Abstract
Background
Achieving balanced gaps is a key surgical goal in total knee arthroplasty, yet most methods rely on subjective surgeon feel and experience to assess and achieve knee balance intraoperatively. Our objective was to evaluate the ability to quantitatively plan and achieve a balanced knee throughout the range of motion using robotic-assisted instrumentation in a tibia-first, gap-balancing technique.
Methods
A robotic-assisted, gap-balancing technique was used in 121 consecutive knees. After resection of the proximal tibia, a computer-controlled tensioning device was inserted into the knee joint and the pre-femoral-resection knee gaps were acquired dynamically throughout flexion under controlled load. Predicted gap profiles were used to plan the femoral implant by adjusting the implant alignment and position within certain boundaries to achieve a balanced knee throughout the range of flexion. Femoral cuts were then made according to this plan using a miniature robotic-assisted cutting guide. The tensioning device used to measure the pre-femoral-resection gaps was then reinserted into the joint to quantify the final gap balance under known tension. The final gap profiles were then compared with the predictive gap plans.
Results
The overall root mean square error between the predicted and achieved gaps was 1.3 mm and 1.5 mm for the medial and lateral sides, respectively. Use of robotic assistance resulted in over 90% of knees having mediolateral balance within 2 mm across the flexion range. Gaps at 0° flexion were 2 mm smaller than the gaps at 90°. This difference decreased to less than 1 mm when comparing the tibiofemoral gaps at 10°, 45°, and 90°.
Conclusions
Imageless, robotic-assisted total knee arthroplasty accurately predicts postoperative gaps before femoral resections. This allows surgeons to virtually plan femoral implant alignment and optimize gap balance throughout the range of motion. The accurate prediction of gaps throughout the arc of motion combined with precise, robotically assisted femoral resection produces accurate postoperative ligament balance consistently.
Keywords: Soft-tissue balance, Total knee arthroplasty, Robotic-assisted, Prediction, Kinematics, Gaps
Introduction
Soft-tissue balance is an important goal toward ensuring the short- and long-term success of total knee arthroplasty (TKA). Proper soft-tissue balance, as traditionally defined by equal and symmetric flexion and extension gaps, has been shown to decrease postoperative instability and stiffness [1], [2], [3], [4], a leading cause of revision [5], [6], and significantly improve patient-reported outcome scores such as Knee Society pain and function scores, Western Ontario and McMaster’s Universities Arthritis index, and patient satisfaction [7], [8]. To achieve postoperative balance, surgeons can perform soft-tissue releases, adjust implant alignment, or use a combination thereof [2], [3], [9], [10]. These methods typically rely on the surgeon’s experience and subjective feel and are not measured by objective tools; thus, they can produce variable and inconsistent results [9], [11].
Instrumentation has been developed to objectively assess soft-tissue balance [9], including manual distraction devices [12], [13], [14], [15] and electronic sensors [16], [17], [18]. These devices provide intraoperative information to assist with planning implant alignment to produce equal or symmetric gaps or quantify postresection balance measurements to aid in soft-tissue releases. While these devices have provided more quantitative analysis of joint balance, adoption in surgery has been slow because of their limited capabilities. Some devices can only measure joint balance in extension and full flexion or they do not allow for measurement with the patella reduced. They can be used before or after femoral resection, but not both. The instrumented devices provide the surgeon with information about joint loads after all bone resections have been completed. They do not predict ligament tension and ligament balance before femoral bone resection nor have the ability to predict ligament balance throughout the full range of motion.
Robotic-assisted instrumentation has been recently introduced that integrates ligament tension with bone resection planning throughout the range of flexion which can facilitate the ability to achieve a well-balanced and aligned knee [19], [20]. Moreover, final ligament balance can be quantified objectively under computer-controlled ligament tension [20]. However, few studies have detailed the accuracy and reproducibility of soft-tissue balance that can be predicted and achieved using such approaches [9], [21], [22], [23]. The primary objective of this study, therefore, was to quantify the accuracy of predicting postoperative gap balance in imageless, robotic-assisted surgery. Secondary objectives were to measure the final medial-to-lateral and flexion-to-extension balance achieved and to evaluate the repeatability of gap assessment under controlled tensioning.
Material and methods
Patients
After institutional review board approval, a consecutive series of patients receiving gap-balancing robotic-assisted TKA by 4 experienced surgeons across 4 institutions were retrospectively reviewed. The study cohort included 129 patients operated on during the first 2 months after introduction of the robotic tensioning system [20] and after a learning period of approximately 3-5 procedures per surgeon. In a separate subset of 24 consecutive patients, the postoperative knee gap measurements were repeated 3 times to evaluate the repeatability of the gap measurement technique using the robotic tensioner. Of the 133 knees reviewed in 129 patients, 12 knees were excluded due to missing computer system log files which resulted in a total of 121 knees in 117 patients included in the study (52 male, mean age: 68 ± 6 years, body mass index: 29 ± 5). Of the 121 knees, 57 were contributed by the first surgeon, 41 cases by the second surgeon, 16 cases by the third surgeon, and 7 cases by the fourth surgeon. The knees included in this study had a preoperative alignment that ranged from 20° varus to 15° valgus and from 9° hyperextension to 22° of flexion contracture.
