Summary
Most marine benthic invertebrates have a pelagic larval phase, after which they settle preferentially on or near conspecific adults, forming aggregations. Although settlement pheromones from conspecific adults have been implicated as critical drivers of aggregation for more than 30 years, surprisingly few have been unambiguously identified. Here we show that in the invasive dreissenid mussel Mytilopsis sallei (an ecological and economic pest), three common purines (adenosine, inosine, and hypoxanthine) released from adults in a synergistic and precise ratio (1:1.125:3.25) serve as an aggregation pheromone by inducing conspecific larval settlement and metamorphosis. Our results demonstrate that simple common metabolites can function as species-specific pheromones when present in precise combinations. This study provides important insights into our understanding of the ecology and communication processes of invasive organisms and indicates that the combination and ratio of purines might be critical for purine-based signaling systems that are fundamental and widespread in nature.
Subject Areas: Ecology, Environmental Science, Marine Organism, Molecular Mechanism of Behavior
Graphical Abstract

Highlights
-
•
M. sallei uses a blend of three common simple purines as an aggregation pheromone
-
•
The three purines synergistically induce M. sallei larvae to settle
-
•
Larvae are highly sensitive to the ratio of purines released by conspecific adults
-
•
Common metabolites in precise combinations can act as species-specific pheromones
Ecology; Environmental Science; Marine Organism; Molecular Mechanism of Behavior
Introduction
Animal aggregation is one of the most striking behaviors in biology that affects many spatial and temporal processes in ecological systems (Toonen and Pawlik, 1994, Parrish and Edelstein-Keshet, 1999). Despite the cost of increased intraspecific competition, for example, for space, food, and oxygen, aggregation has often been viewed as an evolutionarily advantageous state, in which individuals derive the benefits of protection and reproduction (Danchin and Wagner, 2000, Dzierżyńska-Białończyk et al., 2018). Gregarious settlement is a common phenomenon among marine benthic invertebrates, including mussels, barnacles, oysters, and polychaetes. Most benthic marine invertebrates have a pelagic larval phase, after which they settle preferentially on or near conspecific adults, forming aggregations (Toonen and Pawlik, 1994). The transition from a planktonic to a benthic mode of life is generally accepted as a critical point in their life cycle and is fundamental to understanding population and community dynamics (Shikuma et al., 2014). Although many studies have repeatedly implicated a critical role for pheromones from conspecific adults in the induction of larval settlement forming dense aggregates for more than 30 years, surprisingly few settlement pheromones have been isolated and structurally identified (Burke, 1986, Dreanno et al., 2006). The present understanding of aggregation mechanisms and the evolution of aggregation pheromones is limited.
Invasive dreissenid mussels commonly foul submerged structures with typical high-density aggregations and are well-known ecological and economic pests in aquatic ecosystems (Pimentel et al., 2005, Michalak, 2017). These include the zebra mussel Dreissena polymorpha, the quagga mussel Dreissena rostriformis bugensis in North America and Europe (Michalak, 2017, Stokstad, 2007), Mytilopsis leucophaeata in Europe (Kennedy, 2011), and Mytilopsis trautwineana in South America (Aldridge et al., 2008). Dreissenids are dioecious with gametes released directly into the water and fertilized externally (Ram et al., 1996). After a brief free-swimming veliger stage, the pediveliger larvae settle and metamorphose to benthic juveniles, which attach to most substrates with secreted byssal threads leading to fouling. The gregarious settlement of dreissenid mussels causes adverse impacts on aquatic systems and serious cost to industries. The introduction of dreissenid mussels into water pipelines in power plants and water treatment plants causes damage worth billions of dollars in the Great Lakes area (Aldridge et al., 2006). Much research has focused on antifouling compounds in preventing invertebrate settlement (Yebra et al., 2004, Almeida and Vasconcelos, 2015, Qian et al., 2015, Martins et al., 2018). For the control of dreissenid mussels, chlorine has been commonly used in pipelines, but there are environmental concerns about this approach (Meehan et al., 2014). Furthermore, at present no practical technology is available for inhibiting the gregarious settlement and invasion of dreissenid mussels in open waters (Molloy et al., 2013). Research on aggregation mechanisms of dreissenid mussels may shed light on developing environment-friendly and effective methods for their control. Although the formation of aggregates by adults of dreissenid mussels and factors that affect this process have been previously studied (Dzierżyńska-Białończyk et al., 2018, Tošenovský and Kobak, 2016), there is still very little understanding of the biochemical mechanisms of gregarious settlement of dreissenid mussels.
The Caribbean false mussel Mytilopsis sallei (Recluz, 1849) (Figure S1), a close relative of D polymorpha and D. rostriformis bugensis, was introduced into the Pacific via the Panama Canal (Morton, 1981). This dreissenid mussel has wide environmental tolerance, grows rapidly, matures fast, and has a high fecundity, contributing to its success as an invasive species (Morton, 1981, Morton, 1989). M. sallei has been found in Australasia, East Asia, and India (Willan et al., 2000, Wong et al., 2011, Cai et al., 2014). Large aggregations of M. sallei cause serious fouling problems on monsoon drains, concrete walls, floating rafts, aquaculture facilities, and other submerged artificial structures. Here, we hypothesized that M. sallei adults could release aggregation pheromone to induce settlement of conspecific larvae. The aim of the present study was to investigate whether such a pheromone exists in M. sallei adults and to attempt to isolate such an aggregation pheromone by bioassay-guided fractionation to determine its chemical structure. This work may provide insights into our understanding of the population dynamics and ecology of invasive dreissenid mussels and allow the development of methods for their control.
