fgf signaling in incisor tooth germs (Appendix Fig. 5; Zhang et al. 2009; Charles et al. 2011).
Micro-CT, contact microradiography, and histological analysis revealed that ameloblasts and enamel were observed on the endogenous incisors of K5-Ikkβ mice but not on lingual incisors (Figs. 3B, 3C, 4B, 4C). The absence of differentiated ameloblasts was confirmed by the lack of amelogenin (major enamel protein) expression in lingual incisor tooth germs (Fig. 4D). Ameloblasts in incisors are supplied from the labial cervical loops that show a characteristic morphology in wild-type mice (Fig. 4A). K5-Ikkβ lingual incisors, however, had only a thin layer of epithelium at the apical end of the lingual incisors, whereas endogenous incisors in K5-Ikkβ mice showed no significant morphological changes of the cervical loop (Fig. 4B, 4C). Lack of enamel formation in the lingual incisor is thus likely to be caused by abnormal labial cervical loop formation in K5-Ikkβ mice. These findings prompted us to examine whether lingual incisors are able to grow continuously. To address this question, incisors were clipped at the gingival level. Newly formed lingual and endogenous incisors were observed at 2 wk after the cutting, suggesting that lingual incisors retained the ability of continuous growth (Fig. 4E, 4F, 4G).
Figure 3.
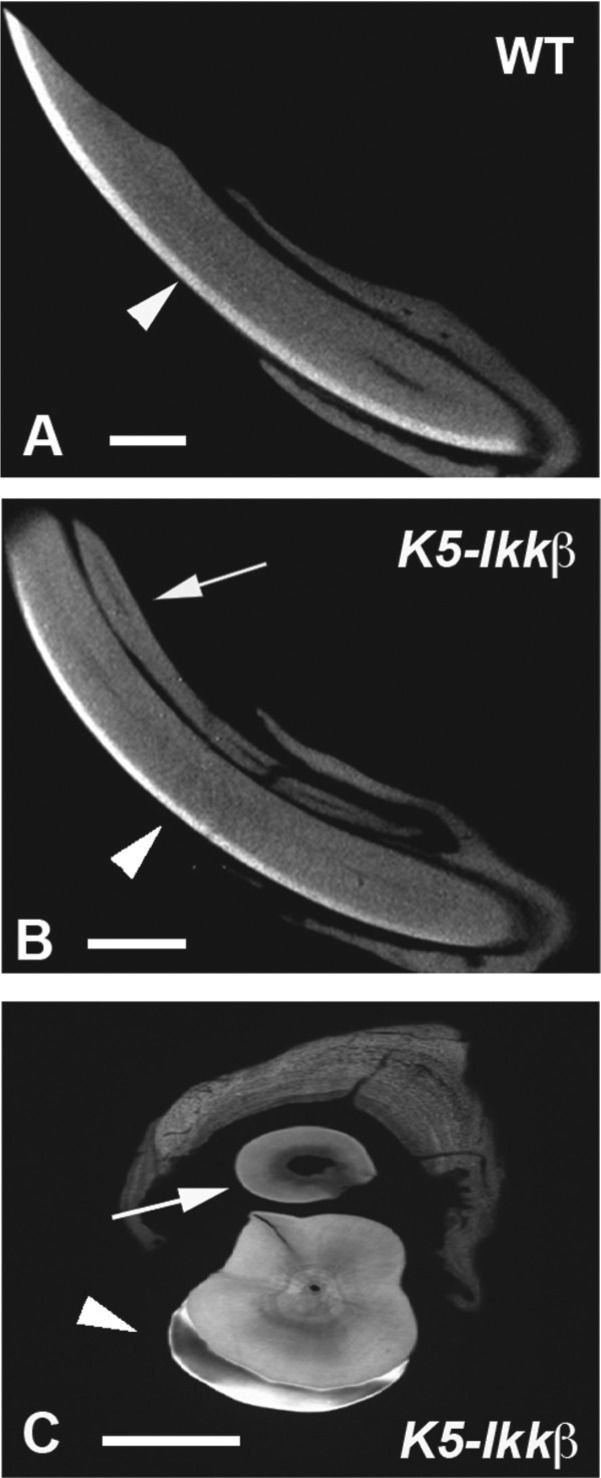
Enamel defects in lingual incisors. (A, B) Sagittal section based on micro–computed tomography scan in lower incisors of wild-type (A) and K5-Ikkβ (B) mice at 6 wk of age. (C) Contact microradiography as the frontal section of K5-Ikkβ mice. Arrows indicate lingual incisors (B, C). Arrowheads indicate enamel. Scale bars: 1.0 mm (A, B) and 500 µm (C).
