Abstract
Background:
Our group has shown that in vivo tau brain binding patterns from FDDNP-PET scans in retired professional football players with suspected chronic traumatic encephalopathy differ from those of tau and amyloid aggregate binding observed in Alzheimer’s disease (AD) patients and cognitively-intact controls.
Objective:
To compare these findings with those from military personnel with histories of mild traumatic brain injury (mTBI).
Methods:
FDDNP-PET brain scans were compared among 7 military personnel and 15 retired players with mTBI histories and cognitive and/or mood symptoms, 24 AD patients, and 28 cognitively-intact controls. Nonparametric ANCOVAs with Tukey-Kramer adjusted post-hoc comparisons were used to test for significant differences in regional FDDNP binding among subject groups.
Results:
FDDNP brain binding was higher in military personnel compared to controls in the amygdala, midbrain, thalamus, pons, frontal and anterior and posterior cingulate regions (p < 0.01–0.0001). Binding patterns in the military personnel were similar to those of the players except for the amygdala and striatum (binding higher in players; p = 0.02–0.003). Compared with the AD group, the military personnel showed higher binding in the midbrain (p = 0.0008) and pons (p = 0.002) and lower binding in the medial temporal, lateral temporal, and parietal regions (all p = 0.02).
Conclusion:
This first study of in vivo tau and amyloid brain signals in military personnel with histories of mTBI shows binding patterns similar to those of retired football players and distinct from the binding patterns in AD and normal aging, suggesting the potential value of FDDNP-PET for early detection and treatment monitoring in varied at-risk populations.
Keywords: Alzheimer’s disease, brain tau and amyloid, chronic traumatic encephalopathy, FDDNP-PET, mild traumatic brain injury, military personnel, retired professional football players
INTRODUCTION
Chronic traumatic encephalopathy (CTE) is a progressive neurodegenerative disease associated with prior brain trauma. Clinically, the disease is marked by cognitive, behavioral, and motor disturbances, and eventually progressive dementia. Neuropathological changes of CTE include widespread accumulation of phosphorylated tau protein as neurofibrillary tangles (similar to those observed in Alzheimer’s disease, AD), astrocytic tangles, neurites, diffuse axonal injury, white matter abnormalities, inflammation, and immune pro-inflammatory cytokine responses in traumatized brain regions. Immunoreactive deposits are found in neocortical, subcortical (e.g., thalamus, caudate, putamen, midbrain, and cerebellar white matter), and medial temporal (hippocampus, entorhinal cortex, and amygdala) regions, where neuronal loss may be observed [1]. TAR DNA-binding protein 43 (TDP-43, transactive response DNA binding protein 43 kDa), is a protein encoded by the TARDBP gene that may accompany tauopathy in CTE cases, predominantly in subcortical areas, and is more prominent in motor neuron disease cases [2]. Amyloid deposition has been reported—typically after age 60—in approximately 40% to 50% of CTE cases and generally consists of sparse diffuse plaques with relatively few neuritic plaques in cortical areas, and rarely in subcortical structures. NINDS criteria for the diagnosis of CTE require p-tau aggregates around small vessels in an irregular pattern at the depths of the cortical sulci and are supported by p-tau-related pathologies in the hippocampus, mammillary bodies and other hypothalamic nuclei, amygdala, nucleus accumbens, thalamus, midbrain tegmentum, and isodendritic core [3].
In vivo imaging of neuropathological, insoluble protein aggregates with positron emission tomography (PET) can aid in the early diagnosis of neurodegenerative diseases before extensive neuronal loss and clinical symptoms become evident [4]. Investigators have studied numerous in vivo imaging probes with purported specificity for amyloid-β; plaques, including [C-11]PiB, [F-18]3-fluoro-PiB ([F-18]flutemetamol), [C-11]SB-13, [F-18]BAY94–9172 ([F-18]florbetaben), [F-18]AV45 ([F-18]florbetapir), [C-11]BF-227, among others [5]; however, only FDDNP has been shown to also detect tau protein deposits in the living human brain. Most recently developed tau-specific ligands used with PET (e.g., [C-11]PBB3, [F-18]THK-523, [F-18]THK-5105, [F-18]THK-5117, [F-18]T808, [F-18]T807) have shown significant non-specific, ”off-target” binding (e.g., T-807 or AV-1451 and others) to MAO-A, MAO-B, neuromelanin-containing neurons, and other tissue targets in AD subjects [6, 7]. In subjects with suspected CTE, with the exception of one publication using T-807 in one National Football League (NFL) subject [7], no other reports have become available [8].
FDDNP is specific for the β-pleated sheet conformation present in amyloidogenic fibrils, both in vitro [9] and in vivo, in AD and mild cognitive impairment (MCI) [10, 11] and in Gerstmann-SträusslerScheinker disease and other prion diseases [12, 13], as well as in tau neuropathology in frontotemporal dementia [14] and progressive supranuclear palsy [15]. Previously, our group used FDDNP-PET to detect brain patterns of tau neuropathology distribution in retired NFL players with suspected CTE consistent with models of concussion and with paired helical filament-tau distribution observed at autopsy in subjects with a history of mild traumatic brain injury (mTBI) and CTE [16, 17].