Results and Discussion
An Aggregation Pheromone Exists in M. sallei
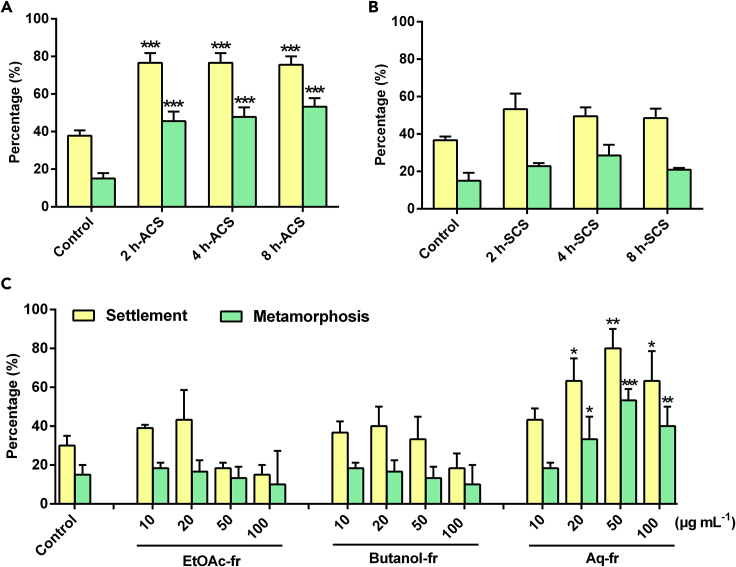
To confirm the existence of the conspecific cue(s), we examined larval settlement and metamorphosis of M. sallei in response to conspecific adult-conditioned seawater (ACS). Larval settlement and metamorphosis were both significantly induced by ACS (Figure 1A, Table 1). The three treatments using ACS prepared by placing M. sallei adults in seawater for 2, 4, and 8 h, respectively, all showed inducing activity. However, treatments using shell-conditioned seawater prepared by placing empty shells of M. sallei adults in seawater for 2, 4, and 8 h, respectively, had no significant impact on larval settlement and metamorphosis compared with the control (Figure 1B, Table 1). These findings demonstrated that M. sallei adults could release chemical cue(s) to induce conspecific larval settlement and metamorphosis, and that the cue(s) were not derived from the shell of the adult M. sallei. We hypothesized that the inducing cue(s) in ACS were derived from mantle cavity fluid (MCF), as MCF is released with exhalant current from adults (Zimmer and Butman, 2000). MCF was successively partitioned with ethyl acetate and n-butanol, and the resultant three fractions, including the residual aqueous fractions, were examined for their effect on settlement and metamorphosis of M. sallei larvae. Only the aqueous fraction was active in inducing larval settlement and metamorphosis (Figure 1C, Table 1), further confirming the existence of waterborne inducing cues.
Figure 1.
M. sallei Adults Release Chemical Cue(s) into Seawater to Induce Conspecific Larval Settlement and Metamorphosis
(A and B) Percentage settlement and metamorphosis of M. sallei larvae after 48-h exposure to conspecific adult-conditioned seawater (A) and conspecific adult shell-conditioned seawater (B). ACS, adult-conditioned seawater; SCS, shell-conditioned seawater. The preparation of 2-, 4-, and 8-h ACS, and that of 2-, 4-, and 8-h SCS, is described in the Methods.
(C) Percentage settlement and metamorphosis of M. sallei larvae after 48-h exposure to three factions of mantle cavity fluid from conspecific adults. Preparation of the three fractions is described in the Methods. EtOAc-fr, the ethyl acetate fraction; butanol-fr, the n-butanol fraction; Aq-fr, the aqueous fraction; Control, filtered (0.22 μm) seawater. Results are shown as mean ± SD (n = 3). Asterisk denotes significant difference compared with the control (*p < 0.05, **p < 0.01, ***p < 0.001, Dunnett's test).
Table 1.
ANOVA Results for the Effect of Chemical Cues on Larval Settlement and Metamorphosis of M. sallei
| Bioassay | Cue | Response | df between Groups | df within Groups | F Value | p Value |
|---|---|---|---|---|---|---|
| Effect of conditioned seawater | ACS | Settlement | 3 | 8 | 55.035 | 0.000 |
| Metamorphosis | 3 | 8 | 22.839 | 0.000 | ||
| SCS | Settlement | 3 | 8 | 1.724 | 0.239 | |
| Metamorphosis | 3 | 8 | 2.303 | 0.154 | ||
| Effect of MCF | EtOAc fraction | Settlement | 3 | 8 | 8.514 | 0.003 |
| Metamorphosis | 3 | 8 | 0.385 | 0.814 | ||
| Butanol fraction | Settlement | 3 | 8 | 3.047 | 0.070 | |
| Metamorphosis | 3 | 8 | 0.771 | 0.568 | ||
| Aqueous fraction | Settlement | 3 | 8 | 8.500 | 0.003 | |