Figure 4.
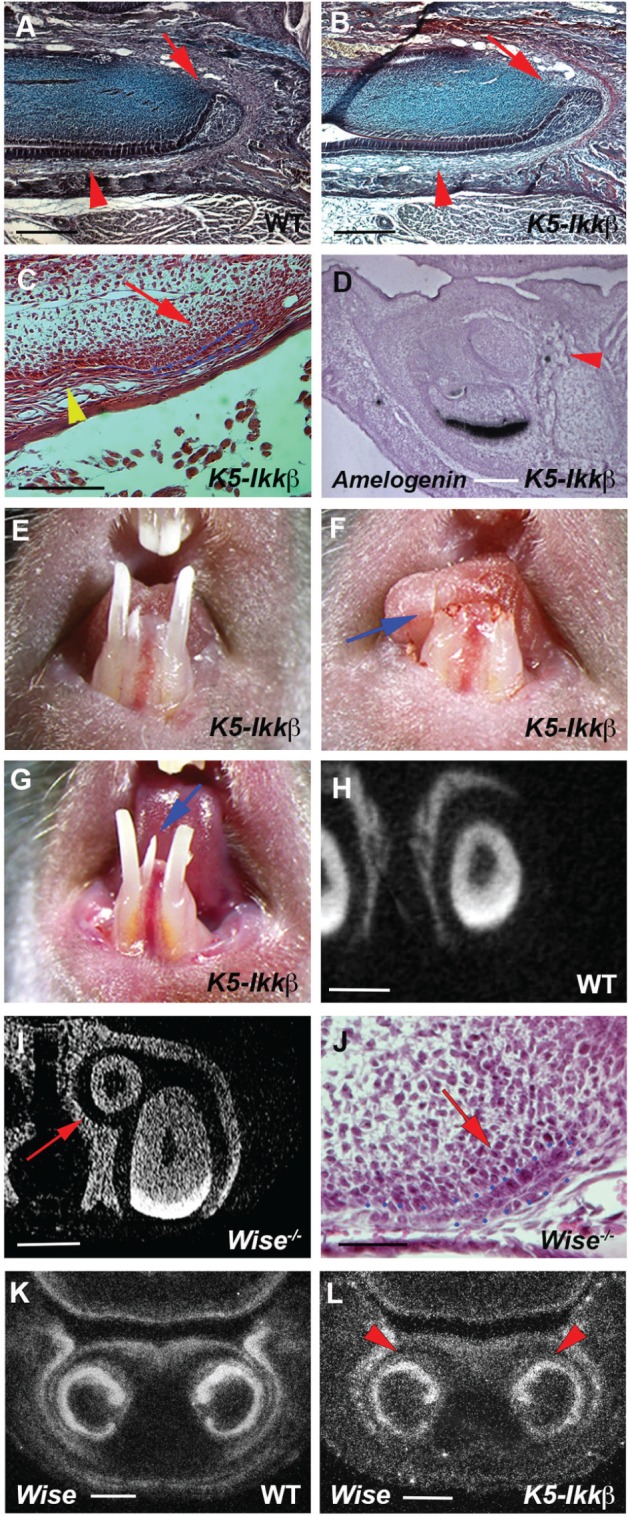
Lingual incisors in K5-Ikkβ mice. (A–C, J) Sagittal sections showing labial cervical loop in endogenous incisor (arrows in A, B) and lingual incisor (arrows in C, J) of wild-type (A), K5-Ikkβ mice (B, C), and Wise mutant (J) at P5. Arrowheads indicating ameloblasts (A, B) and the lack of ameloblasts (C). (D) Sagittal sections showing Amelogenin mRNA expression in K5-Ikkβ mice. Arrowhead indicating lingual incisor (D). (E–G) Endogenous and lingual incisors before clipping (E), just after clipping (arrow in F), and at 2 wk after clipping (G). Arrow indicating newly formed lingual incisor (G). (H, I) Frontal section based on micro–computed tomography scan in lower incisors of wild-type (H) and Wise mutant (I). Arrow indicates lingual incisor (I). (K, L) Wise expression by radioactive in situ hybridization on frontal sections of wild-type (K) and K5-Ikkβ mice (L) at E15.5. Arrowheads showing Wise expression on the lingual side of endogenous incisor tooth germs (L). The labial cervical loop was outlined by blue dots (C, J). Scale bars: 500 µm (A, B, H, I), 300 µm (C), 150 µm (D, J), and 200 µm (K, L).
