Abstract
Regional recurrence of endometrial cancer is a challenging yet potentially curable group of patients without defined standard of care. Our aim is to determine optimal methods of salvage therapy for regionally recurrent endometrial cancer.
Twenty-two cases of nodal, pelvic, or peritoneal cavity recurrences of endometrial cancer were identified from a single institution database. Univariable Cox proportional hazards models were used to estimate the risk of a second recurrence or death. Kaplan-Meier plots were used to estimate the probability of progression free survival and overall survival among patients in three cohorts: Multimodality therapy (surgery, chemotherapy, and external beam radiotherapy [EBRT] +/− vaginal brachytherapy), non-surgery (chemotherapy or EBRT, or both), and surgery cohort (surgery +/− chemotherapy OR EBRT).
Thirteen recurrences (59%) were regional including the pelvic and paraaortic nodes, while nine recurrences (41%) were abdominal. For the entire cohort, the probability of progression free survival at 2 years was 51% (95% CI, 26% - 72%). The 2-year probability of progression free survival was 62% in the multimodality cohort, 40% in the non-surgery cohort, and 38% in the surgery cohort. The 2-year probability of overall survival was 69% (95% CI, 38% - 86%) across our population. At 40 months of follow up, the only living patients belonged to the multimodality cohort.
We found no significant association of a definitive salvage regimen for recurrent endometrial cancer of the pelvis and peritoneal cavity. Aggressive use of multimodality therapy with surgery followed by tumor-directed radiotherapy and chemotherapy offers potentially curative therapy for these patients.
Keywords: Salvage therapy, Recurrent endometrial cancer, Cytoreductive surgery, Multimodality treatment
Highlights
-
•
Multimodality therapy results in fewer recurrences (31%) than surgery alone (50%) or non-surgery regimens (60%).
-
•
Multimodality therapy offers favorable progression free survival with 62% of patients disease free at 24 months.
-
•
Multimodality therapy can result in cure in patients with isolated recurrences to the pelvis and peritoneal cavity.
1. Introduction
In 2018 there were an estimated 63,230 new cases of endometrial cancer and 11,350 deaths due to endometrial cancer (Key Statistics for Endometrial Cancer, n.d.). Recurrence rates range from 2% with early, well-differentiated pathology to 50% with advanced stage or aggressive histology (Howlader et al., 1975-2014; Salani et al., 2017). While adjuvant therapy and surveillance help minimize and detect these recurrences early, a significant number of patients experience disease recurrence.
Vaginal cuff recurrences can be salvaged with radiation therapy with 5 year overall survival of 33–84% and a 5 year disease specific survival of 51%–77%(Jhingran et al., 2003; Sears et al., 1994; Nag et al., 1997; Creutzberg et al., 2003; Chapman et al., 2017; Vargo et al., 2014; Jereczek-Fossa et al., 2000; Baek et al., 2016). Improved outcomes have been shown with the combination of external beam radiotherapy (EBRT) and brachytherapy (Jhingran et al., 2003; Sears et al., 1994; Nag et al., 1997). An evidence-based regimen for treating isolated non-vaginal recurrences of endometrial cancer has not yet been defined. Historically, surgery was reserved for recurrences after radiotherapy and chemotherapy and consisted of partial or complete exenteration (Barakat et al., 1999). In more recent years, however, some have performed surgical cytoreduction earlier in the treatment of recurrences (Turan et al., 2015; Awtrey et al., 2006; Dowdy et al., 2006; Scarabelli et al., 1998). In a study of patients with recurrent endometrial cancer, Bristow et al. demonstrated significantly longer median survival in patients who underwent salvage cytoreductive surgery compared to those treated without surgery (Bristow et al., 2006).
There is limited data on the role of adjuvant chemotherapy, radiation therapy, and combination chemoradiation in this population of non-vaginal pelvic and abdominal recurrences of endometrial cancer. Here we examine cytoreductive surgery and chemoradiation, radiation therapy, and chemotherapy as adjuvant therapies in treating recurrent endometrial cancer. The aim of this study is to report the survival and toxicity outcomes of patients with non-vaginal pelvic and abdominal recurrences of endometrial cancer.
