Abstract
Background:
With 9.1% of all cancer deaths, hepatocellular carcinoma is the second leading cause of cancer deaths worldwide. Due to the increasing prevalence of metabolic syndrome, nonalcoholic fatty liver disease (NAFLD) has evolved into a major risk factor for hepatocellular carcinoma development. Herein, we investigated whether a dietary n-3 polyunsaturated fatty acid (PUFA) supplementation improves the outcome of progressive NAFLD.
Methods:
Feeding three high-fat diets, differing in n-3 and n-6 PUFA contents and ratios (n-3/n-6: 1:8, 1:1, 5:1), the impact of n-3 PUFAs and n-3/n-6 PUFA ratios on NAFLD-related liver fibrosis and tumorigenesis was analyzed in 12- and 20-week-old streptozotocin/high-fat diet (STZ/HFD)-treated mice.
Results:
Feeding of n-3 PUFA-rich diets (1:1 and 5:1) resulted in increased hepatic n-3 PUFA content and n-3/n-6 PUFA ratio with decreased hepatic lipid accumulation. In 20-week-old mice, n-3 PUFA-rich diets alleviated tumor load significantly, with reduced liver/body weight index, tumor size, and tumor number. Finally, these effects were accompanied by a significant improvement of survival of these mice.
Conclusions:
Herein, we showed that increased n-3 PUFA content and n-3/n-6 PUFA ratios lead to improved survival and attenuated tumor progression in STZ/HFD-treated mice. Thus, n-3 PUFAs could be the basis for new therapeutic options against NAFLD-related tumorigenesis.
Keywords: HCC, liver tumors, NAFLD, nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, STAM mice
Introduction
Over the past decades, nonalcoholic fatty liver disease (NAFLD), which is often referred to as hepatic manifestation of the metabolic syndrome, has become the most frequent chronic liver disease.1,2 NAFLD is one of the main causes for liver transplantation,3–5 and was shown to be present in 58.5% of hepatocellular carcinoma (HCC) patients in the United States.6 Hence, progressing from simple steatosis to steatohepatitis (NASH) and cirrhosis, NAFLD is a major risk factor for the development of HCC,7 which is the second leading cause of cancer death worldwide.8 Yet, it is not fully understood which factors cause the progression from steatosis to HCC. However, a chronically inflammatory environment is the basis for hepatocellular carcinogenesis.9 Among other inflammatory cells, macrophages and their secretion of pro-inflammatory cytokines play a crucial role in NAFLD progression, and thus carcinogenesis.10,11 It has long been known that macrophages can polarize towards M1 or M2 phenotypes.12 While M1 polarization is related to progressive NAFLD, M2-polarized macrophages mediate the opposite effect.13
Increasing food intake and sedentary lifestyle contribute substantially to the worldwide epidemic of metabolic syndrome and NAFLD. In recent decades, the dietary n-3/n-6 polyunsaturated fatty acid (PUFA) ratio in industrialized countries has been decreasing dramatically.14 High levels of dietary n-6 PUFA are known to increase inflammation, constrict blood vessels and cause platelet aggregation,15 whereas n-3 PUFAs are well known for their anti-inflammatory properties. N-3 PUFAs have been shown to decrease the risk of, for example, cancer, cardiovascular diseases, diabetes, and obesity.16 N-3 and n-6 PUFAs are endogenously converted to lipid mediators that account for their regulatory properties.17,18 Accordingly, n-3 PUFA-derived specialized proresolving lipid mediators, like resolvins, have been shown to suppress tumor growth.19 Physiologically, the essential fatty acid α-linolenic acid (ALA, n-3) and linoleic acid (LA, n-6) are converted to eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and arachidonic acid (AA), respectively.20 For this process and the subsequent conversion to bioactive lipid mediators, n-3 and n-6 PUFAs compete for the same converting enzymes.17 Consequently, the dietary n-3/n-6 PUFA ratio substantially affects the profile of bioactive lipid mediators and the availability of the fatty acids in tissues.21
Even though numerous positive effects of n-3 PUFAs have been shown in different diseases, including NAFLD, it is still unknown whether increased dietary n-3 PUFA uptake and increased n-3/n-6 PUFA ratios are able to prevent NAFLD-related tumorigenesis. Therefore, we aimed to clarify whether increased dietary n-3 PUFA content and increased n-3/n-6 PUFA ratios alleviate NAFLD-related tumorigenesis.
Material and methods
Mouse model
For assessment of NAFLD-related tumorigenesis, the streptozotocin/high fat diet (STZ/HFD) mouse model was used as described previously by our group.22,23 The mice develop progressive NAFLD and display NASH with light fibrosis at 12 weeks, and liver tumors at 20 weeks of age. In short, male C57BL/6 mice (Charles River, Sulzfeld, Germany) were injected with 200 µg STZ (Sigma-Aldrich, St. Louis, MO, USA) intraperitoneally at day 2 postnatal. At 28 days of age the mice were fed continuous HFDs. The general state of health was monitored daily, and blood glucose levels and body weight were measured weekly. Animals exhibiting normal blood glucose levels were excluded from the experiment. The mice were sacrificed at ages of 12 and 20 weeks and blood and tissue was collected as described previously by our group.23 The experimental design is illustrated in Figure 1(a). The mice were kept on water and food ad libitum at a 12 h light/dark cycle with one to five mice per cage on chipped wood bedding and environmental enrichment in form of a cardboard tube and nesting material. Approval requirements for studies involving animals in Germany are strict and ensure highest scientific, animal welfare, and ethical standards when conducting the studies. Requirements follow federal law laid out in the German Animal Welfare Act (Tierschutzgesetz) and the European Directive 2010/63/EU on the protection of animals used for scientific purposes. Approval to conduct research involving animals is given by the local authority of the state in which the research institution is located (for Mecklenburg-West Pomerania: ‘State Office for Agriculture, Food Safety, and Fishery of Mecklenburg-West Pomerania’, (7221.3-1-022/15)). Compliance with all required and approved standards are enforced on an institutional level by the animal welfare officer of the institution. In addition, regular controls are conducted by the local authorities.
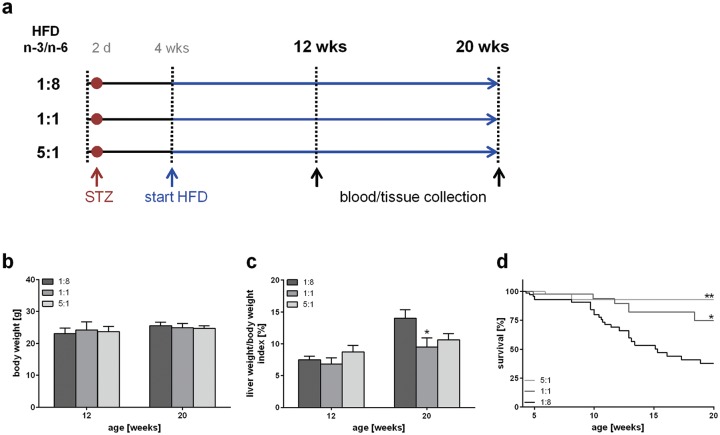
Figure 1.
Experimental design and evaluation of general disease parameters. (a) Experimental design of mice treated with STZ and HFD, differing in n-3 and n-6 PUFA contents and ratios (n-3/n-6: 1:8, 1:1, 5:1). Body weight (b) and liver/body weight index (c) of STZ/HFD-treated mice fed HFDs differing in n-3/n-6 PUFA ratios (1:8, 1:1 and 5:1) (n = 10–12 per group). Values are given as mean ± SEM. Differences between the groups were assessed by two-way ANOVA followed by Tukey’s range test within each time point. *p < 0.05 versus 1:8 fed mice, **p < 0.01 versus 1:8 fed mice. (d) Survival of STZ/HFD-treated mice fed HFDs differing in n-3/n-6 PUFA ratios (1:8, 1:1 and 5:1). Survival curves were created using the product limit method of Kaplan and Meier, and statistical analysis was performed using log-rank test and Bonferroni correction. *p < 0.05 (Bonferroni corrected p < 0.01667) versus 1:8 fed mice (HR: 0.375, 95 % CI; 0.182–0.776), **p < 0.01 (Bonferroni corrected p < 0.0033) versus 1:8 fed mice (HR: 0.129, 95 % CI; 0.129–0.608).