As indicated above, FDDNP is specific for protein motifs having β-sheet pleated sheets, which are present in tau and amyloid neuroaggregates and explain its successful utilization in various neurodegenerative diseases having tau and amyloidcontaining neuroaggregates.
McKee and associates have reported that CTE is a predominant tauopathy and that amyloid, as sparse diffuse amyloid, is only observed in brain cortices of some CTE patients, typically after age 60 [18]. Thus, in the absence of amyloid aggregates, FDDNP appears to serve as a tau-imaging probe in CTE or other tauopathies like progressive supranuclear palsy [15]. Our previous work indicated that FDDNP-PET scans of retired professional football players could be differentiated from those of patients with AD [16]. This tau binding in CTE has recently been confirmed by Omalu and associates, who performed an autopsy on a 63-year-old football player previously scanned with FDDNP, which showed that FDDNP-PET revealed brain binding levels closely correlated with paired helical filament tau (PHF-tau) deposition (Spearman rank-order correlation (rs); rs = 0.59, p = 0.02) [19]. Regardless of whether FDDNP is binding to tau or amyloid, which may not be certain at the time of imaging, it is the regional pattern of FDDNP binding that distinguishes these various neurodegenerative diseases.
Brain trauma in military service members is well documented. Approximately 15% to 23% of returning military service members suffer from mTBI, [20–23] which includes concussion, sub-concussion, and mostly exposures to explosive blasts from improvised explosive devices. Military-related mTBI may be different from mTBI related to sports participation in terms of the manner of causation, including frequency or repetitiveness of impact (i.e., blast versus machine gun) and the type and angle of impact (i.e., massive blasts versus landmines). Unlike the relatively circumscribed types of trauma that occur in sports, military-related mTBI is acquired in a broad variety of ways, including sports and other recreational activities, physical training, falls, and motor vehicle accidents, though, in one study, explosive blast injury accounted for 56% of military-related TBI, of which 80% were mTBI [22]. Injuries from blasts vary depending on the magnitude of the explosive, proximity to the explosive, and the space in which the explosive detonates. McKee and Robinson [24] have commented on the variability of mTBI among military personnel, who may experience single or multiple blast injuries. Blast injury is the result of the rapid transmission of an acoustic wave through the brain tissue and accompanying blast winds that can produce forces similar to multiple, severe concussive impacts occurring over microseconds [25].
There is no consensus at this time on how to grade the severity of military-related mTBI. Militaryrelated mTBI may be further distinguished from sports-related mTBI by its high co-occurrence with posttraumatic stress disorder (PTSD), which occurs in 44% of military personnel who have suffered mTBI and subsequent loss of consciousness [21]. PTSD can be difficult to distinguish from other sequelae of mTBI, post-concussive syndrome, and CTE. All are characterized by executive dysfunction, impulsivity, emotional lability, disinhibition, sleep disturbance, and changes in personality [26, 27]. These common neuropsychiatric features may reflect the increasingly convergent neuropathological findings in the study of post-concussive syndrome, PTSD, and CTE. A tool that could distinguish PTSD due to psychological stress from other trauma-induced conditions such as CTE would be important for developing specific treatments. To address some of these knowledge gaps, we report here the first FDDNP-PET scan findings of brain tau pathology in military personnel with suspected CTE and compare the results to those of retired NFL players, patients with AD and cognitively healthy individuals.
MATERIALS AND METHODS
Neuropsychiatric evaluations were performed on seven military personnel who were recruited for this study because of a history of head trauma and cognitive or mood symptoms. Recruitment of military personnel occurred through word of mouth and individuals who contacted our group after hearing about our previous research through media. We compared results from these subjects with those of 15 retired NFL players, 24 patients with AD, and 28 cognitively intact individuals reported previously [16]. In the current study, their FDDNP-PET scans were reanalyzed using a more complex set of regions of interest (ROIs) as defined below. Subjects in the AD group met the standard diagnostic criteria for AD and dementia [28, 29]. The control group had normal cognitive functioning for their age and did not meet criteria for mild cognitive impairment or AD [30].
Subjects had physical examinations and assessments, screening laboratory tests, and structural imaging scans (computed tomography [CT] or magnetic resonance imaging [MRI]) to rule out major medical problems and other causes of mental symptoms (e.g., stroke, tumor) and for co-registration with PET scans for ROI analyses. Four football players and one veteran underwent CT scans because they could not tolerate MRI (claustrophobia, body metal, body size).
The Mini-Mental State Examination (MMSE) and Hamilton Rating Scales for Depression (HAM-D) and Anxiety (HAM-A) were administered to quantitate degree of cognitive and mood changes as well as to confirm diagnoses [11, 31]. In addition, a neuropsychological test battery was administered to assess memory, language, attention, information-processing speed, executive functioning, and visuospatial ability. We used standard diagnostic criteria for MCI, as previously described to measure memory, executive functioning, attention, and language abilities [11]. Clinical assessments were performed within four weeks of scanning, and clinicians were blinded to scan results. Informed consent was obtained in accordance with UCLA Human Subjects Protection Committee procedures. Cumulative radiation dosimetry was below the mandated maximum annual dose and in compliance with state and federal regulations.
