Abstract
Objective:
To characterize the production and degradation of hyaluronic acid (HA) in menstrual endometrial epithelial cells (EECs) and endometrial stromal cells (ESCs) in women with and without endometriosis. To identify the presence of CD44, the primary receptor of HA, in menstrual EECs and ESCs in women with and without endometriosis.
Design:
In vitro study.
Setting:
Academic center.
Patient(s):
Deidentified patient samples from women with and without endometriosis.
Interventions:
EECs and ESCs were isolated from menstrual endometrial biopsies performed on women with (N = 9) and without (N = 11) endometriosis confirmed by laparoscopy.
Main Outcome Measure:
Real-time polymerase chain reaction, Western blot, and immunohistochemistry were used to assess hyaluronic acid synthase (HAS) isoforms 1, 2, and 3; hyaluronidase (HYAL) isoforms 1 and 2; and standard CD44. Student t test was used to analyze the results.
Results:
There was no significant difference in messenger RNA (mRNA) or protein expression of HAS2, HAS3, HYAL1, or HYAL2 in EECs or ESCs from women with or without endometriosis. HAS1 mRNA was variably detected, whereas HAS1 protein was similarly expressed in EECs and ESCs from women with and without endometriosis. Standard CD44 was expressed in both cell types, and expression did not differ in cells from women with or without endometriosis.
Conclusions:
The HA system is expressed in eutopic menstrual ESCs and EECs from women with and without endometriosis. There are no differences in expression in HA production or degradation enzymes in EECs or ESCs from women with and without endometriosis. Standard CD44 expression does not differ in eutopic menstrual endometrial cells from women with and without endometriosis.
Keywords: endometriosis, hyaluronic acid, CD44, endometrium
Introduction
Endometriosis is a common gynecologic disease affecting up to 10% of reproductive-age women.1 Women with endometriosis report numerous symptoms that decrease their quality of life and productivity.2 Endometriosis develops primarily by retrograde menstruation and implantation of endometrial cells on peritoneal or ovarian tissue. Increasing evidence suggests that cell adherence and inflammation are critically involved in the development of the early endometriotic lesion.3
Hyaluronic acid (HA) is a glycosaminoglycan that is important in cell-to-cell adhesion, migration, survival, and apoptosis.4 CD44, a transmembrane glycoprotein with multiple isoforms, is the primary receptor for HA.5 Standard CD44 (CD44s) is the smallest isoform that does not contain variants produced by alternative splicing of intervening exons. We have previously shown, using in vitro and in vivo models, that the HA/CD44 system is involved in the development of the early endometriotic lesion.6–8 Other studies have shown that inhibition of HA in a murine model decreases endometriotic lesion formation.6 Specifically, in menstrual endometrium, CD44 splice variants 6, 7, 8, and 9 are increased in women with endometriosis compared to women without endometriosis.14 However, other components of the HA/CD44 system have not been well characterized in menstrual endometrial cells that have the potential to develop into endometriosis.
Hyaluronic acid is produced by HA synthases (HAS) 1, 2, and 3. Most of the degradation of HA is by 2 major hyaluronidases (HYAL) 1 and 2. HA is an independent, unfavorable prognostic factor in gynecological malignancy.9 HAS3 was shown to be increased in endometrioid endometrial carcinoma compared to normal endometrium.9 Expression of HAS1 and HAS3 has also been associated with poor prognosis in breast cancer.10 Other studies showed that HYAL2 was more highly expressed than HYAL1 in endometrial cancer when compared to normal endometrium and that decreased HYAL1 expression is associated with early disease recurrence.11,12
In this study, we investigate the expression of HA/CD44 system in eutopic menstrual endometrial epithelial cells (EECs) and endometrial stromal cells (ESCs) from women with and without endometriosis to further assess the HA/CD44 system’s involvement in the development of the early endometriotic lesion.
