Abstract
Increased subchondral trabecular bone turnover due to imbalanced bone-resorbing and bone-forming activities is a hallmark of osteoarthritis (OA). Wnt5a/Ror2 signaling, which can derive from bone marrow stromal cells (BMSCs), takes a role in modulating osteoblast and osteoclast formation. We showed previously that experimentally unilateral anterior crossbites (UACs) elicited OA-like lesions in mice temporomandibular joints (TMJs), displaying as subchondral trabecular bone loss. Herein, we tested the role of BMSC-derived Wnt5a/Ror2 signaling in regulating osteoclast precursor migration and differentiation in this process. The data confirmed the decreased bone mass, increased tartrate-resistant acid phosphatase (TRAP)–positive cell number, and enhanced osteoclast activity in TMJ subchondral trabecular bone of UAC-treated rats. Interestingly, the osteoblast activity in the tissue of TMJ subchondral trabecular bone of these UAC-treated rats was also enhanced, displaying as upregulated expressions of osteoblast markers and increased proliferation, migration, and differentiation capabilities of the locally isolated BMSCs. These BMSCs showed an increased CXCL12 protein expression level and upregulated messenger RNA expressions of Rankl, Wnt5a, and Ror2. Ex vivo data showed that their capacities of inducing migration and differentiation of osteoclast precursors were enhanced, and these enhanced capabilities were restrained after blocking their Ror2 signaling using small interfering RNA (siRNA) assays. Reducing Ror2 expression in the BMSC cell line by siRNA or blocking the downstream signalings with specific inhibitors also demonstrated a suppression of the capacity of the BMSC cell line to promote Wnt5a-dependent migration (including SP600125 and cyclosporine A) and differentiation (cyclosporine A only) of osteoclast precursors. These findings support the idea that Wnt5a/Ror2 signaling in TMJ subchondral BMSCs enhanced by UAC promoted BMSCs to increase Cxcl12 and Rankl expression, in which JNK and/or Ca2+/NFAT pathways were involved and therefore were engaged in enhancing the migration and differentiation of osteoclast precursors, leading to increased osteoclast activity and an overall TMJ subchondral trabecular bone loss in the UAC-treated rats.
Keywords: bone marrow stromal cells, osteoarthritis, osteoclast, bone resorption, mandibular condyle, traumatic dental occlusion
Introduction
Aberrant subchondral trabecular bone remodeling, due to imbalanced bone-resorbing and bone-forming activities, is a hallmark in the development of osteoarthritis (OA) (Ivanovska and Dimitrova 2011; Mahjoub et al. 2012), which is most often caused by mechanical stimulation (Zarb and Carlsson 1999). Loss of subchondral trabecular bone occurs in the early stage of OA (Bolbos et al. 2008). Recently, we developed an animal model of temporomandibular joint (TMJ) OA using unilateral anterior crossbite (UAC) prostheses. UAC-treated TMJs displayed severe cartilage degeneration (Zhang et al. 2013; Wang et al. 2014) and subchondral trabecular bone loss with increased osteoclast activity (Lu et al. 2014) at the early stage of OA development.
Osteoblastic and osteoclastic lineage cells interact closely to maintain skeletal homeostasis during mechanical stimulated remodeling. Osteoblasts exert bone-forming functions (Pittenger et al. 1999), while osteoclasts produce bone-resorbing activities. Early stage cells of the osteoblastic lineage can promote osteoclast precursor differentiation in their proximity by producing cytokines, such as the receptor activator of nuclear factor–κB ligand (RANKL), osteoprotegerin (OPG) and the macrophage colony-stimulating factor (M-CSF) (Oshita et al. 2011; Tsubaki et al. 2012). Co-cultured bone marrow stromal cells (BMSCs) promoted differentiation of hematopoietic progenitors into fully functional osteoclasts without additional exogenous cytokines (Mbalaviele et al. 1999).
Wnt5a, a noncanonical Wnt-secreted ligand, forms a ternary complex with Frizzled (Fzd) and the receptor tyrosine kinase-like orphan receptor 2 (Ror2) to activate downstream signaling responses (Kikuchi et al. 2012), such as the Ca2+/NFAT pathway in tumor and kidney cells, as well as osteoblasts, and the planar cell polarity (PCP)/convergent extension (CE) pathway, which involves JNK and so on (Oishi et al. 2003; Kikuchi et al. 2012). Although overexpression of Ror2 promotes differentiation of human mesenchymal stromal cells into osteoblasts by inducing an osteogenic transcription factor osterix (Liu et al. 2007), mice deficient in either Wnt5a or Ror2 showed impaired osteoclast formation (Maeda et al. 2012). These data suggest that Wnt5a/Ror2 signaling critically regulates the differentiation and function of not only osteoblasts but also osteoclasts.
In the present study, we tested Wnt5a/Ror2 signaling in BMSCs isolated from subchondral trabecular bone marrow of UAC-treated rats and investigated the role of BMSC-derived Wnt5a/Ror2 signaling in UAC-induced TMJ subchondral trabecular bone loss at the early stage by promoting migration and differentiation of osteoclast precursors.
Materials and Methods
Animals and Dental Operation
Six-week-old female Sprague-Dawley (SD) rats (weighing 140 to 160 g) were obtained from the Animal Center of the Fourth Military Medical University and randomly divided into sham-operated control (Con) and UAC groups (n = 20 rats/group). Animal experiments were performed in accordance with the institutional guidelines set by the University Ethics Committee. UACs were applied by dental prostheses as previously described (Zhang et al. 2013; Wang et al. 2014) and are briefly introduced in the Appendix.
Culture of BMSCs and Osteoclast Precursors
To culture BMSCs, rat TMJ subchondral bones between the osteochondral junction and the condyle’s sigmoid notch were dissected, minced, and flushed with Dulbecco’s modified Eagle’s medium (DMEM)/F12 medium (Hyclone, Logan, UT, USA). Adherent bone marrow cells were cultured until 80% confluence and replated by tryptic digestion. Cells from passages 3 to 6 were used in this study. An adult C57BL/6 mice origin BMSC cell line (CL-BMSCs; Texas A&M Health Science Center, Round Rock, TX, USA) (Benoit and Boutin 2012) was maintained in DMEM/F12 medium.
For osteoclast precursor culture, bone marrow cells were flushed from the femora and tibiae of rats and cultured with α-MEM medium (Hyclone) overnight. Nonadherent cells were harvested and cultured with 20 ng/mL recombinant murine macrophage colony-stimulating factor (M-CSF; PeproTech, Offenbach, Germany) for 3 d, which were used as bone marrow macrophages (BMMs). RAW264.7 cell line was used as osteoclast precursors (Han et al. 2005; Aharon and Bar-Shavit 2006) and grown in DMEM medium (Hyclone).
