Abstract
The prepartum transition from a soft to ripening cervix is an inflammatory process that occurs well before birth when systemic progesterone is near peak concentration. This 2-part study first determined that stromal fibroblasts but not macrophages in the cervix have progesterone receptors (PRs). Neither the number of PR cells in cervix sections nor the relative abundance or ratio of nuclear PR isoforms (PR-A/PR-B) were diminished in mice between day 15 of pregnancy and term. Second in mice lacking PR-B (Pgrtm20mc), the number of cells that expressed the PR-A isoform were maintained during this period of prepartum cervix remodeling. Thus, progesterone effects to sustain pregnancy, as well as soften and ripen the cervix, are mediated by a stable stromal cell population that expresses PR-A and, through interactions with resident macrophages, are likely to mediate inflammatory ripening processes in preparation for birth.
Keywords: ripening, cervix, Pgrtm20mc mice, parturition, macrophage, collagen degradation
Introduction
The cervix serves as a barrier to shelter the fetus from the external environment during pregnancy. However, overcoming this obstacle for birth involves structural remodeling of the cervix such that this gate for parturition opens for the newborn to pass through the birth canal. From studies in rodents, prepartum remodeling occurs in 3 phases: (1) softening, during which the extracellular matrix reorganizes and expands; (2) ripening, which includes reduced cross-linked collagen structure, and inflammation characterized by an increased density of resident macrophages; and (3) dilation, which is temporally in sequence with uterine contractions of labor.1,2 The timing for this transformation is key for birth in 2 respects. First, delayed or incomplete remodeling is associated with dystocia that may require surgical intervention to prevent maternal or newborn injury.3 Second, premature ripening of the cervix typically precedes preterm birth, and early delivery significantly increases risks of neonatal morbidity with the potential for lifelong consequences.4 Thus, the timing of cervix remodeling is critical for the successful outcome of pregnancy.
Progesterone is well recognized as essential to maintain pregnancy and sustain an unremodeled cervix.5-8 This consensus is based, in part, upon evidence that across a variety of mammals, progesterone withdrawal triggers the premature prepartum shift from a soft to ripe cervix and advances the conclusion of pregnancy.9-13 Specifically, premature birth results from ovariectomy, which removes the principal source of systemic progesterone in rodents, while treatment with PR modulators/antagonists (mifepristone/RU486 or onapristone) disrupts or blocks progesterone signaling. By contrast, progesterone or progesterone receptor (PR) agonists delay preterm birth after ovariectomy, promote cervix softening, suppress further remodeling, and inhibit inflammatory processes.14-16 For the prepartum transition of the cervix from soft to ripening before term, systemic progesterone is near or at peak concentrations across a variety of species.2 Thus, loss of progesterone efficacy has been conceived, since at least 1956, as an essential component of a final common mechanism for labor and birth.17 Mesiano and colleagues have since proposed that regulation of PR expression may mediate a functional progesterone withdrawal in women at term.18-20 However, extension of the concept for loss of the ability of progesterone to sustain an unripe cervix while concentrations remain elevated in circulation during the transitions from soft to ripening has yet to be a focus of study. Thus, the principal objectives of this study were to determine whether macrophages or stromal cells in the cervix have classic nuclear receptors for progesterone and whether the census of these cells or the abundance of PR isoforms change as pregnancy nears term. The hypothesis tested was that reduced numbers of PR cells and a predominance of the PR-A isoform in the cervix characterize a local withdrawal of progesterone effects for remodeling and parturition.
Materials and Methods
Experimental procedures involving mice were in accordance with the National Institute of Health guidelines and were approved by the Loma Linda University Institutional Animal Care and Use Committee. Mice were asphyxiated with CO2 prior to tissue collection. Timed pregnant and nonpregnant (NP) CD-1 mice were obtained from Harlan Laboratories (Livermore, California) and housed in a room under controlled conditions (temperature 70°F, humidity 55%, and photoperiod 12 hours light beginning 7 am). Time-dated pregnant Pgrtm20mc mice (PRB−/− on a Balb/C 129S7/SvEvBrd-Hprtb-m2 background) were bred at Loma Linda University (founder pair gifted by Dr Orla Conneely, Baylor University College of Medicine). Genotype was confirmed by the presence of the mutant sequence 5′ to 3′ CTTTGCCTGGAGCTCAGTCAG compared to CTTTGCCAGCAGCTCAGTCAT in wild-type controls (TransnetYX; Cordova, Tennessee).