2. Materials and methods
2.1. Data acquisition
A retrospective review protocol was approved by the Loyola University Medical Center Institutional Review Board. Our database was analyzed from 2007 to 2018 with 22 cases of nodal, pelvic, or peritoneal cavity recurrences identified. Vaginal cuff recurrences were excluded. Isolated recurrences to the pelvic sidewall, pelvic or paraaortic lymph nodes, and sigmoid colon were grouped as greater pelvis. Recurrences to the surface of the liver, omentum, and abdominal wall were classified as abdomen. Concurrent pelvic and abdominal recurrences were included with the abdomen group. We collected data on variables including patient demographics, histology, stage, tumor characteristics, microsatellite instability status, and adjuvant therapy received at initial diagnosis. Additional data were gathered at the time of recurrence on surgical management and adjuvant therapy including whether the chemotherapy was administered concurrently with radiation (chemoradiation) or following radiation (sequential chemo). Regarding salvage therapy, the non-surgery cohort included patients who received chemotherapy alone, EBRT alone, or chemotherapy and EBRT. The surgery cohort included patients who underwent surgery alone, surgery and EBRT, or surgery and chemotherapy. The multimodality cohort included patients who underwent a combination of surgery, EBRT, and chemotherapy with or without vaginal brachytherapy.
2.2. Statistical analyses
Univariable Cox proportional hazards models were used to estimate the risk of a second recurrence or mortality as a function of patient and disease factors. In these models, time was measured in months from the date of patients' first recurrence to the date of second recurrence or death, whichever came first. Living patients not experiencing a second recurrence or death were censored at the last known time they were alive and in remission.
For each model, the proportional hazards assumption was assessed graphically using Martingale residuals (Lin et al., 1993). Due to observing few events, all conclusions were confirmed using Fisher exact tests for nominal comparisons and exact Wilcoxon rank-sum tests for continuous and ordinal comparisons. Univariable Cox proportional hazards models were also used to compare the risk of recurrence or mortality as well as the overall risk of mortality among these three cohorts. As before, Fisher's exact tests were used to confirm model conclusions, and Kaplan-Meier plots were used to estimate event-free probabilities. All analyses were completed using SAS version 9.4 (Cary, NC).
3. Results
Twenty-two patients met inclusion criteria. The median age at initial surgery was 63.6 years (interquartile range [IQR] 60.2–67.6 years) and at first recurrence was 65.4 years (IQR 63.1–69.5 years) (Table 1). Seventy-three percent (16/22) of patients received adjuvant therapy after their initial surgery. Nine (40.9%) patients received adjuvant vaginal brachytherapy, nine (40.9%) received EBRT, and four (18.2%) received chemotherapy as adjuvant therapy. Combinations of adjuvant therapies received are listed in Table 1. Additional demographics of initial diagnosis including histology, stage, lymphovascular space invasion, and microsatellite instability status are found in Table 2. Patients were divided into three cohorts based on the salvage treatment received (Table 2). Thirteen (59.1%) of 22 patients were included in the multimodality cohort, 4 (18.2%) in the surgery cohort, and 5 (22.7%) in the non-surgery cohort (Table 2). None of the patients received bevacizumab or immunotherapies for salvage treatment of their first recurrence. Patient outcomes are described in supplemental table 1.
Table 1.