Co-culture of BMSCs and RAW264.7 Cells
The 2 × 104 BMSCs or CL-BMSCs and 6 × 104 RAW264.7 cells were co-cultured in 24-well plates with or without Wnt5a (100 ng/mL), JNK inhibitor SP600125 (10 µM), or Ca2+/NFAT inhibitor cyclosporine A (1 µM) in the presence of 10 nM 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] (Cayman Chemical, Ann Arbor, MI, USA) for 10 d. The media were changed on days 4 and 7. Multinucleated (≥3 nuclei) osteoclasts were counted after tartrate-resistant acid phosphatase (TRAP) staining and 4′,6-diamidino-2-phenylindole (DAPI) counterstaining, presented as the percentage of the RAW264.7 cells seeded. Images were merged from the light microscope images of TRAP staining and fluorescent images of DAPI counterstaining within the same field of the microscope.
Appendix Materials and Methods
The methods of micro–computed tomography (CT) and hematoxylin and eosin (H&E) analysis, TRAP and immunohistochemistry staining, phenotypic analysis and cell sorting of BMSCs, real-time polymerase chain reaction (PCR), Western blot, methyl-thiazolyl-tetrazolium (MTT), cell cycle analysis, colony-forming unit–fibroblast (CFU-F), small interfering RNA (siRNA), cell differentiation and migration, and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining are shown in the Appendix.
Statistical Analysis
Data are presented as means ± standard deviation (SD). As our data showed equal variances, unpaired Student’s t test and one-way analysis of variance (ANOVA) followed by Dunnett’s test were used to compare data between one or multiple experimental groups and the control by SPSS 13.0 software (SPSS, Inc., an IBM Company, Chicago, IL, USA), respectively. P values <0.05 were considered statistically significant. Power was calculated by SAS 9.3 software (SAS Institute, Cary, NC, USA). All experiments were repeated at least 3 times.
Results
UACs Caused Subchondral Trabecular Bone Loss in Mandibular Condyles and Enhanced Osteoclast and Osteoblast Activities
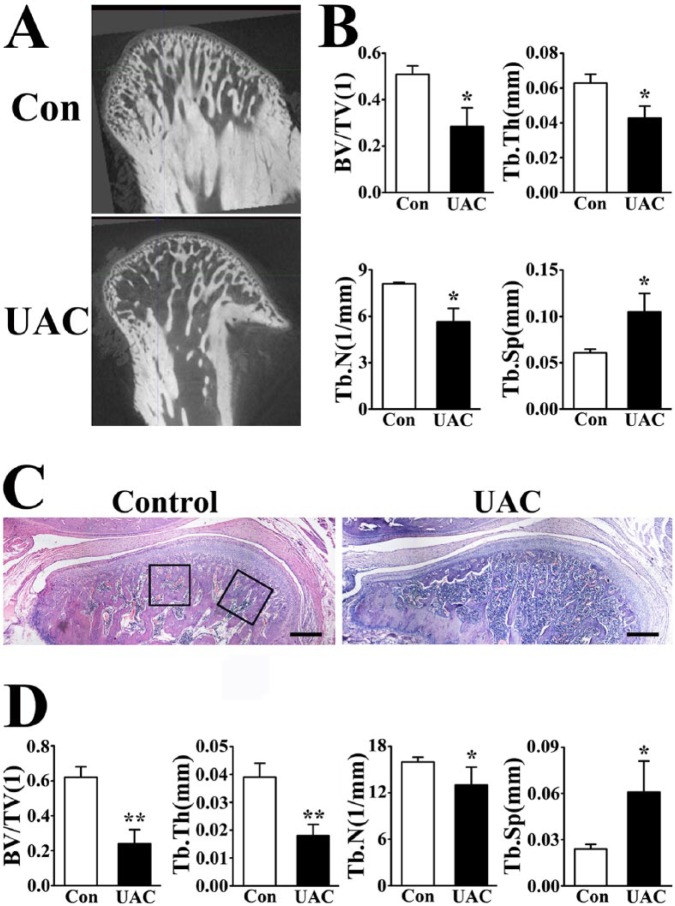
Micro-CT analysis showed that bone volume to tissue volume (BV/TV), trabecular number (Tb.N), and trabecular thickness (Tb.Th) were significantly decreased, while trabecular separation (Tb.Sp) doubled in mandibular condyles of UAC-treated versus control rats (Fig. 1A, B), indicating severe subchondral trabecular bone loss.
Figure 1.
Unilateral anterior crossbite (UAC) induced subchondral trabecular bone loss in temporomandibular joint (TMJ). (A) Micro–computed tomography (CT) images and (B) quantifications of subchondral trabecular bone parameters in the mandibular condyles from control (Con) and UAC-treated (UAC) rats 4 wk after operation. Power = 0.84 (bone volume to tissue volume [BV/TV]), 0.87 (trabecular thickness [Tb.Th]), 0.86 (trabecular number [Tb.N]), and 0.86 (trabecular separation [Tb.Sp]). (C) Hematoxylin and eosin staining and (D) histomorphologic parameter analysis of TMJ mandibular condyles. The black frames showed the selected regions for histomorphologic parameter analysis. Bars = 400 µm. Power = 0.83 (BV/TV), 0.88 (Tb.Th), 0.55 (Tb.N), and 0.88 (Tb.Sp). Data are presented as means ± SD. *P < 0.05. **P < 0.01.
Consistent with the micro-CT results, histomorphometric analysis also showed decreased BV/TV, Tb.Th, and Tb.N but increased Tb.Sp in UAC-treated versus control TMJ subchondral trabecular bone (Fig. 1C, D).
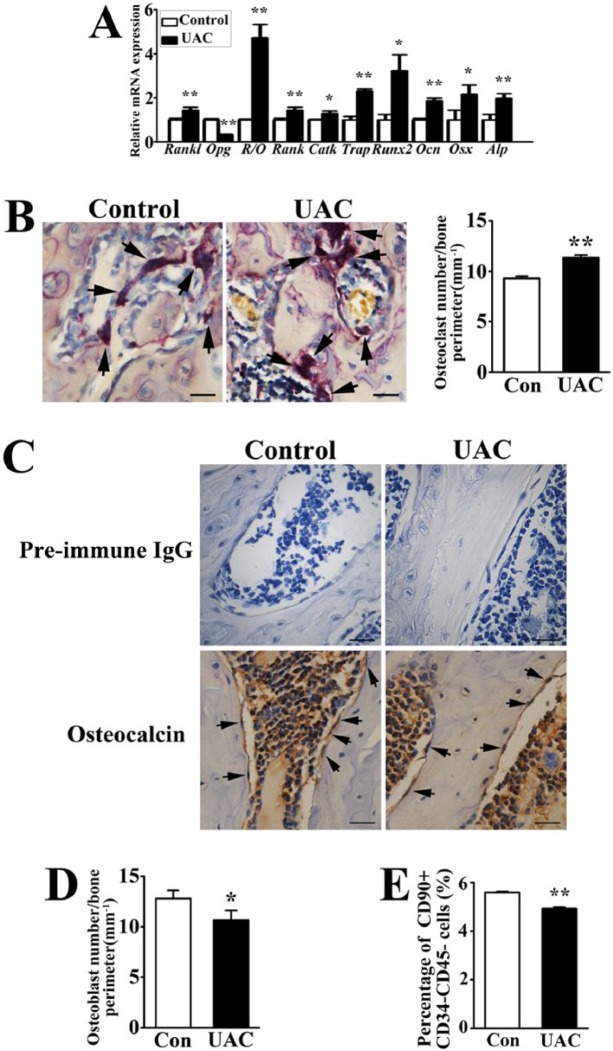
Real-time PCR analyses showed decreased Opg messenger RNA (mRNA) level and increased Rankl expression and Rankl/Opg ratio (R/O) in the tissue of TMJ subchondral bones of UAC-treated versus control rats, accompanied by increased expressions of osteoclast markers Rank, cathepsin K, and Trap (Fig. 2A). The number of TRAP-positive osteoclasts was larger in UAC-treated versus control TMJs (Fig. 2B), supporting that UACs caused bone loss by increasing bone resorption.