Supernumerary incisors have been reported in mice with mutation of the Wnt signal inhibitor, Wise (Sostdc, Ectodin), which initiates from the epithelium of the embryonic endogenous incisor tooth germs at the most anterior/lingual extent and show increased Wnt signaling and no apoptotic activity (Murashima-Suginami et al. 2007; Ohazama et al. 2008; Munne et al. 2009; Ahn et al. 2010). Timing and location of initiation and molecular changes in lingual incisor formation of K5-Ikkβ mice were thus similar to those in Wise mutants. We therefore examined supernumerary incisors in Wise mutant mice. In common with K5-Ikkβ mice, no enamel formation and abnormal morphology of the labial cervical loop were observed in Wise mutant supernumerary incisors (Fig. 4I, 4J). Lingual incisors in K5-Ikkβ mice thus are a phenocopy to those in Wise mutants. However, no obvious changes in Wise expression were evident in K5-Ikkβ incisors (Fig. 4L).
Discussion
The overexpression of Ikkβ from the K5 promoter induced extra odontogenic activity in embryonic incisor tooth epithelium, which resulted in lingual incisor formation. The ectopic lingual incisors had no enamel that is likely to be caused by an abnormal epithelial stem cell niche, although epithelial cells are seen. Despite these abnormalities, lingual incisors in K5-Ikkβ mice exhibited continuous growth, suggesting that full function of the cervical loop is dispensable for incisor continuous growth. It is believed that there is interaction between the cervical loop epithelial cells and mesenchymal cells. The continuous growth and normal appearance of the dentine and pulp suggest that the incisor mesenchymal stem cell niche is unaffected (Lapthanasupkul et al. 2012).
Change of tooth number is a significant evolutionary adaptation to accommodate novel feeding strategies. Reduction in tooth number is a well-known evolutionary trend of the dentition within eutherians (Rose and Archibald 2005). In some primitive fossil Glires, a second pair of incisors was present in both the upper and lower jaws (Asher et al. 2005). Lingual incisors initiate from endogenous incisor epithelium, and apoptosis is observed in wild-type rodent incisor epithelium where the lingual tooth initiates (Munne et al. 2009). Lack of the apoptotic activity is found to result in extra incisor formation of K5-Ikkβ mice and Wise mutant mice. It is thus likely that additional odontogenic activity is usually inhibited in extant rodent incisor epithelium that was acquired during evolution, and mice retain the evolutionary lost odontogenic activity. In addition to lack of apoptosis, increased cell proliferation in incisor epithelium is likely to enlarge the lingual aspect of endogenous incisor tooth germs. Lingual incisors could not be detected in the maxillae of K5-Ikkβ mice, whereas Sprouty2/4, Wise, and Lrp4 mutant mice show extra incisors in both maxillae and mandibles (Murashima-Suginami et al. 2007, Ohazama et al. 2008; Munne et al. 2009; Ahn et al. 2010; Charles et al. 2011). Inhibitory mechanisms of odontogenesis are thus different between lower and upper incisors.