All scans (Siemens Biograph TruePoint PET/CT scanner) were performed with participants in a supine position and with the imaging plane parallel to the orbitomeatal line as detailed previously [11, 17, 32]. In brief, subjects were injected with 10 mCi of FDDNP. FDDNP binding data were quantified using Logan graphical analysis: The slope of the linear portion of the Logan plot is the relative distribution volume (DVR) of the tracer in an ROI divided by that in the reference region (cerebellum) [33]. ROIs were traced on co-registered MRI or CT scans for subcortical, limbic (amygdala, striatum, thalamus, subthalamus, midbrain, pons), and cortical (frontal, parietal, occipital, anterior cingulate, posterior cingulate, and medial and lateral temporal) regions [11, 17, 32]. Each DVR or binding value was expressed as an average of left and right regions. All scans were read and ROIs drawn by individuals blinded to clinical assessments.
Statistical analyses
Demographic and clinical measures were compared between groups using Kruskal-Wallis tests for continuous measures (followed by MannWhitney tests for pair-wise comparisons) and Fishers exact tests for categorical measures. Nonparametric ANCOVAs (using ranked FDDNP binding levels rather than the raw DVR values; controlling for age) with Tukey-Kramer adjusted post-hoc comparisons were used to test for statistically significant differences in regional FDDNP binding (DVR values) among the four subject groups. We also examined whether inclusion of sex, APOE4 status, and educational level as covariates changed any of the findings. A significance level of p < 0.05 (two-tailed) was used for all inferences.
RESULTS
Demographic and clinical data are shown in Table 1. Military personnel and football players were exclusively men, whereas the AD and cognitively healthy control groups had a substantial proportion of women. Military personnel were significantly younger than the other three groups. Four of the seven military personnel were cognitively normal, and three had MCI (Supplementary Table 1). All but two football players had a diagnosis of MCI; one player was cognitively normal and one had dementia (Supplementary Table 2). While the difference in education levels among subject groups did not reach statistical significance, pairwise comparisons indicated that military personnel had significantly lower education levels than both players and cognitively healthy individuals. Military personnel and players had significantly higher levels of depression and anxiety than did controls and AD subjects but military personnel and players did not differ significantly. Three military personnel and five players had moderate to severe depression, while one military and two players had moderate to severe anxiety. One military serviceman had also played football and suffered concussions.
Table 1.
Demographic and clinical characteristics of the study groups
| Characteristic* | Militar y(N = 7) | Players (N = 15) | AD AD (N = 24) | Cognitively Intact | Statistics**, p-value |
|---|---|---|---|---|---|
| (N = 28) | |||||
| Age (y) | 45 (33–57) | 54 (40–86) | 77 (50–86) | 62.5 (30–84) | 24.1,<0.0001 |
| Education (y) | 14 (12–16) | 16 (14–28) | 16 (6–22) | 18 (12–22) | 7.5, 0.06 |
| Females | 0 (0) | 0 (0) | 12 (50) | 10 (36) | 0.0009 |
| Family History of Dementia | 1 (14) | 5 (33) | 10 (43) | 14 (50) | 0.4 |
| APOE4 carriers$ | 3 (43) | 2 (13) | 11 (58) | 12 (44) | 0.06 |
| MMSE score | 29 (24–29) | 27 (17–30) | 22 (4–27) | 30 (26–30) | 49.6, <0.0001 |
| HAM-D score | 17 (1–39) | 15 (0–33) | 1 (0–15) | 2 (0–9) | 29.3, <0.0001 |
| HAM-A score | 16 (2–32) | 13 (0–31) | 2 (0–17) | 3 (0–9) | 21.1, <0.0001 |
Median (range) or number (%).
Kruskal-Wallis χ2(3) (continuous measures) or Fisher’s exact test p-value (categorical measures). Mann-Whitney tests for pair-wise comparisons: Subjects with Alzheimer’s disease (AD) were significantly older than controls (U = 758, p = 0.03), players (U = 179, p = 0.001) and military personnel (U = 30.5, p = 0.0006). Military personnel were younger than players (U = 45.5, p = 0.02) and controls (U = 52, p = 0.005). Military personnel had lower educational level than players (U = 44, p = 0.02) and controls (U = 65.5, p = 0.02). The AD group had lower Mini-Mental State Examination (MMSE) scores than all other groups (U = 174–411, p = 0.002–0.0001); controls had significantly higher MMSE scores compared to players (U = 178, p = 0.0002) and military (U = 59, p = 0.005). The players and military personnel had higher scores on the 1) Hamilton Depression Rating Scale (HAM-D) compared to AD (players: U = 371, p = 0.0006; military: U = 148, p = 0.005) and controls (players: U = 500.5, p < 0.0001; military: U = 207.5, p = 0.002); 2) Hamilton Anxiety Rating Scale (HAM-A) compared to AD (players: U = 379, p = 0.0025; military: U = 155.5, p = 0.008) and controls (players: U = 455, p = 0.001; military: U = 193.5, p = 0.005). There were no other pair-wise difference between groups.
Missing for 5 AD and 1 cognitively intact subject.