Figure 2.
Effects of unilateral anterior crossbites (UACs) on osteoblast and osteoclast activities in mandibular condyles. (A) Real-time polymerase chain reaction analysis for the expressions of osteoblast and osteoclast markers in mandibular condyles from control (Con) and UAC-treated rats. R/O, the ratio of Rankl over Opg expression; CatK, cathepsin K; Ocn, osteocalcin; Osx, osterix; Alp, alkaline phosphatase. Power = 0.65 (Rankl), 0.70 (Opg), 0.70 (R/O), 0.65 (Rank), 0.66 (Catk), 0.70 (Trap), 0.70 (Runx2), 0.70 (Ocn), 0.66 (Osx), 0.66 (Alp). (B) Tartrate-resistant acid phosphatase (TRAP) staining was performed to indicate multinucleated osteoclasts (black arrow) and their numbers per bone surface (mm–1) (in histogram) in the subchondral trabecular bone of mandibular condyles from Con and UAC-treated rats. Bars = 50 µm. Power = 0.71. (C, D) Immunohistochemical staining was performed to indicate Ocn-positive osteoblasts (black arrow) and their numbers per bone surface (mm–1) (in histogram). Preimmune immunoglobulin G (IgG) was used as negative controls, and no positive cells were shown in the subchondral trabecular bone. Bars = 50 µm. Power = 0.56. (E) Percentage of CD90+CD34–CD45– bone marrow stromal cells (BMSCs) to the subchondral bone marrow cells of mandibular condyles from Con and UAC-treated rats was analyzed by flow cytometry. Power = 0.73. Data are presented as means ± SD. *P < 0.05. **P < 0.01.
Interestingly, the number of Ocn-positive osteoblasts was decreased in UAC-treated versus control mandibular condyles (Fig. 2C, D), along with increased mRNA expressions of Runx2, osteocalcin, osterix, and alkaline phosphatase (Fig. 2A), suggesting that UACs induced osteogenic activities on a per-cell basis. To determine whether the reduced osteoblast number by UACs was attributed partially to a smaller local BMSC number, we counted BMSCs (CD90+CD34–CD45– cells) (Dominici et al. 2006; Bian et al. 2009; Zhang and Chan 2010) from TMJ subchondral trabecular bone marrow cells. The percentage of BMSCs in the UAC-treated group was slightly but significantly lower than the controls (Fig. 2E).
The above observations suggested that even though UACs reduced numbers of BMSCs and Ocn-positive osteoblasts and stimulated TMJ subchondral trabecular bone loss, it enhanced local mRNA levels of osteogenic markers and Rankl expression, which was capable of promoting osteoclast differentiation, showing an increased number of TRAP-positive osteoclasts.
UACs Enhanced Differentiation of BMSCs into Osteoblastic Lineage
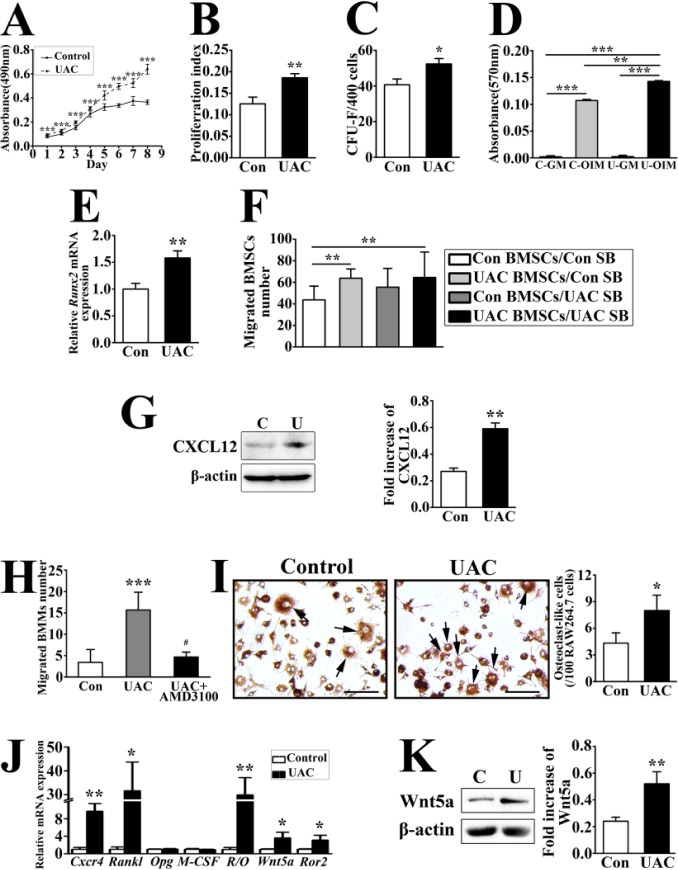
The current locally isolated cells from TMJ subchondral trabecular bone were defined as BMSCs (Dominici et al. 2006) because they were positive for CD54 and CD90 but negative for CD34 and CD45 (Pittenger et al. 1999; Agata 2013; Lin et al. 2013) (Appendix Fig. 1A), showed plastic-adherent fibroblast-like morphology, and were able to differentiate into osteoblasts or adipocytes (Appendix Fig. 1B). As assessed by MTT assays, UAC-treated BMSCs grew faster than the controls (Fig. 3A). In agreement with this, the proliferation index of BMSCs determined by cell cycle analysis (Fig. 3B) and the number of BMSC-derived colony-forming unit–fibroblasts (CFU-Fs) (Fig. 3C) were increased in UAC-treated versus control group.
Figure 3.