| Metamorphosis | 3 | 8 | 12.458 | 0.001 | ||
| Effect of subfractions obtained during the bioassay-guided fractionation of MCF | F1 | Settlement | 4 | 10 | 8.859 | 0.003 |
| Metamorphosis | 4 | 10 | 4.400 | 0.026 | ||
| F2 | Settlement | 4 | 10 | 6.100 | 0.009 | |
| Metamorphosis | 4 | 10 | 1.150 | 0.388 | ||
| F3 | Settlement | 4 | 10 | 7.597 | 0.004 | |
| Metamorphosis | 4 | 10 | 10.750 | 0.001 | ||
| F3-1 | Settlement | 4 | 10 | 0.849 | 0.526 | |
| Metamorphosis | 4 | 10 | 0.885 | 0.507 | ||
| F3-2 | Settlement | 4 | 10 | 0.827 | 0.537 | |
| Metamorphosis | 4 | 10 | 0.512 | 0.729 | ||
| F3-3 | Settlement | 4 | 10 | 2.226 | 0.139 | |
| Metamorphosis | 4 | 10 | 1.726 | 0.220 | ||
| F3-4 | Settlement | 4 | 10 | 7.264 | 0.005 | |
| Metamorphosis | 4 | 10 | 16.469 | 0.000 | ||
| F3-4-1 | Settlement | 4 | 10 | 0.394 | 0.809 | |
| Metamorphosis | 4 | 10 | 0.310 | 0.865 | ||
| F3-4-2 | Settlement | 4 | 10 | 13.676 | 0.000 | |
| Metamorphosis | 4 | 10 | 9.342 | 0.002 | ||
| F3-4-3 | Settlement | 4 | 10 | 18.015 | 0.000 | |
| Metamorphosis | 4 | 10 | 12.375 | 0.001 | ||
| F3-4-4 | Settlement | 4 | 10 | 3.438 | 0.052 | |
| Metamorphosis | 4 | 10 | 1.314 | 0.329 | ||
| F3-4-3-1 | Settlement | 4 | 10 | 15.886 | 0.000 | |
| Metamorphosis | 4 | 10 | 11.452 | 0.001 | ||
| F3-4-3-2 | Settlement | 4 | 10 | 32.947 | 0.000 | |
| Metamorphosis | 4 | 10 | 17.717 | 0.000 | ||
| F3-4-3-3 | Settlement | 4 | 10 | 5.857 | 0.011 | |
| Metamorphosis | 4 | 10 | 1.521 | 0.269 | ||
| Effect of purine compounds | Ado | Settlement | 6 | 14 | 13.991 | 0.000 |
| Metamorphosis | 6 | 14 | 16.123 | 0.000 | ||
| Ino | Settlement | 6 | 14 | 26.119 | 0.000 | |
| Metamorphosis | 6 | 14 | 18.028 | 0.000 | ||
| Hyp | Settlement | 6 | 14 | 13.604 | 0.000 | |
| Metamorphosis | 6 | 14 | 18.028 | 0.000 | ||
| Ade | Settlement | 6 | 14 | 1.127 | 0.396 | |
| Metamorphosis | 6 | 14 | 0.673 | 0.673 | ||
| Xan | Settlement | 6 | 14 | 2.497 | 0.074 | |
| Metamorphosis | 6 | 14 | 0.220 | 0.964 | ||
| Effect of dilution of ACS | Different dilutions of ACS | Settlement | 4 | 10 | 10 | 0.000 |
| Metamorphosis | 4 | 10 | 10 | 0.000 | ||
| Synergistic effect of Ado, Ino, and Hyp | Mixture of Ado, Ino and Hyp, and individual components | Settlement | 5 | 12 | 12 | 0.000 |
| Metamorphosis | 5 | 12 | 12 | 0.000 | ||
| Effects of different ratios of Ado, Ino, and Hyp | Different ratios of Ado, Ino and Hyp | Settlement | 34 | 70 | 70 | 0.000 |
| Metamorphosis | 34 | 70 | 70 | 0.000 |
ACS, adult-conditioned seawater; SCS, shell-conditioned seawater; MCF, mantle cavity fluid; Ado, adenosine; Ino, inosine; Hyp, hypoxanthine; Ade, adenine; Xan, xanthine; EtOAc, ethyl acetate; df, degree of freedom.
M. sallei Uses Three Common Simple Purines as Aggregation Pheromone
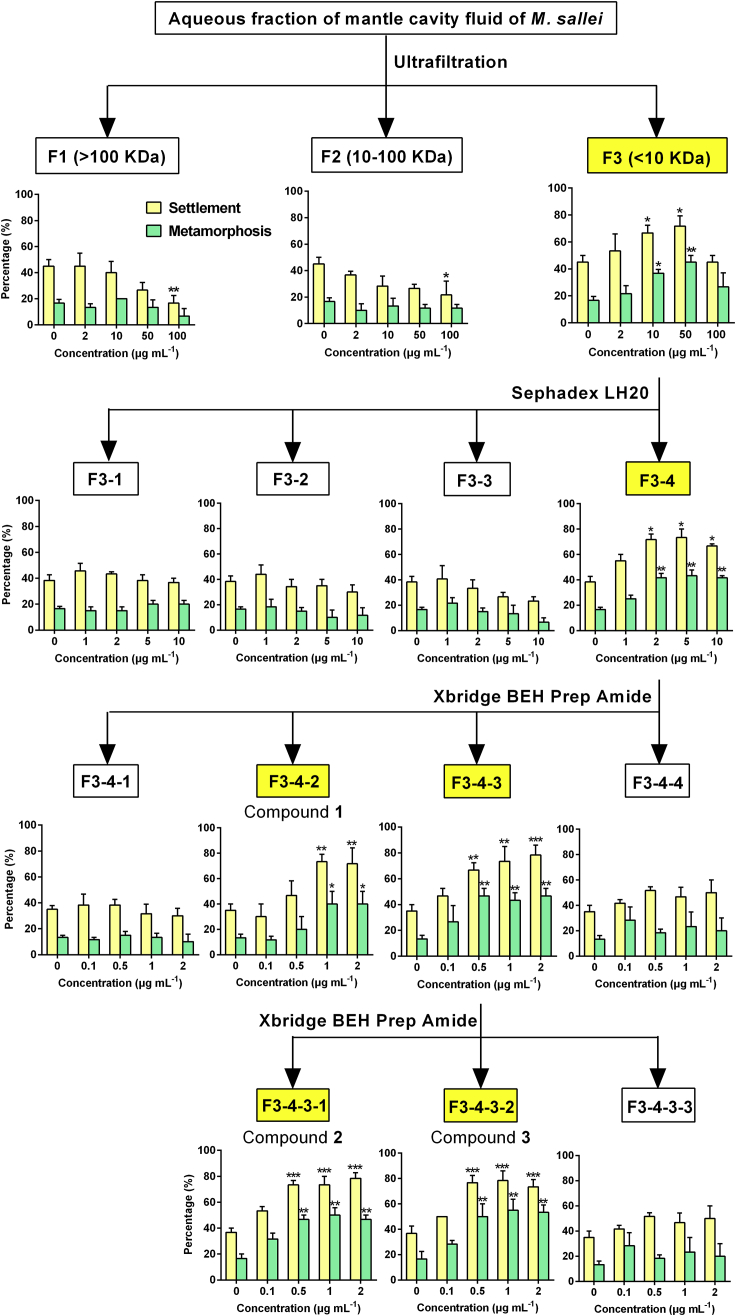