Experiment 1: Do Macrophages in the Cervix Express PRs
The cervix was excised from CD-1 mice on postbreeding day (D) 15 or D18 (n = 3/group) with NP cervix as baseline controls (n = 3). In brief, the cervix was fixed for 24 hours in 4% paraformaldehyde, embedded in paraffin, and sectioned at 10 μm. Cervix sections from each postbreeding group and from NP mice were processed by immunohistochemistry to stain cells for PR-A and PR-B isoforms (1:1000; #8757; Cell Signaling Technology, Inc, Danvers, Massachusetts) and macrophages (F4/80; 1:800 dilution; T-2006; BMA Biomedicals, Switzerland). A 5% nickel and 5% cobalt solution was added to the 3,3′-diaminobenzidine step to enhance the density of blackish–gray nuclear PR stain (adapted from13,21). Sections were then counterstained with methyl green solution in acetate buffer (pH 7.2) to identify cell nuclei. Macrophages were identified by a cytoplasmic brown stain surrounding a methyl green-colored nucleus. Positive controls in this and subsequent experiments included sections stained for macrophages or PR alone, while cervix sections processed without primary antisera served as negative controls in each immunohistochemistry run.
Experiment 2: Is the Cervix Transition From Soft to Ripening Associated With a Decrease in Density of Cells That Express PRs?
The cervix was excised from CD-1 mice on postbreeding D15, D16, D17, D18, and PP (n = 8-15/group) with NP cervix as baseline controls (n = 5). In brief, the cervix was fixed for 24 hours in 4% paraformaldehyde and embedded in paraffin. Longitudinal 10-μm sections of cervix that included vaginal and uterine tissues for anatomical reference were processed by immunohistochemistry to label PR-A and PR-B isoforms (1:1000; #8757; Cell Signaling Technology, Inc) and counterstain cell nuclei with methyl green solution in acetate buffer (pH 7.2). Controls in each immunohistochemistry run included cervix sections stained for macrophages or PRs alone as well as sections processed without primary antisera, respectively, positive and negative controls for staining. To count cell nuclei and densities of cells that stain for PRs, 8 representative photomicrographs from 2 adjacent cervix sections (up to 4 each) were captured of the stroma (16 images total 1.25 × 105 µm2/region) from the lower os up to the transition zone of striated fibers and smooth muscle before leading into the uterine body and bicornate horns. Lumen, epithelium, blood vessels, and other atypical structures were excluded, as best as possible, from photomicrographs and areas that were further analyzed. The PRs were clearly identified by dense dark brown deposits overlay of the methyl green-stained cell nucleus. Intensity of stain, which may vary among sections within or between mice or runs, was not a consideration for cell identification. Cells were counted with NIH ImageJ and normalized to cell nuclei/area to account for heterogeneous morphology, edema, and cellular hypertrophy that occur with the progress of pregnancy. Additional sections were stained with a picrosirius red stain kit (PK-4000; Polysciences, Warrington, Pennsylvania) to assess extracellular collagen organization and counterstained with hematoxylin (SH26; Fisher Scientific, Asheville, NC) as described previously.12,22 The optical density of birefringence of stained sections under circular polarized light was analyzed using the Rodbard transformation, an assessment that is inversely related to cross-linked collagen structure.
Experiment 3: Is There a Change in the PR-A–PR-B Ratio in Cervix Stromal Cells During the Soft-to-Ripening Transition
The cervix was obtained from CD-1 mice on postbreeding D15, D16, D16.5, D17, D18, and PP (n = 4/group) as well as from NP mice as controls (n = 4). Each cervix carefully trimmed of vaginal tissue, adherent fat, and the caudal attachment of the uterine horns. The cervix was flash frozen with liquid nitrogen and stored at −80°C until immunoblot analyses to quantify expression of PR isoforms in whole cervix using standard protocols. A monoclonal antibody was used (SP-2, MA5-14505; ThermoFisher Scientific, Fremont, CA Santa Cruz Biotechnology, Santa Cruz, CA)19 because the polyclonal cell signaling antibody from experiments 1 and 2 did not identify PR in Western immunoblots. The SP-2 antibody was optimally titred and a protocol standardized for processing thawed tissues to minimized band variability in immunoblots. Standard controls included molecular weight markers and identification of the GAPDH housekeeping protein (1:300, sc-32233; Santa Cruz Biotechnology). Intensity of bands was assessed by digital densitometry (ImageJ, National Institutes of Health, http://imagej.nih.gov/ij/) as previously described.22
Experiment 4: In PR-B Deficient Mice, Do Changes in Density of PR-A Cells or Abundance of PR-A Characterize the Period for Cervix Ripening
In PRB−/− mice, the duration of pregnancy, time course for cervix remodeling (temporal pattern of changes in cell nuclei, cross-linked collagen, and macrophage densities), and parturition at term are the same as in wild-type controls.23 The novel objective of this study was to assess whether PR-A cell density is temporally associated with remodeling as pregnancy nears term. Accordingly, the cervix from pregnant PRB−/− mice was obtained in groups on D15, D16.5, and D18 postbreeding (n = 4 each). Cervix sections were processed and analyzed as described previously.