Unilateral anterior crossbites (UACs) induced bone marrow stromal cells (BMSCs) to grow, differentiate, and migrate in cultures and enhanced the capacity of these cells to promote migration and differentiation of osteoclast precursors. BMSCs were isolated from temporomandibular joint (TMJ) subchondral trabecular bone of control (Con) and UAC-treated (UAC) rats and cultured for the following assays. (A) Methyl-thiazolyl-tetrazolium (MTT) assays indicated the time courses of growth of BMSCs. Power = 0.96, 0.87, 0.86, 0.81, 0.87, 0.93, 0.90, and 0.88 (days 1 to 8, respectively). (B) Proliferation index (PI) of the cultured BMSCs was analyzed by flow cytometry. PI = (S + G2/M) ÷ (G0/G1 + S + G2/M). Power = 0.66. (C) Numbers of colonies (>1 mm2) were determined by colony-forming unit–fibroblast (CFU-F) assays. Power = 0.65. (D) Matrix mineralization was quantified by Alizarin red staining in cultured BMSCs grown in osteogenic induction medium (OIM) or growth medium (GM) for 2 wk. Power = 0.75 (Con vs. UAC in OIM). (E) The messenger RNA expression of Runx2 in BMSCs cultured in osteogenic medium for 7 d was analyzed by real-time polymerase chain reaction (PCR). Power = 0.69. (F) The BMSCs and TMJ subchondral bone fragments from Con and UAC-treated rats were seeded into the upper and lower compartments of transwell chambers to produce 4 experimental configurations (upper/lower): 1) control-BMSC/control-bone (Con BMSCs/Con SB), 2) UAC-BMSCs/control-bone (UAC BMSCs/Con SB), 3) Con-BMSCs/UAC-bone (Con BMSCs/UAC SB), and 4) UAC-BMSC/UAC-bone (UAC BMSCs/UAC SB). Migrated BMSCs were counted after 4′,6-diamidino-2-phenylindole (DAPI) nuclear dye staining. Power = 0.95, 0.75 (2 and 4 vs. 1, respectively). (G) Immunoblotting of protein lysates from BMSCs cultured from mandibular condyles of Con and UAC-treated (UAC) rats was performed to detect the protein level of CXCL12. The histogram shows quantifications of immunoreactive signals from 3 batches of cells. Power = 0.41. (H) Bone marrow macrophages (BMMs) (4 × 103 cells/well) and control (Con) or UAC-treated BMSCs (2.5 × 104 cells/well) were seeded into the upper and lower compartments of transwell chambers, respectively, in the absence or presence of CXCR4 antagonist AMD3100 (1 µg/mL). Migrated BMMs were counted and averaged. Power = 0.80 (UAC vs. control), 0.64 (UAC + AMD3100 vs. UAC). (I) Tartrate-resistant acid phosphatase staining (TRAP) staining in the mixed cultures of RAW264.7 cells (6 × 104 cell/well) and Con or UAC-treated BMSCs (2 × 104 cells/well). Cells were counterstained with DAPI nuclear dye. The images were merged from TRAP staining of light microscope and DAPI staining of fluorescence microscope in the same field. The histogram shows quantifications of TRAP-positive multinucleated (>3 nuclei) cells (black arrows). Bars = 40 µm. Power = 0.87. (J) Real-time PCR was performed on RNAs extracted from control versus UAC-treated BMSCs to assess the expressions of Cxcr4, Rankl, Opg, M-CSF, Wnt5a, and Ror2 messenger RNA and the Rankl/Opg ratio. Power = 0.69 (Cxcr4), 0.63 (Rankl), 0.69 (R/O), 0.60 (Wnt5a), and 0.62 (Ror2). (K) Western blot analysis of Wnt5a from control (Con) and UAC-treated (UAC) BMSCs. The histogram shows quantifications of immunoreactive signals from 3 batches of cells. Power = 0.71. Data are presented as means ± SD. *P < 0.05. **P < 0.01. ***P < 0.001.
The results of osteogenic differentiation assays showed increases in mineralizing potential of UAC-treated versus control BMSCs (Fig. 3D), and mRNA expression of an osteogenic transcription factor, Runx2, was increased in the former cells (Fig. 3E). In addition, adipogenic differentiation potential was decreased in UAC-treated BMSCs, as indicated by their reduced abilities to produce Oil red O–positive lipid vacuoles (Appendix Fig. 2).
The migration capability of UAC-treated BMSCs was also found enhanced, as demonstrated by transwell assays. BMSCs and TMJ subchondral bone fragments from the UAC-treated or control group were plated in the upper and lower compartments of transwell chambers, respectively, to produce 4 configurations. More robust migration was noticed in UAC-treated than in control BMSCs regardless of the sources of bone (Fig. 3F). The mRNA expression of Cxcr4, the receptor of CXCL12, was increased in UAC-treated versus control BMSCs (Fig. 3J).
These data suggested an enhanced osteogenic potential and an increased migration capability of UAC-treated BMSCs.
UACs Promoted BMSC-induced Migration and Differentiation of Osteoclast Precursors
BMSCs from UAC-treated rats showed a profound increase in the expression of CXCL12 protein compared to the controls (Fig. 3G). We then tested whether the increased CXCL12 expression in UAC-treated BMSCs functions as a chemoattractant to promote migration of BMMs and found a significant increase in the number of migrated BMMs when co-cultured with UAC-treated versus control BMSCs in transwell chambers. This enhancement of BMM migration was inhibited by CXCR4 antagonist AMD3100 (Fig. 3H), supporting a critical role of CXCL12 from UAC-treated BMSCs in recruiting BMMs.
UACs greatly increased Rankl expression and Rankl/Opg ratio in BMSCs but displayed no significant changes in Opg and M-CSF expressions in comparison with the controls (Fig. 3J). In agreement with this, BMSCs from UAC-treated rats displayed a stronger capability in inducing osteoclast formation than control rats when co-cultured with RAW264.7 cells (Fig. 3I).
Wnt5a Enhanced BMSCs to Induce Migration and Differentiation of Osteoclast Precursors
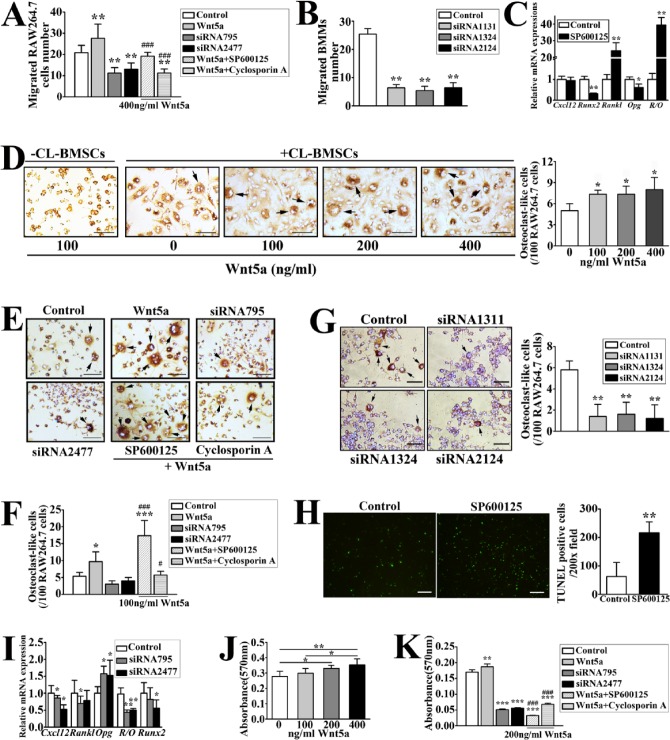
Wnt5a and Ror2 mRNA expression (Fig. 3J) and Wnt5a protein level (Fig. 3K) were increased in UAC-treated versus control BMSCs. It has been shown that Wnt5a/Ror2 signaling enhances RANKL-dependent osteoclast formation in mouse BMM cultures (Maeda et al. 2012). We then adopted CL-BMSCs and RAW264.7 cells for in vitro signaling tests. We first confirmed, by reverse transcriptase (RT)-PCR analyses, the expression of Ror2 in subchondral BMSCs and CL-BMSCs but not in BMMs and RAW264.7 cells (Appendix Fig. 3A). The 2 mouse Ror2 siRNAs (siRNA795 and siRNA2477) and 3 rat Ror2 siRNAs (siRNA1311, siRNA1324, and siRNA2124) inhibited Ror2 expression in CL-BMSCs and UAC-treated BMSCs, respectively (Appendix Fig. 3B, C). Additions of recombinant Wnt5a (400 ng/mL) in transwell co-cultures of CL-BMSCs and RAW264.7 cells increased migration of the latter cells, and knockdown of Ror2 in CL-BMSCs reduced migration level of RAW264.7 cells (Fig. 4A) with decreased Cxcl12 expression in CL-BMSCs (Fig. 4I). In agreement with this, Ror2 siRNA in UAC-treated BMSCs decreased BMM migration toward UAC-treated BMSCs (Fig. 4B). Incubating the co-cultures with SP600125 and cyclosporine A, inhibitors of signaling molecules downstream of Ror2, JNK, and Ca2+/NFAT pathways, respectively, blocked the effects of recombinant Wnt5a on RAW264.7 cell migration (Fig. 4A). The results showed an even lower migration level by cyclosporine A than by SP600125. However, after incubation with SP600125 for 24 h, Cxcl12 mRNA expression showed no change in CL-BMSCs (Fig. 4C). These results suggested that JNK, especially the Ca2+/NFAT pathway, is involved in Wnt5a/Ror2 signaling-enhanced migration of osteoclast precursors toward BMSCs.