Materials and Methods
This study was approved by the institutional review board of the University of Texas Health at San Antonio, and written consent was obtained from all patients for the tissue bank. Eutopic endometrial tissue samples from reproductive-age women with laparoscopy documenting the presence or absence of endometriosis were chosen from samples in our deidentified endometriosis tissue bank. Peritoneal mesothelial cells were obtained from a commercially available LP-9 cell line (NIH Aging Cell Repository, Coriell Institute for Medical Research, Camden, New Jersey) as described previously.13
Endometrial Cell Collection and Preparation
Menstrual endometrium was obtained from women by aspiration biopsy using a pipelle. Both EECs and ESCs were separated and cultured as described previously.8,14 Briefly, the endometrium was minced and enzymatically digested with collagenase type 3 and DNase. The EECs were separated from ESCs using gravity sedimentation. The ESC-rich supernatant was plated in a culture flask, allowed to adhere, and washed. The EEC-rich cell pellet was reconstituted and plated in flasks. The nonadherent EEC-rich supernatant was recovered and plated in a new flask. These cells were frozen and stored after passages 0 to 4.
RNA Extraction and Real-Time Polymerase Chain Reaction
Total RNA was extracted by Trizol (Trizol reagent, Sigma-Aldrich, St Louis, Missouri). Expression of HAS1, HAS2, HAS3, HYAL1, HYAL2, and CD44 was evaluated using real-time polymerase chain reaction (RT-PCR). RNA from endometrial cells was purified with Tri-Reagent using an RNA isolation kit (Direct-Zol RNA miniprep, Zymo Research, Irvine, California). SYBR green was used to detect amplification of the genes of interest. The relative expression of each sample was normalized to the housekeeping control gene, β-actin, and was calculated by 2ΔΔ method. Products were purified and sequenced to confirm identity.
Western Blot
Western blot was performed using standard technique.15 Protein was extracted from endometrial cells using radioimmunoprecipitation assay buffer. Protein concentration was determined using Bradford assay method. Equal amounts of protein (45 µg or more) from each sample were separated on a 10% (or 12% for HAS1) polyacrylamide gel and transferred to a nitrocellulose membrane. Specific binding was visualized using species-specific immunoglobulin G followed by enhanced chemiluminescent detection (ECL kit; Pierce Biotechnology, Illinois) and exposed to ECL X-ray film. Rabbit-specific anti-HAS1 (Ab198846; Abcam Biotechnology, Inc, Cambridge, Massachusetts), anti-HAS2 (H-60; Santa Cruz Biotechnology, Inc, Dallas, Texas), anti-HAS3 (H-69; Santa Cruz Biotechnology, Inc), anti-HYAL1 (HPA002112; Sigma-Aldrich), anti-HYAL2 (Ab68608; Abcam Biotechnology, Inc), anti-CD44s (BS-4916R; Bioss Antibodies, Woburn, Massachusetts), HA-binding protein (HAbp; HABP2; Abcam Biotechnology, Inc),11 and anti-actin (sc1616; Santa Cruz Biotechnology, Inc) were used. Expression of the housekeeping gene β-actin was used for normalization.16
Immunohistochemistry
Immunohistochemistry (IHC) was performed utilizing Vectastain Elite ABC kits (Vector Laboratories, Burlingame, CA) as described previously.17 Fresh ESCs and EECs were plated and fixed with formaldehyde. The cells were incubated with diluted normal blocking serum to minimize nonspecific staining. The primary rabbit antibodies as described earlier were diluted to a 1:100 concentration. Their respective biotinylated secondary antibodies were used, followed by the Vectastain Elite ABC reagent. After washing the cells, the peroxidase substrate was added until the development of the desired staining intensity. The cells were examined under the 20× light microscope. The IHC expression was described as present or not present. Mouse antihuman nuclei monoclonal antibody (MAB1281; Sigma-Aldrich) served as a positive control. Omission of the primary antibody was the negative control. Cytokeratin and vimentin were used to confirm that separated endometrial cells were either epithelial or stromal in nature.
Statistics
The relative messenger RNA (mRNA) expression of HAS1, HAS2, HAS3, HYAL1, HYAL2, and CD44 was compared in women with and without endometriosis by unpaired Student t test. A P value <.05 was considered statistically significant.
Results
Both ESCs and EECs from women with (N = 9) and without (N = 11) endometriosis expressed HAbp by Western blot analysis (Figure 1). Densitometric readings, performed with Image J (Version 1.49m) , demonstrated no difference in expression (Figure 1). Expression of HAbp was confirmed by IHC in individual EECs and ESCs. There was no difference in HA expression among fresh and passaged cells (data not shown).
Figure 1.
HA Western blot analysis. This representative Western blot figure showed HAbp is present in EECs and ESCs; densitometric analysis showed no difference in protein from multiple samples differences among cells from women with and without endometriosis. HA indicates hyaluronic acid; HAbp, HA-binding protein.