HFD, high-fat diet; PUFA, polyunsaturated fatty acid; SEM, standard error of the mean; STZ, streptozotocin.
Diets
At 28 days of age, the mice were assigned to different groups receiving different HFDs [60 kJ% fat; D12492(II) modified experimental diet; Ssniff, Soest, Germany]. Three diets were fed, which differed in their n-3/n-6 PUFA ratio and n-3 PUFA content (Figure 1a). While the control HFD exhibited an n-3/n-6 PUFA ratio of 1:8, the other two HFD were fish-oil-supplemented, leading to an n-3/n-6 PUFA ratio of 1:1 and 5:1, respectively. An n-6 PUFA oversupply, which is typical for western diets, is reflected by the 1:8 diet, whereas the fish-oil-supplemented diets allow the assessment of a well balanced n-3/n-6 PUFA ratio (1:1) and a marked oversupply of n-3 PUFA (5:1). A fatty acid profiling analysis of the diets (Table 1) confirmed that the 1:1 diet differed from the control diet (1:8) only in its n-3/n-6 PUFA ratio, while the composition of saturated fatty acid (SFA), monounsaturated fatty acid (MUFA), and PUFA was similar. The 5:1 diet contained less SFA and MUFA but more PUFA compared with the 1:8 diet due to high amounts of EPA and DHA, leading to an increased n-3/n-6 PUFA ratio of 5:1 and higher n-3 PUFA content.
Table 1.
Composition of the diets. Protein, fat, fibre, ash and energy content of the diets as provided by the manufacturer. SFA, MUFA and PUFA proportions in the experimental diets (given as percentage of total fatty acids) measured by gas chromatography.
| 1:8 diet | 1:1 diet | 5:1 diet | ||
|---|---|---|---|---|
| Crude protein (%) | 24.4 | 24.4 | 24.4 | |
| Crude fat (%) | 34.6 | 34.6 | 34.6 | |
| Crude fibre (%) | 6.0 | 6.0 | 6.0 | |
| Crude ash (%) | 5.4 | 5.5 | 5.5 | |
| Metabolizable energy (MJ/kg) | 21.6 | 21.5 | 21.5 | |
| SFA (% of total fatty acids) |
C12:0 | 0.09 | 0.11 | 0.18 |
| C14:0 | 3.03 | 3.71 | 5.18 | |
| C15:0 | 0.46 | 0.51 | 0.52 | |
| C16:0 | 26.18 | 26.34 | 21.03 | |
| C17:0 | 1.07 | 1.09 | 0.80 | |
| C18:0 | 21.89 | 21.11 | 12.88 | |
| C20:0 | <0.01 | <0.01 | <0.01 | |
| C22:0 | <0.01 | <0.01 | <0.01 | |
| Sum SFAa | 53.19 | 53.32 | 41.33 | |
| MUFA (% of total fatty acids) |
C16:1cis-9 | 2.84 | 3.60 | 5.39 |
| C18:1cis-9 | 31.58 | 29.79 | 19.17 | |
| C18:1cis-11 | 1.30 | 1.49 | 2.18 | |
| C18:1trans-11 | 1.82 | 1.88 | 0.88 | |
| Sum MUFAb | 38.81 | 38.19 | 29.30 | |
| PUFA (% of total fatty acids) |
C18:2n-6 (LA) | 6.68 | 2.58 | 2.99 |
| C18:3n-3 (ALA) | 0.92 | 0.38 | 0.79 | |
| C18:3n-6 | <0.01 | <0.01 | <0.01 | |
| C20:2n-6 | <0.01 | <0.01 | <0.01 | |
| C20:3n-6 | <0.01 | <0.01 | <0.01 | |
| C20:4n-6 | <0.01 | <0.01 | <0.01 | |
| C20:5n-3 (EPA) | <0.01 | 2.86 | 14.00 | |
| C22:4n-6 | 0.04 | 0.10 | 0.22 | |
| C22:5n-3 | <0.01 | <0.01 | <0.01 | |
| C22:6n-3 (DHA) | <0.01 | 1.58 | 7.70 | |
| Sum PUFAc | 8.00 | 8.48 | 29.37 | |
| Sum n-3 PUFAd | 0.94 | 5.28 | 24.69 | |
| Sum n-6 PUFAe | 6.79 | 2.93 | 4.51 |
Sum SFA: 10:0+11:0+12:0+13:0+14:0+15:0+16:0+17:0+18:0+20:0+21:0+22:0+23:0+24:0.
Sum MUFA: 14:1+15:1+16:1+17:1+18:1t+18:1c9+C18:1c11+C22:1+C24:1.
Sum PUFA: 18:2tr-9,tr-12+18:2n-6+18:3n-3+18:4n-3+20:3n-6+20:4n-6+20:5n-3+22:1+22:4n-6+22:5n-3+22:6n-3+c9,tr11CLA+18:3n-6+20:2n-6+20:3n-3+22:2n-6.
Sum n-3 PUFA: 20:3n-3+22:6n-3+22:5n-3+20:5n-3+18:4n-3+18:3n-3.
Sum n-6 PUFA: 22:2n-6+20:2n-6+18:3n-6+22:4n-6+20:3n-6+18:2n-6+20:4n-6.
MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; SFA, saturated fatty acid.
Hematological measurements and plasma analyses
Alanine aminotransferase (ALT) and glutamate dehydrogenase (GLDH) activity in EDTA plasma, blood glucose levels, and plasma triglyceride content was measured as described previously by our group.23
Histology and immunohistochemistry
Formalin-fixed liver tissue was embedded in paraffin and cut into 5 µm thick sections. The sections were then stained with hematoxylin/eosin (H&E) and Sirius red, respectively. All histological analyses were performed in a blinded manner. NAFLD Activity Score was determined as proposed by Kleiner and colleagues.24 The score for each section was determined by three independent observers. For analysis of Sirius red staining at least 20 photomicrographs were consecutively taken using a 20× objective and a polarization filter. The stained area was then quantified using Adobe Photoshop CS5 Extended 12.0.4 (Adobe, San José, CA, USA). For tumor analysis, photomicrographs of H&E-stained liver sections were taken using a 1.25× objective. The micrographs were then combined with a picture of the whole liver section. Areas of neoplastic foci or tumors, as well as the area of the whole section, were selected and measured using ImageJ 1.47v (Wayne Rasband, National Institutes of Health, Bethesda, MA, USA).
Oil Red O staining was performed as described previously by our group.23 At least 10 photomicrographs were taken per section using a 20× objective. The red stained area was quantified with ImageJ 1.47v.
Immunohistochemical staining against F4/80 was conducted as described previously by our group.23,25 For analysis of the staining, at least 30 consecutive photomicrographs were taken using a 40× objective. The red stained F4/80 positive area was quantified using the color threshold tool in ImageJ 1.47v.