Figure 4.
Wnt5a-induced signaling responses in the bone marrow stromal cell line (CL-BMSCs) promoted migration and differentiation of RAW264.7 cells in co-cultures and strengthened mineralization of CL-BMSCs. (A) Migration of RAW264.7 cells (4 × 103 cells/well) in co-cultures with CL-BMSCs (2.5 × 104/well), which were preincubated with control or Ror2 small interfering RNA (siRNA) (siRNA795 or siRNA 2477; 20 pmol/5 × 104 cells) in transwell plates in the absence or presence of Wnt5a (400 ng/mL) with or without SP600125 (10 µM) or cyclosporine A (1 µM), was assessed. Power = 0.64, 0.94, 0.90 (Wnt5a, siRNA795, and siRNA2477 vs. control, respectively) and 0.8, 0.94 (SP600125 and cyclosporine A vs. Wnt5a, respectively). (B) Migration of bone marrow macrophages (BMMs) (4 × 103 cells/well) in co-cultures with UAC-treated BMSCs (2.5 × 104/well), which were preincubated with control or Ror2 siRNA (siRNA1131, siRNA1324, or siRNA 2124; 20 pmol/5 × 104 cells), was assessed. Power = 0.98. (C) CL-BMSCs were incubated with 10 µM SP600125 for 24 h. Real-time polymerase chain reaction (PCR) analysis was performed to detect the messenger RNA expressions of Cxcl12, Runx2, Rankl, Opg, and the ratio of Rankl to Opg (R/O) in CL-BMSCs. Power = 0.78 (Runx2), 0.77 (Rankl), 0.64 (Opg), and 0.88 (R/O). (D) Images and quantifications of tartrate-resistant acid phosphatase staining (TRAP)–positive osteoclasts (black arrows) in the co-cultures of CL-BMSCs and RAW264.7 cells were obtained 10 d after they were cultured with various concentrations of Wnt5a (0 to 400 ng/mL). Bars = 40 µm. No multinucleated osteoclasts were found in cultures of RAW264.7 cells without CL-BMSCs but with 100 ng/mL Wnt5a. Power = 0.90, 0.86, and 0.56 (100, 200, and 400 vs. 0, respectively). (E) Images and (F) quantifications of TRAP-positive osteoclasts (black arrows) in the co-cultures of CL-BMSCs pretreated with control or Ror2 siRNA (siRNA795 or siRNA 2477; 20 pmol/5 × 104 cells) and RAW264.7 cells were assessed 10 d after they were treated in the absence or presence of Wnt5a (100 ng/mL) and SP600125 (10 µM) or cyclosporine A (1 µM). Bars = 40 µm. Power = 0.84 (Wnt5a vs. control), 0.84, and 0.83 (SP600125 and cyclosporine A vs. Wnt5a). (G) Images and quantifications of TRAP-positive osteoclasts (black arrows) in the co-cultures of UAC-treated BMSCs pretreated with control or Ror2 siRNA (siRNA1131, siRNA1324, or siRNA 2124; 20 pmol/5 × 104 cells) and RAW264.7 cells. Bars = 40 µm. Power = 0.98. (H) Image and calculation of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)–positive cells per 200× field. RAW264.7 cells were incubated with 10 µM SP600125 for 7 d. TUNEL assay was performed to determine the apoptosis of osteoclast precursors. Bars = 50 µm. Power = 0.96. (I) Quantitative PCR was performed on RNAs extracted from CL-BMSCs pretreated with control or Ror2 siRNA (siRNA795 or siRNA 2477; 20 pmol/5 × 104 cells) to assess changes in the expressions of Cxcl12, Rankl, Opg, and Runx2 messenger RNA and the Rankl/Opg ratio (R/O) in the cells. Power = 0.23, 0.6 (Cxcl12 siRNA795 and siRNA 2477); 0.28 (Rankl siRNA795); 0.6, 0.42 (Opg siRNA795 and siRNA 2477); 0.7, 0.7 (Rankl/Opg siRNA795 and siRNA 2477); and 0.42 (Runx2 siRNA 2477). (J) Matrix mineralization of CL-BMSCs was analyzed by Alizarin red staining after the cells were cultured in osteogenic induction medium (OIM) with various concentrations (0 to 400 ng/mL) of Wnt5a for 2 wk. Power = 0.58, 0.68 (200 and 400 vs. 0, respectively). (K) Matrix mineralization of CL-BMSCs, which were pretreated with control or Ror2 siRNA (siRNA795 or siRNA 2477; 20 pmol/5 × 104 cells) and cultured in OIM containing Wnt5a (200 ng/mL) in the absence or presence of SP600125 (10 µM) or cyclosporine A (1 µM) for 2 wk, was determined by Alizarin red. Power = 0.60, 0.75, 0.80 (Wnt5a, siRNA795, and siRNA2477 vs. control, respectively); 0.83, 0.81 (SP600125 and cyclosporine A vs. Wnt5a). Data are presented as means ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control; #P < 0.05, ###P < 0.001 vs. Wnt5a group.