Demographics related to initial surgery.
| Median age at initial surgery (Interquartile range) | 63.6 (60.2–67.6) |
|---|---|
| Median age at recurrence (Interquartile range) | 65.4 (63.1–69.5) |
| Initial Stage | |
| IA | 13 (59.1%) |
| IB | 3 (13.6%) |
| II | 3 (13.6%) |
| IIIA | 2 (9.1%) |
| IIIC1 | 1 (4.5%) |
| Histology | |
| Endometrioid | 16 (72.7%) |
| Serous | 2 (9.1%) |
| Clear Cell | 2 (9.1%) |
| Carcinosarcoma | 2 (9.1%) |
| Lymphovascular Space Invasion | |
| Present | 6 (27.3%) |
| Absent | 16 (72.7%) |
| Microsatellite Instability | |
| Intact | 3 (13.6%) |
| MLH1, PMS2 lost | 5 (22.7%) |
| PMS2 lost | 1 (4.5%) |
| No data reported | 13 (59.1%) |
| Adjuvant Therapy | |
| None | 6 (27.3%) |
| Vaginal Brachytherapy Alone | 5 (22.7%) |
| External Beam Radiation Therapy Alone | 3 (13.6%) |
| Hormonal Therapy Alone | 1 (4.5%) |
| Vaginal Brachytherapy, Chemotherapy | 1 (4.5%) |
| Vaginal Brachytherapy, External Beam Radiation Therapy | 3 (13.6%) |
| External Beam Radiation Therapy, Chemotherapy | 2 (9.1%) |
| Vaginal Brachytherapy, External Beam Radiation Therapy, Chemotherapy | 1 (4.5%) |
Note: N = 22. MLH1 = MLH1 gene. PMS2 = PMS2 gene. Chemotherapy = systemic chemotherapy.
Table 2.
Demographics related to salvage treatment.
| Location of Recurrence | |
|---|---|
| Greater Pelvis | 13 (59.1%) |
| Abdomen | 9 (40.9%) |
| Salvage Therapy Regimens | |
| Multimodality therapy | 13 (59.1%) |
| Surgery (with or without chemotherapy or external beam radiation therapy) | 4 (18.2%) |
| Non-surgery (chemotherapy or external beam radiation therapy, or both) | 5 (22.7%) |
| Patients who received chemotherapy | |
| Yes | 19 (86.4%) |
| No | 3 (13.6%) |
| Patients who received radiotherapy (external beam radiation therapy +/− vaginal brachytherapy) | |
| Yes | 17 (77.3%) |
| No | 5 (22.7%) |
| Received chemoradiation | |
| Yes | 10 (45.5%) |
| No | 12 (54.5%) |
| Surgical Procedures Performed (n = 17) | |
| Excision of abdominal wall/rectus mass | 4 (23.5%) |
| Tumor debulking | 7 (41.2%) |
| Lymphadenectomy | 6 (35.3%) |
| Vaginal cuff resection | 1 (5.9%) |
| Bowel resection | 5 (29.4%) |
| Intraoperative radiation | 1 (5.9%) |
| Partial cystectomy | 1 (5.9%) |
| Transurethral resection of bladder tumor | 1 (5.9%) |
Note: N = 22 unless otherwise specified. Multimodality therapy includes surgery, chemotherapy, and radiotherapy with or without vaginal brachytherapy.
Recurrence in the pelvis or abdomen was diagnosed at a median of 16.0 months (IQR 8.2–23.2 months) after the completion of surgery or adjuvant therapy. Twelve (54.5%) recurrences were diagnosed based on symptoms that prompted imaging, 3 (13.6%) patients presented with symptoms that prompted biopsy, 5 (22.7%) had rising CA125 followed by imaging, and 2 (9.1%) recurrences were incidentally found on imaging. Thirteen patients (59.1%) recurred in the greater pelvis, whereas 9 (40.1%) had recurrences in the abdomen. Twenty-six surgical procedures were performed in 17 operations as described in Table 2.