Surgical technique
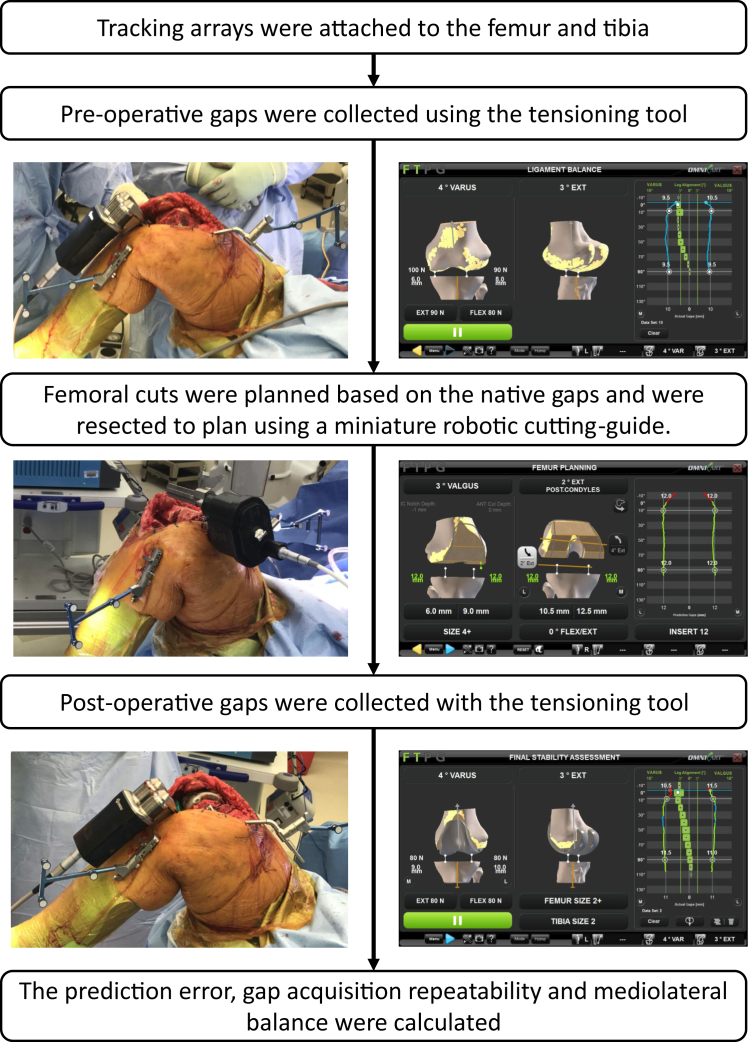
A standard medial parapatellar approach was used to expose the knee joint. An imageless robotic-assisted TKA system (OMNIBotics, Corin, Raynham, MA) was employed using a tibial-cut first gap-balancing technique for all cases (Fig. 1) [20], [24]. Tracking arrays were rigidly attached to the femur and tibia. Three-dimensional models of the femur and tibia were created using a bone-morphing algorithm that deforms a statistical knee shape model to match the patient’s femoral and tibial bone surfaces without preoperative imaging [25]. Preresection varus-valgus and flexion-extension knee kinematics were evaluated and recorded with the tracking system. The tibia was resected perpendicular to the mechanical axis and with 2°-4° of posterior slope, and the posterior cruciate ligament was excised. All knees were within 1.5° of their planned slope; however, the software utilized the validated cut instead of the planned cut to reduce the error caused by not achieving the correct cuts. A miniature robotic-assisted ligament-tensioning tool (BalanceBot, Corin, Raynham, MA) was inserted into the joint space, and the patella was reduced [20]. The tensioning tool was set to apply equal load on the medial and lateral compartments that ranged between 70N and 100N using the application software. The force used for each patient was determined by the surgeon, based on the patient’s weight and soft-tissue properties. The applied load was individualized for a patient’s particular phenotype by quantitatively evaluating the degree of residual laxity at the selected load. This is accomplished by applying a specific amount of pretension to the ligaments and then locking the tensioner at that height. The surgeon then performed a varus-valgus stress test while assessing the degree of medial and lateral condylar liftoff using the gap values represented on the screen. The “prefemoral resection” gap profiles were captured dynamically as the surgeon ranged the knee from flexion to extension and as the tensioner applied equal mediolateral load to the collateral ligaments throughout the range of motion. A virtual gap algorithm computed and displayed the predicted gap profiles throughout the range of flexion based on the prefemoral resection gap profiles generated by the tensioning device and the planned virtual femoral implant position and size. Using the virtual gap algorithm, the surgeon adjusted the femoral implant alignment in all planes such that the predicted gap profiles were as equal and symmetric as possible between 10° and 90° of flexion while maintaining certain implant anatomic alignment limits (3° varus to 2° valgus relative to the mechanical axis; 0-4° flexion relative to the mechanical axis; 0-8° of external