The four groups differed significantly in all of their regional FDDNP binding levels, controlling for age (F(3,69) = 4.4–44.0; all p-values <0.0001 except for occipital, p = 0.007) (Table 2). FDDNP binding levels were significantly higher in military personnel compared to cognitively intact individuals in the following ROIs: amygdala, midbrain, thalamus, pons, frontal, anterior cingulate gyrus, and posterior cingulate gyrus (p < 0.01–0.0001) (Table 2, Figs. 1 and 2). Binding patterns in the military personnel were similar to those of the football players except in the amygdala and striatum, where the players had higher binding than the military personnel (p = 0.02–0.003). Compared with the AD group, the military personnel showed higher binding in the midbrain (p = 0.0008) and pons (p = 0.002) and lower binding in the medial temporal, lateral temporal, and parietal regions (all p = 0.02) (Table 2). All the above findings were similar with inclusion of sex, APOE4 status, or educational level as a covariate.
Table 2.
Mean group 2-(1- 6-[(2-[F-18]fluoroethyl) (methyl)amino]-2-naphthyl ethylidene)malononitrile relative distribution volumes (standard deviation values given in parentheses) and comparisons between groups
| Amygd | Midb | HypoTh | Thal | Pons | Str | MTL | LTL | F | p | ACG | PCG | O | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Military Personnel | 1.30 | 1.26 | 1.32 | 1.37 | 1.27 | 1.33 | 1.14 | 1.11 | 1.12 | 1.08 | 1.17 | 1.13 | 1.03 |
| n = 7 | (0.09) | (0.14) | (0.13) | (0.14) | (0.14) | (0.10) | (0.09) | (0.07) | (0.05) | (0.05) | (0.07) | (0.06) | (0.03) |
| Players | 1.39 | 1.34 | 1.42 | 1.51 | 1.31 | 1.53 | 1.18 | 1.12 | 1.14 | 1.09 | 1.22 | 1.16 | 1.00 |
| n = 15 | (0.10) | (0.07) | (0.08) | (0.10) | (0.07) | (0.10) | (0.04) | (0.05) | (0.05) | (0.05) | (0.07) | (0.08) | (0.05) |
| Alzheimer’s disease | 1.24 | 1.15 | 1.23 | 1.32 | 1.16 | 1.35 | 1.19 | 1.16 | 1.11 | 1.15 | 1.14 | 1.18 | 1.06 |
| n = 24 | (0.06) | (0.06) | (0.06) | (0.11) | (0.06) | (0.09) | (0.02) | (0.02) | (0.03) | (0.03) | (0.07) | (0.02) | (0.06) |
| Cognitively Intact | 1.16 | 1.14 | 1.23 | 1.25 | 1.15 | 1.32 | 1.11 | 1.06 | 1.04 | 1.05 | 1.09 | 1.08 | 1.02 |
| n = 28 | (0.04) | (0.05) | (0.05) | (0.09) | (0.08) | (0.07) | (0.02) | (0.03) | (0.03) | (0.03) | (0.04) | (0.04) | (0.05) |
| Nonparametric ANCOVA* | 36.25 | 26.51 | 16.42 | 19.2 | 20.13 | 21.35 | 28 | 29.34 | 43.97 | 23.88 | 16.54 | 25.71 | 4.39 |
| Tukey-Kramer adjusted pairwise Mestp-values | |||||||||||||
| Players versus Military | 0.02 | 0.5 | 0.1 | 0.2 | 0.8 | 0.003 | 0.07 | 0.9 | 0.8 | 0.9 | 0.6 | 0.9 | 0.4 |
| Cognitively intact versus Military | 0.001 | 0.0003 | 0.1 | 0.01 | 0.0007 | 0.5 | 0.3 | 0.07 | O.0001 | 0.2 | 0.004 | 0.007 | 0.9 |
| Alzheimer’s disease versus Military | 0.9 | 0.0008 | 0.1 | 0.3 | 0.002 | 0.5 | 0.02 | 0.02 | 0.7 | 0.02 | 0.3 | 0.4 | 0.8 |
| Cognitively intact versus Players | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.6 | <0.0001 | <0.0001 | 0.5 |
Amygd, amygdala; Midb, midbrain; HypoTh, hypothalamus; Thal, Thalamus; Str, striatum (caudate nucleus and putamen); MTL, medial temporal lobe; LTL, lateral temporal lobe; F, frontal; P, parietal; PCG, posterior cingulate gyrus; ACG, anterior cingulate gyrus; O, occipital.
F(3,69) statistics; all p-values<0.0001 except for O (p = 0.007), controlling for age
Fig. 1.
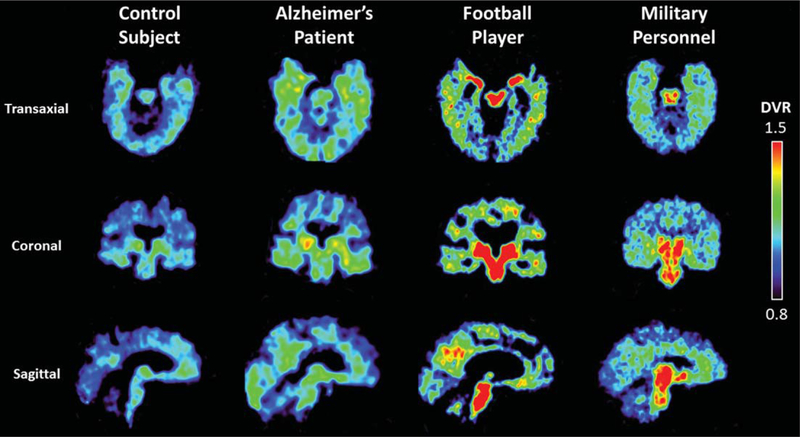
Examples of FDDNP-PET DVR transaxial, coronal, and sagittal images of a cognitively healthy individual, Alzheimer’s disease patient, football player, and a military subject. The Alzheimer’s disease patient shows higher DVR signals in parietal, temporal and frontal regions compared to the healthy individual, who may have neuropathology deposition given the age of the subject (80 years) [11]. The football player and military subject show higher amygdala, midbrain, and other subcortical binding compared with the healthy individual and Alzheimer’s disease patient. The healthy individual showed some mild cortical binding typical of other healthy individuals age 70 and older (or darker shades in greyscale) [10]. Warmer colors (or darker shades in greyscale) indicate higher FDDNP binding.