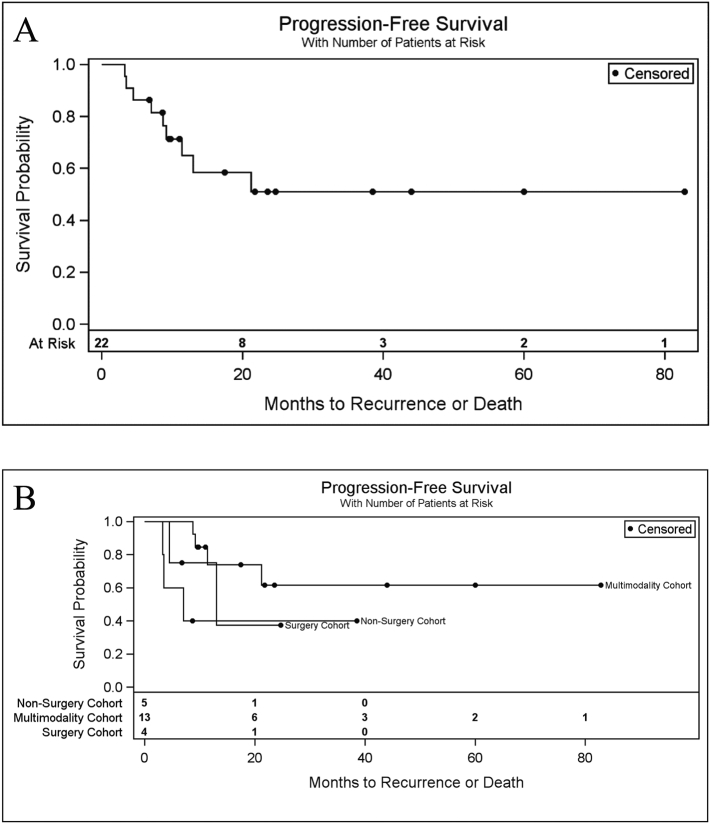
Two-year progression free survival (PFS) for all recurrences in both the pelvis and peritoneal cavity was 51% (95% confidence interval [CI]: 26–72%) (Fig. 1A). Two-year PFS was greater in the multimodality cohort than in the non-surgery and surgery cohorts (Fig. 1B). The 2-year PFS in patients receiving multimodality therapy was 62% compared to 40% in the non-surgery cohort and 38% in the surgery cohort. Of the 13 patients in the multimodality cohort, 2 patients reached 5 years without recurrence or death (Fig. 1).
Fig. 1.
Cumulative probability of progression free survival at two years was 51%. A. Progression free survival of study population. B. Progression free survival by salvage therapy cohort. The multimodality cohort had increased progression free survival compared to non-surgery or surgery cohorts.
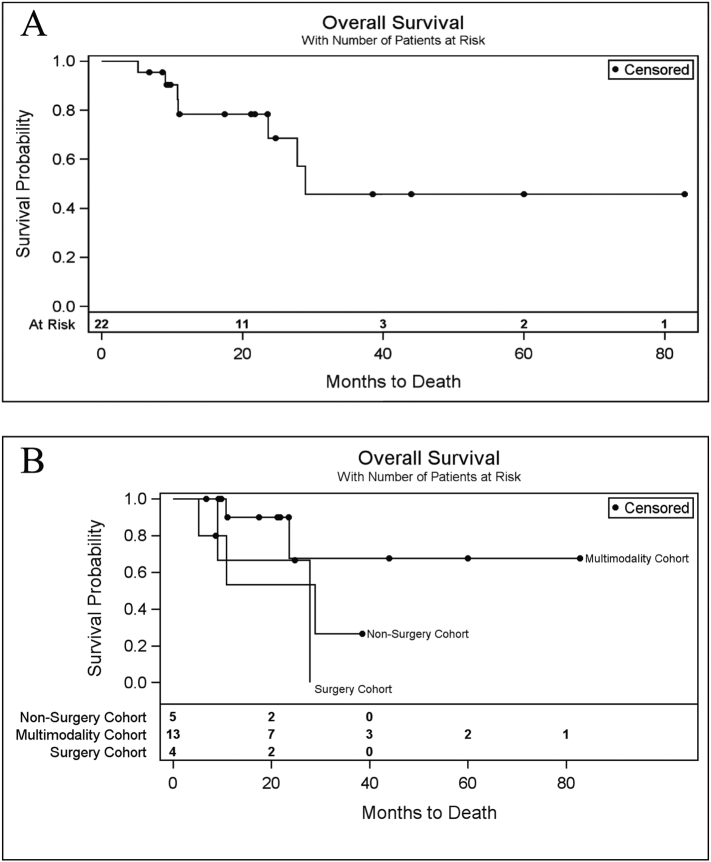
Two-year overall survival (OS) was estimated to be 69% (95% CI: 38–86%) (Fig. 2A). Similar 2-year OS was seen in the sample of patients receiving multimodality therapy (68%) and surgery (67%), but OS was lower among patients in the non-surgery cohort (53%) (Fig. 2B). At 40 months of follow up, the only living patients belonged to the multimodality cohort.