rotation relative to the posterior condyles). If balance gaps could not be achieved using these values, then the software proposed an alignment within these parameters that minimized the medial-to-lateral gap difference as well as the flexion-to-extension gap difference. The surgeon then had the option to proceed or perform releases to address the gap difference. The planned femoral cuts were preformed using a miniature robotic-assisted cutting guide that attaches to the medial side of the distal femur using the same 2 pins that are used to affix the femoral tracker. The robotic cutting-guide automatically positions a single saw guide for all 5 femoral resections and has a reported resection accuracy within ±1 mm and ±1° [26]. Medial and lateral inserts matching the femoral implant were attached to the robotic tensioner to mimic the tibial insert. With the femoral trial component implanted (Apex Knee System, Corin, Raynham, MA), the robotic tensioner was reinserted into the joint. The postoperative gap profiles were collected throughout the range of motion with the patella reduced using the same loading profile as used for the prefemoral resection gap acquisition. The postoperative implant gap was defined as the distance between the tibial cut surface and the closest point on the medial and lateral surface of the femoral trial (Fig. 2C).
Figure 1.
Diagram of the surgical and data capture workflow.
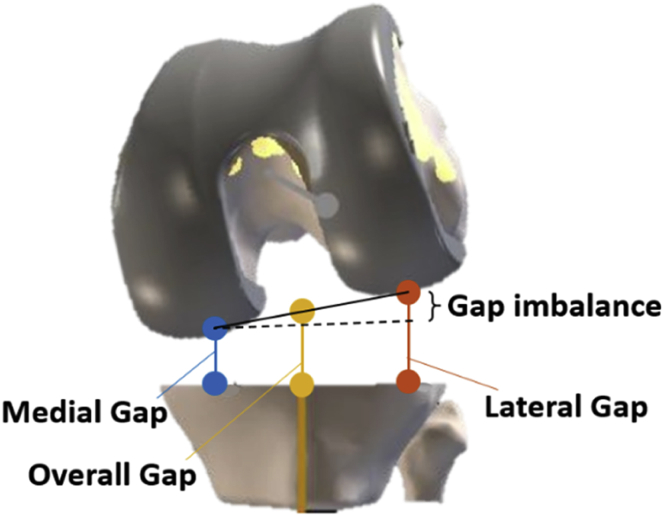
Figure 2.
Calculation of the medial (blue), lateral (red), and overall (yellow) tibiofemoral gaps.
The root mean square (RMS) error between the predicted and measured postoperative gaps was calculated at every 10° of flexion and across the range of flexion for the medial and lateral sides. Paired t-tests were used to identify significant differences between the predicted and measured gaps.
The percentage of knees with mediolateral balanced among 0-1 mm, 1-2 mm, and >2 mm was calculated at 0, 10, 45, and 90°. The medial, lateral, and overall gap (Fig. 2) at 0, 10, and 45° was compared with that at 90° to determine the gap variation between extension, mid-flexion, and flexion. Paired t-tests were used to identify significant differences between the gap at 0°, 10°, 45°, and 90° of flexion.
The repeatability in acquiring the postoperative gaps was assessed by performing 3 repeated gap measurements throughout the range of motion in 24 knees. The mean gap and the variation from the mean were calculated for the 3 iterations at each flexion angle for each subject. The median, first quarter (q1), third quarter (q3), minimum, maximum, and outlier variation was calculated for all the subjects from 0° to 90° and displayed in a box plot. Data points are plotted as outliers if they are greater or less than q3 ± 2.7σ × (q3−q1).