Lipid extraction and fatty acid analysis
After homogenization of frozen liver samples, and the addition of C19:0 as an internal standard, total liver lipids were extracted in duplicate using chloroform/methanol (2:1, v/v) by the use of Ultra Turrax T25 (IKA, Staufen, Germany), 3 × 15 sec, 15,780 × g, at room temperature. The detailed sample preparation procedure has been described previously.26
The fatty acid analysis of the liver lipids was performed using capillary GC with a CP-Sil 88 CB column (100 m × 0.25 mm, Agilent, Santa Clara, CA, USA) that was installed in a PerkinElmer gas chromatograph CLARUS 680 with a flame ionization detector and split injection (PerkinElmer Instruments, Shelton, CT, USA). The detailed GC conditions were recently described.27 Fatty acid concentrations are displayed as the percentage of total fatty acid content in liver tissue.
RT-PCR
RT-PCR analyses were performed as described previously.28 Primers used for amplification are: Collagen 1α forward 5′-TGGACCTCCGGCTCCTGCTC-3′ and reverse 5′-TCGCACACAGCCGTGCCATT-3′, tumor necrosis factor-α (TNF-α) forward 5′-AGGCTCTGGAGAACAGCACAT-3′ and reverse 5′-TGGCTTCTCTTCCTGCACCAAA-3′ and RPS18 forward 5′-AGGATGTGAAGGATGGGAAG-3′ and reverse 5′-TTGGATACACCCACAGTTCG-3′.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 6.05 (GraphPad Software, La Jolla, CA, USA). Differences between the groups were assessed by two-way ANOVA followed by Tukey’s range test within each time point. Statistical significance was set at p < 0.05. Survival curves were created using the product limit method of Kaplan and Meier, and statistical analysis was tested using log-rank test and Bonferroni correction (Bonferroni corrected p-value for statistical significance: p < 0.01667). All data are presented as mean ± SEM.
Results
General aspects
No differences were detectable between the groups regarding body weight (Figure 1b) and blood glucose levels, which were constantly elevated about the entire observation period (~20 mmol/l) (data not shown). While liver weight/body weight index at 12 weeks of age was similar between the groups, the control-diet-fed mice exhibited a higher weight/body weight index (14%) than 1:1 (9.5%) and 5:1 (10.6%) fed mice at an age of 20 weeks (Figure 1c). This reduction was even significant in 1:1 fed mice compared with 1:8 fed mice. Of most interest, the dietary fatty acid composition affected the survival of the mice significantly (Figure 1d). While mice receiving the control diet had a survival of only 37% at 20 weeks, feeding of the 1:1 diet resulted in a significantly improved survival of 75% at 20 weeks. The protective effect of n-3 PUFA enriched diets was even more pronounced upon feeding of the 5:1 diet, resulting in a survival of 93% at 20 weeks (Figure 1d). Nevertheless, the extent of liver injury was not markedly affected by the different diets, as no significant differences in the plasma activity of ALT and GLDH were observed (Table 2). Plasma triglyceride analysis revealed a slight reduction at 20 weeks of age upon feeding n-3 PUFA-rich diets, whereas no differences between the groups were observed at an age of 12 weeks (Table 2).
Table 2.
Plasma analyses. Analysis of triglyceride concentration, ALT and GLDH activities in plasma of 12 and 20 week old STZ/HFD-treated mice receiving HFDs differing in n-3/n-6 PUFA ratios (1:8, 1:1 and 5:1) (n = 6–12 per group). Values are given as mean ± SEM.
| 12 weeks |
20 weeks |
|||||
|---|---|---|---|---|---|---|
| 1:8 | 1:1 | 5:1 | 1:8 | 1:1 | 5:1 | |
| Triglycerides [mg/dL] | 423 ± 151 | 301 ± 116 | 384 ± 153 | 596 ± 228 | 540 ± 174 | 353 ± 156 |
| ALT activity [U/L] | 73 ± 11 | 60 ± 5 | 120 ± 25 | 208 ± 34 | 144 ± 49 | 145 ± 72 |
| GLDH activity [U/L] | 142 ± 73 | 36 ± 7 | 97 ± 21 | 139 ± 32 | 146 ± 85 | 143 ± 84 |
ALT, Alanine aminotransferase; GLDH, glutamate dehydrogenase ; HFD, high-fat diet; PUFA, polyunsaturated fatty acid; STZ, streptozotocin.
Fatty acid profiling
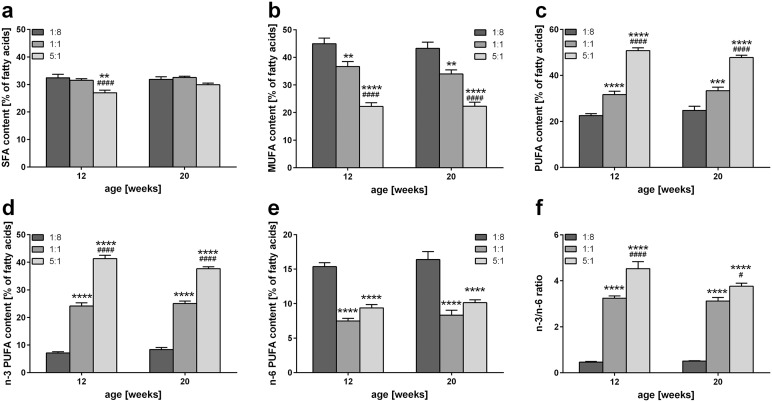
Analysis of fatty acid composition of liver tissue revealed highly significant impact of the diet composition on hepatic fat composition, which was independent of age and disease stage (Table 3, Figure 2). Percentage of SFA of all fatty acids in the liver did not change upon feeding of the 1:1 diet compared with the control diet, whereas feeding of the 5:1 diet decreased SFA percentage at 12 and 20 weeks (Figure 2a). The difference was highly significant at 12 weeks compared with the 1:1 and 1:8 diet. Interestingly, even though the control and 1:1 diet per se did not differ in terms of SFA, MUFA, and PUFA content, the livers of 1:1 and 5:1 fed mice exhibited a significantly lower percentage of MUFA (Figure 2b) and a significantly higher percentage of PUFA (Figure 2c) compared with control mice fed the 1:8 diet. This difference was more pronounced in livers of 5:1 fed mice, which contained half as much MUFA, and twice as much PUFA compared with the liver of mice receiving the control diet. These differences were not only highly significant compared with 1:8 fed mice, but also compared with 1:1 fed mice.
Table 3.
Hepatic fatty acid composition. Hepatic SFA, MUFA and PUFA content of STZ/HFD-treated mice fed HFDs differing in n-3/n-6 PUFA ratios (1:8, 1:1 and 5:1) (n = 10 per group). The values are given as mean ± SEM in % of total fatty acids.