Statistical Analysis
Comparisons were made by 1-way analysis of variance (ANOVA) or Student t test using GraphPad Prism (GraphPad Software, Inc, La Jolla, California) with Tukey or Dunnett post hoc analyses for individual comparisons. Data were assessed for normal distribution and outliers using the Levene and Grubb tests, respectively. P < .05 was considered significant. For analyses of Western blot data, the density of bands was normalized to the housekeeping protein GADPH as described previously22,24 to enhance sensitivity and accuracy of comparisons of band densities for replicates and among different experimental groups across gels.
Results
Experiment 1: Do Macrophages in the Cervix Express PRs
In sections processed with both antibodies (PRs and F4/80 macrophages), cells in the cervix stroma that expressed PRs had a gray-black stain that typically covered the cell nucleus and obscured the methyl green counterstain (Figure 1, blue arrows). This pattern of cellular PR staining was similar to that in sections processed solely with the PR antibody or in combination with the F4/80 antibody. Among pregnant and NP mice, the vast majority of PR-stained cells were dispersed in stromal fibroblasts across various groups. Cells that stained for PR were occasionally found in luminal and subepithelial areas of the cervix. By contrast, F4/80-stained macrophages were brown, in association with a prominent methyl green-counterstained nucleus, and distributed in no particular association with PR cells (Figure 1, red arrows). Across all groups and irrespective of pregnancy, only a few or no cells per cervix were found with dark brown stain that covered the cell nucleus. Such densely stained brown cells (Figure 1, inset) were not present in every mouse or more common at any particular day postbreeding—a finding observed in sections stained solely for F4/80 macrophages in this study (controls) and in previous reports.
Figure 1.
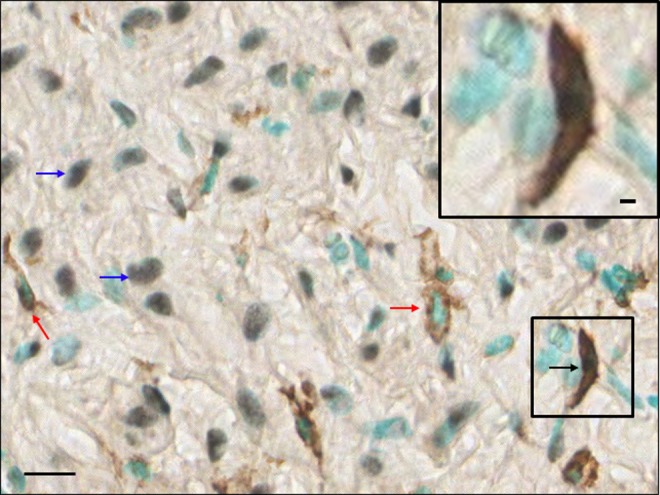
Photomicrograph of a cervix section from a day 18 postbreeding mouse stained for progesterone receptors (PR-A and PR-B isoforms), as well as macrophages and cell nuclei. Examples of distinct cells stained for PRs (blue arrows points to black stain that obscures methyl green-counterstained cell nuclei) or macrophages (red arrows of brown-stained cellular stain around cell nucleus). Inset is a magnified rare example of a densely stained macrophage with darkened cell nuclei. The methyl green-stained cell nucleus remains evident. Scale bars are 25 and 10 µm in inset.
Experiment 2: Is the Cervix Transition From Soft to Ripening Associated With a Decrease in Density of Cells That Express PRs
Cells whose nuclei stained for PRs were predominantly in the stroma (Figure 2, top) and sparsely in the luminal epithelia. The stromal PR cells appeared interspersed with other methyl green-stained cell nuclei in each field of view in all cervix sections. Cell density in the stroma decreased as pregnancy progressed. Compared to NP mice, the density of cell nuclei in cervix stroma decreased by D15 postbreeding (0.15 × 10−2 ± 0.16 × 10−3 vs 0.47 × 10−3 ± 0.38 × 10−4 /area, respectively; NP vs D15, n = 5-10/group, P < .05, ANOVA) and remained reduced for the duration of pregnancy as well as in PP mice (data not shown for D16-PP groups, mean range 0.35 − 0.50 × 10−3 ± 0.27 − 0.31 × 10−4 /area; P > .05, a finding comparable to that in previous studies2,12). For PR-stained cells normalized to cell nuclei area to account for reduced density of cells/area, the census was unchanged in the cervix from NP, pregnant, and PP mice (Figure 2, middle).
Figure 2.