Results
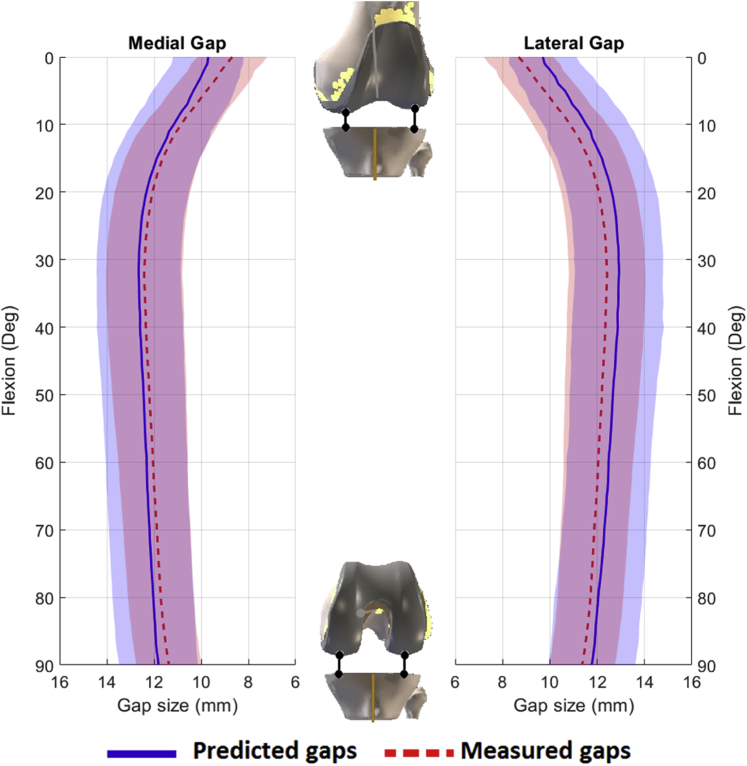
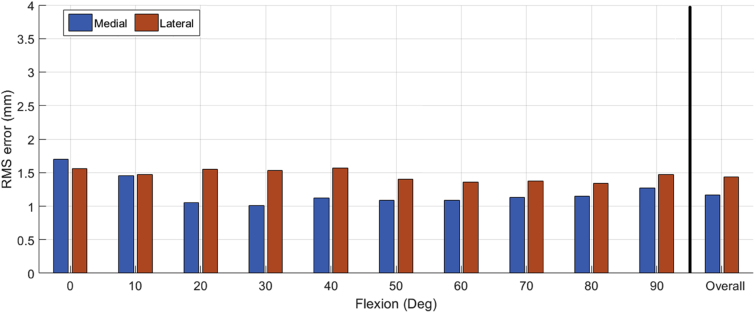
Femoral alignment was planned to produce equal and symmetric gaps at 10° and 90° of flexion which resulted in a predicted gap profile that was approximately 2 mm tighter in full extension (0°) and 0.5-1 mm more lax at approximately 30° of flexion (Fig. 3). The measured postoperative gap profiles were similar in shape and magnitude to the predicted gaps with tighter gaps in extension and a slight increase in laxity at 30° mid-flexion. The difference between the predicted and measured postoperative gap was not significantly different throughout flexion (P = .8). The overall RMS error for the medial and lateral sides was 1.3 mm and 1.5 mm, respectively (Fig. 4). The maximum RMS error was 1.6 mm medially (at 0° flexion) and 1.5 mm laterally (at 20° flexion), Figure 4.
Figure 3.
Average predicted (blue) and measured (red) postoperative tibiofemoral gaps for the medial and lateral gaps. Shaded areas represent ± one standard deviation.
Figure 4.
Root mean square (RMS) error between the predicted and measured tibiofemoral gaps across the flexion range for the medial (blue) and lateral (orange) gaps. The overall RMS error for the medial and lateral gaps is shown to the right.
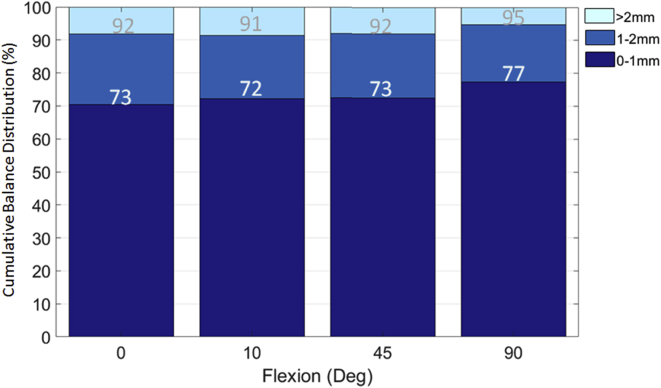
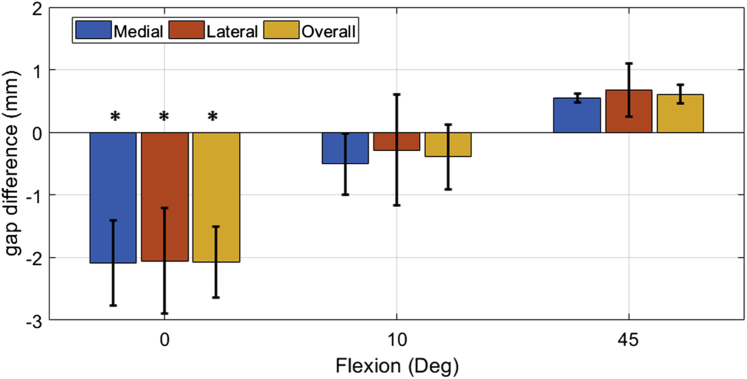
The percentage of knees balanced to within 1 mm mediolaterally ranged from 72%-77%, with the lowest percentage occurring at 10° of flexion and the highest percentage at 90° of flexion (Fig. 5). Over 90% of knees were balanced to within 2 mm mediolaterally across the flexion range (Fig. 5) with maximum imbalance of 3.1, 3.2, 3.0, and 3.3 mm for 0°, 10°, 45°, and 90°, respectively. For flexion-extension balance, the mean gap difference between 90° of flexion and 10° and 45° of flexion was within ±1 mm for the medial, the lateral, and the overall gap (Fig. 6). This resulted in over 90% of cases having a balanced flexion-to-extension gap (10° vs 90°) within 1 mm. The extension gaps at 0° were approximately 2 mm tighter than the flexion gaps at 90° for the medial, lateral, and overall gap (Fig. 6). Gap differences across flexion were only significant at 0° and 90° (P < .05).