Fig. 2.
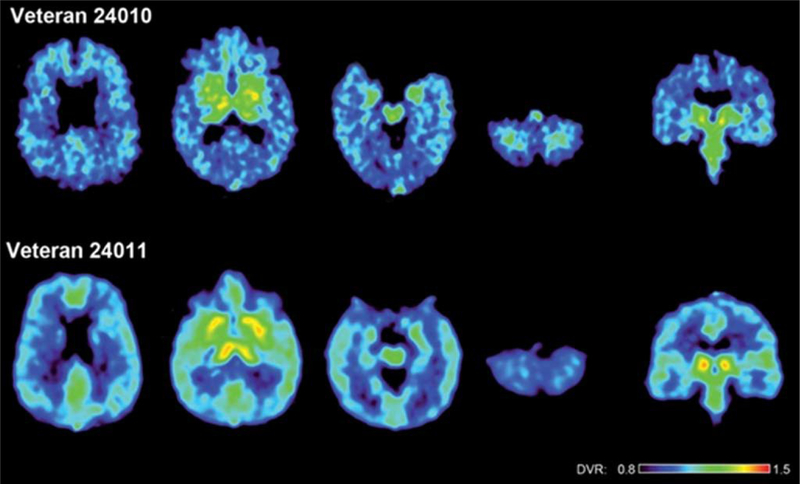
FDDNP DVR parametric images of the brains of two war veterans with histories of multiple blast concussions (mTBIs) during their war zone deployment. The upper row shows a 48-year-old man (veteran 24010) and the lower row a 36-year-old man (veteran 24011). Left four images in each row show transaxial brain images from top of the brain to the bottom. The right image shows a coronal cut through the midbrain. (This figure was originally published in Barrio et al., 2015 [16].)
DISCUSSION
This study is the first to report in vivo FDDNP binding patterns of brain tau and amyloid protein deposits in military personnel with suspected CTE and compare them to those of retired football players with suspected CTE, as well as FDDNP tau and amyloid binding patterns in patients with AD and cognitively intact individuals. As predicted, FDDNP binding patterns in military personnel with suspected CTE were similar to those of retired football players with suspected CTE, but different in certain brain regions from those with AD, and distinct from those with normal cognition.
The FDDNP binding patterns in these military personnel show high levels of binding in brain regions demonstrating tau accumulation found in postmortem studies of individuals with CTE [19, 34]. While athletes have been the focus of similar studies, the present investigation points to the potential utility of this technology in other at-risk populations. Only one subject (football player) had dementia, suggesting that FDDNP may potentially detect CTE-related neuropathology well before individuals suffer from major cognitive impairment.
Efforts toward developing tau-specific PET ligands have not thus far yielded useful results, as the developed probes have shown a lack of in vivo specificity and a lack of correlation with in vitro tau deposition due to binding to other tissue targets leading to significant ‘off-target’ binding [35]. FDDNP is the only available, well-studied tau-PET ligand that, thus far, has been used in the PET imaging of any tauopathy, including progressive supranuclear palsy or CTE, and has been shown to differentiate CTE from AD and normal cognition [15, 16]. We have previously shown that FDDNP binds to both fibrillary tau and amyloid, abnormal protein deposits that accumulate in AD [10, 11]. Since neuropathological determinations indicate that CTE is a predominant tauopathy [34] and amyloid cortical deposition—as diffuse amyloid—is observed as a function of age later in the development of the disease (typically in subjects older than 60 years of age) [1], our imaging results indicate that the FDDNP signal in our cases of suspected CTE likely represent fibrillary tau deposition, as confirmed in our recent work. Further, our results in the present study confirm our group’s findings that the pattern of abnormal brain tau deposition is the key feature of FDDNP-PET that offers a potentially useful biomarker for differentiating these and other important forms of neurodegeneration [16].
The military and retired football player groups showed similar FDDNP tau binding patterns although there were some regional distinctions, specifically in the amygdala and striatum. The distinctions in FDDNP binding values between these two groups with suspected CTE may reflect the differences of the concussion-based CTE development versus the blast-based mechanism of CTE (Blast-Variant CTE) [16]. The subjects in the military group are typically subjected to impacts that may be fairly powerful but less frequent than the concussive and sub-concussive injuries occurring while playing football. Among athletes with concussion-based CTE, our prior research[16] suggests a progression of neuropathology over four stages of severity (T1-T4) [24], whereas in blast-mediated CTE experienced by military personnel, such a progressive pattern of neuropathological change has not yet been described.