| Fatty acid | 12 weeks |
20 weeks |
|||||
|---|---|---|---|---|---|---|---|
| 1:8 | 1:1 | 5:1 | 1:8 | 1:1 | 5:1 | ||
| SFA | C12:0 | 0.07 ± 0.02 | 0.06 ± 0.02 | 0.08 ± 0.03 | 0.05 ± 0.01 | 0.06 ± 0.02 | 0.07 ± 0.02 |
| C14:0 | 0.72 ± 0.23 | 0.62 ± 0.20 | 0.71 ± 0.22 | 0.54 ± 0.17 | 0.58 ± 0.18 | 0.67 ± 0.21 | |
| C15:0 | 0.25 ± 0.08 | 0.24 ± 0.08 | 0.23 ± 0.07 | 0.21 ± 0.07 | 0.22 ± 0.07 | 0.24 ± 0.08 | |
| C16:0 | 22.31 ± 7.05 | 21.97 ± 6.95 | 16.70 ± 5.28 | 21.03 ± 6.65 | 21.65 ± 6.85 | 20.17 ± 6.38 | |
| C17:0 | 0.68 ± 0.21 | 0.53 ± 0.17 | 0.49 ± 0.16 | 0.63 ± 0.20 | 0.58 ± 0.18 | 0.59 ± 0.19 | |
| C18:0 | 9.38 ± 2.97 | 7.43 ± 2.35 | 8.17 ± 2.58 | 8.80 ± 2.78 | 8.72 ± 2.76 | 8.99 ± 2.84 | |
| C20:0 | 0.19 ± 0.06 | 0.29 ± 0.09 | 0.25 ± 0.08 | 0.19 ± 0.06 | 0.26 ± 0.08 | 0.22 ± 0.07 | |
| C22:0 | 0.08 ± 0.03 | 0.08 ± 0.03 | 0.06 ± 0.02 | 0.08 ± 0.03 | 0.08 ± 0.03 | 0.08 ± 0.03 | |
| Sum SFAa | 33.98 ± 10.75 | 31.50 ± 9.96 | 26.96 ± 8.53 | 31.84 ± 10.07 | 32.50 ± 10.28 | 31.33 ± 9.91 | |
| MUFA | C16:1cis-9 | 1.38 ± 0.44 | 1.95 ± 0.62 | 1.46 ± 0.46 | 1.22 ± 0.39 | 1.51 ± 0.48 | 1.39 ± 0.44 |
| C18:1cis-9 | 35.11 ± 11.10 | 31.95 ± 10.10 | 18.54 ± 5.86 | 38.52 ± 12.18 | 29.78 ± 9.42 | 27.66 ± 8.75 | |
| C18:1cis-11 | 1.44 ± 0.45 | 1.35 ± 0.43 | 1.03 ± 0.33 | 1.73 ± 0.55 | 1.28 ± 0.41 | 1.20 ± 0.38 | |
| C18:1trans-11 | 0.43 ± 0.13 | 0.35 ± 0.11 | 0.25 ± 0.08 | 0.34 ± 0.11 | 0.32 ± 0.10 | 0.34 ± 0.11 | |
| Sum MUFAb | 39.59 ± 12.52 | 36.67 ± 11.59 | 22.20 ± 7.02 | 43.23 ± 13.67 | 33.99 ± 10.75 | 31.64 ± 10.00 | |
| PUFA | C18:2n-6 | 9.29 ± 2.94 | 3.92 ± 1.24 | 4.42 ± 1.40 | 7.20 ± 2.28 | 4.09 ± 1.29 | 5.76 ± 1.82 |
| C18:3n-3 | 0.34 ± 0.11 | 0.19 ± 0.06 | 0.49 ± 0.15 | 0.56 ± 0.18 | 0.16 ± 0.05 | 0.34 ± 0.11 | |
| C18:3n-6 | 0.43 ± 0.14 | 0.07 ± 0.02 | 0.06 ± 0.02 | 0.19 ± 0.06 | 0.05 ± 0.02 | 0.16 ± 0.05 | |
| C20:2n-6 | 0.10 ± 0.03 | 0.04 ± 0.01 | 0.06 ± 0.02 | 0.12 ± 0.04 | 0.05 ± 0.02 | 0.07 ± 0.02 | |
| C20:3n-6 | 0.94 ± 0.30 | 0.34 ± 0.11 | 0.26 ± 0.08 | 0.98 ± 0.31 | 0.48 ± 0.15 | 0.51 ± 0.16 | |
| C20:4n-6 | 7.05 ± 2.23 | 3.03 ± 0.96 | 4.44 ± 1.40 | 7.49 ± 2.37 | 3.54 ± 1.12 | 4.90 ± 1.55 | |
| C20:5n-3 | 0.33 ± 0.11 | 4.79 ± 1.51 | 8.59 ± 2.72 | 0.27 ± 0.09 | 4.88 ± 1.54 | 4.60 ± 1.46 | |
| C22:4n-6 | 0.28 ± 0.09 | 0.06 ± 0.02 | 0.11 ± 0.03 | 0.36 ± 0.12 | 0.07 ± 0.02 | 0.15 ± 0.05 | |
| C22:5n-3 | 0.51 ± 0.16 | 2.01 ± 0.64 | 3.11 ± 0.98 | 0.42 ± 0.13 | 2.06 ± 0.65 | 1.87 ± 0.59 | |
| C22:6n-3 | 6.79 ± 2.15 | 17.01 ± 5.38 | 28.88 ± 9.13 | 6.90 ± 2.18 | 17.81 ± 5.63 | 18.32 ± 5.79 | |
| Sum PUFAc | 26.26 ± 8.30 | 31.63 ± 10.00 | 50.77 ± 16.05 | 24.75 ± 7.83 | 33.34 ± 10.54 | 36.90 ± 11.67 | |
| Sum n-3 PUFAd | 8.13 ± 2.57 | 24.13 ± 7.63 | 41.31 ± 13.06 | 8.36 ± 2.64 | 25.01 ± 7.91 | 25.29 ± 8.00 | |
| Sum n-6 PUFAe | 18.12 ± 5.73 | 7.48 ± 2.36 | 9.38 ± 2.97 | 16.38 ± 5.18 | 8.31 ± 2.63 | 11.58 ± 3.66 | |
HFD, high-fat diet; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; SEM, standard error of the mean; SFA, saturated fatty acid; STZ, streptozotocin.
Sum SFA: 10:0+11:0+12:0+13:0+14:0+15:0+16:0+17:0+18:0+20:0+21:0+22:0+23:0+24:0.
Sum MUFA: 14:1+15:1+16:1+17:1+18:1t+18:1c9+C18:1c11+C22:1+C24:1.
Sum PUFA: 18:2tr-9,tr-12+18:2n-6+18:3n-3+18:4n-3+20:3n-6+20:4n-6+20:5n-3+22:1+22:4n-6+22:5n-3+22:6n-3+c9,tr11CLA+18:3n-6+20:2n-6+20:3n-3+22:2n-6.
Sum n-3 PUFA: 20:3n-3+22:6n-3+22:5n-3+20:5n-3+18:4n-3+18:3n-3.
Sum n-6 PUFA: 22:2n-6+20:2n-6+18:3n-6+22:4n-6+20:3n-6+18:2n-6+20:4n-6.
Figure 2.
Influence of n-3 PUFA rich diets on hepatic fatty acid composition. Quantitative analysis of hepatic SFA (a), MUFA (b), PUFA (c), n-3 PUFA (d), and n-6 PUFA (e) content of STZ/HFD-treated mice fed HFDs differing in n-3/n-6 PUFA ratios (1:8, 1:1 and 5:1) given as percentage of total fatty acids (n = 10 per group). (f) Hepatic n-3/n-6 ratio of STZ/HFD-treated mice fed HFDs differing in n-3/n-6 PUFA ratios (1:8, 1:1 and 5:1) (n = 10 per group). Values are given as mean ± SEM. Differences between the groups were assessed by two way ANOVA followed by Tukey’s range test within each time point. **p < 0.01 versus 1:8 fed mice, ****p < 0.0001 versus 1:8 fed mice, #p < 0.05 versus 1:1 fed mice, ####p < 0.0001 versus 1:1 fed mice.
HFD, high-fat diet; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; SEM, standard error of the mean; SFA, saturated fatty acid; STZ, streptozotocin.
Furthermore, highly significant differences in hepatic n-3 PUFA content were observed in correlation with the composition of the diet. Thus, n-3 PUFA content was increased in livers of 1:1 (~25 %) and 5:1 (~40 %) fed mice compared with livers of 1:8 fed control animals (~8 %) (Figure 2d). Accordingly, the hepatic amount of n-6 PUFAs was strongly reduced in 1:1 and 5:1 fed mice compared with mice receiving the 1:8 control diet, with no difference being present between 1:1 and 5:1 fed mice (Figure 2e). Interestingly, compared with control mice feeding of the 1:1 and 5:1 diet resulted in a significantly higher hepatic n-3/n-6 PUFA ratio of approximately 3:1 and 4:1, respectively (Figure 2f).