Figure 5.
Percentage of knees with medial-to-lateral balance among 0-1 mm, 1-2 mm, and >2 mm at 0°, 10°, 45°, and 90°.
Figure 6.
Difference between the flexion gaps at 90° and the gaps at 0°, 10°, and 45° for the medial (blue), lateral (orange), and overall (yellow) gaps. *Statistically significant difference (P < .05).
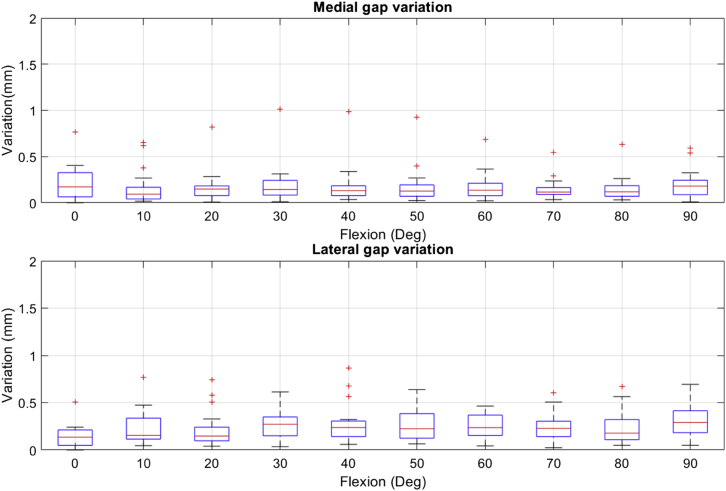
Gap assessment repeatability under controlled ligament tension was within 0.5 mm across all 4 surgeons when excluding outliers (Fig. 7). Including outliers, the maximum variation in gap assessment of an individual subject was 1 mm and 0.9 mm for the medial and lateral gaps, respectively.
Figure 7.
Medial and lateral gap measurement repeatability showing the range of deviation, 1st and 3rd quarter data spread and outliers for the 24 cases.
Discussion
This study investigated the ability of a robotic-assisted method that uses a computer-controlled ligament-tensioning tool to predict and achieve postoperative gap balance. The tibiofemoral gaps were planned to be as equal as possible at 10° and 90° of flexion. This technique produced predicted gaps with similar patterns to those measured in the native knee with tighter gaps in full extension and looser gaps in mid-flexion [20], [27], [28], [29]. The postoperative gaps achieved were similar to the predicted gaps throughout the flexion range. The results support the hypothesis that postoperative knee gaps can be accurately predicted and achieved with low RMS error across the flexion range when using a robotic-assisted, gap-tensioning and planning algorithm in conjunction with a precise, robotic bone resection guide. Femoral cuts within 1 to 1.5 mm of the planned cuts were accepted during surgery based on surgeon discretion. The prediction algorithm assumes that all femoral cuts are made to the plan, and the predicted gaps are not adjusted when the femoral cuts differ from the planned cuts. Therefore, some of the minor differences between the predicted and achieved tibiofemoral gaps could be due to small variations in the femoral cuts relative to the plan.
Postoperative balance was planned to have 0 mm residual gap between 10° and 90° of flexion to match the prefemoral resection gap laxity and to reduce mid-flexion laxity caused by planning for equal gaps at 0° and 90° [20]. Studies have shown that knee laxity decreases dramatically from 20° to 0° degree of flexion due to the screw-home mechanism and the engagement of the posterior capsule [20], [27], [30], [31], [32]. The screw-home mechanism and planning for equal and symmetric gaps between 10° and 90° of flexion could explain the 2 mm difference in postoperative gap between 0° and 90° flexion for the medial, lateral, and overall gap (Fig. 6).