Changes in Fgf signal activity by Sprouty2/4 mutations have been shown to result in mandible supernumerary incisor formation (Charles et al. 2011). The location of the supernumerary incisors in Sprouty2/4 mutants is, however, different from those in K5-Ikkβ incisors, and Fgf signaling showed no significant changes in K5-Ikkβ mice. It is possible that inhibitory mechanisms of odontogenesis in incisor tooth germs are controlled by several different pathways, and lingual incisors in K5-Ikkβ mice might not involve Fgf signaling. In common with K5-Ikkβ mice, Wise mutants show supernumerary incisors lingual to endogenous incisors (Munne et al. 2009), and extra incisors in both K5-Ikkβ mice and Wise mutant mice initiate from similar regions of embryonic incisor tooth epithelium. Up-regulation of the Wnt signaling pathway is observed in embryonic incisor bud epithelium in both K5-Ikkβ mice and Wise mutant mice. Lingual incisors in K5-Ikkβ mice thus appear to phenocopy those in Wise mutants. K5-Ikkβ mouse lingual incisors retained Wise expression, suggesting that Wise is not the target of the NF-κB pathway in lingual incisor formation. It is possible that Wnt signaling activity in wild-type embryonic incisor bud epithelium is physiologically reduced by Wise, which can be activated by excess NF-κB signaling, since NF-κB signaling has been shown to activate the Wnt pathway (Kaler et al. 2009). Incisors lingual to endogenous incisors are also present in extant Lagomorpha, which, however, show enamel formation (Hirschfeld et al. 1973). No enamel formation in the lingual incisors of both K5-Ikkβ and Wise mutant mice indicates that lingual incisor formation by up-regulation of Wnt signaling is insufficient to lead to full development of evolutionary lost incisor teeth. No enamel formation in K5-Ikkβ lingual incisors might be caused by a defect in the cervical loop, and Wnt signaling has been shown to be not involved in cervical loop formation (Suomalainen and Thesleff 2010).
In addition to the role of Ikkβ in NF-κB activation, Ikkβ is also known to modulate cellular responses in a manner independent of NF-κB (Lee et al. 2007; Xia et al. 2009). Although we show that NF-κB activity is increased, we cannot exclude the possibility that lingual incisor formation is caused by Ikkβ-NF-κB–independent activity in K5-Ikkβ mice.
Author Contributions
J. Blackburn, K. Kawasaki, T. Porntaveetus, M. Kawasaki, Y. Otsuka-Tanaka, Y. Miake, M. Watanabe, M. Hishinuma, T. Nomoto, S. Oommen, S. Ghafoor, F. Harada, K. Nozawa-Inoue, T. Maeda, contributed to conception, design, data analysis, and interpretation, drafted and critically revised the manuscript; M.S. Ota, J. Inoue, T. Akiyama, R. Schmidt-Ullrich, B. Liu, Y. Hu, A. Page, Á. Ramirez, contributed to conception, design, data acquisition, and interpretation, drafted and critically revised the manuscript; R. Peterková, H. Lesot, P.T. Sharpe, A. Ohazama, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Acknowledgments
We would like to thank Tony Brain for scanning electron microscope analysis, Chris Healy for micro-CT analysis, Dr. Koyama for HistoneH4C plasmid, Alasdair Edgar and Alex Huhn for their technical help, and K. Lochovska, S. Vojtechova, M. Hovorakova, S. Kieffer, and T. Boran for help with 3-dimensional reconstructions.