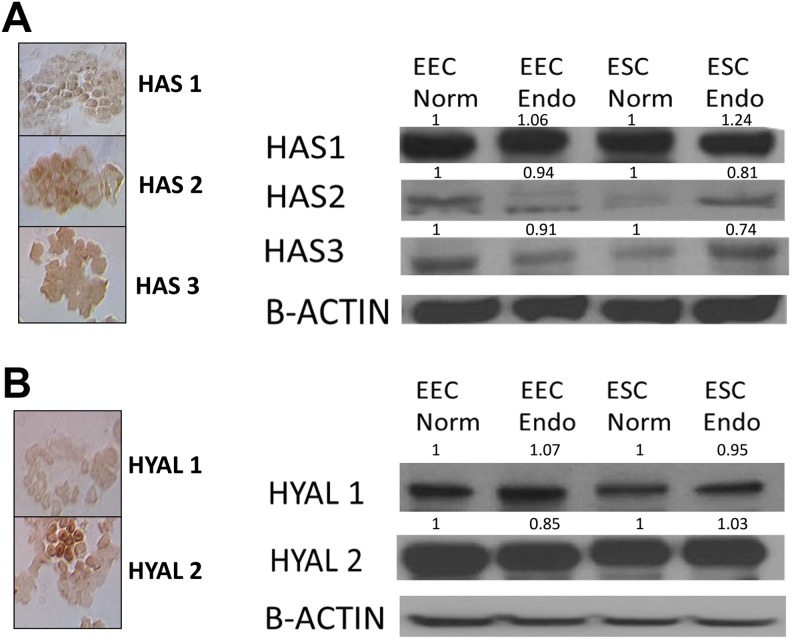
HAS1 mRNA was variably detected by RT-PCR with large cycle numbers required likely due to limited expression. HAS1 protein was detected by Western blot analysis in ESCs and EECs from women with and without endometriosis. Immunohistochemistry confirmed expression of HAS1. Sequencing and blasting the primers also confirmed expression of HAS1. There were no differences in HAS2 and HAS3 mRNA expression in EECs and ESCs from women with and without endometriosis, and this was confirmed by Western blot with densitometric analysis (Table 1 and Figure 2A).
Table 1.
RT-PCR Expression Normalized to Normal Endometrium.
| EEC With Endometriosis | EEC Without Endometriosis | ESC With Endometriosis | ESC Without Endometriosis | |
|---|---|---|---|---|
| HAS2 | 0.99 | 1 | 0.92 | 1 |
| HAS3 | 0.97 | 1 | 0.92 | 1 |
| HYAL1 | 1.08 | 1 | 0.97 | 1 |
| HYAL2 | 1.00 | 1 | 0.96 | 1 |
| CD44 | 1.03 | 1 | 0.92 | 1 |
Abbreviations: RT-PCR, real-time polymerase chain reaction; EECs, endometrial epithelial cells; ESCs, endometrial stromal cells; HAS, hyaluronic acid synthase; HYAL, hyaluronidase.
Figure 2.
A, Western blot and IHC localization of HAS1, HAS2, and HAS3. This representative Western blot figure showed all HAS enzymes were present in EECs and ESCs; densitometric analysis showed no difference in protein from multiple samples among cells from women with and without endometriosis. B, Western blot and IHC localization of HYAL1 and HYAL2. Both HYAL enzymes were present in EECs and ESCs. This representative Western blot figure showed all HAS enzymes were present in EECs and ESCs; densitometric analysis showed no difference in protein from multiple samples among cells from women with and without endometriosis. IHC indicates immunohistochemistry; HAS, hyaluronic acid synthase; HYAL, hyaluronidase; EECs, endometrial epithelial cells; ESCs, endometrial stromal cells.
The mRNA expression of HYAL1 and HYAL2 did not differ in EECs or ESCs from women with and without endometriosis (Table 1). HYAL1 and HYAL2 were confirmed at the protein level by Western blot analysis and IHC (Figure 2B). Densitometer readings confirmed no difference in expression (Figure 2B).