The recombinant Wnt5a (from 100 ng/mL) enhanced osteoclast precursor differentiation when RAW264.7 cells were co-cultured with CL-BMSCs but not when cultured RAW264.7 cells alone (Fig. 4D). Knockdown of Ror2 decreased Rankl expression and the Rankl/Opg ratio and increased Opg expression in CL-BMSCs (Fig. 4I). In agreement with this, Ror2 siRNA in CL-BMSCs led to a decreased trend, although without significance, on differentiation of the co-cultured RAW264.7 cells (Fig. 4E, F). Similarly, knockdown of Ror2 in UAC-treated BMSCs decreased differentiation of the co-cultured RAW264.7 cells (Fig. 4G). The promoting effect of the recombinant Wnt5a on RAW264.7 cell differentiation when co-cultured with CL-BMSCs was blocked by cyclosporine A but greatly enhanced by SP600125 (Fig. 4E, F). SP600125 greatly upregulated Rankl mRNA level and the Rankl/Opg ratio in CL-BMSCs after a 24-h incubation (Fig. 4C) and increased the number of TUNEL-positive RAW264.7 cells after a 7-d incubation (Fig. 4H), suggesting that in this model, blockade of JNK activation promoted osteoclast formation by upregulating the Rankl/Opg ratio in BMSCs but not through inhibiting RANKL-induced JNK-mediated apoptosis of differentiating osteoclasts as reported by others (Bharti et al. 2004; Otero et al. 2008). Taken together, Wnt5a/Ror2 signaling may enhance BMSCs’ capacity to promote osteoclast precursor migration and differentiation by activating JNK and/or Ca2+/NFAT signaling.
Wnt5a Enhanced Mineralization of BMSCs
Incubations of CL-BMSCs with recombinant Wnt5a (200 to 400 ng/mL) significantly increased the mineral deposition (Fig. 4J), while silencing Ror2 expression by siRNA markedly inhibited their mineralization (Fig. 4K), along with a reduced expression of Runx2, a transcription factor critical for osteoblast differentiation (Fig. 4I). Furthermore, culturing CL-BMSCs with SP600125 blocked Wnt5a-induced mineralization to a greater degree than that with cyclosporine A (Fig. 4K), and SP600125 significantly downregulated Runx2 expression in CL-BMSCs (Fig. 4C), supporting the involvement of Ca2+/NFAT and especially JNK signaling in Wnt5a-induced differentiation of BMSCs into osteoblasts.
Discussion
Previously, we showed that UACs elicited OA-like lesions in TMJs, displaying as increased MMP-3 but decreased MMP-9, MMP-13, and TIMP-1 mRNA expressions in rat cartilage at 4 and 8 wk (Wang et al. 2014) and subchondral trabecular bone loss (Lu et al. 2014; Yang et al. 2014). At 2 wk, the osteoclast and osteoblast numbers were decreased in UAC-treated TMJ subchondral trabecular bone (Yang et al. 2014) without Wnt5a and Ror2 mRNA changes (data not shown). However, at 4 and 8 wk (Figs. 1, 2 and Appendix Fig. 4, respectively), the osteoclast number was increased, but osteoblast number was decreased. It seems that the 8-wk results are a continuation of the 4-wk results. Thus, we chose 4 wk as the time point in this study to explore the role of BMSC-derived Wnt5a/Ror2 signaling in mediating TMJ subchondral trabecular bone remodeling.
The current data indicated that the locally isolated BMSCs from the UAC-treated subchondral bone marrow displayed a higher capability of recruiting osteoclast precursors with upregulated CXCL12 protein level and promoting osteoclast precursor differentiation with increased expression of Rankl, which were mediated, at least partially, by Wnt5a/Ror2 signaling as discussed below. This contributed to an explanation for the current resorptive subchondral trabecular bone phenotype when the osteogenic activities of BMSCs in the UAC-treated subchondral trabecular bone were increased, which seems insufficient to override the concurrent robust osteoclastic activity.
It was reported that CXCL12 is a dominant chemokine in bone marrow and is involved in skeletal repair of rheumatoid arthritis and bone fractures by recruiting BMSCs to the site of injury (Kitaori et al. 2009). CXCR4 is highly expressed not only by BMSCs but also by osteoclast precursors (Yu et al. 2003; Sordi et al. 2005). Hence, CXCL12 produced by BMSCs may attract BMSCs as well as circulating osteoclast precursors (Wright et al. 2005). We currently provided the evidence that Wnt5a/Ror2 signaling took a role in recruiting osteoclast precursors by increasing CXCL12 expression in local BMSCs, which explained the increased osteoclast number, where there was an upregulated osteogenic activity. Of course, numerous chemokines may be potentially involved in the migration of BMSCs or osteoclast precursors. Whether there are more chemokines participating in Wnt5a/Ror2-induced migration of BMSCs and osteoclast precursors needs further investigation.
In the present study, Ror2 expression was detected in BMSCs, such as BMSCs derived from rat TMJ subchondral bone and the BMSC cell line, but was undetectable in both rat BMMs and RAW264.7 cells. These findings suggested that BMSCs but not osteoclast precursors were the major cells responding to Wnt5a. This observation in RAW264.7 cells was compatible with the findings by Santiago and colleagues (2012). However, Maeda et al. (2012) reported weak Ror2 expression in BMMs from C57BL/6 mice. Nevertheless, the results that we observed with rat BMMs and RAW264.7 cells are all supportive of our assessment of the Wnt5a/Ror2 signaling responses in BMSCs.
A recent study showed that Wnt5a/Ror2 signaling is an important pathway between osteoblast-lineage cells and osteoclast precursors (Maeda et al. 2012). In agreement with that, the current data demonstrated that UACs increase osteoclast activities by activating Wnt5a/Ror2 signaling in local BMSCs. This notion was supported by the results that 1) UACs increased osteoclast precursor migration and differentiation in co-cultures of local BMSCs and osteoclast precursors, with upregulated Wnt5a and Ror2 expressions in these BMSCs; 2) Wnt5a promoted osteoclast precursor migration and differentiation in co-cultures of CL-BMSCs and RAW264.7 cells, while Ror2 knockdown in CL-BMSCs or UAC-treated BMSCs suppressed these capabilities and decreased expression levels of Cxcl12 and Rankl in CL-BMSCs; and 3) inhibitors of Ror2-induced signalings, such as cyclosporine A and SP600125, blunted the effects of Wnt5a on migration and/or differentiation of RAW264.7 cells when co-cultured with CL-BMSCs.
Notably, JNK inhibitor promoted osteoclast formation by upregulating Rankl expression in CL-BMSCs, which contradicted the results from current models that Wnt5a promoted the co-cultured osteoclast precursor differentiation by upregulating Rankl expression in BMSCs. We noticed that Wnt5a/Ror2 activated not only JNK but also the Ca2+/NFAT pathway (Oishi et al. 2003; Kikuchi et al. 2012). Moreover, we observed that Ca2+/NFAT inhibitor inhibited osteoclast precursor differentiation and that silencing Ror2 downregulated Rankl expression in CL-BMSCs. Considering RANKL activates the Ca2+/NFAT pathway in osteoclast precursors to enhance their differentiation (Takayanagi et al. 2002; Chen and Pan 2013), we inferred that it is the Wnt5a upregulated Rankl expression in BMSCs that activated the Ca2+/NFAT pathway in osteoclast precursors to promote osteoclast formation. The mechanisms of Wnt5a-upregulated Rankl expression in BMSCs seem not to be mediated by the JNK pathway and remain to be explored. However, cyclosporine A treatment may also negatively influence osteoclast precursor differentiation, regardless of CL-BMSC action.