Using a predictive gap algorithm and a robotic-assisted cutting guide resulted in a high percentage of knees that were balanced to within 1 mm from medial to lateral. In some cases, the femoral component was rotated relative to the posterior condylar axis to account for the mismatch between the medial and lateral gaps in flexion. The femoral rotation could have caused uneven tension in the posterior capsule at full extension which can influence the mediolateral gap balance [33]. Another factor that could have affected the medial-to-lateral gap balance is the tibial implant position. Variation in the tibial implant rotation may affect the gaps due to the relatively conforming tibiofemoral design. In this study, the percentage of cases within 2 mm of mediolateral balance was appreciably higher than that reported in another study using manual instruments (90% compared to 51%) [9]. Griffin et al [34] reported similar mediolateral balance to the present study using manual instrumentation; however, that study used subjective methods for applying tension to assess balance, employed less accurate means for measuring gaps, and only reported balance in flexion and extension. In the present study, in which the knee was planned to have a zero-residual gap, the 0.3 mm average difference between the 90° flexion and 10° extension gaps was smaller than the 2.5 mm average difference reported in the literature [35]. In addition, 90% of cases in this study had flexion-to-extension gaps balanced to within 1 mm compared with another study which reported balance in only 47-57% of cases using a manual technique [34]. This suggests that an imageless, robotic-assisted system can achieve postoperative mediolateral and flexion-extension balance successfully which may produce more consistent surgical outcomes.
The accuracy of the predicted and final gap measurements depends on the system reproducibility. Gap measurements using the robotic tensioner were highly repeatable between acquisitions. The variation calculated between acquisitions was within the optical tracking system accuracy. Therefore, these variations are likely due to the noise in the system. The small intrauser variability indicates a high reliability of the gap measurements and supports the use of this type of data to facilitate intraoperative decision-making in achieving a stable and well-balanced joint.
The technique used in this study allows for quantitative analysis of the joint state while current modern-day techniques rely on qualitative and subjective analysis of the joint state. Modern methods rely on either surgeon feel to assess the joint and to determine the femoral cuts required to produce equal gaps or on manual gap-balancing devices that are used to plan femoral cuts sequentially without visualizing the gap profiles throughout the entire range of motion before executing resections. The technique employed in this study applies equal and precise loads to the medial and lateral side. The information is then used to determine the femoral cuts that will produce equal postoperative gaps. The method also allows for quantitative assessment of postoperative gap results to ensure that medial-lateral and flexion-extension gap balance was achieved.
Potential study bias and conflict of interest is minimal as the study utilized quantitative measurements from a robotic device and did not rely on any subjective data that could have been influenced by bias or conflict of interest. However, the study has several limitations. There was no control cohort using a manual TKA technique. Therefore, we could not directly compare the intraoperative balance achieved between the manual and robotic-assisted techniques for the same surgeon. While the data were compared with the gap-balance data from the literature, a comparison between the 2 techniques for the same user would provide a better understating of the advantages of robotic-assisted TKA. This study measured the knee joint balance obtained intraoperatively but did not measure any patient-reported outcomes. To better interpret the results, future studies are needed to determine the effect of balance on clinical outcomes. An ongoing study is analyzing the correlations between gap balancing and improvements in knee joint function, quality of life, and patient satisfaction.
Conclusions
The present study has shown that using an imageless, robotic-assisted system with ligament tensioning and balancing tools can accurately predict TKA postoperative joint gaps before making any femoral cuts. The ability to predict postoperative gaps allows surgeons to better optimize their surgical plan and minimize soft-tissue releases. Using robotic-assistance in TKA resulted in over 90% of cases having a mediolateral balance and flexion-to-extension balance within 2 mm. In addition, the gap profiles presented in this study help increase our understanding of the gap patterns that occur in both conventional and computer-assisted gap-balancing techniques. Future research is needed to assess the variation in achieving balanced gaps using the robotic-assisted device in direct comparison to conventional manual devices, as well as understanding the effect of improvements in soft-tissue balancing and its reproducibility on knee function, patient satisfaction, and quality of life.
Acknowledgments
The authors would like to acknowledge Corin Ltd. for their support in this research.
Footnotes
One or more of the authors of this paper have disclosed potential or pertinent conflicts of interest, which may include receipt of payment, either direct or indirect, institutional support, or association with an entity in the biomedical field whichmay be perceived to have potential conflict of interest with this work. For full disclosure statements refer to https://doi.org/10.1016/j.artd.2019.07.003.