Footnotes
This research was funded by grant MRC-GR-200709, Grant Agency of the Czech Republic (CZ:GA CR:14-37368G); Thailand Research Fund (TRG5780209); Ratchadaphiseksomphot Endowment Fund (RES560530253-AS) and Faculty Research Grant (DRF56018) of Faculty of Dentistry Chulalongkorn University; and the Japan Society for the Promotion of Science (JSPS; 26293421). Y.O.-T. is supported by Nihon University. M.K. and K.K. are supported by the JSPS International Program for Young Researcher Overseas Visits.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Ahn Y, Sanderson BW, Klein OD, Krumlauf R. 2010. Inhibition of Wnt signaling by Wise (Sostdc1) and negative feedback from Shh controls tooth number and patterning. Development. 137(19):3221–3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher RJ, Meng J, Wible JR, McKenna MC, Rougier GW, Dashzeveg D, Novacek MJ. 2005. Stem lagomorpha and the antiquity of Glires. Science. 307(5712):1091–1094. [DOI] [PubMed] [Google Scholar]
- Blackburn J, Ohazama A, Kawasaki K, Otsuka-Tanaka Y, Liu B, Honda K, Rountree RB, Hu Y, Kawasaki M, Birchmeier W, et al. 2012. The role of Irf6 in tooth epithelial invagination. Dev Biol. 365(1):61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles C, Hovorakova M, Ahn Y, Lyons DB, Marangoni P, Churava S, Biehs B, Jheon A, Lesot H, Balooch G, et al. 2011. Regulation of tooth number by fine-tuning levels of receptor-tyrosine kinase signaling. Development. 138(18):4063–4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada H, Kettunen P, Jung HS, Mustonen T, Wang YA, Thesleff I. 1999. Localization of putative stem cells in dental epithelium and their association with Notch and FGF signaling. J Cell Biol. 147(1):105–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschfeld Z, Weinreb MM, Michaeli Y. 1973. Incisors of the rabbit: morphology, histology, and development. J Dent Res. 52(2):377–384. [DOI] [PubMed] [Google Scholar]
- Järvinen E, Salazar-Ciudad I, Birchmeier W, Taketo MM, Jernvall J, Thesleff I. 2006. Continuous tooth generation in mouse is induced by activated epithelial Wnt/beta-catenin signaling. Proc Natl Acad Sci USA. 103(49):18627–18632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaler P, Godasi BN, Augenlicht L, Klampfer L. 2009. The NF-kappaB/AKT-dependent induction of Wnt signaling in colon cancer cells by macrophages and IL-1beta. Cancer Microenviron. 2:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassai Y, Munne P, Hotta Y, Penttilä E, Kavanagh K, Ohbayashi N, Takada S, Thesleff I, Jernvall J, Itoh N. 2005. Regulation of mammalian tooth cusp patterning by ectodin. Science. 309(5743):2067–2070. [DOI] [PubMed] [Google Scholar]
- Lapthanasupkul P, Feng J, Mantesso A, Takada-Horisawa Y, Vidal M, Koseki H, Wang L, An Z, Miletich I, Sharpe PT. 2012. Ring1a/b polycomb proteins regulate the mesenchymal stem cell niche in continuously growing incisors. Dev Biol. 367(2):140–153. [DOI] [PubMed] [Google Scholar]
- Lee DF, Kuo HP, Chen CT, Hsu JM, Chou CK, Wei Y, Sun HL, Li LY, Ping B, Huang WC, et al. 2007. IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 130(3):440–455. [DOI] [PubMed] [Google Scholar]
- Lesot H, Vonesch JL, Peterka M, Turecková J, Peterková R, Ruch JV. 1996. Mouse molar morphogenesis revisited by three-dimensional reconstruction. II. Spatial distribution of mitoses and apoptosis in cap to bell staged first and second upper molar teeth. Int J Dev Biol. 40(5):1017–1031. [PubMed] [Google Scholar]
- Liu F, Dangaria S, Andl T, Zhang Y, Wright AC, Damek-Poprawa M, Piccolo S, Nagy A, Taketo MM, Diekwisch TG, et al. 2010. beta-Catenin initiates tooth neogenesis in adult rodent incisors. J Dent Res. 89(9):909–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomada D, Liu B, Coghlan L, Hu Y, Richie ER. 2007. Thymus medulla formation and central tolerance are restored in IKKalpha-/- mice that express an IKKalpha transgene in keratin 5+ thymic epithelial cells. J Immunol. 178(2):829–837. [DOI] [PubMed] [Google Scholar]