Limitations to interpretation of our findings include the small number of military personnel studied, the absence of women in the two groups with mTBI, the lack of specificity of injury to military personnel, and lack of comparison with individuals with mTBI but no symptoms or signs of CTE. Previous studies have not reported any gender based differences observed in FFDNP binding. One subject from the military personnel group also had an athletic background and may have suffered mTBI related to sports and/or military experience. Several subjects in the military and player groups suffered from moderate to severe depression and anxiety. In a previous study of individuals with MCI and without a history of mTBI, our group found that depression and trait anxiety correlated with cortical FDDNP binding [36], suggesting that current findings may also be attributable to psychopathology unrelated to mTBI. Other investigators posit that inflammation, which has been implicated along with tau and amyloid in the cascade of causative events in neurodegenerative diseases, could also play a role in depression and posttraumatic stress disorder (PTSD) associated with TBI [37, 38]. We did not screen for PTSD, which is highly prevalent among military personnel with mTBI and may in itself be correlated with neuropathology [39, 40]. Other factors that could influence the results would be differences in cerebrovascular health, substance use, and genetic risk among subjects with suspected CTE and normal cognition. Given these limitations, our findings warrant further study before they can be applied to clinical settings. Despite such limitations, our findings are consistent with our a priori hypothesis and the binding patterns in subjects with suspected CTE—both the players and the military personnel—are consistent with known neuropathological deposition patterns in multiple cases of CTE confirmed through neuropathological examination at autopsy [1, 2].
Further study of CTE biomarkers in at-risk individuals, both symptomatic and asymptomatic, is warranted to better characterize disease progression and develop effective strategies to treat CTE in military personnel, athletes, and other at-risk groups. It is also critically important to avoid premature assumptions of CTE based on nonspecific symptomatology, and it is here that objective biomarkers may prove very useful clinically. Longitudinal studies of FDDNP and other CTE biomarkers can objectively quantify injury severity, predict patient outcome and risk for future neurodegeneration, identify injury mechanisms and therapeutic targets, aid in assessment of therapeutic efficacy, and monitor recovery or decline, though the relationship between neuropathological burden and symptom severity has not yet been established. The current results suggest that FDDNP-PET may have the potential to become a valuable biomarker for early detection, treatment monitoring, and prevention of CTE in varied at-risk populations. Given the large number of people at risk, the public health impact of such a biomarker would be considerable.
Supplementary Material
ACKNOWLEDGMENTS
We thank John Williams, University of California, Los Angeles, Nuclear Medicine Clinic, for performing the PET scans; Vladimir Kepe, Cleveland Clinic, for developing the FDDNP PET analysis procedures for proteinopathies in patients with neurodegenerative diseases, including CTE; and Billy West, and Bus Cook for guidance with subject referral and for their helpful input. This study was supported by the Fran and Ray Stark Foundation Fund for Alzheimer’s Disease Research; the Parlow-Solomon (GWS) and Plott (JRB) Professorships; Bob and Marion Wil-son; the Ahmanson Foundation; and the following grants: P01-AG025831, AG13308, P50 AG 16570, MH/AG58156, MH52453, AG10123, and M01-RR00865 from the National Institutes of Health; and the UCLA CTSI (grant no. UL1TR000124). CCG is supported by grants from NCAA, U.S. Department of Defense, NIH/NINDS, UCLA Steve Tisch Brain-SPORT Program, the UCLA Easton Labs for Brain Injury and Avanir Pharmaceuticals. These agencies were not involved in the approval and review of the manuscript or in the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The University of California, Los Angeles, owns a U.S. patent (6,274,119) entitled “Methods for Labeling β-Amyloid Plaques and Neurofibrillary Tangles;” which has been licensed to TauMark, LLC. Drs. Satyamurthy, Huang, Omalu, Barrio and Small are among the inventors.
Footnotes
Presented in part at the International Psychogeriatrics Asso-ciation Annual Meeting in September, 2016 in San Francisco, CA.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/17-1152r3).