The active aqueous fraction was then subjected to bioassay-guided fractionation by ultrafiltration and column chromatography, which gave three pure active compounds (1, 2, and 3) (Figure 2). They all significantly induced settlement and metamorphosis of M. sallei larvae (Table 1). Based on analysis of their electrospray ionization mass spectrometry and nuclear magnetic resonance spectral data (Supplemental Information), compounds 1, 2, and 3 were identified as the purines, hypoxanthine (Hyp), inosine (Ino), and adenosine (Ado), respectively (Chenon et al., 1975, Saladino et al., 2006, Abou-Hussein et al., 2007, Ghose, 2009). This study identifies purines as pheromones for larval settlement or metamorphosis of a marine invertebrate. To further confirm the activity of compounds for larval settlement of M. sallei under hydrodynamic conditions, as in the natural environment, we performed larval bioassays using Ado (as an example of inductive purines) in a racetrack flume with seawater at a flow rate of 10.8–16.2 L min−1 (Figure 3A). More than twice the number of M. sallei larvae were found to settle on substrates, which slowly released Ado compared with the controls (Figures 3B–3D), demonstrating the inducing activity of Ado for larval settlement under flow conditions.
Figure 2.
Bioassay-Guided Fractionation of Compounds that Can Induce M. sallei Larval Settlement and Metamorphosis from Mantle Cavity Fluid of Conspecific Adults
Results are shown as mean ± SD (n = 3). Asterisk denotes significant difference compared with the control (*p < 0.05, **p < 0.01, ***p < 0.001, Dunnett's test).
Figure 3.
Adenosine Induced Larval Settlement of M. sallei under Flow Condition
(A) Experimental apparatus used in this study, with seawater in the racetrack flume at a flow rate of 10.8–16.2 L min-1.
(B–D) Number of settled larvae on the treated substrates that slowly released adenosine (Ado) and control substrates after 12 h (B), 24 h (C), and 48 h (D). Results are shown as mean ± SD (n = 3). Asterisk denotes significant difference compared with the control (*p < 0.05, Student's t test).
Purines as signal molecules have been reported in fish and polychaetes. In fish, purines such as ATP, ADP, AMP, and Ado evoke attraction or feeding (appetitive) responses, whereas hypoxanthine 3-N-oxide can evoke avoidance or alarm (Wakisaka et al., 2017, Shamchuk et al., 2018). In polychaetes, the purine metabolites uric acid and Ino serve as the sperm- and egg-release pheromones, respectively, and were the first identified gamete-release pheromones in marine invertebrates (Zeeck et al., 1998a, Zeeck et al., 1998b). Here we demonstrate the use of purines for intraspecific communication in the phylum Mollusca. Purines are common metabolites in all organisms. Utilizing already existing purine metabolites as pheromones might be more energetically favorable than producing entirely new molecules. However, as suggested in a recent study (Shamchuk et al., 2018), the role of purines as chemical communication molecules in animals is substantially underestimated.
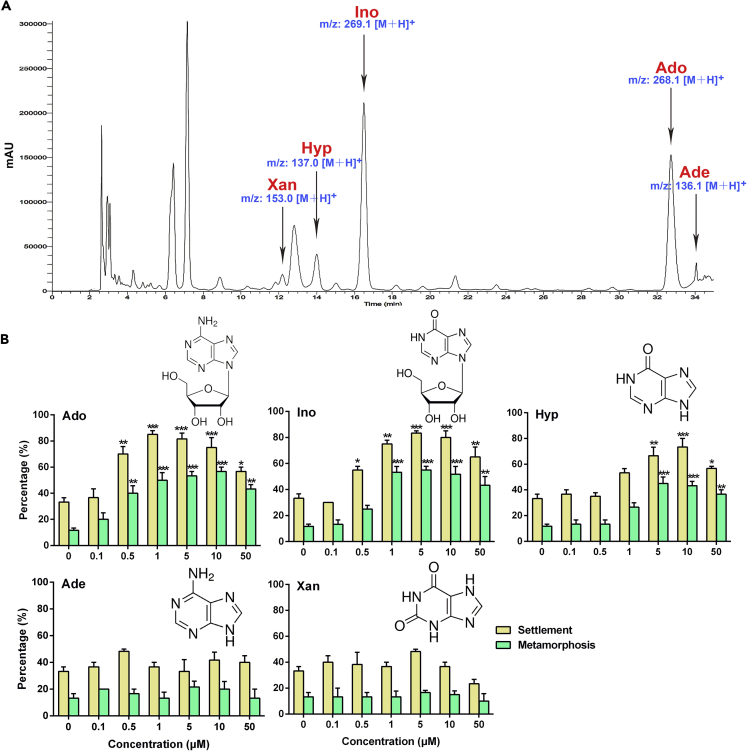
As Hyp, Ino, and Ado are purine metabolites, we wondered whether there were other active purine metabolites in M. sallei MCF. By using liquid chromatography-mass spectrometry, five purine metabolites, namely, Hyp, Ino, Ado, xanthine (Xan), and adenine (Ade), were detected in MCF of M. sallei (Figure 4A). However, unlike the significant inducing activity exhibited by Hyp, Ino, and Ado for M. sallei larval settlement and metamorphosis, Xan and Ade showed no significant effects (Figure 4B, Table 1). This finding further confirmed the efficiency of the bioassay-guided fractionation procedure for identification of active compounds used here.