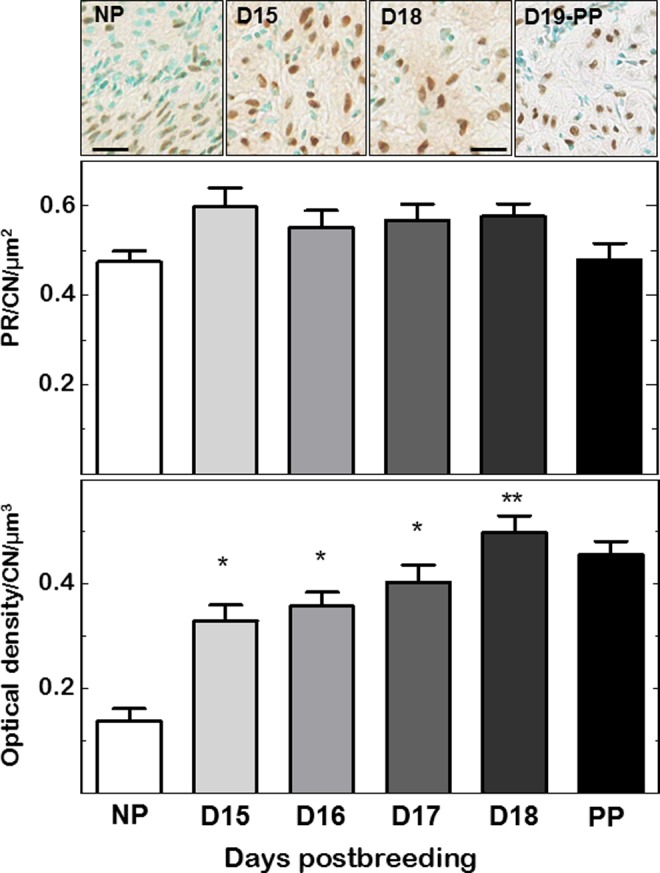
Top: Photomicrographs of progesterone receptor (PR) stained cells and counterstained cell nuclei (CN) in cervix sections from nonpregnant (NP), pregnant (days 15 and 18 postbreeding), and day of birth postpartum (PP) mice. Scale bar is 25 µm. Middle: Density of PR cells normalized to CN per area to account for variability in cell nuclei density due to heterogeneity of tissue morphology within and among sections in individuals, as well as within and among groups. Data are the mean ± SE (n = 5-21/group). Bottom: As inversely related to cross-linked collagen in the extracellular matrix,2 optical density of birefringence of picrosirius red-stained cervix sections cells was normalized to CN per area. Data are mean ± SE (n = 4-10/group; *P < .05 vs NP mice or **vs NP, day 15 and day 16 postbreeding groups by 1-way ANOVA). See Methods for details about staining and analyses. SE indicates standard error; ANOVA, analysis of variance.
Optical density analysis of picrosirius red birefringence indicated a significant decline in extracellular cross-linked collagen in cervix sections from D15, D16.5, and D17 pregnant mice relative to NP controls (Figure 2, bottom). With optical density of birefringence inversely related to cross-linked collagen, optical densities in cervix sections from groups of pregnant mice were significantly increased compared to that from NP mice. By D18 postbreeding, the optical density of picrosirius-stained collagen birefringence further increased compared to that in cervix sections from mice on D15 and D16 of pregnancy. Thus, cross-linked collagen was reduced in the cervix from pregnant mice and further declined by the D18 of pregnancy, the day before birth, as in previous studies.2,12
Experiment 3: Is There a Change in the Ratio of PR-A and PR-B in Cervix Stromal Cells During the Soft-to-Ripening Transition
The PR-A and, to a lesser extent, PR-B were present in immunoblots of cervix from mice on all days postbreeding. No change in expression of the PR-A-to-PR-B ratio was evident as pregnancy progressed to term (Figure 3; P > .05, ANOVA). Additionally, abundance of PR-A normalized to the GAPDH did not change across pregnancy during the period of cervix ripening.
Figure 3.
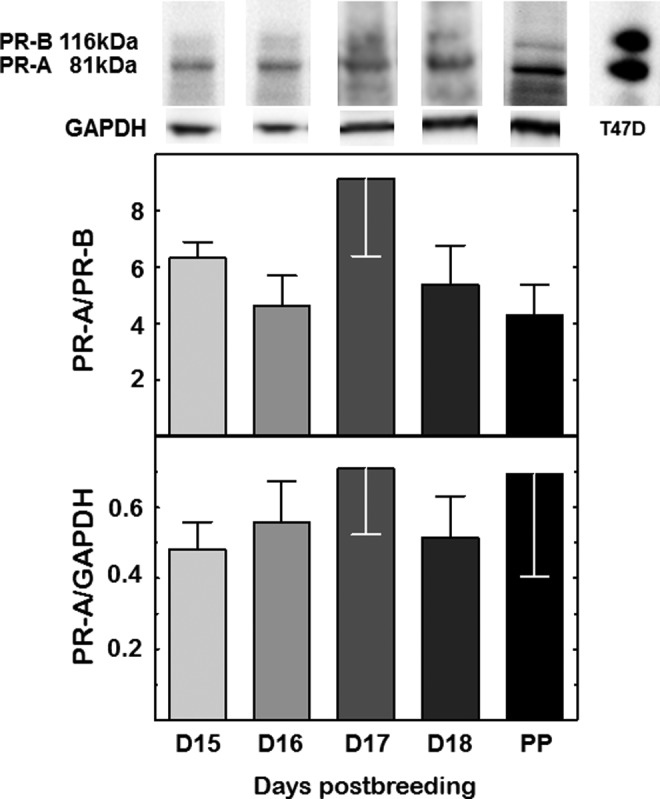
Representative immunoblot of cervix protein extract from pregnant mice on postbreeding days 15 to 18 and the day of birth (PP). Histograms are the optical density of the PR-A-to-PR-B ratio or optical density of the PR-A band normalized to the GAPDH loading control. Data are the mean ± SE (n = 4/group, P > .05; 1-way ANOVA). See Methods for details. PR indicates progesterone receptor; SE, standard error; ANOVA, analysis of variance.