Apart from mediating osteoclast formation, our current data also showed that 1) local BMSCs displayed an increased migration and osteogenesis capability despite a reduced number of them in UAC-treated rats, 2) knockdown of Ror2 in CL-BMSCs inhibited the expression of Runx2 and blunted the mineralizing function in CL-BMSCs, and 3) inhibitors of Wnt5a/Ror2-mediated signalings suppressed the osteoblast differentiation of CL-BMSCs. In the current study, JNK signaling stimulated Runx2 activity, which had been reported to promote osteogenic differentiation of BMSCs (Cárcamo-Orive et al. 2010; Huang et al. 2012). Activation of the Wnt5a/Ror2 pathway by interleukin (IL)–1β had been reported to promote mesenchymal stem cell differentiation into osteoblasts (Sonomoto et al. 2012). It seems Wnt5a/Ror2 signaling in BMSCs promotes their osteogenesis, which provides an explanation for an overall increase in the expressions of osteoblast markers in UAC-treated subchondral bone and UAC-treated BMSCs.
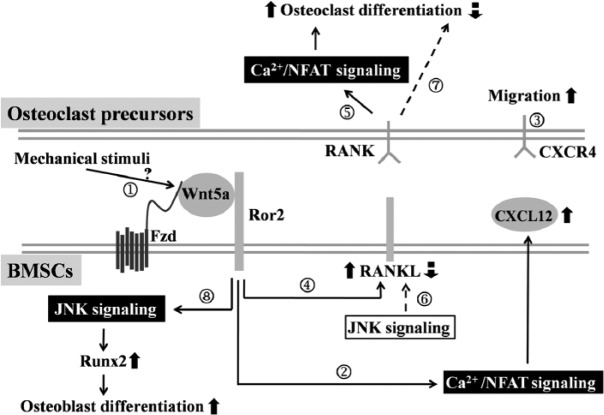
The following working model is proposed (Fig. 5). In response to mechanical dental stimulation of UACs, local BMSCs in TMJ subchondral trabecular bone increase their Wnt5a/Ror2 signaling through unknown mechanisms that remain to be defined (①). The activation of Wnt5a/Ror2 in BMSCs may increase their expression of CXCL12 mainly through the Ca2+/NFAT pathway (②), and CXCL12 may exert autocrine/paracrine effects to promote BMSC migration toward the bone surface. The increased CXCL12 may also attract osteoclast precursors to bone-remodeling units (③). Activation of Wnt5a/Ror2 signaling in BMSCs also increases their expression of Rankl (④), which may activate RANK and its downstream Ca2+/NFAT signaling cascade in osteoclast precursors to promote their differentiation (⑤). Meanwhile, JNK signaling downregulates Rankl expression (⑥) and inhibits osteoclast formation (⑦). However, this effect seems weaker than that of the RANKL-activated Ca2+/NFAT pathway, leading to an enhanced osteoclast differentiation. Furthermore, stimulation of Wnt5a/Ror2 signaling promotes Runx2 expression and osteoblast differentiation, which may be associated with the activation of the JNK pathway in BMSCs (⑧). Overall, the main effect of osteoclastic activity to resorb bone leads to bone loss in TMJ subchondral trabecular bone at the early stage of UAC-treated rats.
Figure 5.
Sketch map of the Wnt5a/Ror2 signaling pathway in bone marrow stromal cells (BMSCs) that derives osteoclast precursor migration and differentiation.
Author Contributions
T. Yang, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; J. Zhang, contributed to data acquisition and analysis, drafted the manuscript; Y. Cao, contributed to data acquisition and interpretation, drafted the manuscript; M. Zhang, L. Jing, contributed to data acquisition, drafted the manuscript; K. Jiao, contributed to design, drafted the manuscript; S. Yu, contributed to data analysis and interpretation, drafted the manuscript; W. Chang, D. Chen, contributed to data analysis and interpretation, drafted and critically revised the manuscript; M. Wang, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Acknowledgments
The authors thank Dr. Shiqiang Yu and Ms. Shujing Cai at the Fourth Military Medical University for technical support. The authors also thank Verhonda Hearon Eggleston for her assistance in preparing the manuscript.
Footnotes
This study was supported by the National Natural Science Foundation of China (grant No. 81271169).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Agata H. 2013. Isolation of bone marrow stromal cells: cellular composition is technique-dependent. In: Andrades JA. editor. Regenerative medicine and tissue engineering. Rijeka (Croatia): InTech. pp. 37–50. [Google Scholar]
- Aharon R, Bar-Shavit Z. 2006. Involvement of aquaporin 9 in osteoclast differentiation. J Biol Chem. 281(28):19305–19309. [DOI] [PubMed] [Google Scholar]
- Benoit DS, Boutin ME. 2012. Controlling mesenchymal stem cell gene expression using polymer-mediated delivery of siRNA. Biomacromolecules. 13(11):3841–3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti AC, Takada Y, Shishodia S, Aggarwal BB. 2004. Evidence that receptor activator of nuclear factor (NF)–kappaB ligand can suppress cell proliferation and induce apoptosis through activation of a NF-kappaB–independent and TRAF6-dependent mechanism. J Biol Chem. 279(7):6065–6076. [DOI] [PubMed] [Google Scholar]
- Bian ZY, Li G, Gan YK, Hao YQ, Xu WT, Tang TT. 2009. Increased number of mesenchymal stem cell–like cells in peripheral blood of patients with bone sarcomas. Arch Med Res. 40(3):163–168. [DOI] [PubMed] [Google Scholar]
- Bolbos RI, Zuo J, Banerjee S, Link TM, Ma CB, Li X, Majumdar S. 2008. Relationship between trabecular bone structure and articular cartilage morphology and relaxation times in early OA of the knee joint using parallel MRI at 3 T. Osteoarthritis Cartilage. 16(10):1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárcamo-Orive I, Gaztelumendi A, Delgado J, Tejados N, Dorronsoro A, Fernández-Rueda J, Pennington DJ, Trigueros C. 2010. Regulation of human bone marrow stromal cell proliferation and differentiation capacity by glucocorticoid receptor and AP-1 crosstalk. J Bone Miner Res. 25(10):2115–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Pan M. 2013. NFAT signaling and bone homeostasis. J Hematol Thromb. 1(1):102–108. [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E. 2006. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 8(4):315–317. [DOI] [PubMed] [Google Scholar]
- Han SY, Lee NK, Kim KH, Jang IW, Yim M, Kim JH, Lee WJ, Lee SY. 2005. Transcriptional induction of cyclooxygenase-2 in osteoclast precursors is involved in RANKL-induced osteoclastogenesis. Blood. 106(4):1240–1245. [DOI] [PubMed] [Google Scholar]
- Huang YF, Lin JJ, Lin CH, Su Y, Hung SC. 2012. c-Jun N-terminal kinase 1 negatively regulates osteoblastic differentiation induced by BMP2 via phosphorylation of Runx2 at Ser104. J Bone Miner Res. 27(5):1093–1105. [DOI] [PubMed] [Google Scholar]