Fig. 2.
Cumulative probability of overall survival at two years was 69%. A. Overall survival of study population. B. Overall survival by salvage therapy cohort. The multimodality cohort had improved overall survival compared to non-surgery or surgery cohorts.
Univariable analyses did not reveal any statistically significant factors associated with the risk of recurrence or death. Advanced stage trended toward being hazardous (hazard ratio [HR] = 3.08, p = .10) as was the presence of lymphovascular space invasion (HR = 3.41, p = .12). Similarly, in this sample receipt of use of chemoradiation for adjuvant salvage therapy trended toward a decreased risk of progression or death for chemoradiation (HR = 0.37, p = .17) as well as for sequential chemotherapy (HR = 0.38, p = .16). The salvage treatment received was not statistically significant (overall p = .17), but patients in the multimodality cohort experienced lower crude rates recurrences (31%) than those in the surgery cohort (50%) and non-surgery cohort (60%) [Supplemental Table 2]. Additional univariable analyses are included in Supplemental Table 3.
Toxicity of salvage therapy was limited. Radiation toxicities were all grade 1–2 acute toxicities as listed in Supplemental Table 4. Only grade 1 late toxicity was noted. Eight of 19 patients undergoing chemotherapy required dose reduction due to hematologic toxicities or neuropathy (Supplemental Table 4).
4. Discussion
Pelvic and abdominal recurrence of surgically managed endometrial cancer is a challenging and life-threatening condition. No meaningful association of a definitive salvage regimen and survival benefit for recurrent endometrial cancer of the pelvis and peritoneal cavity was determined, though this study was underpowered due to limited patient numbers given the scarcity of this pattern of recurrence. PFS was favorable in the multimodality cohort, who received surgery, chemotherapy, and EBRT +/− vaginal brachytherapy, with 62% of patients being disease free at 24 months. Additionally, toxicity of multimodality salvage therapy was limited. There is limited available data on the outcomes of patients with non-vaginal pelvic or abdominal recurrences without distant metastases regarding survival and toxicity outcomes, and clinical trials would be warranted to explore to optimal combination of multimodality therapy. To our knowledge this is the first study that focuses on the role of multi-modality therapy and potential impact on survival outcomes.
Turan et al. (Turan et al., 2015) examined 34 patients with recurrent endometrial cancer, 22 of whom underwent laparotomy. Receipt of adjuvant therapy and optimal surgical debulking of the recurrence were associated with improved overall survival. Patients optimally debulked had an overall survival of 53 months compared to 5 months in those not optimally debulked indicating the role for surgery in patients for whom optimal debulking is possible (Turan et al., 2015).
Ren et al. (Ren et al., 2014) retrospectively analyzed outcomes for 75 patients with recurrent endometrial cancer. Thirty-five of 75 (46.7%) patients had an isolated site of recurrence, which is similar to our patient population. Sixty-four percent (48/75) received chemotherapy, 8% (6/75) received radiotherapy, and 10.7% (8/75) received chemotherapy and radiotherapy as salvage therapy after surgery for recurrence. In this study, 62.7% (47/75) of patients had disease progression, and median survival was 18 months. During follow up 41.3% (31/75) patients were alive without disease (Ren et al., 2014). Even patients who are optimally debulked are highly likely to harbor microscopic disease in high-risk regions. The high rate of disease progression in this study by Ren et al. may be related to a low rate of radiotherapy in their series, and comparatively, more patients underwent multimodality salvage therapy in our study (59%).