Experiment 4: In PR-B-Deficient Mice, Do Changes in Density of PR-A Cells or Abundance of PR-A Characterize the Period for Cervix Ripening?
In the cervix stroma of PRB−/− mice, PRs were clearly evident as brown stain over the cell nuclei (Figure 4). The density of cells with PR-A was not different among prepartum days postbreeding, despite reduced cell nuclei density (D15, D17, D18, 0.623 ± 0.037, 0.545 ± 0.05, 0.578 ± 0.026, respectively; n = 10-15/group, P > .05, ANOVA). Finally, analyses of immunoblots indicated the absence of a molecular weight band at 116 kDa, confirmation that PR-B was deficient in PRB−/− mice, while the band at 81 kDa suggested that PR-A was exclusively present (Figure 5). Comparison of band densities across groups found no change in PR-A expression in the cervix of pregnant PR-B−/− mice from D15 to D18 postbreeding (P > .05, ANOVA). These different approaches similarly indicate no change in PR-A in the cervix during the period of cervix ripening.
Figure 4.
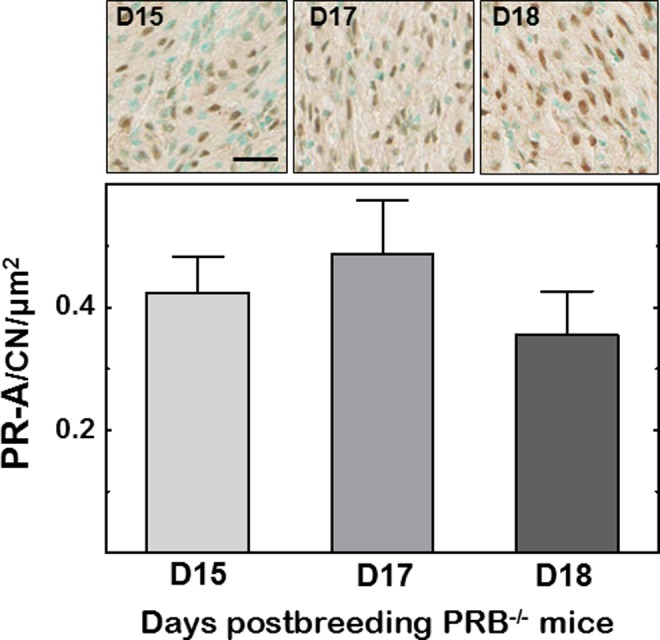
Photomicrographs of stroma in cervix sections from PRB−/− mice on days 15, 17, and 18 postbreeding with only PR-A isoform-stained cells and counterstained cell nuclei. Histogram is the numbers of PR-A cell normalized to cell nuclei/area. Data are the mean ± SE (n = 4/group, P > .05; 1-way ANOVA). Scale bar = 50 µm. PR indicates progesterone receptor; SE, standard error; ANOVA, analysis of variance.
Figure 5.
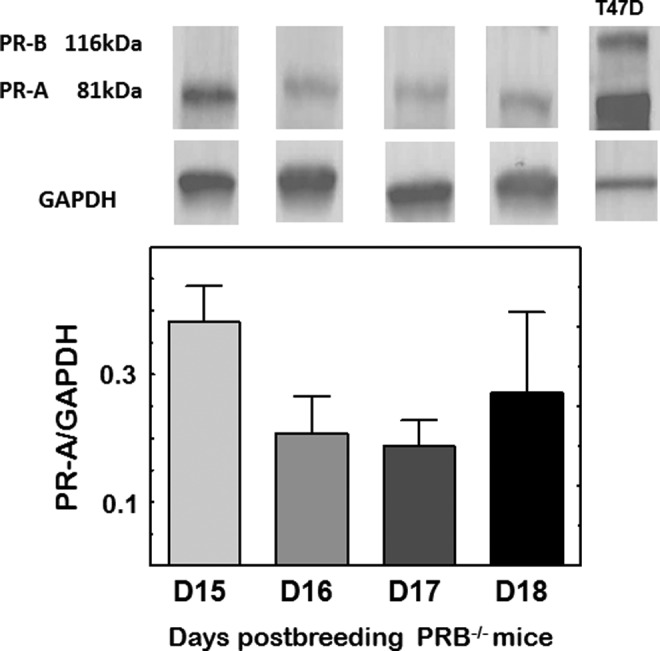
Representative immunoblots from the cervix of a pregnant PRB−/− mouse on days 15, 16, 17, and 18 postbreeding. With PR-B absent (no band at 116 kDa), only PR-A optical density was normalized to GAPDH (mean ± SE; n = 3/group, P > .05; 1-way ANOVA). PR indicates progesterone receptor; SE, indicates standard error; ANOVA, analysis of variance.