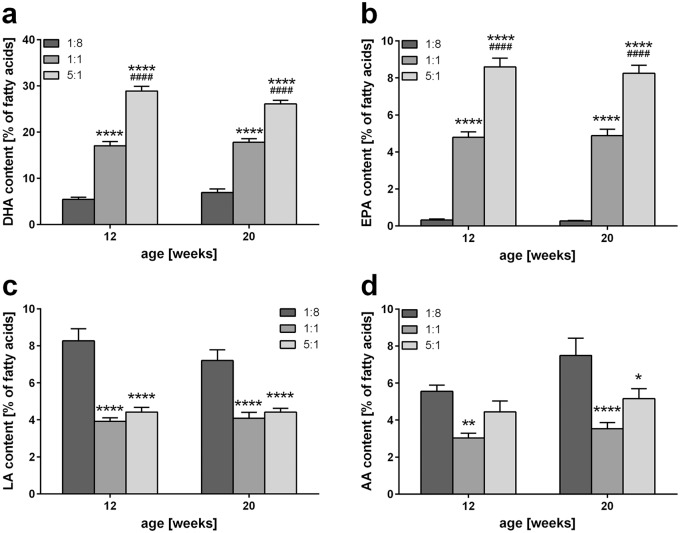
Specific analysis of PUFAs revealed that DHA and EPA were the most abundant n-3 PUFAs observed in liver tissue. As also observed for the n-6 PUFAs LA and AA, profiles of these individual PUFAs (Figure 3) reflected the total hepatic content of n-3 and n-6 PUFAs displayed in Figure 2(d and e). Thus, hepatic DHA and EPA contents (n-3) increased strongly with rising n-3/n-6 PUFA ratio of the diet (Figure 3a and b), while the content of LA and AA (n-6) was reduced upon feeding of the 1:1 and 5:1 diet compared with mice receiving the control diet, with no difference between 1:1 and 5:1 fed mice (Figure 3c and d).
Figure 3.
Analysis of hepatic n-3 and n-6 PUFAs. Quantitative analysis of hepatic DHA (a), EPA (b), LA (c), and AA (d) content of STZ/HFD-treated mice fed HFDs differing in n-3/n-6 PUFA ratios (1:8, 1:1, and 5:1) given as percentage of total fatty acids (n = 10 per group). Values are given as mean ± SEM. Differences between the groups were assessed by two-way ANOVA followed by Tukey’s range test within each time point. *p < 0.05 versus 1:8 fed mice, **p < 0.01 versus 1:8 fed mice, ****p < 0.0001 versus 1:8 fed mice, ####p < 0.0001 versus 1:1 fed mice.
AA, arachidonic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; HFD, high-fat diet; LA, linoleic acid; SEM, standard error of the mean; STZ, streptozotocin.
NAFLD severity
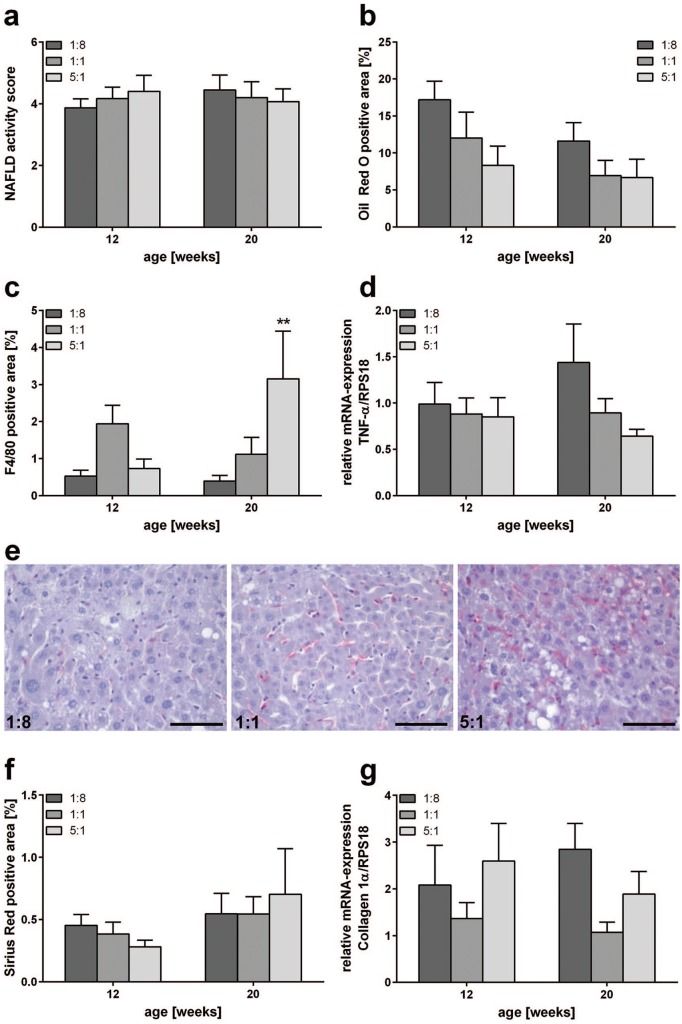
Severity of NAFLD was assessed using the NAFLD activity score, showing no significant differences between the groups at both time points (Figure 4a). Simultaneously, feeding of the 1:1 and 5:1 diet resulted in a marked reduction of fat accumulation (Oil Red O positive area), at both time points, but particularly pronounced at 12 weeks of age (Figure 4b). Analysis of liver macrophages by means of F4/80 staining revealed an increased F4/80 positive area in n-3 PUFA-rich-fed mice at an age of 20 weeks (Figure 4c and e). While liver sections of 1:8 fed control mice exhibited 0.4% F4/80 positive area, in liver sections of 1:1 and 5:1 fed mice, 1.1% and 3.2% of the total area was positively stained, respectively. The difference was even significant in 5:1 fed mice compared with mice receiving the control diet. Even though liver sections of 1:1 fed mice displayed a slightly larger F4/80 positive area, there were no significant differences between the groups at 12 weeks. Of interest, no differences in TNF-α mRNA levels were detected between the groups at 12 weeks of age, while TNF-α mRNA expression declined with increasing n-3 PUFA content of the diet at an age of 20 weeks (Figure 4d). Assessment of collagen deposition by Sirius red staining revealed a slight, but nonsignificant, reduction at the fibrosis stage (12 weeks) upon feeding of the 5:1 diet (Figure 4f). Additionally, there were no significant differences in collagen 1α mRNA levels between the groups at 12 weeks, but, at 20 weeks of age, collagen 1α mRNA levels of n-3 PUFA-rich-fed mice were slightly decreased compared with mice receiving the n-6 PUFA-rich control diet (Figure 4g).
Figure 4.
Assessment of NAFLD progression, inflammation and fibrosis. (a) NAFLD activity score of STZ/HFD-treated mice fed HFDs differing in n-3/n-6 PUFA ratios (1:8, 1:1 and 5:1) (n = 10–12 per group). Histomorphometric quantification of Oil Red O (b), F4/80 (c), and Sirius red (f)-stained liver sections of STZ/HFD-treated mice fed HFDs differing in n-3/n-6 PUFA ratios (1:8, 1:1 and 5:1) (n = 8–12 per group). Values are given as mean ± SEM. Differences between the groups were assessed by two-way ANOVA followed by Tukey’s range test within each time point. **p < 0.01 versus 1:8 fed mice. Quantitative RT-PCR analysis of hepatic TNF-α (d) and Collagen 1α (g) expression of STZ/HFD-treated mice receiving HFDs differing in n-3/n-6 PUFA ratios (1:8, 1:1 and 5:1) (n = 10–12 per group). Values are given as mean ± SEM. Differences between the groups were assessed by two-way ANOVA followed by Tukey’s range test within each time point. **p < 0.01 versus 1:8 fed mice. (e) Representative photomicrographs of F4/80 stained liver sections of 20-week-old STZ/HFD-treated mice receiving HFDs with n-3/n-6 PUFA ratios of 1:8, 1:1 and 5:1, respectively. Scale bar represents 50 µm.