Bristow et al. (Bristow et al., 2006) evaluated 61 patients and determined that salvage surgery and residual disease status were correlated with post-recurrence survival in patients with recurrent endometrial cancer. Patients treated with surgery had a median survival after recurrence of 28 months compared to 13 months for those with recurrence treated without surgery (p < .0001). In their study, of those undergoing surgery, 57.1% (20/35) received systemic chemotherapy, 42.9% (15/35) received radiation, and 6 of the 35 received both chemotherapy and radiation therapy. None of these studies thoroughly evaluate the impact of adjuvant therapy on survival and disease progression outcomes.
In a meta-analysis of 14 studies and 672 patients with advanced or recurrent endometrial cancer, Barlin et al. (Barlin et al., 2010) determined that complete surgical cytoreduction and adjuvant radiation were significantly associated with increased overall survival. For each 10% incremental increase in patients receiving postoperative radiation, there was an 11 month increase in overall survival. Receipt of adjuvant chemotherapy was associated with decreased survival (Barlin et al., 2010). An important distinguishing point of this study is that it is not limited only to patients with recurrent disease.
We acknowledge that multimodality therapy is not appropriate for all patients with recurrent endometrial cancer. We would recommend against multi-modal therapy for patients with disseminated disease not amenable to surgical resection or disease that cannot be treated within a reasonable tumor-directed radiotherapy plan. Patients with contraindications to surgery (i.e significant co-morbidities), chemotherapy (i.e. blood dyscrasia or significant chemotherapy exposure), or radiotherapy (i.e. prior in field radiotherapy) may not be good candidates or should be referred to a tertiary care center for opinion.
The greatest strength of our study is the inclusion of multiple cases of recurrent endometrial cancer isolated to the pelvis or peritoneal cavity excluding vaginal cuff recurrences and a relatively high use of multimodality adjuvant therapy in our experience. These recurrences are rare, and while challenging to treat, can result in long term survival if aggressively treated. All cases were treated at the same institution by the same gynecologic and radiation oncology teams. These small numbers prohibited performance of multivariate analyses or deriving significant associations between variables leaving the study underpowered for survival endpoints. Additionally, only 22 patients met inclusion criteria over the study period of 11 years. We acknowledge that treatment options and approaches to treatment may have evolved over the course of the study period. This study also has the potential for selection bias as patients with lower burden of disease at recurrence are more likely to undergo resection, and healthier and younger patients may be more likely to receive multimodality adjuvant therapy.
Several hypotheses can be generated from this study. Multimodality therapy with surgery, EBRT, and chemotherapy is feasible and well-tolerated in selected patients with isolated recurrences to the pelvis and peritoneal cavity. Reasonable rates of both progression-free and overall survival with multimodality adjuvant therapy can be achieved in this population, and such an approach should be considered for patients who can tolerate aggressive adjuvant therapy following optimal debulking surgery, and as a result this approach is now standard at our institution. Multi-institutional and/or prospective studies are warranted to help develop an evidenced-based optimal treatment regimen for patients with isolated pelvic or peritoneal cavity endometrial cancer recurrences.
Conflict of interests
All authors indicate that they do not have any financial or personal relationships that could inappropriate influence this work.
Author contributions
Lindsey A. McAlarnen: study design, data analysis, wrote original draft, edited manuscript.
Kelly Ryan: study design, data analysis, edited manuscript.
William Adams: study design, data analysis, figure design, wrote original draft, edited manuscript.
Adam Gliniewicz: data analysis, wrote original draft, edited manuscript.
Abigail D. Winder: edited manuscript, provided supervision and study oversight.
Margaret R. Liotta: edited manuscript, provided supervision and study oversight.
Ronald K. Potkul: edited manuscript, provided supervision and study oversight, provided departmental support.
William Small, Jr.: study design, edited manuscript, provided supervision and study oversight, provided departmental support.
Matthew M. Harkenrider: principal investigator, study design, data analysis, wrote original draft, edited manuscript, provided supervision and study oversight.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gore.2019.05.002.