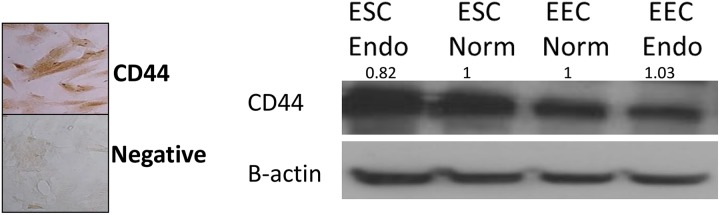
CD44s mRNA was similarly expressed in ESCs and EECs from women with and without endometriosis (Figure 3). CD44s protein expression was present in ESCs and EECS from women with and without endometriosis.
Figure 3.
Western blot and IHC localization of CD44. Standard CD44 was expressed in both EECs and ESCs. This representative Western blot figure showed no difference; densitometric analysis showed no difference in protein from multiple samples among cells from women with and without endometriosis. IHC indicates immunohistochemistry; EECs, endometrial epithelial cells; ESCs, endometrial stromal cells, Endo, endometriosis; norm, normal.
Discussion
Endometrial cells from women with endometriosis have increased adherence to peritoneal cells.14 The HA/CD44 system is involved in adherence of endometrial cells to peritoneum. The HA/CD44 system's involvement in adherence has been shown in multiple in vitro studies using inhibitors and in a murine knockout model.8,13,18 Specifically, for CD44, previous studies investigated CD44 variants in menstrual endometrium including CD44 variants and also at different aspects of the development of the early endometriotic lesion.6–8,14 To further elucidate the role of the HA system, we looked specifically at the synthesis and degradation of HA and its primary ligand, CD44s, in menstrual endometrium.
The HA system is expressed in eutopic menstrual ESCs and EECs from women with and without endometriosis. We found no significant differences in mRNA or protein expression of HAbp, HAS, HYAL, or CD44s among cells from women with and without endometriosis.
There are no differences in mRNA expression of HAS2, HAS3, HYAL1, or HYAL2 in ESCs and EECs from women with and without endometriosis. The HA-binding protein and HA enzymatic markers, HAS2, HAS3, HYAL1, and HYAL2, are similarly expressed in both endometrial cell types as assessed by Western blot analysis.
HAS2 and HAS3 have higher enzymatic activity than HAS1.19 In our study, HAS1 was variably present at the mRNA level. Nykopp et al reported that endometrial cancer transcripts of HAS1 were detected at such a low level that “reliable quantification was not consistently possible.”9 Other studies have reported difficulty assessing HAS1 even in highly expressed tissues due to variability in the sensitivity of methods and its dependence on cells type and other factors.19,20 Paiva et al reported HAS1 expression in endometrial cancer cells using a polyclonal antibody for HAS1, HAS2, and HAS3.11 The HAS1 expression also appears dependent on the presence of proinflammatory cytokines, HAS2 or HAS3, and possibly CD44.4,19 A BLASTN search revealed that the primers we used recognized 2 of the known variants of HAS1 (v1 and v2).
Six hyaluronidase-like genes have been reported with HYAL1 and HYAL2 being the major HYAL expressed in human somatic tissues.11,21 HYAL3 is present mainly in the bone marrow and testis and may be important for stem cell regeneration. In our study, both HYAL1 and HYAL2 were expressed at the mRNA and protein level. There was no difference in expression in EECs and ESCs from women with and without endometriosis.
Hyaluronic acid can have nonspecific binding. Therefore, HAbp was used to assess the presence of HA because it is more specific than HA antibodies.22,23 There are different HA biologically active molecules, based on molecular weight and cell type, which contribute to nonspecific binding of HA.24 We used a previously studied HAbp antibody to confirm HA expression in separated ESCs and EECs. 25
Menstrual endometrium for women with endometriosis has greater expression of the CD44 variants v6, v7, v8, and v9 compared to women without endometriosis.14 In this study, we used an antibody exclusively for CD44s which does not detect the splice variants of CD44 to investigate whether CD44s, not the variants, was different in menstrual endometrium in women with and without endometriosis. We found that CD44s is expressed in menstrual eutopic ESCs and EECs, and there is no difference in expression of CD44s mRNA or protein in menstrual ESCs and EECs from women with and without endometriosis. Further studies are needed to assess the role of CD44 variants’ regulation of the HA/CD44 system in the development of the early endometriotic lesion.26
Our study’s strength is the number of primary cell samples evaluated and the ability to separate EECs and ESCs. One limitation is lack of paired samples of eutopic menstrual and ectopic tissue. Expression was evaluated through RT-PCR, as this technique permits less subjective quantification than IHC. Immunohistochemistry was performed to confirm the presence of protein and cellular localization. These findings may offer further therapeutic options in the future through targeted therapy for endometriosis.