Appendix A. Supplementary data
References
- 1.Insall J.N., Binazzi R., Soudry M., Mestriner L.A. Total knee arthroplasty. Clin Orthop Relat Res. 1985;(192):13. [PubMed] [Google Scholar]
- 2.Whiteside L.A., Saeki K., Mihalko W.M. Functional medical ligament balancing in total knee arthroplasty. Clin Orthop Relat Res. 2000;(380):45. doi: 10.1097/00003086-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Mihalko W.M., Whiteside L.A., Krackow K.A. Comparison of ligament-balancing techniques during total knee arthroplasty. J Bone Joint Surg Am. 2003;85-A(Suppl 4):132. doi: 10.2106/00004623-200300004-00018. [DOI] [PubMed] [Google Scholar]
- 4.Meloni M.C., Hoedemaeker R.W., Violante B., Mazzola C. Soft tissue balancing in total knee arthroplasty. Joints. 2014;2(1):37. [PMC free article] [PubMed] [Google Scholar]
- 5.Fehring T.K., Odum S., Griffin W.L., Mason J.B., Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;(392):315. doi: 10.1097/00003086-200111000-00041. [DOI] [PubMed] [Google Scholar]
- 6.Postler A., Lutzner C., Beyer F., Tille E., Lutzner J. Analysis of total knee arthroplasty revision causes. BMC Musculoskelet Disord. 2018;19(1):55. doi: 10.1186/s12891-018-1977-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gustke K.A., Golladay G.J., Roche M.W., Elson L.C., Anderson C.R. Primary TKA patients with quantifiably balanced soft-tissue achieve significant clinical gains sooner than unbalanced patients. Adv Orthop. 2014;2014:628695. doi: 10.1155/2014/628695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gustke K.A., Golladay G.J., Roche M.W., Jerry G.J., Elson L.C., Anderson C.R. Increased satisfaction after total knee replacement using sensor-guided technology. Bone Joint J. 2014;96-B(10):1333. doi: 10.1302/0301-620X.96B10.34068. [DOI] [PubMed] [Google Scholar]
- 9.Joseph J., Simpson P.M., Whitehouse S.L., English H.W., Donnelly W.J. The use of navigation to achieve soft tissue balance in total knee arthroplasty - a randomised clinical study. Knee. 2013;20(6):401. doi: 10.1016/j.knee.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Daines B.K., Dennis D.A. Gap balancing vs. measured resection technique in total knee arthroplasty. Clin Orthop Surg. 2014;6(1):1. doi: 10.4055/cios.2014.6.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elmallah R.K., Mistry J.B., Cherian J.J. Can we really “feel” a balanced total knee arthroplasty? J Arthroplasty. 2016;31(9 Suppl):102. doi: 10.1016/j.arth.2016.03.054. [DOI] [PubMed] [Google Scholar]
- 12.Asano H., Hoshino A., Wilton T.J. Soft-tissue tension total knee arthroplasty. J Arthroplasty. 2004;19(5):558. doi: 10.1016/j.arth.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Bathis H., Perlick L., Tingart M., Luring C., Perlick C., Grifka J. Flexion gap configuration in total knee arthroplasty following high tibial osteotomy. Int Orthop. 2004;28(6):366. doi: 10.1007/s00264-004-0595-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsumoto T., Muratsu H., Tsumura N. Joint gap kinematics in posterior-stabilized total knee arthroplasty measured by a new tensor with the navigation system. J Biomech Eng. 2006;128(6):867. doi: 10.1115/1.2354201. [DOI] [PubMed] [Google Scholar]
- 15.Heesterbeek P.J.C., Haffner N., Wymenga A.B., Stifter J., Ritschl P. Patient-related factors influence stiffness of the soft tissue complex during intraoperative gap balancing in cruciate-retaining total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2017;25(9):2760. doi: 10.1007/s00167-015-3694-5. [DOI] [PubMed] [Google Scholar]
- 16.Roche M., Elson L., Anderson C. Dynamic soft tissue balancing in total knee arthroplasty. Orthop Clin North Am. 2014;45(2):157. doi: 10.1016/j.ocl.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Morris B.A., D'Lima D.D., Slamin J. e-Knee: evolution of the electronic knee prosthesis. Telemetry technology development. J Bone Joint Surg Am. 2001;83-A(Suppl 2 Pt 1):62. doi: 10.2106/00004623-200100021-00013. [DOI] [PubMed] [Google Scholar]
- 18.Camarata D.A. Soft tissue balance in total knee arthroplasty with a force sensor. Orthop Clin North Am. 2014;45(2):175. doi: 10.1016/j.ocl.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Werner S.D., Stonestreet M., Jacofsky D.J. Makoplasty and the accuracy and efficacy of robotic-assisted arthroplasty. Surg Technol Int. 2014;24:302. [PubMed] [Google Scholar]