- Ivanovska N, Dimitrova P. 2011. Bone resorption and remodeling in murine collagenase–induced osteoarthritis after administration of glucosamine. Arthritis Res Ther. 13(2):R44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi A, Yamamoto H, Sato A, Matsumoto S. 2012. Wnt5a: its signalling, functions and implication in diseases. Acta Physiol (Oxf). 204(1):17–33. [DOI] [PubMed] [Google Scholar]
- Kitaori T, Ito H, Schwarz EM, Tsutsumi R, Yoshitomi H, Oishi S, Nakano M, Fujii N, Nagasawa T, Nakamura T. 2009. Stromal cell–derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum. 60(3):813–823. [DOI] [PubMed] [Google Scholar]
- Lin CY, Yang JR, Teng SL, Tsai S, Chen MH. 2013. Microarray analysis of gene expression of bone marrow stem cells cocultured with salivary acinar cells. J Formos Med Assoc. 113(10):742–749. [DOI] [PubMed] [Google Scholar]
- Liu Y, Bhat RA, Seestaller-Wehr LM, Fukayama S, Mangine A, Moran RA, Komm BS, Bodine PV, Billiard J. 2007. The orphan receptor tyrosine kinase Ror2 promotes osteoblast differentiation and enhances ex vivo bone formation. Mol Endocrinol. 21(2):376–387. [DOI] [PubMed] [Google Scholar]
- Lu L, Huang J, Zhang X, Zhang J, Zhang M, Jing L, Yu S, Wang M. 2014. Changes of temporomandibular joint and Semaphorin 4D/Plexin-B1 expression in TMJ in mice with experimental incisor crossbite relation. J Oral Facial Pain Headache. 28(1):68–79. [DOI] [PubMed] [Google Scholar]
- Maeda K, Kobayashi Y, Udagawa N, Uehara S, Ishihara A, Mizoguchi T, Kikuchi Y, Takada I, Kato S, Kani S, et al. 2012. Wnt5a-Ror2 signaling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis. Nat Med. 18(3):405–412. [DOI] [PubMed] [Google Scholar]
- Mahjoub M, Berenbaum F, Houard X. 2012. Why subchondral bone in osteoarthritis? The importance of the cartilage bone interface in osteoarthritis. Osteoporos Int. 23(Suppl 8):S841–S846. [DOI] [PubMed] [Google Scholar]
- Mbalaviele G, Jaiswal N, Meng A, Cheng L, Van Den Bos C, Thiede M. 1999. Human mesenchymal stem cells promote human osteoclast differentiation from CD34+ bone marrow hematopoietic progenitors. Endocrinology. 140(8):3736–3743. [DOI] [PubMed] [Google Scholar]
- Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC, et al. 2003. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 8(7):645–654. [DOI] [PubMed] [Google Scholar]
- Oshita K, Yamaoka K, Udagawa N, Fukuyo S, Sonomoto K, Maeshima K, Kurihara R, Nakano K, Saito K, Okada Y, et al. 2011. Human mesenchymal stem cells inhibit osteoclastogenesis through osteoprotegerin production. Arthritis Rheum. 63(6):1658–1667. [DOI] [PubMed] [Google Scholar]
- Otero JE, Dai S, Foglia D, Alhawagri M, Vacher J, Pasparakis M, Abu-Amer Y. 2008. Defective osteoclastogenesis by IKK beta-null precursors is a result of receptor activator of NF-kappaB ligand (RANKL)–induced JNK-dependent apoptosis and impaired differentiation. J Biol Chem. 283(36):24546–24553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. 1999. Multilineage potential of adult human mesenchymal stem cells. Science. 284(5411):143–147. [DOI] [PubMed] [Google Scholar]
- Santiago F, Oguma J, Brown AM, Laurence J. 2012. Noncanonical Wnt signaling promotes osteoclast differentiation and is facilitated by the human immunodeficiency virus protease inhibitor ritonavir. Biochem Biophys Res Commun. 417(1):223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonomoto K, Yamaoka K, Oshita K, Fukuyo S, Zhang X, Nakano K, Okada Y, Tanaka Y. 2012. Interleukin-1beta induces differentiation of human mesenchymal stem cells into osteoblasts via the Wnt-5a/receptor tyrosine kinase-like orphan receptor 2 pathway. Arthritis Rheum. 64(10):3355–3363. [DOI] [PubMed] [Google Scholar]
- Sordi V, Malosio ML, Marchesi F, Mercalli A, Melzi R, Giordano T, Belmonte N, Ferrari G, Leone BE, Bertuzzi F, et al. 2005. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood. 106(2):419–427. [DOI] [PubMed] [Google Scholar]
- Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, et al. 2002. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 3(6):889–901. [DOI] [PubMed] [Google Scholar]
- Tsubaki M, Satou T, Itoh T, Imano M, Yanae M, Kato C, Takagoshi R, Komai M, Nishida S. 2012. Bisphosphonate- and statin-induced enhancement of OPG expression and inhibition of CD9, M-CSF, and RANKL expressions via inhibition of the Ras/MEK/ERK pathway and activation of p38MAPK in mouse bone marrow stromal cell line ST2. Mol Cell Endocrinol. 361(1–2):219–231. [DOI] [PubMed] [Google Scholar]
- Wang YL, Zhang J, Zhang M, Lu L, Wang X, Guo M, Zhang X, Wang MQ. 2014. Cartilage degradation in temporomandibular joint induced by unilateral anterior crossbite prosthesis. Oral Dis. 20(3):301–306. [DOI] [PubMed] [Google Scholar]
- Wright LM, Maloney W, Yu X, Kindle L, Collin-Osdoby P, Osdoby P. 2005. Stromal cell–derived factor-1 binding to its chemokine receptor CXCR4 on precursor cells promotes the chemotactic recruitment, development and survival of human osteoclasts. Bone. 36(5):840–853. [DOI] [PubMed] [Google Scholar]
- Yang T, Zhang J, Cao Y, Zhang M, Jing L, Jiao K, Yu S, Wang M. 2014. Decreased bone marrow stromal cells activity involves in unilateral anterior crossbite-induced early subchondral bone loss of temporomandibular joints. Arch Oral Biol. 59(9):962–969. [DOI] [PubMed] [Google Scholar]
- Yu X, Huang Y, Collin-Osdoby P, Osdoby P. 2003. Stromal cell–derived factor-1 (SDF-1) recruits osteoclast precursors by inducing chemotaxis, matrix metalloproteinase-9 (MMP-9) activity, and collagen transmigration. J Bone Miner Res. 18(8):1404–1418. [DOI] [PubMed] [Google Scholar]
- Zarb GA, Carlsson GE. 1999. Temporomandibular disorders: osteoarthritis. J Orofac Pain. 13(4):295–306. [PubMed] [Google Scholar]
- Zhang L, Chan C. 2010. Isolation and enrichment of rat mesenchymal stem cells (MSCs) and separation of single-colony derived MSCs. J Vis Exp. (37):pii:1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Dai J, Lu L, Zhang J, Zhang M, Wang Y, Guo M, Wang X, Wang M. 2013. Experimentally created unilateral anterior crossbite induces a degenerative ossification phenotype in mandibular condyle of growing Sprague-Dawley rats. J Oral Rehabil. 40(7):500–508. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.