Figure 4.
Adenosine, Inosine, and Hypoxanthine Are Inducing Compounds from M. sallei Adults for Settlement and Metamorphosis of Conspecific Larvae
(A) Detection by liquid chromatography-mass spectrometry of purines in the mantle cavity fluid (MCF) of adults.
(B) Chemical structures of the purines detected in MCF, and percentages of settlement and metamorphosis of M. sallei larvae after 48-h exposure to each purine compound. Xan, xanthine; Hyp, hypoxanthine; Ino, inosine; Ado, adenosine; Ade, adenine. Results are shown as mean ± SD (n = 3). Asterisk denotes significant difference compared with the control (*p < 0.05, **p < 0.01, ***p < 0.001, Dunnett's test).
Pheromone Components Synergistically Induce Larval Settlement and Metamorphosis
To serve as effective pheromone signals in the natural environment, Hyp, Ino, and Ado should be released into seawater by M. sallei adults at effective concentrations. As ACS was active for conspecific larval settlement and metamorphosis, whether the three purines were released into ACS and responsible for its inducing activity was determined as follows. Figure 5A showed that when ACS was diluted with seawater, the inducing activity of ACS decreased with increasing dilution. A 5-fold dilution of ACS (5-d ACS) was the highest dilution to show inducing activity. Ado, Ino, and Hyp were found to be present in the 5-d ACS with concentrations of 8, 9, and 26 nM, respectively (Figures 5B and S2B), which were much lower than the lowest effective concentration of each compound when tested individually (0.5 μM for Ado, 0.5 μM for Ino, and 1.0 μM for Hyp, Figure 4B), indicating that there might be a synergistic effect of these three purines on M. sallei. To determine whether there was a synergistic effect, the response of larval settlement and metamorphosis to a mixture of the three purines (8 nM for Ado, 9 nM for Ino, and 26 nM for Hyp) was investigated, and compared with the response to individual compounds under the same concentrations of each compound separately in the mixture. The 5-d ACS was used as positive control. Interestingly, the blend of the purines produced a significant response, whereas each individual component showed no inducing activity under the concentration tested in this bioassay (Figure 5C, Table 1), which strongly suggests a synergistic effect of Ado, Ino, and Hyp in ACS. By comparing the effective concentration of the mixture and that of the individual components, the mixture was 38.5- to 62.5-fold more potent than the individual compounds. Furthermore, the synthetic mixture and the 5-d ACS were equally effective at inducing settlement and metamorphosis.
Figure 5.
Pheromone Components Synergistically Induce Larval Settlement and Metamorphosis
(A) Percentage of larval settlement and metamorphosis in response to different dilutions of adult-conditioned seawater (ACS). Filtered seawater was used as control.
(B) Concentrations of adenosine (Ado), inosine (Ino), and hypoxanthine (Hyp) in the 5-fold dilution of ACS determined by high-performance liquid chromatography.
(C) Percentage of larval settlement and metamorphosis of M. sallei in response to 8 nM Ado, 9 nM Ino, 26 nM Hyp, and mixture of the three purines. Filtered seawater was used as a negative control. The 5-fold dilution of ACS (5-d ACS) was used as a positive control. Results are shown as mean ± SD (n = 3). Asterisk denotes significant difference compared with the negative control (**p < 0.01, ***p < 0.001, Dunnett's test).
Pheromone Components Function in a Precise Ratio
As Ado, Ino, and Hyp are common metabolites found in aquatic environments (Cunliffe, 2015), we wondered how M. sallei larvae can distinguish conspecific adult-derived purine signals from background levels. The use of a blend of pheromone components that act synergistically has also been reported in insects (Meier et al., 2016), although there are few published examples for marine organisms (Li et al., 2018). Usually the ratio of the pheromone components is highly species specific in insects, allowing effective communication among related sympatric species (Symonds and Elgar, 2008). For example, two Helicoverpa (moth) species use the same sex pheromone components (Z)-11-hexadecenal and (Z)-9-hexadecenal in nearly reverse ratios, 100:2 is used by Helicoverpa armigera and 6:100 is used by Helicoverpa assulta, thus ensuring segregation in nature (Wang et al., 2005). To determine whether M. sallei larvae had specific preference for particular ratios of Ado, Ino, and Hyp, thirty-three treatments with different ratios but with the same total concentration of compounds (50 nM) were prepared using simplex lattice mixture design (Figure 6A) (50 nM was chosen because the sum of concentrations of Ado, Ino, and Hyp in the effective 5-d ACS was close to 50 nM). These mixtures were then tested for larval response to settlement and metamorphosis. Results showed that different ratios produced quite different larval responses (Figures 6B–6E, Table 1). Twenty-five mixtures had no significant effect on settlement and metamorphosis. Six treatments with ratios of Ado, Ino, and Hyp of 0:4:1, 1:3:1, 0:1:4, 5:1:19, 1:5:19, and 1:1:3, gave a significant inducing activity (Figures 6B and 6C). The highest rates of settlement and metamorphosis were observed with a ratio of 1:1:3, which, interestingly was similar to the ratio of 8:9:26 (1:1.125:3.25) for Ado, Ino, and Hyp in natural ACS (Figures 5B, 6D, and 6E). The finding that M. sallei larvae are highly sensitive only to a particular ratio of Ado, Ino, and Hyp suggested that M. sallei larvae may have evolved species-specific responses to this ratio.