- 20.Shalhoub S., Moschetti W.E., Dabuzhsky L., Jevsevar D.S., Keggi J.M., Plaskos C. Laxity profiles in the native and replaced knee-application to robotic-assisted gap-balancing total knee arthroplasty. J Arthroplasty. 2018;33(9):3043. doi: 10.1016/j.arth.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Winemaker M.J. Perfect balance in total knee arthroplasty: the elusive compromise. J Arthroplasty. 2002;17(1):2. doi: 10.1054/arth.2002.29321. [DOI] [PubMed] [Google Scholar]
- 22.Viskontas D.G., Skrinskas T.V., Johnson J.A., King G.J., Winemaker M.J., Chess D.G. Computer-assisted gap equalization in total knee arthroplasty. J Arthroplasty. 2007;22(3):334. doi: 10.1016/j.arth.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 23.Lee D.H., Park J.H., Song D.I., Padhy D., Jeong W.K., Han S.B. Accuracy of soft tissue balancing in TKA: comparison between navigation-assisted gap balancing and conventional measured resection. Knee Surg Sports Traumatol Arthrosc. 2010;18(3):381. doi: 10.1007/s00167-009-0983-x. [DOI] [PubMed] [Google Scholar]
- 24.Clark T.C., Schmidt F.H. Robot-assisted navigation versus computer-assisted navigation in primary total knee arthroplasty: efficiency and accuracy. ISRN Orthop. 2013;2013:794827. doi: 10.1155/2013/794827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perrin N., Stindel E., Roux C. BoneMorphing versus freehand localization of anatomical landmarks: consequences for the reproducibility of implant positioning in total knee arthroplasty. Comput Aided Surg. 2005;10(5-6):301. doi: 10.3109/10929080500389845. [DOI] [PubMed] [Google Scholar]
- 26.Koulalis D., O'Loughlin P.F., Plaskos C., Kendoff D., Cross M.B., Pearle A.D. Sequential versus automated cutting guides in computer-assisted total knee arthroplasty. Knee. 2011;18(6):436. doi: 10.1016/j.knee.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Roth J.D., Howell S.M., Hull M.L. Native knee laxities at 0 degrees, 45 degrees , and 90 degrees of flexion and their relationship to the goal of the gap-balancing alignment method of total knee arthroplasty. J Bone Joint Surg Am. 2015;97(20):1678. doi: 10.2106/JBJS.N.01256. [DOI] [PubMed] [Google Scholar]
- 28.Mayman D., Plaskos C., Kendoff D., Wernecke G., Pearle A.D., Laskin R. Ligament tension in the ACL-deficient knee: assessment of medial and lateral gaps. Clin Orthop Relat Res. 2009;467(6):1621. doi: 10.1007/s11999-009-0748-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Damme G., Defoort K., Ducoulombier Y., Van Glabbeek F., Bellemans J., Victor J. What should the surgeon aim for when performing computer-assisted total knee arthroplasty? J Bone Joint Surg Am. 2005;87(Suppl 2):52. doi: 10.2106/JBJS.E.00447. [DOI] [PubMed] [Google Scholar]
- 30.Blankevoort L., Huiskes R., de Lange A. The envelope of passive knee joint motion. J Biomech. 1988;21(9):705. doi: 10.1016/0021-9290(88)90280-1. [DOI] [PubMed] [Google Scholar]
- 31.Verstraete M.A., Meere P.A., Salvadore G., Victor J., Walker P.S. Contact forces in the tibiofemoral joint from soft tissue tensions: implications to soft tissue balancing in total knee arthroplasty. J Biomech. 2017;58:195. doi: 10.1016/j.jbiomech.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 32.Minoda Y., Nakagawa S., Sugama R. Intraoperative assessment of midflexion laxity in total knee prosthesis. Knee. 2014;21(4):810. doi: 10.1016/j.knee.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 33.Mitsuyasu H., Matsuda S., Fukagawa S. Enlarged post-operative posterior condyle tightens extension gap in total knee arthroplasty. J Bone Joint Surg Br. 2011;93(9):1210. doi: 10.1302/0301-620X.93B9.25822. [DOI] [PubMed] [Google Scholar]
- 34.Griffin F.M., Insall J.N., Scuderi G.R. Accuracy of soft tissue balancing in total knee arthroplasty. J Arthroplasty. 2000;15(8):970. doi: 10.1054/arth.2000.6503. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto T., Muratsu H., Kawakami Y. Soft-tissue balancing in total knee arthroplasty: cruciate-retaining versus posterior-stabilised, and measured-resection versus gap technique. Int Orthop. 2014;38(3):531. doi: 10.1007/s00264-013-2133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.