Discussion
Although progesterone is essential to maintain pregnancy and soften the cervix, parturition appears to reflect a loss in progesterone efficacy and considered an inflammatory process. With progesterone concentrations at or near peak concentrations, the transition from soft to ripening cervix is characterized by reduced stromal cell nuclei density, degradation of collagen in cross-linked structure, and increased presence of macrophages.2 The present findings indicate that cells in the stroma, but not macrophages, express PRs. Moreover, during this period of cervix ripening, the density of cells that express PRs/area of stroma does not change. This result is corroborated to an extent by Western analyses evidence that the abundance of PR isoforms and their ratio are also unchanged in the cervix. Evidence in PRB−/− mice that lacks the PR-B isoform further reveals that neither the number of PR-A expressing cells nor abundance of PR-A in the cervix reflects loss of responsiveness to progesterone or drive to increase in the presence of macrophages.12,23 Thus, evidence suggests that progesterone effects to promote a soft unripe cervix and loss of progesterone efficacy during the critical period for ripening occur with an undiminished number of stromal cells and abundance of PR-A isoform protein prior to term.
These novel findings focus attention on the capability of stromal cells in the prepartum cervix to integrate a variety of physiological signals to regulate 2 critical aspects of PR-mediated activities for ripening. First, the absence of PRs in macrophages in the cervix of pregnant or NP mice or rats13,25 indicates that progesterone is unlikely to directly regulate their activities or differentiation. Rather, stroma cells as the predominant expresser of PRs in the cervix would directly mediate effects of progesterone on extracellular matrix structure given that this cell is a source of enzymes that degrades collagen.26 Second, although paracrine signals from stromal cells that may guide functions by resident macrophages have yet to be defined, effector molecules produced by macrophages can promote ripening in preparation for birth.27-29 These macrophage products include prostaglandins, nitric oxide, cytokines, and transcription factors like nuclear factor-κB that drive inflammatory activities, degradation of extracellular collagen structure, and vascular permeability. Thus, cross talk between stromal cells and macrophages may be essential to assess the progression of pregnancy and readiness of the cervix for vaginal delivery in anticipation of appropriate timing for labor and birth.30
This study further indicates that the presence of PR-A alone sustains reproductive functions related to pregnancy and cervix remodeling. This conclusion replicates our previous report in homozygous PRB−/− mice that PR-B is not required for cervix softening or ripening, labor, or birth.23 These findings do not exclude the possibility for post-transcriptional modification of PR-A. Enhanced histone acetylation has been associated with increased PR-A in the myometrium and labor.31 Moreover, PR isoforms are implicated in the regulation of uterine responsiveness to progesterone.32 Evidence by Amini et al suggests that phosphorylation of PR-A at serine-345 in the term human myometrium process blocks anti-inflammatory actions of progesterone mediated by PR-B.19 Though the cervix has yet to be studied, this conclusion raises the possibility that post-translational modification of the PR-A receptor may be essential for cervix ripening, as well as function of the myometrium for labor and birth in mice.
By contrast, findings in transgenic Srd5a1−/− mice, implicated the catabolism of progesterone, may be important for a local loss in progesterone efficacy and cervix ripening.33,34 However, evidence in these studies indicates that concentrations of progesterone remain elevated in both serum and cervix while the inactive 20α-hydroxyprogesterone catabolite is unchanged in transgenic mice and wild-type controls and before D17 of pregnancy, that is, when the shift to ripening occurs.12 In other respects, whether cervix remodeling may be influenced by binding of progesterone to the glucocorticoid receptor35 also seems unlikely. Use of a pure PR agonist or antagonist, respectively, delay or induce preterm birth through suppression or enhancement of the density of macrophages in the cervix.12,13 Moreover in these studies, increased presence of macrophages in the peripartum cervix was similarly associated with PR-antagonist-induced preterm birth and in controls that delivered at term.
Overall, these findings advance understanding of the mechanism for progesterone actions during pregnancy in support of a common mechanism for parturition that involve a stromal cell PR-A-mediated loss of progesterone efficacy to advance a macrophage-driven transition from a soft to ripening cervix and promote myometrial contractility for preterm and term birth. Understanding the genomic regulation of stromal cell-derived factors that regulate progesterone effects on stromal cells, macrophage activities, and extracellular cross-linked collagen may provide useful endpoints for diagnostic assessment of the ripening process. Opportunities for therapeutic interventions that alter the cross talk between stromal cells and macrophages may reduce the risk of either preterm birth when remodeling is premature or to mitigate fetal and maternal comorbidities associated with dystocia or when pregnancy extends past term.