Figure 6.
Specific Ratios of Adenosine, Inosine, and Hypoxanthine Induce Conspecific Larval Settlement and Metamorphosis
(A) Thirty three different ratios of Ado, Ino, and Hyp were designed based on simplex lattice mixture design. The test levels used for each purine compound were designed as 0, 1/25, 1/5, and 1. Fractions of each level were used, and the sum of contributory fractions for each ratio treatment was one. The positions of the coded levels are shown in the triangle, and the corresponding ratios are shown in the table on the right. All treatments were at the same total concentration of compounds (50 nM). Scale bar, 2 nM (i.e., 1/25 of total concentration).
(B and C) Percentage of larval settlement (B) and metamorphosis (C) of M. sallei in response to different ratios of Ado, Ino, and Hyp. Filtered seawater was used as a negative control (NC). The 5-fold dilution of adult-conditioned seawater was used as a positive control (PC). Results are shown as mean ± SD (n = 3). Asterisk denotes significant difference compared with the negative control (*p < 0.05, **p < 0.01, ***p < 0.001, Dunnett's test).
(D and E) Mixture contour plots show the effect of different ratios of Ado, Ino, and Hyp on settlement (D) and metamorphosis (E) of M. sallei larvae. Star shows the measured ratio of Ado:Ino:Hyp in ACS, i.e., 1:1.125:3.25.
We further measured the ratios of these three purines in the conditioned seawater of the mussel Perna viridis and the oyster Crassostrea angulata (Figures S2B and S2C), two molluscan species with ecological niches similar to M. sallei, and found that the ratio of Ado, Ino, and Hyp in ACS was 6:6:1 for P. viridis and 1:10:5 for C. angulata. The clear difference of these ratios and that of M. sallei (1:1.125:3.25) supports the hypothesis that M. sallei larvae recognize conspecific adult-derived pheromones from background levels of purines, by only responding to a particular ratio of pheromone components. The other noteworthy finding was that two ratios of Ado, Ino, and Hyp (20:4:1 and 20:1:4) significantly inhibited settlement of M. sallei (Figure 6B), suggesting that even under the same total concentration of compounds, the larval response to certain mixtures could change from induction of settlement to its prevention. The discovery that changing the ratio of the pheromone components can result in the opposite observed effect is reported here. It suggests a potential method for interfering with the gregarious settlement of M. sallei and other dreissenid mussels by simply changing the ratio of components in an aggregation pheromone blend.
Purines have received increasing attention as intracellular and intercellular signaling messengers (Massé et al., 2007, Idzko et al., 2014, Verkhratsky and Burnstock, 2014). Sensitivity to purines is widespread across prokaryotes, plants, and animals. The purinergic signaling system is not only ancient in evolution but also omnipresent across species and tissues, involved in highly diverse functions (Verkhratsky and Burnstock, 2014). This signaling system is essential in living organisms because it mediates numerous cellular processes, including neurotransmission, neuromodulation, immune responses, cell proliferation, differentiation and death in development, regeneration, wound healing, cancer, and aging (Burnstock, 2012). Purines as signaling molecules in internal tissues of organisms are now widely accepted. Purinergic communication between individuals has also been found in a few organisms such as fish and polychaetes as mentioned above. As purines are most likely to occur as mixtures both in internal tissues and external environments (Shamchuk et al., 2018), our finding that the combination and ratio of purines is critical for communication in aquatic mussels has significant implications for the study of purinergic signaling in general.
We have shown that M. sallei MCF contains three purines, Ado, Ino, and Hyp, which can induce conspecific larval settlement and metamorphosis. Our discovery that the aggregation pheromone of M. sallei consists of a synergistic blend of these three purines, most active in a specific ratio allows us to begin to understand how different species of marine bivalve can communicate effectively in the same environment. We further suggest that a blend of Hyp, Ino, and Ado at the optimum ratio induces M. sallei larvae to settle and is a critical driver for the development of dense aggregations of this bivalve in the natural environment. Similar pheromone-driven aggregation mechanisms are likely to exist in other invasive dreissenid mussels. Throughout the chemical ecology literature, pheromone molecules are commonly reported to be unique compounds (Wyatt, 2014). However, this work indicates that further study of simple common metabolites that can act as species-specific pheromones when present in specific combinations, like a chemical combination lock, is warranted. In addition, the discovery of settlement inhibition by specific mixtures of purines suggests approaches to the prevention of marine biofouling.
Limitations of the Study
Our results suggested that M. sallei adults release a blend of three purines as the aggregation pheromone to induce settlement and metamorphosis of conspecific larvae. However, the mechanism of the synergetic effect of the three purines, Ado, Ino, and Hyp, on settlement of M. sallei larvae is unknown. Moreover, whether this pheromone plays an important role in M. sallei aggregation in the complex and dynamic natural environment remains to be confirmed, although we have demonstrated that Ado could induce larval settlement of M. sallei under flow conditions in laboratory. Further study will be needed to address these issues.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This research was supported by the National Natural Science Foundation of China under grant 41276127, the National Key Research and Development Program of China under grant 2018YFC1407505, the National Marine Economic Development Demonstration Project in Xiamen under grant 16CZB023SF12, the Science and Technology Project of Fujian Province under grant 2018Y0076, and the Project of Subsidy Funds for Marine Economic Development in Fujian Province under grant FJHJF-L-2018-2. We thank S. Tang for help in structural identification of compounds.
Author Contributions
J.H., D.F., P.S., M.H., and C.K. designed research. J.H., D.F., Q.D., Y.Q., Z.W., and Q.F. performed research. J.G.B. provided constructive suggestions. All authors wrote the paper.