Conclusion
The HA system is present in menstrual eutopic separated endometrial cells in women with and without endometriosis. Although CD44 variants 6 to 9 are expressed more in menstrual endometrium in women with endometriosis compared to women without, we found no differences in the synthesis and degradation of HA and its primary ligand, CD44s. More prospective research is needed to elucidate the mechanism of endometrial HA system changes that may affect development of the early endometriotic lesion.
Supplemental Material
Supplemental_Table_1 for The Hyaluronic Acid System is Intact in Menstrual Endometrial Cells in Women With and Without Endometriosis by Jennifer F. Knudtson, Jessica E. McLaughlin, Marlen Tellez Santos, Peter A. Binkley, Rajeshwar R. Tekmal, and Robert S. Schenken in Reproductive Sciences
Footnotes
Authors’ Note: This work was presented at the 2016 Society for Reproductive Investigation meeting in Montréal, Canada. The material contained in the manuscript has not been published, has not been submitted, or is not being submitted elsewhere for publication. All authors are in agreement to submission of this manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Capsule: The hyaluronan system is expressed in eutopic menstrual separated endometrial cells from women with and without endometriosis. Expression does not differ in women with or without endometriosis.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant KL2 TR001118 (JFK). The project was also supported by the American Society for Reproductive Medicine grant (JFK), Long School of Medicine, Women's health pilot grant (RRT) and Endometriosis Foundation of America grant (JFK).
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Wheeler JM. Epidemiology of endometriosis-associated infertility. J Reprod Med. 1989;34(1):41–46. [PubMed] [Google Scholar]
- 2. Simoens S, Dunselman G, Dirksen C, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod (Oxford, England). 2012;27(5):1292–1299. [DOI] [PubMed] [Google Scholar]
- 3. Bulun SE. Endometriosis. New Engl J Med. 2009;360(3):268–279. [DOI] [PubMed] [Google Scholar]
- 4. Siiskonen H, Karna R, Hyttinen JM, Tammi RH, Tammi MI, Rilla K. Hyaluronan synthase 1 (HAS1) produces a cytokine-and glucose-inducible, CD44-dependent cell surface coat. Exp Cell Res. 2014;320(1):153–163. [DOI] [PubMed] [Google Scholar]
- 5. Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61(7):1303–1313. [DOI] [PubMed] [Google Scholar]
- 6. Hasegawa A, Yoshino O, Osuga Y, et al. Hyaluronic acid reagent suppressed endometriotic lesion formation in a mouse model. Fertil Steril. 2010;93(8):2757–2759. [DOI] [PubMed] [Google Scholar]
- 7. Knudtson JF, Tekmal RR, Santos MT, et al. Impaired development of early endometriotic lesions in CD44 knockout mice. Reprod Sci (Thousand Oaks, Calif.). 2016;23(1):87–91. [DOI] [PubMed] [Google Scholar]
- 8. Dechaud H, Witz CA, Montoya-Rodriguez IA, Degraffenreid LA, Schenken RS. Mesothelial cell-associated hyaluronic acid promotes adhesion of endometrial cells to mesothelium. Fertil Steril. 2001;76(5):1012–1018. [DOI] [PubMed] [Google Scholar]
- 9. Nykopp TK, Rilla K, Tammi MI, et al. Hyaluronan synthases (HAS1-3) and hyaluronidases (HYAL1-2) in the accumulation of hyaluronan in endometrioid endometrial carcinoma. BMC Cancer. 2010;10:512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Auvinen P, Rilla K, Tumelius R, et al. Hyaluronan synthases (HAS1-3) in stromal and malignant cells correlate with breast cancer grade and predict patient survival. Breast Cancer Res Treat. 2014;143(2):277–286. [DOI] [PubMed] [Google Scholar]
- 11. Paiva P, Van Damme MP, Tellbach M, Jones RL, Jobling T, Salamonsen LA. Expression patterns of hyaluronan, hyaluronan synthases and hyaluronidases indicate a role for hyaluronan in the progression of endometrial cancer. Gynecol Oncol. 2005;98(2):193–202. [DOI] [PubMed] [Google Scholar]