HFD, high-fat diet; NAFLD, nonalcoholic fatty liver disease; RT-PCD, reverse transcriptase-polymerase chain reaction; SEM, standard error of the mean; STZ, streptozotocin; TNF-α, tumor necrosis factor-α.
Tumor analysis
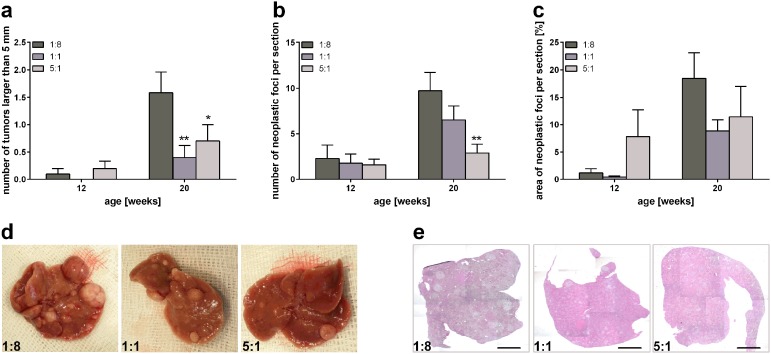
To evaluate the impact of n-3 PUFAs on tumor progression, comprehensive macroscopic and microscopic analyses of tumor load were performed. While only a few mice developed liver tumors at an age of 12 weeks, 20-week-old mice frequently developed numerous liver tumors (Figure 5). Thus, there were no apparent differences between the groups in 12-week-old mice reflecting tumor load (liver weight/body weight index, number of surficial tumors larger than 5 mm, and number of neoplastic foci per section), whereas considerable differences were observed in 20-week-old mice. On average, 20-week-old control diet (1:8) fed mice developed 1.6 surficial tumors larger than 5 mm, whereas 1:1 and 5:1 fed mice developed significantly fewer surficial tumors larger than 5 mm, with only 0.4 and 0.7 tumors being observed, respectively (Figure 5a and d). Concordantly, the number of neoplastic foci per liver section was reduced with increasing n-3 PUFA content of the diet compared with control animals (Figure 5b and e). While the liver of control-diet-fed animals exhibited 9.7 neoplastic foci per section, only 7.0 and 2.9 neoplastic foci per section were counted in 1:1 and 5:1 fed mice, respectively. This decrease was significant in 5:1 fed mice compared with control animals. Additionally, the area of neoplastic foci per section was reduced at 20 weeks of age upon feeding of the n-3 PUFA rich diets compared with mice receiving the 1:8 control diet (Figure 5c).
Figure 5.
Macroscopic and microscopic analyses of tumor load. Assessment of surficial liver tumors larger than 5 mm (a), number of neoplastic foci per liver section (b), and area of neoplastic foci per liver section (c) in STZ/HFD-treated mice receiving HFDs differing in n-3/n-6 PUFA ratios (1:8, 1:1 and 5:1) (n = 10–11 per group). Values are given as mean ± SEM. Differences between the groups were assessed by two-way ANOVA followed by Tukey’s range test within each time point. *p < 0.05 versus 1:8 fed mice, **p < 0.01 versus 1:8 fed mice. Representative images of livers (d) and photomicrographs of H&E stained liver sections (e) of 20-week-old STZ/HFD-treated mice receiving HFDs differing in n-3/n-6 PUFA ratios (1:8, 1:1 and 5:1). Scale bar represents 2 mm.
HFD, high-fat diet; H&E, hematoxylin/eosin; PUFA, polyunsaturated fatty acid; SEM, standard error of the mean; STZ, streptozotocin.
Discussion
NAFLD is an increasingly important risk factor for HCC, and one of the major causes for liver transplantation in the United States.3–5,7 Over the past decades, dietary habits and lifestyle have changed dramatically, leading to increasing prevalence of obesity and NAFLD worldwide.1,14 Thus, prevalence of NAFLD-related end-stage liver disease is estimated to further increase.3 It is therefore of great importance to find new strategies and therapies against NAFLD progression. The results presented herein show that n-3 PUFA-enriched diets and high dietary n-3/n-6 PUFA ratios alleviate NAFLD-related tumorigenesis.
Availability of n-3 PUFAs in the liver is detrimental for hepatic production of n-3 PUFA-derived lipid mediators, and, thus, its positive effects on NAFLD progression. Herein, we showed that dietary fatty acids also change the hepatic lipid profile in n-3 PUFA-rich HFD-fed mice. Interestingly, hepatic fatty acid composition reflects the fatty acid composition of the diet. Similarly, other studies reported altered hepatic fatty acid composition upon increased dietary n-3 PUFA contents.29,30 Furthermore, lipidomic analyses of patient liver tissue showed increased SFA levels, decreased levels of DHA and EPA, and decreased n-3/n-6 PUFA ratio in NASH patients compared with patients with simple steatosis.31 Together, these findings indicate a connection between NAFLD severity and hepatic fatty acid composition.
Interestingly, it has already been shown that fat-1 transgenic mice, which endogenously convert n-6 PUFAs to n-3 PUFAs, have increased hepatic contents of anti-inflammatory mediators.21 Therefore, it could be suggested that increased contents of n-3 PUFAs and increased n-3/n-6 PUFA ratios, as seen in mice receiving n-3 PUFA-rich HFDs, probably increase hepatic anti-inflammatory lipid mediator concentration. Thus, high amounts of n-3 PUFAs may contribute to an overall attenuated inflammatory state in NAFLD. Inflammation is an important feature of NAFLD progression. Beside other cells, Kupffer cells and monocyte-derived macrophages play a crucial role in NASH and NAFLD progression.10,32 For a long time it has been known that macrophages can polarize towards M1 or M2 phenotypes.12 While M1-polarized macrophages produce high amounts of pro-inflammatory cytokines, M2-polarized macrophages secrete anti-inflammatory cytokines and phagocyte more efficiently. Thus, M1-polarized macrophages promote inflammation while M2-polarized macrophages decrease inflammation and promote resolution of inflammation. In this study, we report an increasing number of macrophages in liver tissue with increasing n-3 PUFA content and n-3/n-6 PUFA ratio of the diet. Despite a higher number of macrophages, we observed decreasing TNF-α mRNA levels in the liver tissue upon feeding of n-3 PUFA-rich diets, suggesting accumulation of M2-polarized macrophages in the livers of n-3 PUFA-rich-fed mice. Interestingly, it is known that M2-polarized macrophages infiltrate inflamed tissue during resolution of inflammation and phagocyte cell debris without stimulating further inflammation.33 Feeding a standard HFD promotes a chronic inflammatory environment by induction of liver macrophage M1 polarization.34 Contrarily, n-3 PUFAs and their lipid mediators promote M2 polarization of macrophages in adipose tissue of HFD-fed mice.35,36 Furthermore, endogenously increased n-3 PUFA levels of fat-1 transgenic mice promote M2 polarization of bone marrow macrophages.37 Concordantly, protective effects of n-3 PUFA treatment of ischemia reperfusion injury of the liver are also mediated by M2 polarization of liver macrophages.38–40
Dietary consumption of n-3 PUFAs is well known to be protective regarding different diseases like diabetes, cardiovascular disease, and NAFLD.41,42 Furthermore, fish consumption is independently correlated with reduced risk of HCC.43 Herein, we observed reduced liver tumor number and size in n-3 PUFA-rich-fed mice, indicating inhibition of NAFLD-related tumorigenesis and tumor growth. Reduced tumorigenesis upon increased n-3 PUFA tissue levels has been reported for NAFLD-independent DEN-induced liver tumors in fat-1 transgenic mice.21 Furthermore, dietary n-3 PUFAs and increased n-3/n-6 PUFA ratios were reported to decrease DEN-induced tumorigenesis in rats and in a rat multi-organ cancer model.44,45 Nevertheless, Enos and colleagues reported in 2015 that different dietary n-3/n-6 PUFA ratios (1:1 and 1:20) had no effect on NAFLD.46 In contrast to our study, Enos and colleagues used ALA to supplement the diet and to increase the n-3/n-6 PUFA ratio. Interestingly, only minor amounts of ALA are endogenously converted to EPA and DHA.47 As the HFDs used in the present study were enriched with EPA and DHA, we suppose that the positive effects of increased n-3/n-6 PUFA ratios are mediated by DHA and EPA rather than ALA.