Declaration of Interests
The authors declare no competing interests.
Published: September 27, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.08.022.
Contributor Information
Caihuan Ke, Email: chke@xmu.edu.cn.
Danqing Feng, Email: dqfeng@xmu.edu.cn.
Supplemental Information
References
- Abou-Hussein D.R., Badr J.M., Youssef D.T.A. Nucleoside constituents of the Egyptian tunicate Eudistoma laysani. Nat. Prod. Sci. 2007;13:229–233. [Google Scholar]
- Aldridge D.C., Salazar M., Serna A., Cock J. Density-dependent effects of a new invasive false mussel, Mytilopsis trautwineana (Tryon 1866), on shrimp, Litopenaeus vannamei (Boone 1931), aquaculture in Colombia. Aquaculture. 2008;281:34–42. [Google Scholar]
- Aldridge D.C., Elliott P., Moggridge G.D. Microencapsulated biobullets for the control of biofouling zebra mussels. Environ. Sci. Technol. 2006;40:975–979. doi: 10.1021/es050614+. [DOI] [PubMed] [Google Scholar]
- Almeida J.R., Vasconcelos V. Natural antifouling compounds: effectiveness in preventing invertebrate settlement and adhesion. Biotechnol. Adv. 2015;33:343–357. doi: 10.1016/j.biotechadv.2015.01.013. [DOI] [PubMed] [Google Scholar]
- Burke R.D. Pheromones and gregarious settlement of marine invertebrate larvae. Bull. Mar. Sci. 1986;39:323–331. [Google Scholar]
- Burnstock G. Purinergic signalling: its unpopular beginning, its acceptance and its exciting future. Bioessays. 2012;34:218–225. doi: 10.1002/bies.201100130. [DOI] [PubMed] [Google Scholar]
- Cai L.Z., Hwang J.S., Dahms H.U., Fu S.J., Zhuo Y., Guo T. Effect of the invasive bivalve Mytilopsis sallei on the macrofaunal fouling community and the environment of Yundang Lagoon, Xiamen, China. Hydrobiologia. 2014;741:101–111. [Google Scholar]
- Chenon M.T., Pugmire R.J., Grant D.M., Panzica R.P., Townsend L.B. Carbon-13 magnetic resonance. XXVI. A quantitative determination of the tautomeric populations of certain purines. J. Am. Chem. Soc. 1975;97:4636–4642. doi: 10.1021/ja00849a028. [DOI] [PubMed] [Google Scholar]
- Cunliffe M. Purine catabolic pathway revealed by transcriptomics in the model marine bacterium Ruegeria pomeroyi DSS-3. FEMS Microbiol. Ecol. 2015;92:1–6. doi: 10.1093/femsec/fiv150. [DOI] [PubMed] [Google Scholar]
- Danchin E., Wagner R.H. Benefits of membership. Science. 2000;287:803. doi: 10.1126/science.287.5454.803e. [DOI] [PubMed] [Google Scholar]
- Dreanno C., Matsumura K., Dohmae N., Takio K., Hirota H., Kirby R.R., Clare A.S. An α2-macroglobulin-like protein is the cue to gregarious settlement of the barnacle Balanus amphitrite. Proc. Natl. Acad. Sci. U S A. 2006;103:14396–14401. doi: 10.1073/pnas.0602763103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzierżyńska-Białończyk A., Skrzypczak A., Kobak J. Happy together? Avoidance of conspecifics by gregarious mussels. Curr. Zool. 2018;64:53–61. doi: 10.1093/cz/zox022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghose R. Interaction of adenosine, guanosine and inosine with ruthenium hydride complexes. J. Chem. Res. 2009;2009:52–55. [Google Scholar]
- Idzko M., Ferrari D., Eltzschig H.K. Nucleotide signalling during inflammation. Nature. 2014;509:310–317. doi: 10.1038/nature13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy V.S. The invasive dark false mussel Mytilopsis leucophaeata (Bivalvia: Dreissenidae): a literature review. Aquat. Ecol. 2011;45:163–183. [Google Scholar]
- Li K., Buchinger T.J., Li W. Discovery and characterization of natural products that act as pheromones in fish. Nat. Prod. Rep. 2018;35:501–513. doi: 10.1039/c8np00003d. [DOI] [PubMed] [Google Scholar]
- Martins S.E., Fillmann G., Lillicrap A., Thomas K. Reviews: ecotoxicity of organic and organo-metallic antifouling co-biocides and implications for environmental hazard and risk assessments in aquatic ecosystems. Biofouling. 2018;34:34–52. doi: 10.1080/08927014.2017.1404036. [DOI] [PubMed] [Google Scholar]
- Massé K., Bhamra S., Eason R., Dale N., Jones E.A. Purine-mediated signalling triggers eye development. Nature. 2007;449:1058–1062. doi: 10.1038/nature06189. [DOI] [PubMed] [Google Scholar]
- Meehan S., Gruber B., Lucy F.E. Zebra mussel control using Zequanox® in an Irish waterway. Manag. Biol. Invasion. 2014;5:279–286. [Google Scholar]
- Meier L.R., Zou Y., Millar J.G., Mongold-Diers J.A., Hanks L.M. Synergism between enantiomers creates species-specific pheromone blends and minimizes cross-attraction for two species of cerambycid beetles. J. Chem. Ecol. 2016;42:1181–1192. doi: 10.1007/s10886-016-0782-z. [DOI] [PubMed] [Google Scholar]
- Michalak A.M. Environmental sciences: troubled waters on the Great Lakes. Nature. 2017;543:488. [Google Scholar]