Acknowledgments
The authors thank Talar Kechichian for Western blot analyses (UTMB) as well as Brigitte Vazquez for data analysis and manuscript preparation (LLU) and John Hough for histology services. With his passing in May 2017, this article is dedicated to Donald R. Chase, MD, Professor Emeritus of Pathology and Human Anatomy, past Director of the California Tumor Tissue Registry at Loma Linda University in gratitude for help with microscopy.
Authors’ Note: Anne C. Heuerman is primary author who wrote and contributed to revision of manuscript, performed studies, tissue processing, data analyses, graphics, and supervised Mr. Hollinger’s efforts. Trevor T. Hollinger is an undergraduate student who did image analyses and data acquisition. Ramkumar Menon provided data, lab performed and analyzed Western blots of cervix PR protein from PR-B receptor knockout mice, wrote relevant sections of manuscript, and revised drafts of this report.Sam Mesiano is a consultant on experimental design who provided progesterone receptor protein data, performed and analyzed Western blots of cervix progesterone receptor protein from CD-1 wild-type mice, wrote relevant sections of manuscript, and revised drafts of this report. Steven M. Yellon is a principal investigator who supervised project including design, implementation of experimental plans, data analysis, graphing, collaborative communications, and writing. Presentation: Abstracts of data were previously presented at the 2015 Reproductive Sciences meeting and the 2016 Biology of Reproduction meeting.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Grant support includes NIH HD954931 (SY) and NIH HD084532 (RM).
ORCID iD: Steven M. Yellon, PhD  https://orcid.org/0000-0003-4850-3590
https://orcid.org/0000-0003-4850-3590
References
- 1. Word RA, Li XH, Hnat M, Carrick K. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin Reprod Med. 2007;25(1):69–79. [DOI] [PubMed] [Google Scholar]
- 2. Yellon SM. Contributions to the dynamics of cervix remodeling prior to term and preterm birth. Biol Reprod. 2017;96(1):13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kjaergaard H, Olsen J, Ottesen B, Nyberg P, Dykes AK. Obstetric risk indicators for labour dystocia in nulliparous women: a multi-centre cohort study. BMC Pregnancy Childbirth. 2008;8:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blencowe H, Cousens S, Chou D, et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health. 2013;10(suppl 1):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Merlino AA, Welsh TN, Tan H, et al. Nuclear progesterone receptors in the human pregnancy myometrium: evidence that parturition involves functional progesterone withdrawal mediated by increased expression of progesterone receptor-A. J Clin Endocrinol Metab. 2007;92(5):1927–1933. [DOI] [PubMed] [Google Scholar]
- 6. Lockwood CJ, Stocco C, Murk W, Kayisli UA, Funai EF, Schatz F. Human labor is associated with reduced decidual cell expression of progesterone, but not glucocorticoid, receptors. J Clin Endocrinol Metab. 2010;95(5):2271–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Swaggart KA, Pavlicev M, Muglia LJ. Genomics of preterm birth. Cold Spring Harb Perspect Med. 2015;5(2):a023127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pieber D, Allport VC, Hills F, Johnson M, Bennett PR. Interactions between progesterone receptor isoforms in myometrial cells in human labour. Mol Hum Reprod. 2001;7(9):875–879. [DOI] [PubMed] [Google Scholar]
- 9. Hapangama D, Neilson JP. Mifepristone for induction of labour. Cochrane Database Syst Rev. 2009;(3):CD002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Denison FC, Riley SC, Elliott CL, Kelly RW, Calder AA, Critchley HO. The effect of mifepristone administration on leukocyte populations, matrix metalloproteinases and inflammatory mediators in the first trimester cervix. Mol Hum Reprod. 2000;6(6):541–548. [DOI] [PubMed] [Google Scholar]
- 11. Clark K, Ji H, Feltovich H, Janowski J, Carroll C, Chien EK. Mifepristone-induced cervical ripening: structural, biomechanical, and molecular events. Am J Obstet Gynecol. 2006;194(5):1391–1398. [DOI] [PubMed] [Google Scholar]