- Mikkola ML, Thesleff I. 2003. Ectodysplasin signaling in development. Cytokine Growth Factor Rev. 14(3-4): 211–224. [DOI] [PubMed] [Google Scholar]
- Munne PM, Tummers M, Järvinen E, Thesleff I, Jernvall J. 2009. Tinkering with the inductive mesenchyme: Sostdc1 uncovers the role of dental mesenchyme in limiting tooth induction. Development. 136(3):393–402. [DOI] [PubMed] [Google Scholar]
- Murashima-Suginami A, Takahashi K, Kawabata T, Sakata T, Tsukamoto H, Sugai M, Yanagita M, Shimizu A, Sakurai T, Slavkin HC, et al. 2007. Rudiment incisors survive and erupt as supernumerary teeth as a result of USAG-1 abrogation. Biochem Biophys Res Commun. 359(3):549–555. [DOI] [PubMed] [Google Scholar]
- Mustonen T, Pispa J, Mikkola ML, Pummila M, Kangas AT, Pakkasjärvi L, Jaatinen R, Thesleff I. 2003. Stimulation of ectodermal organ development by ectodysplasin-A1. Dev Biol. 259(1):123–136. [DOI] [PubMed] [Google Scholar]
- Oeckinghaus A, Hayden MS, Ghosh S. 2011. Crosstalk in NF-κB signaling pathways. Nat Immunol. 12(8):695–708. [DOI] [PubMed] [Google Scholar]
- Ohazama A, Hu Y, Schmidt-Ullrich R, Cao Y, Scheidereit C, Karin M, Sharpe PT. 2004. A dual role for Ikk alpha in tooth development. Dev Cell. 6(2):219–227. [DOI] [PubMed] [Google Scholar]
- Ohazama A, Johnson EB, Ota MS, Choi HY, Porntaveetus T, Oommen S, Itoh N, Eto K, Gritli-Linde A, Herz J, et al. 2008. Lrp4 modulates extracellular integration of cell signaling pathways in development. PLoS One. 3(12):e4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page A, Navarro M, Garín M, Pérez P, Casanova ML, Moreno R, Jorcano JL, Cascallana JL, Bravo A, Ramírez A. 2010. IKKbeta leads to an inflammatory skin disease resembling interface dermatitis. J Invest Dermatol. 130(6):1598–1610. [DOI] [PubMed] [Google Scholar]
- Peterková R1, Kristenová P, Lesot H, Lisi S, Vonesch JL, Gendrault JL, Peterka M. 2002. Different morphotypes of the tabby (EDA) dentition in the mouse mandible result from a defect in the mesio-distal segmentation of dental epithelium. Orthod Craniofac Res. 5(4):215–226. [DOI] [PubMed] [Google Scholar]
- Rose KD, Archibald JD. 2005. The rise of placental mammals: origins and relationships of the major extant clades. Rose KD, Archibald JD, editors. Baltimore (MD): Johns Hopkins University Press. [Google Scholar]
- Satokata I, Maas R. 1994. Msx1 deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nat Genet. 6(4):348–356. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich R, Aebischer T, Hülsken J, Birchmeier W, Klemm U, Scheidereit C. 2001. Requirement of NF-kappaB/Rel for the development of hair follicles and other epidermal appendices. Development. 128(19):3843–3853. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich R, Mémet S, Lilienbaum A, Feuillard J, Raphaël M, Israel A. 1996. NF-kappaB activity in transgenic mice: developmental regulation and tissue specificity. Development. 122(7):2117–2128. [DOI] [PubMed] [Google Scholar]
- Suomalainen M, Thesleff I. 2010. Patterns of Wnt pathway activity in the mouse incisor indicate absence of Wnt/beta-catenin signaling in the epithelial stem cells. Dev Dyn. 239(1):364–372. [DOI] [PubMed] [Google Scholar]
- Xia Y, Padre RC, De Mendoza TH, Bottero V, Tergaonkar VB, Verma IM. 2009. Phosphorylation of p53 by IkappaB kinase 2 promotes its degradation by beta-TrCP. Proc Natl Acad Sci USA. 106(8):2629–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Lan Y, Chai Y, Jiang R. 2009. Antagonistic actions of Msx1 and Osr2 pattern mammalian teeth into a single row. Science. 323(5918):1232–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.