- Molloy D.P., Mayer D.A., Giamberini L., Gaylo M.J. Non-target trials with Pseudomonas fluorescens strain CL145A, a lethal control agent of dreissenid mussels (Bivalvia: Dreissenidae) Manag. Biol. Invasion. 2013;4:71–79. doi: 10.1016/j.jip.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Morton B. The biology and functional morphology of Mytilopsis sallei (Recluz) (Bivalvia: Dreissenacea) fouling Visakhapatnam Harbour, Andhra Pradesh, India. J. Molluscan Stud. 1981;47:25–42. [Google Scholar]
- Morton B. Life-history characteristics and sexual strategy of Mytilopsis sallei (Bivalvia: Dreissenacea), introduced into Hong Kong. J. Zool. 1989;219:469–485. [Google Scholar]
- Parrish J.K., Edelstein-Keshet L. Complexity, pattern, and evolutionary trade-offs in animal aggregation. Science. 1999;284:99–101. doi: 10.1126/science.284.5411.99. [DOI] [PubMed] [Google Scholar]
- Pimentel D., Zuniga R., Morrison D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 2005;52:273–288. [Google Scholar]
- Qian P.Y., Li Z., Xu Y., Li Y., Fusetani N. Mini-review: marine natural products and their synthetic analogs as antifouling compounds: 2009–2014. Biofouling. 2015;31:101–122. doi: 10.1080/08927014.2014.997226. [DOI] [PubMed] [Google Scholar]
- Ram J.L., Fong P.P., Garton D.W. Physiological aspects of zebra mussel reproduction: maturation, spawning, and fertilization. Am. Zool. 1996;36:326–338. [Google Scholar]
- Saladino R., Crestini C., Neri V., Ciciriello F., Costanzo G., Di Mauro E. Origin of informational polymers: the concurrent roles of formamide and phosphates. Chembiochem. 2006;7:1707–1714. doi: 10.1002/cbic.200600139. [DOI] [PubMed] [Google Scholar]
- Shamchuk A.L., Blunt B.J., Lyons D.D., Wang M.Q., Gasheva A., Lewis C.R., Tomlin K., Hazard E.S., Hardiman G., Tierney K.B. Nucleobase-containing compounds evoke behavioural, olfactory, and transcriptional responses in model fishes. FACETS. 2018;3:79–102. [Google Scholar]
- Shikuma N.J., Pilhofer M., Weiss G.L., Hadfield M.G., Jensen G.J., Newman D.K. Marine tubeworm metamorphosis induced by arrays of bacterial phage tail–like structures. Science. 2014;343:529–533. doi: 10.1126/science.1246794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokstad E. Feared quagga mussel turns up in western United States. Science. 2007;315:453. doi: 10.1126/science.315.5811.453. [DOI] [PubMed] [Google Scholar]
- Symonds M.R., Elgar M.A. The evolution of pheromone diversity. Trends. Ecol. Evol. 2008;23:220–228. doi: 10.1016/j.tree.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Toonen R.J., Pawlik J.R. Foundations of gregariousness. Nature. 1994;370:511–512. [Google Scholar]
- Tošenovský E., Kobak J. Impact of abiotic factors on aggregation behaviour of the zebra mussel Dreissena polymorpha. J. Molluscan Stud. 2016;82:55–66. [Google Scholar]
- Verkhratsky A., Burnstock G. Biology of purinergic signalling: its ancient evolutionary roots, its omnipresence and its multiple functional significance. Bioessays. 2014;36:697–705. doi: 10.1002/bies.201400024. [DOI] [PubMed] [Google Scholar]
- Willan R.C., Russell B.C., Murfet N.B., Moore K.L., McEnnulty F.R., Horner S.K., Hewitt C.L., Dally G.M., Campbell M.L., Bourke S.T. Out-break of Mytilopsis sallei (Recluz, 1849)(Bivalvia: Dreissenidae) in Australia. Molluscan Res. 2000;20:25–30. [Google Scholar]
- Wakisaka N., Miyasaka N., Koide T., Masuda M., Hiraki-Kajiyama T., Yoshihara Y. An adenosine receptor for olfaction in fish. Curr. Biol. 2017;27:1437–1447. doi: 10.1016/j.cub.2017.04.014. [DOI] [PubMed] [Google Scholar]
- Wang H.L., Zhao C.H., Wang C.Z. Comparative study of sex pheromone composition and biosynthesis in Helicoverpa armigera, H. assulta and their hybrid. Insect Biochem. Mol. Biol. 2005;35:575–583. doi: 10.1016/j.ibmb.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Wong Y.T., Meier R., Tan K.S. High haplotype variability in established Asian populations of the invasive Caribbean bivalve Mytilopsis sallei (Dreissenidae) Biol. Invasions. 2011;13:341–348. [Google Scholar]
- Wyatt T.D. Cambridge University Press; 2014. Pheromones and Animal Behavior: Chemical Signals and Signatures. [Google Scholar]
- Yebra D.M., Kiil S., Dam-Johansen K. Antifouling technology—past, present and future steps towards efficient and environmentally friendly antifouling coatings. Progr. Org. Coating. 2004;50:75–104. [Google Scholar]
- Zeeck E., Harder T., Beckmann M. Uric acid: the sperm-release pheromone of the marine polychaete Platynereis dumerilii. J. Chem. Ecol. 1998;24:13–22. [Google Scholar]
- Zeeck E., Harder T., Beckmann M. Inosine, L-glutamic acid and L-glutamine as components of a sex pheromone complex of the marine polychaete Nereis succinea (Annelida: Polychaeta) Chemoecology. 1998;8:77–84. [Google Scholar]
- Zimmer R.K., Butman C.A. Chemical signaling processes in the marine environment. Biol. Bull. 2000;198:168–187. doi: 10.2307/1542522. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.