Interestingly, DHA and EPA derived lipid mediators (resolvin D1, D2, and E1) have been shown to reduce tumor growth, especially in the presence of cell debris in the tumor.19 This effect was reported to be macrophage-dependent, as no effect of resolvins was observed in macrophage-depleted mice. Concordantly, we showed that the number of macrophages increased with a simultaneous decrease in TNF-α mRNA levels in n-3 PUFA-rich-fed mice with NAFLD-related liver tumors.
Finally, and of most importance, n-3 PUFA-rich diets and increased n-3/n-6 PUFA ratios had a significant impact on the survival rate of the mice, in that they almost prevent mortality in the STZ/HFD mouse model.
A limitation of the study is the mouse model used, as the mice are lean and thus, do not reflect the human situation of obesity. Although many models of NAFLD exist that mimic human metabolic syndrome better, mouse models of liver tumors from progressive NAFLD are rare, and often come with various limitations like slow disease progression and low tumor incidence.48–50 Hence, the NASH-tumor mouse model is a broadly used model to study NAFLD progression and tumorigenesis as it reflects various stages of NAFLD resulting in tumors with a high incidence in a short period of time.51–56 The results obtained from this mouse model need to be evaluated carefully, but still give new insights on possible therapeutic approaches to limit NAFLD progression to tumors.
Conclusion
In conclusion, high dietary n-3/n-6 PUFA levels and n-3 PUFA contents alleviate NAFLD-caused tumorigenesis and tumor growth, which drastically improves survival in STZ/HFD-treated mice. This effect is accompanied by pronounced changes in hepatic fatty acid composition and accumulation of macrophages in the liver. Further studies have to be conducted to clarify mechanisms and pathways and the role of individual fatty acids like EPA and DHA. This anti-inflammatory approach may be a treatment option of NAFLD that reduces the risk for NAFLD-related tumorigenesis.
Acknowledgments
We thank Alexander Hartmann, Berit Blendow, Chantal von Hörsten, Dorothea Frenz, Eva Lorbeer, Ilona Klammfuß, Laura Grüner, Mareike Degner, and Maren Nerowski for their excellent technical assistance. The authors wish to thank Birgit Jentz and Maria Dahm of the Institute of Muscle Biology and Growth who collaborated in terms of sample preparation and gas chromatography measurements.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and publication of this article: This work was supported by the Deutsche Forschungsgemeinschaft, Bonn-Bad Godesberg, Germany [AB 453/2-1].
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Kerstin Abshagen  https://orcid.org/0000-0002-9541-9239
https://orcid.org/0000-0002-9541-9239
Contributor Information
Marie Liebig, Institute for Experimental Surgery, University Medicine Rostock, Germany.
Dirk Dannenberger, Institute of Muscle Biology and Growth, Leibniz Institute for Farm Animal Biology, Dummerstorf, Germany.
Brigitte Vollmar, Institute for Experimental Surgery, University Medicine Rostock, Germany.
Kerstin Abshagen, Institute for Experimental Surgery, University Medicine Rostock, Germany.
References
- 1. Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 2013; 10: 686–690. [DOI] [PubMed] [Google Scholar]
- 2. Younossi ZM, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018; 15: 11–20. [DOI] [PubMed] [Google Scholar]
- 3. Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology 2014; 59: 2188–2195. [DOI] [PubMed] [Google Scholar]
- 4. Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015; 148: 547–555. [DOI] [PubMed] [Google Scholar]
- 5. Charlton MR, Burns JM, Pedersen RA, et al. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology 2011; 141: 1249–1253. [DOI] [PubMed] [Google Scholar]
- 6. Sanyal A, Poklepovic A, Moyneur E, et al. Population-based risk factors and resource utilization for HCC: US perspective. Curr Med Res Opin 2010; 26: 2183–2191. [DOI] [PubMed] [Google Scholar]
- 7. Wong VWS, Chitturi S, Wong GL-H, et al. Pathogenesis and novel treatment options for non-alcoholic steatohepatitis. Lancet Gastroenterol Hepatol 2016; 1: 56–67. [DOI] [PubMed] [Google Scholar]
- 8. Wallace MC, Preen D, Jeffrey GP, et al. The evolving epidemiology of hepatocellular carcinoma: a global perspective. Expert Rev Gastroenterol Hepatol 2015; 9: 765–779. [DOI] [PubMed] [Google Scholar]
- 9. Luedde T, Schwabe RF. NF-κB in the liver - linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2011; 8: 108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reid DT, Reyes JL, McDonald BA, et al. Kupffer cells undergo fundamental changes during the development of experimental NASH and are critical in initiating liver damage and inflammation. PLoS One 2016; 11: e0159524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tacke F. Targeting hepatic macrophages to treat liver diseases. J Hepatol 2017; 66: 1300–1312. [DOI] [PubMed] [Google Scholar]
- 12. Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 2014; 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wan J, Benkdane M, Teixeira-Clerc F, et al. M2 Kupffer cells promote M1 Kupffer cell apoptosis: a protective mechanism against alcoholic and nonalcoholic fatty liver disease. Hepatology 2014; 59: 130–142. [DOI] [PubMed] [Google Scholar]
- 14. Simopoulos AP. The importance of the Omega-6/Omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med 2008; 233: 674–688. [DOI] [PubMed] [Google Scholar]
- 15. Patterson E, Wall R, Fitzgerald GF, et al. Health implications of high dietary Omega-6 polyunsaturated fatty acids. J Nutr Andm 2012; 2012: 539426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saini RK, Keum Y-S. Omega-3 and omega-6 polyunsaturated fatty acids: dietary sources, metabolism, and significance: a review. Life Sci 2018; 203: 255–267. [DOI] [PubMed] [Google Scholar]
- 17. Marventano S, Kolacz P, Castellano S, et al. A review of recent evidence in human studies of n-3 and n-6 PUFA intake on cardiovascular disease, cancer, and depressive disorders: does the ratio really matter? Int J Food Sci Nutr 2015; 66: 611–622. [DOI] [PubMed] [Google Scholar]
- 18. Serhan CN, Levy BD. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest 2018; 128(7): 2657–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sulciner ML, Serhan CN, Gilligan MM, et al. Resolvins suppress tumor growth and enhance cancer therapy. J Exp Med 2018; 215: 115–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Salem N, Pawlosky R, Wegher B, et al. In vivo conversion of linoleic acid to arachidonic acid in human adults. Prostaglandins, Leukot Essent Fat Acids 1999; 60: 407–410. [DOI] [PubMed] [Google Scholar]
- 21. Weylandt KH, Krause LF, Gomolka B, et al. Suppressed liver tumorigenesis in fat-1 mice with elevated omega-3 fatty acids is associated with increased omega-3 derived lipid mediators and reduced TNF-α. Carcinogenesis 2011; 32: 897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fujii M, Shibazaki Y, Wakamatsu K, et al. A murine model for non-alcoholic steatohepatitis showing evidence of association between diabetes and hepatocellular carcinoma. Med Mol Morphol 2013; 46: 141–152. [DOI] [PubMed] [Google Scholar]