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-171152
REFERENCES
- [1].McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, Santini VE, Lee HS, Kubilus CA, Stern RA (2009) Chronic traumatic encephalopathy in athletes: Progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol 68, 709–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].McKee AC, Gavett BE, Stern RA, Nowinski CJ, Cantu RC, Kowall NW, Perl DP, Hedley-Whyte ET, Price B, Sullivan C, Morin P, Lee HS, Kubilus CA, Daneshvar DH, Wulff M, Budson AE (2010) TDP-43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy. J Neuropathol Exp Neurol 69, 918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].McKee AC, Cairns NJ, Dickson DW, Folkerth RD, Keene CD, Litvan I, Perl DP, Stein TD, Vonsattel JP, Stewart W, Tripodis Y, Crary JF, Bieniek KF, Dams-O’Connor K, Alvarez VE, Gordon WA (2016) The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol 131, 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Small GW, Bookheimer SY, Thompson PM, Cole GM, Huang SC, Kepe V, Barrio JR (2008) Current and future uses of neuroimaging for cognitively impaired patients. Lancet Neurol 7, 161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rowe CC, Villemagne VL (2011) Brain amyloid imaging. J Nucl Med 52, 1733–1740. [DOI] [PubMed] [Google Scholar]
- [6].Marquie M, Normandin MD, Meltzer AC, Siao Tick Chong M, Andrea NV, Anton-Fernandez A, Klunk WE, Mathis CA, Ikonomovic MD, Debnath M, Bien EA, Vanderburg CR, Costantino I, Makaretz S, DeVos SL, Oakley DH, Gomperts SN, Growdon JH, Domoto-Reilly K, Lucente D, Dickerson BC, Frosch MP, Hyman BT, Johnson KA, Gomez-Isla T (2017) Pathological correlations of [F-18]-AV-1451 imaging in non-alzheimer tauopathies. Ann Neurol 81, 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gandy S, Ikonomovic MD, Mitsis E, Elder G, Ahlers ST, Barth J, Stone JR, DeKosky ST (2014) Chronic traumatic encephalopathy: Clinical-biomarker correlations and current concepts in pathogenesis. Mol Neurodegener 9, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hall B, Mak E, Cervenka S, Aigbirhio FI, Rowe JB, O’Brien JT (2017) In vivo tau PET imaging in dementia: Pathophysiology, radiotracer quantification, and a systematic review of clinical findings. Ageing Res Rev 36, 50–63. [DOI] [PubMed] [Google Scholar]
- [9].Smid LM, Vovko TD, Popovic M, Petric A, Kepe V, Barrio JR, Vidmar G, Bresjanac M (2006) The 2,6-disubstituted naphthalene derivative FDDNP labeling reliably predicts Congo red birefringence of protein deposits in brain sections of selected human neurodegenerative diseases. Brain Pathol 16, 124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shoghi-Jadid K, Small GW, Agdeppa ED, Kepe V, Ercoli LM, Siddarth P, Read S, Satyamurthy N, Petric A, Huang SC, Barrio JR (2002) Localization of neurofibrillary tangles and beta-amyloid plaques in the brains of living patients with Alzheimer disease. Am J Geriatr Psychiatry 10, 24–35. [PubMed] [Google Scholar]
- [11].Small GW, Kepe V, Ercoli LM, Siddarth P, Bookheimer SY, Miller KJ, Lavretsky H, Burggren AC, Cole GM, Vinters HV, Thompson PM, Huang SC, Satyamurthy N, Phelps ME, Barrio JR (2006) PET of brain amyloid and tau in mild cognitive impairment. N Engl J Med 355, 2652–2663. [DOI] [PubMed] [Google Scholar]
- [12].Boxer AL, Rabinovici GD, Kepe V, Goldman J, Furst AJ, Huang SC, Baker SL, O’Neil JP, Chui H, Geschwind MD, Small GW, Barrio JR, Jagust W, Miller BL (2007) Amyloid imaging in distinguishing atypical prion disease from Alzheimer disease. Neurology 69, 283–290. [DOI] [PubMed] [Google Scholar]
- [13].Kepe V, Ghetti B, Farlow MR, Bresjanac M, Miller K, Huang SC, Wong KP, Murrell JR, Piccardo P, Epperson F, Repovs G, Smid LM, Petric A, Siddarth P, Liu J, Satyamurthy N, Small GW, Barrio JR (2010) PET of brain prion protein amyloid in Gerstmann-Straussler-Scheinker disease. Brain Pathol 20, 419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Barrio JR, Satyamurthy N, Huang SC, Petric A, Small GW, Kepe V, Smith J (2009) Dissecting molecular mechanisms in the living brain of dementia patients. Acc Chem Res 42, 842–850. [DOI] [PubMed] [Google Scholar]
- [15].Kepe V, Bordelon Y, Boxer A, Huang SC, Liu J, Thiede FC, Mazziotta JC, Mendez MF, Donoghue N, Small GW, Barrio JR (2013) PET imaging of neuropathology in tauopathies: Progressive supranuclear palsy. J Alzheimers Dis 36, 145– 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Barrio JR, Small GW, Wong KP, Huang SC, Liu J, Merrill DA, Giza CC, Fitzsimmons RP, Omalu B, Bailes J, Kepe V (2015) In vivo characterization of chronic traumatic encephalopathy using [F-18]FDDNP PET brain imaging. Proc Natl Acad SciUSA 112, E2039–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Small GW, Kepe V, Siddarth P, Ercoli LM, Merrill DA, Donoghue N, Bookheimer SY, Martinez J, Omalu B, Bailes J, Barrio JR (2013) PET scanning of brain tau in retired national football league players: Preliminary findings. Am J Geriatr Psychiatry 21, 138–144. [DOI] [PubMed] [Google Scholar]