- 12. Kirby MA, Heuerman AC, Custer M, et al. Progesterone receptor-mediated actions regulate remodeling of the cervix in preparation for preterm parturition. Reprod Sci. 2016;23(11):1473–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yellon SM, Dobyns AE, Beck HL, Kurtzman JT, Garfield RE, Kirby MA. Loss of progesterone receptor-mediated actions induce preterm cellular and structural remodeling of the cervix and premature birth. PLoS One. 2013;8(12):e81340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yellon SM, Burns AE, See JL, Lechuga TJ, Kirby MA. Progesterone withdrawal promotes remodeling processes in the nonpregnant mouse cervix. Biol Reprod. 2009;81(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lucovnik M, Kuon RJ, Chambliss LR, et al. Progestin treatment for the prevention of preterm birth. Acta Obstet Gynecol Scand. 2011;90(10):1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yellon SM, Oldford EJ, Heuerman AC. Progesterone effects on cervix ripening and preterm birth in a murine model for progesterone withdrawal. Reprod Sci. 2018;25:183A. [Google Scholar]
- 17. Zakar T, Hertelendy F. Progesterone withdrawal: key to parturition. Am J Obstet Gynecol. 2007;196(4):289–296. [DOI] [PubMed] [Google Scholar]
- 18. Ackerman WET, Summerfield TL, Mesiano S, Schatz F, Lockwood CJ, Kniss DA. Agonist-dependent downregulation of progesterone receptors in human cervical stromal fibroblasts. Reprod Sci. 2016;23(1):112–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Amini P, Michniuk D, Kuo K, et al. Human parturition involves progesterone receptor-A phosphorylation at serine-345 in myometrial cells. Endocrinology. 2016;157(11):4434–4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mesiano S, Chan EC, Fitter JT, Kwek K, Yeo G, Smith R. Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. J Clin Endocrinol Metab. 2002;87(6):2924–2930. [DOI] [PubMed] [Google Scholar]
- 21. Lopez V, Wagner CK. Progestin receptor is transiently expressed perinatally in neurons of the rat isocortex. J Comp Neurol. 2009;512(1):124–139. [DOI] [PubMed] [Google Scholar]
- 22. Yellon SM, Heuerman AC, Kirby MA. Utility of optical density of picrosirius red birefringence for analysis of collagen. Integr Gynecol Obstet J. 2018;1(2):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yellon SM, Oshiro BT, Chhaya TY, et al. Remodeling of the cervix and parturition in mice lacking the progesterone receptor B isoform. Biol Reprod. 2011;85(3):498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Invitrogen. Normalization in western blotting to obtain relative quantitation. Thermo Fisher Scientific. 2018. https://assets.thermofisher.com/TFS-Assets/BID/Technical-Notes/ibright-normalization-western-blotting-relative-quantitation-technical-note.pdf. Accessed October 5, 2018.
- 25. Miller L, Hunt JS. Sex steroid hormones and macrophage function. Life Sci. 1996;59(1):1–14. [DOI] [PubMed] [Google Scholar]
- 26. Yoshida M, Sagawa N, Itoh H, et al. Prostaglandin F(2alpha), cytokines and cyclic mechanical stretch augment matrix metalloproteinase-1 secretion from cultured human uterine cervical fibroblast cells. Mol Hum Reprod. 2002;8(7):681–687. [DOI] [PubMed] [Google Scholar]
- 27. Keelan JA. Intrauterine inflammatory activation, functional progesterone withdrawal, and the timing of term and preterm birth. J Reprod Immunol. 2018;125:89–99. [DOI] [PubMed] [Google Scholar]
- 28. Miller L, Hunt JS. Regulation of TNF-alpha production in activated mouse macrophages by progesterone. J Immunol. 1998;160(10):5098–5104. [PubMed] [Google Scholar]
- 29. Collins JJ, Usip S, McCarson KE, Papka RE. Sensory nerves and neuropeptides in uterine cervical ripening. Peptides. 2002;23(1):167–183. [DOI] [PubMed] [Google Scholar]
- 30. Longo LD, Yellon S. Biological timekeeping during pregnancy and the role of circadian rhythms in parturition In: Kunzel W, Jensen A, eds. The Endocrine Control of the Fetus. Berlin, Germany: Springer-Verlag; 1988:173–191. [Google Scholar]
- 31. Chai SY, Smith R, Fitter JT, et al. Increased progesterone receptor A expression in labouring human myometrium is associated with decreased promoter occupancy by the histone demethylase JARID1A. Mol Hum Reprod. 2014;20(5):442–453. [DOI] [PubMed] [Google Scholar]
- 32. Patel B, Elguero S, Thakore S, Dahoud W, Bedaiwy M, Mesiano S. Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Hum Reprod Update. 2015;21(2):155–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mahendroo MS, Cala KM, Russell DW. 5 alpha-reduced androgens play a key role in murine parturition. Mol Endocrinol. 1996;10(4):380–392. [DOI] [PubMed] [Google Scholar]
- 34. Mahendroo MS, Porter A, Russell DW, Word RA. The parturition defect in steroid 5alpha-reductase type 1 knockout mice is due to impaired cervical ripening. Mol Endocrinol. 1999;13(6):981–992. [DOI] [PubMed] [Google Scholar]
- 35. Lei K, Chen L, Georgiou EX, et al. Progesterone acts via the nuclear glucocorticoid receptor to suppress IL-1beta-induced COX-2 expression in human term myometrial cells. PLoS One. 2012;7(11):e50167. [DOI] [PMC free article] [PubMed] [Google Scholar]