- 23. Liebig M, Hassanzada A, Kämmerling M, et al. Microcirculatory disturbances and cellular changes during progression of hepatic steatosis to liver tumors. Exp Biol Med 2018; 243: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41: 1313–1321. [DOI] [PubMed] [Google Scholar]
- 25. Abshagen K, Eipel C, Kalff JC, et al. Loss of NF-kappaB activation in Kupffer cell-depleted mice impairs liver regeneration after partial hepatectomy. Am J Physiol Gastrointest Liver Physiol 2007; 292: G1570–G1577. [DOI] [PubMed] [Google Scholar]
- 26. Dannenberger D, Nuernberg K, Nuernberg G, et al. Different dietary protein and PUFA interventions alter the fatty acid concentrations, but not the meat quality, of porcine muscle. Nutrients 2012; 4: 1237–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dannenberger D, Nuernberg G, Nuernberg K, et al. Effects of diets supplemented with n–3 or n–6 PUFA on pig muscle lipid metabolites measured by non-targeted LC–MS lipidomic profiling. J Food Compos Anal 2017; 56: 47–54. [Google Scholar]
- 28. Reetz J, Genz B, Meier C, et al. Development of adenoviral delivery systems to target hepatic stellate cells in vivo. PLoS One 2013; 8: e67091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen X, Du X, Shen J, et al. Effect of various dietary fats on fatty acid profile in duck liver: efficient conversion of short-chain to long-chain omega-3 fatty acids. Exp Biol Med 2017; 242: 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nuernberg K, Breier BH, Jayasinghe SN, et al. Metabolic responses to high-fat diets rich in n-3 or n-6 long-chain polyunsaturated fatty acids in mice selected for either high body weight or leanness explain different health outcomes. Nutr Metab (Lond) 2011; 8: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Puri P, Baillie RA, Wiest MM, et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology 2007; 46: 1081–1090. [DOI] [PubMed] [Google Scholar]
- 32. Tosello-Trampont A-C, Landes SG, Nguyen V, et al. Kuppfer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-α production. J Biol Chem 2012; 287: 40161–40172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Serhan CN. Novel lipid mediators and resolution mechanisms in acute inflammation. Am J Pathol 2010; 177: 1576–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Luo W, Xu Q, Wang Q, et al. Effect of modulation of PPAR-γ activity on Kupffer cells M1/M2 polarization in the development of non-alcoholic fatty liver disease. Sci Rep 2017; 7: 44612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Titos E, Rius B, Gonzalez-Periz A, et al. Resolvin D1 and its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization toward an M2-like phenotype. J Immunol 2011; 187: 5408–5418. [DOI] [PubMed] [Google Scholar]
- 36. Li J, Li FR, Wei D, et al. Endogenous ω-3 PUFAs production confers resistance to obesity, dyslipidemia, and diabetes in mice. Mol Endocrinol 2014; 28: 1316–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Song M-Y, Wang J, Lee Y, et al. Enhanced M2 macrophage polarization in high n-3 polyunsaturated fatty acid transgenic mice fed a high-fat diet. Mol Nutr Food Res 2016; 60: 2481–2492. [DOI] [PubMed] [Google Scholar]
- 38. Zhang T, Shu H-H, Chang L, et al. Resolvin D1 protects against hepatic ischemia/reperfusion injury in rats. Int Immunopharmacol 2015; 28: 322–327. [DOI] [PubMed] [Google Scholar]
- 39. Raptis DA, Perparim L, Jang JH, et al. GPR120 on Kupffer cells mediates hepatoprotective effects of ω3-fatty acids. J Hepatol 2014; 60: 625–632. [DOI] [PubMed] [Google Scholar]
- 40. Kang JW, Lee SM. Resolvin D1 protects the liver from ischemia/reperfusion injury by enhancing: M2 macrophage polarization and efferocytosis. Biochim Biophys Acta 2016; 1861: 1025–1035. [DOI] [PubMed] [Google Scholar]
- 41. Parker HM, Johnson NA, Burdon CA, et al. Omega-3 supplementation and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol 2012; 56: 944–951. [DOI] [PubMed] [Google Scholar]
- 42. Nettleton JA, Katz R. n-3 long-chain polyunsaturated fatty acids in type 2 diabetes: a review. J Am Diet Assoc 2005; 105: 428–440. [DOI] [PubMed] [Google Scholar]
- 43. Gao M, Sun K, Guo M, et al. Fish consumption and n-3 polyunsaturated fatty acids, and risk of hepatocellular carcinoma: systematic review and meta-analysis. Cancer Causes Control 2015; 26: 367–376. [DOI] [PubMed] [Google Scholar]
- 44. Kim Y, Ji SK, Choi H. Modulation of liver microsomal monooxygenase system by dietary n-6/n-3 ratios in rat hepatocarcinogenesis. Nutr Cancer 2000; 37: 65–72. [DOI] [PubMed] [Google Scholar]
- 45. Toriyama-Baba H, Iigo M, Asamoto M, et al. Organotropic chemopreventive effects of n-3 unsaturated fatty acids in a rat multi-organ carcinogenesis model. Jpn J Cancer Res 2001; 92: 1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Enos RT, Velázquez KT, McClellan JL, et al. Lowering the dietary omega-6:omega-3 does not hinder non-alcoholic fatty-liver disease development in a murine model. Nutr Res 2015; 35: 449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Burdge G. Alpha-linolenic acid metabolism in men and women: nutritional and biological implications. Curr Opin Clin Nutr Metab Care 2004; 7: 137–144. [DOI] [PubMed] [Google Scholar]
- 48. Nakagawa H. Recent advances in mouse models of obesity- and nonalcoholic steatohepatitis-associated hepatocarcinogenesis. World J Hepatol 2015; 7: 2110–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Riordan JD, Nadeau JH. Modeling progressive non-alcoholic fatty liver disease in the laboratory mouse. Mamm Genome 2014; 25: 473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Heindryckx F, Colle I, Van Vlierberghe H. Experimental mouse models for hepatocellular carcinoma research. Int J Exp Pathol 2009; 90: 367–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Saito K, Uebanso T, Maekawa K, et al. Characterization of hepatic lipid profiles in a mouse model with nonalcoholic steatohepatitis and subsequent fibrosis. Sci Rep 2015; 5: 12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jojima T, Tomotsune T, Iijima T, et al. Empagliflozin (an SGLT2 inhibitor), alone or in combination with linagliptin (a DPP-4 inhibitor), prevents steatohepatitis in a novel mouse model of non-alcoholic steatohepatitis and diabetes. Diabetol Metab Syndr 2016; 8: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ishikawa H, Takaki A, Tsuzaki R, et al. L-carnitine prevents progression of non-alcoholic steatohepatitis in a mouse model with upregulation of mitochondrial pathway. PLoS One 2014; 9: e100627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Takaki Y, Saito Y, Takasugi A, et al. Silencing of microRNA-122 is an early event during hepatocarcinogenesis from non-alcoholic steatohepatitis. Cancer Sci 2014; 105: 1254–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Konishi H, Shirabe K, Nakagawara H, et al. Suppression of silent information regulator 1 activity in noncancerous tissues of hepatocellular carcinoma: possible association with non-B non-C hepatitis pathogenesis. Cancer Sci 2015; 106: 542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Thomson JP, Ottaviano R, Unterberger EB, et al. Loss of Tet1-associated 5-hydroxymethylcytosine is concomitant with aberrant promoter hypermethylation in liver cancer. Cancer Res 2016; 76: 3097–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]