Appendix A. Supplementary data
Supplemental Table 1: Patient outcomes of the 22 patients included in the analysis. Details of chemotherapy and radiation for recurrence are included. EBRT=external beam radiation therapy. Chemo=systemic chemotherapy. VBT=vaginal brachytherapy. IORT=intraoperative radiation therapy. HDR BT=High dose rate brachytherapy. Gy=Gray. Supplemental Table 2: Univariable progression free survival estimates. While none were statistically significant, the treatment cohorts of surgery and chemo had increased hazard ratios suggesting benefit of the multimodality regimen. Supplemental Table 3: Additional univariable analyses. Supplemental Table 4: Radiation and chemotherapy toxicities.
References
- Awtrey C.S., Cadungog M.G., Leitao M.M., Alektiar K.M., Aghajanian C., Hummer A.J., Barakat R.R., Chi D.S. Surgical resection of recurrent endometrial carcinoma. Gynecol. Oncol. 2006;102:480–488. doi: 10.1016/j.ygyno.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Baek S., Isohashi F., Yamaguchi H., Mabuchi S., Yoshida K., Kotsuma T., Yamazaki H., Tanaka E., Sumida I., Tamari K., Otani K., Seo Y., Suzuki O., Yoshioka Y., Kimura T., Ogawa K. Salvage high-dose-rate brachytherapy for isolated vaginal recurrence of endometrial cancer. Brachytherapy. 2016;15:812–816. doi: 10.1016/j.brachy.2016.08.005. [DOI] [PubMed] [Google Scholar]
- Barakat R.R., Goldman N.A., Patel D.A., Venkatraman E.S., Curtin J.P. Pelvic exenteration for recurrent endometrial cancer. Gynecol. Oncol. 1999;75:99–102. doi: 10.1006/gyno.1999.5536. [DOI] [PubMed] [Google Scholar]
- Barlin J.N., Puri I., Bristow R.E. Cytoreductive surgery for advanced or recurrent endometrial cancer: a meta-analysis. Gynecol. Oncol. 2010;118:14–18. doi: 10.1016/j.ygyno.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Bristow R.E., Santillan A., Zahurak M.L., Gardner G.J., Giuntoli R.L., Armstrong D.K. Salvage cytoreductive surgery for recurrent endometrial cancer. Gynecol. Oncol. 2006;103:281–287. doi: 10.1016/j.ygyno.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Chapman C.H., Maghsoudi K., Littell R.D., Chen L.-M., Hsu I.-C. Salvage high-dose-rate brachytherapy and external beam radiotherapy for isolated vaginal recurrences of endometrial cancer with no prior adjuvant therapy. Brachytherapy. 2017;16:1152–1158. doi: 10.1016/j.brachy.2017.07.002. [DOI] [PubMed] [Google Scholar]
- Creutzberg C.L., van Putten W.L.J., Koper P.C., Lybeert M.L.M., Jobsen J.J., Wárlám-Rodenhuis C.C., De Winter K.A.J., Lutgens L.C.H.W., van den Bergh A.C.M., van der Steen-Banasik E., Beerman H., van Lent M., PORTEC Study Group Survival after relapse in patients with endometrial cancer: results from a randomized trial. Gynecol. Oncol. 2003;89:201–209. doi: 10.1016/s0090-8258(03)00126-4. [DOI] [PubMed] [Google Scholar]
- Dowdy S.C., Mariani A., Cliby W.A., Haddock M.G., Petersen I.A., Sim F.H., Podratz K.C. Radical pelvic resection and intraoperative radiation therapy for recurrent endometrial cancer: technique and analysis of outcomes. Gynecol. Oncol. 2006;101:280–286. doi: 10.1016/j.ygyno.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Howlader N., Noone A.M., Krapcho M., Miller D., Bishop K., Kosary C.L., Yu M., Ruhl J., Tatalovich Z., Mariotto A., Lewis D.R., Chen H.S. In: SEER Cancer Statistics Review. Feuer E.J., Cronin K.A., editors. 1975–2014. (n.d) [Google Scholar]