- 12. Nykopp TK, Pasonen-Seppanen S, Tammi MI, et al. Decreased hyaluronidase 1 expression is associated with early disease recurrence in human endometrial cancer. Gynecol Oncol. 2015;137(1):152–159. [DOI] [PubMed] [Google Scholar]
- 13. Rodgers AK, Nair A, Binkley PA, Tekmal R, Schenken RS. Inhibition of CD44 N- and O-linked glycosylation decreases endometrial cell lines attachment to peritoneal mesothelial cells. Fertil Steril. 2011;95(2):823–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Griffith JS, Liu YG, Tekmal RR, Binkley PA, Holden AE, Schenken RS. Menstrual endometrial cells from women with endometriosis demonstrate increased adherence to peritoneal cells and increased expression of CD44 splice variants. Fertil Steril. 2010;93(6):1745–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nair HB, Luthra R, Kirma N, et al. Induction of aromatase expression in cervical carcinomas: effects of endogenous estrogen on cervical cancer cell proliferation. Cancer Res. 2005;65(23):11164–11173. [DOI] [PubMed] [Google Scholar]
- 16. Tekmal RR, Liu YG, Nair HB, et al. Estrogen receptor alpha is required for mammary development and the induction of mammary hyperplasia and epigenetic alterations in the aromatase transgenic mice. J Steroid Biochem Mol Biol. 2005;95(1-5):9–15. [DOI] [PubMed] [Google Scholar]
- 17. Sun M, Ramirez M, Challis JR, Gibb W. Immunohistochemical localization of the glucocorticoid receptor in human fetal membranes and decidua at term and preterm delivery. J Endocrinol. 1996;149(2):243–248. [DOI] [PubMed] [Google Scholar]
- 18. Knudtson JF, Tekmal RR, Santos MT, et al. Impaired development of early endometriotic lesions in CD44 knockout mice. Reprod Sci (Thousand Oaks, Calif.). 2016;23(1):87–91. [DOI] [PubMed] [Google Scholar]
- 19. Siiskonen H, Oikari S, Pasonen-Seppanen S, Rilla K. Hyaluronan synthase 1: a mysterious enzyme with unexpected functions. Front Immunol. 2015;6:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rilla K, Oikari S, Jokela TA, et al. Hyaluronan synthase 1 (HAS1) requires higher cellular UDP-GlcNAc concentration than HAS2 and HAS3. J Biol Chem. 2013;288(8):5973–5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Csoka AB, Frost GI, Stern R. The six hyaluronidase-like genes in the human and mouse genomes. Matrix Biol. 2001;20(8):499–508. [DOI] [PubMed] [Google Scholar]
- 22. Ripellino JA, Klinger MM, Margolis RU, Margolis RK. The hyaluronic acid binding region as a specific probe for the localization of hyaluronic acid in tissue sections. Application to chick embryo and rat brain. J Histochem Cytochem. 1985;33(10):1060–1066. [DOI] [PubMed] [Google Scholar]
- 23. Shibutani T, Imai K, Kanazawa A, Iwayama Y. Use of hyaluronic acid binding protein for detection of hyaluronan in ligature-induced periodontitis tissue. J Periodontal Res. 1998;33(5):265–273. [DOI] [PubMed] [Google Scholar]
- 24. Cyphert JM, Trempus CS, Garantziotis S. Size matters: molecular weight specificity of hyaluronan effects in cell biology. Int J Cell Biol. 2015;2015:563818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shinohara T, Izawa T, Mino-Oka A, et al. Hyaluronan metabolism in overloaded temporomandibular joint. J Oral Rehabil. 2016;43(12):921–928. [DOI] [PubMed] [Google Scholar]
- 26. Koo YH, Na YJ, Ahn MY, Jeon HN, Yeom JI, Lee KS. Expression of CD44 in endometrial stromal cells from women with and without endometriosis and its effect on the adherence to peritoneal mesothelial cells. Obstet Gynecol Sci. 2013;56(2):102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental_Table_1 for The Hyaluronic Acid System is Intact in Menstrual Endometrial Cells in Women With and Without Endometriosis by Jennifer F. Knudtson, Jessica E. McLaughlin, Marlen Tellez Santos, Peter A. Binkley, Rajeshwar R. Tekmal, and Robert S. Schenken in Reproductive Sciences