- [18].McKee AC, Stein TD, Kiernan PT, Alvarez VE (2015) The neuropathology of chronic traumatic encephalopathy. Brain Pathol 25, 350–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Omalu B, Small GW, Bailes J, Ercoli LM, Merrill DA, Wong KP, Huang SC, Satyamurthy N, Hammers JL, Lee J, Fitzsimmons RP, Barrio JR (2018) Postmortem autopsyconfirmation of antemortem [F-18]FDDNP-PET scans in a football player with chronic traumatic encephalopathy. Neurosurgery 82, 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wojcik BE, Stein CR, Bagg K, Humphrey RJ, Orosco J (2010) Traumatic brain injury hospitalizations of U.S. army soldiers deployed to Afghanistan and Iraq. Am J Prev Med 38, S108–116. [DOI] [PubMed] [Google Scholar]
- [21].Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA (2008) Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N Engl J Med 358, 453–463. [DOI] [PubMed] [Google Scholar]
- [22].Bell RS, Vo AH, Neal CJ, Tigno J, Roberts R, Mossop C, Dunne JR, Armonda RA (2009) Military traumatic brain and spinal column injury: A 5-year study of the impact blast and other military grade weaponry on the central nervous system. J Trauma 66, S104–111. [DOI] [PubMed] [Google Scholar]
- [23].Terrio H, Brenner LA, Ivins BJ, Cho JM, Helmick K, Schwab K, Scally K, Bretthauer R, Warden D (2009) Traumatic brain injury screening: Preliminary findings in a US Army Brigade Combat Team. J Head Trauma Rehabil 24, 14–23. [DOI] [PubMed] [Google Scholar]
- [24].McKee AC, Robinson ME (2014) Military-related traumatic brain injury and neurodegeneration. Alzheimers Dement 10, S242–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Goldstein LE, Fisher AM, Tagge CA, Zhang XL, Velisek L, Sullivan JA, Upreti C, Kracht JM, Ericsson M, Wojnarowicz MW, Goletiani CJ, Maglakelidze GM, Casey N, Moncaster JA, Minaeva O, Moir RD, Nowinski CJ, Stern RA, Cantu RC, Geiling J, Blusztajn JK, Wolozin BL, Ikezu T, Stein TD, Budson AE, Kowall NW, Chargin D, Sharon A, Saman S, Hall GF, Moss WC, Cleveland RO, Tanzi RE, Stanton PK, McKee AC (2012) Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med 4, 134ra160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Elder GA, Mitsis EM, Ahlers ST, Cristian A (2010) Blastinduced mild traumatic brain injury. Psychiatr Clin North Am 33, 757–781. [DOI] [PubMed] [Google Scholar]
- [27].Vasterling JJ, Verfaellie M, Sullivan KD (2009) Mild traumatic brain injury and posttraumatic stress disorder in returning veterans: Perspectives from cognitive neuroscience. Clin Psychol Rev 29, 674–684. [DOI] [PubMed] [Google Scholar]
- [28].McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34, 939–944. [DOI] [PubMed] [Google Scholar]
- [29].American Psychiatric Association; (2000) Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision American Psychiatric Association Publishing, Washington, DC. [Google Scholar]
- [30].Petersen RC (2004) Mild cognitive impairment as a diagnostic entity. J Intern Med 256, 183–194. [DOI] [PubMed] [Google Scholar]
- [31].Lezak M, Howieson D, Loring D (2004) Neuropsychological Assessment, Fourth edition. University Press, New York. [Google Scholar]
- [32].Small GW, Siddarth P, Kepe V, Ercoli LM, Burggren AC, Bookheimer SY, Miller KJ, Kim J, Lavretsky H, Huang SC, Barrio JR (2012) Prediction of cognitive decline by positron emission tomography of brain amyloid and tau. Arch Neurol 69, 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL (1996) Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab 16, 834–840. [DOI] [PubMed] [Google Scholar]
- [34].McKee AC, Stern RA, Nowinski CJ, Stein TD, Alvarez VE, Daneshvar DH, Lee HS, Wojtowicz SM, Hall G, Baugh CM, Riley DO, Kubilus CA, Cormier KA, Jacobs MA, Martin BR, Abraham CR, Ikezu T, Reichard RR, Wolozin BL, Budson AE, Goldstein LE, Kowall NW, Cantu RC (2013) The spectrum of disease in chronic traumatic encephalopathy. Brain 136, 43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Vermeiren C, Mercier J, Viot D, Mairet-Coello G, Hannestad J, Courade J-P, Smith J (2015) T807, a reported selective tau tracer, binds with nanomolar affinity to monoamine oxi-dase A. Alzheimers Dement 11(Suppl), P283. [Google Scholar]
- [36].Lavretsky H, Siddarth P, Kepe V, Ercoli LM, Miller KJ, Burggren AC, Bookheimer SY, Huang SC, Barrio JR, Small GW (2009) Depression and anxiety symptoms are associated with cerebral FDDNP-PET binding in middle-aged and older nondemented adults. Am J Geriatr Psychiatry 17, 493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Muller N (2014) Immunology of major depression. Neuroimmunomodulation 21, 123–130. [DOI] [PubMed] [Google Scholar]
- [38].Wieck A, Grassi-Oliveira R, Hartmann do Prado C, Teixeira AL, Bauer ME (2014) Neuroimmunoendocrine interactions in post-traumatic stress disorder: Focus on long-term implications of childhood maltreatment. Neuroimmunomodulation 21, 145–151. [DOI] [PubMed] [Google Scholar]
- [39].Rabinak CA, Angstadt M, Welsh RC, Kenndy AE, Lyubkin M, Martis B, Phan KL (2011) Altered amygdala resting-state functional connectivity in post-traumatic stress disorder. Front Psychiatry 2, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sripada RK, King AP, Garfinkel SN, Wang X, Sripada CS, Welsh RC, Liberzon I (2012) Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J Psychiatry Neurosci 37, 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.