- Jereczek-Fossa B., Badzio A., Jassem J. Recurrent endometrial cancer after surgery alone: results of salvage radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2000;48:405–413. doi: 10.1016/s0360-3016(00)00642-8. [DOI] [PubMed] [Google Scholar]
- Jhingran A., Burke T.W., Eifel P.J. Definitive radiotherapy for patients with isolated vaginal recurrence of endometrial carcinoma after hysterectomy. Int. J. Radiat. Oncol. Biol. Phys. 2003;56:1366–1372. doi: 10.1016/s0360-3016(03)00414-0. [DOI] [PubMed] [Google Scholar]
- Key Statistics for Endometrial Cancer 2019. https://www.cancer.org/cancer/endometrial-cancer/about/key-statistics.html
- Lin D.Y., Wei L.J., Ying Z. Checking the cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80:557–572. [Google Scholar]
- Nag S., Martínez-Monge R., Copeland L.J., Vacarello L., Lewandowski G.S. Perineal template interstitial brachytherapy salvage for recurrent endometrial adenocarcinoma metastatic to the vagina. Gynecol. Oncol. 1997;66:16–19. doi: 10.1006/gyno.1997.4722. [DOI] [PubMed] [Google Scholar]
- Ren Y., Shan B., Shi D., Wang H. Salvage cytoreductive surgery for patients with recurrent endometrial cancer: a retrospective study. BMC Cancer. 2014;14 doi: 10.1186/1471-2407-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salani R., Khanna N., Frimer M., Bristow R.E., Chen L.-M. An update on post-treatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncology (SGO) recommendations. Gynecol. Oncol. 2017;146:3–10. doi: 10.1016/j.ygyno.2017.03.022. [DOI] [PubMed] [Google Scholar]
- Scarabelli C., Campagnutta E., Giorda G., DePiero G., Sopracordevole F., Quaranta M., DeMarco L. Maximal Cytoreductive surgery as a reasonable therapeutic alternative for recurrent endometrial carcinoma. Gynecol. Oncol. 1998;70:90–93. doi: 10.1006/gyno.1998.5017. [DOI] [PubMed] [Google Scholar]
- Sears J.D., Greven K.M., Hoen H.M., Randall M.E. Prognostic factors and treatment outcome for patients with locally recurrent endometrial cancer. Cancer. 1994;74:1303–1308. doi: 10.1002/1097-0142(19940815)74:4<1303::aid-cncr2820740420>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Turan T., Tasci T., Karalok A., Ureyen I., Kocak O., Turkmen O., Basaran D., Tulunay G. Salvage Cytoreductive surgery for recurrent endometrial Cancer. Int. J. Gynecol. Cancer. 2015;25:1623–1632. doi: 10.1097/IGC.0000000000000543. [DOI] [PubMed] [Google Scholar]
- Vargo J.A., Kim H., Houser C.J., Berhane H., Sukumvanich P., Olawaiye A.B., Kelley J.L., Edwards R.P., Comerci J.T., Huang M., Courtney-Brooks M., Beriwal S. Definitive salvage for vaginal recurrence of endometrial cancer: the impact of modern intensity-modulated-radiotherapy with image-based HDR brachytherapy and the interplay of the PORTEC 1 risk stratification. Radiother. Oncol. 2014;113:126–131. doi: 10.1016/j.radonc.2014.08.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Patient outcomes of the 22 patients included in the analysis. Details of chemotherapy and radiation for recurrence are included. EBRT=external beam radiation therapy. Chemo=systemic chemotherapy. VBT=vaginal brachytherapy. IORT=intraoperative radiation therapy. HDR BT=High dose rate brachytherapy. Gy=Gray. Supplemental Table 2: Univariable progression free survival estimates. While none were statistically significant, the treatment cohorts of surgery and chemo had increased hazard ratios suggesting benefit of the multimodality regimen. Supplemental Table 3: Additional univariable analyses. Supplemental Table 4: Radiation and chemotherapy toxicities.