Short abstract
Background
The association of peripapillary retinal nerve fibre layer (pRNFL) and ganglion cell-inner plexiform layer (GCIPL) thickness with neurodegeneration in multiple sclerosis (MS) is well established. The relationship of the adjoining inner nuclear layer (INL) with inflammatory disease activity is less well understood.
Objective
The objective of this paper is to investigate the relationship of INL volume changes with inflammatory disease activity in MS.
Methods In this longitudinal, multi-centre study, optical coherence tomography (OCT) and clinical data (disability status, relapses and MS optic neuritis (MSON)) were collected in 785 patients with MS (68.3% female) and 92 healthy controls (63.4% female) from 11 MS centres between 2010 and 2017 and pooled retrospectively. Data on pRNFL, GCIPL and INL were obtained at each centre.
Results
There was a significant increase in INL volume in eyes with new MSON during the study (N = 61/1562, β = 0.01 mm3, p < .001). Clinical relapses (other than MSON) were significantly associated with increased INL volume (β = 0.005, p = .025). INL volume was independent of disease progression (β = 0.002 mm3, p = .474).
Conclusion
Our data demonstrate that an increase in INL volume is associated with MSON and the occurrence of clinical relapses. Therefore, INL volume changes may be useful as an outcome marker for inflammatory disease activity in MSON and MS treatment trials.
Keywords: Inflammation, inner nuclear layer, multiple sclerosis, optical coherence tomography
Introduction
Thinning of the inner retinal layers, as observed with the use of optical coherence tomography (OCT), is a common finding in multiple sclerosis (MS) patients.1 Retinal OCT has been suggested as a structural imaging biomarker for neuroaxonal degeneration, as reduced thickness of both the peripapillary retinal nerve fibre layer (pRNFL, consisting of axons) and the combined thickness of the ganglion cell layer and inner plexiform layer (GCIPL, consisting of mainly ganglion cells) have shown to be associated with grey and white matter atrophy in patients with MS.2–6 Although the association of pRNFL and GCIPL thickness with neurodegeneration in MS is well established, a more complex situation is observed for the adjoining inner nuclear layer (INL). A histological study demonstrated that the INL, representing a neuronal network of bipolar, amacrine and horizontal cells, shows signs of atrophy but the presence of inflammatory cells was also described.7 Nevertheless, the INL seems not to be susceptible to retrograde degeneration caused by MS-related optic neuritis (MSON), as it does not show the extensive neuro-axonal injury in eyes with MSON as observed in the pRNFL and GCIPL.1,8 Rather than reflecting neurodegeneration like the innermost pRNFL and GCIPL, the INL may rather be a biomarker for inflammatory processes. In 2012, Gelfand et al. first described the presence of microcystic macular oedema (MMO) in the INL and the relationship with disability.9 Furthermore, a retrospective study by Saidha and colleagues reported that increased thickness of the combined INL and outer plexiform layer (OPL) was associated with disease activity in MS.10 More recently, Knier et al. reported that successful use of disease-modifying treatment (DMT) is associated with sustained reduction of INL volume,11 suggesting that the INL could serve as a biomarker to monitor central nervous system inflammation. Therefore, the aim of this study was to investigate the relationship of INL volume changes over time with local and global inflammatory disease activity in a large cohort of patients with MS.
Methods
Study design and participants
We used longitudinal data from the International Multiple Sclerosis Visual System Consortium (IMSVISUAL) database (www.imsvisual.org). Patients were recruited from 11 centres in the Netherlands (Amsterdam N = 165), Germany (Berlin N = 81, Düsseldorf N = 15, Munich (Universität München) N = 11, Munich (Technische Universität München) N = 169), Kuwait (Kuwait-City N = 98), Spain (Barcelona CEMCAT N = 39, Barcelona IDIBAPS N = 69), Italy (Milan N = 56), the United States (New York City N = 10) and France (Lille N = 72). Healthy control individuals (HCs) were recruited from three centres (Amsterdam N = 41, Munich N = 17 and Berlin N = 34). All patients and HCs participated in local observational studies and provided written informed consent for participation in their respective studies. Data were pooled retrospectively. All data from reported and ongoing cohort studies at MS centres were stored in the IMSVISUAL repository. The raw dataset is available from IMSVISUAL on request.
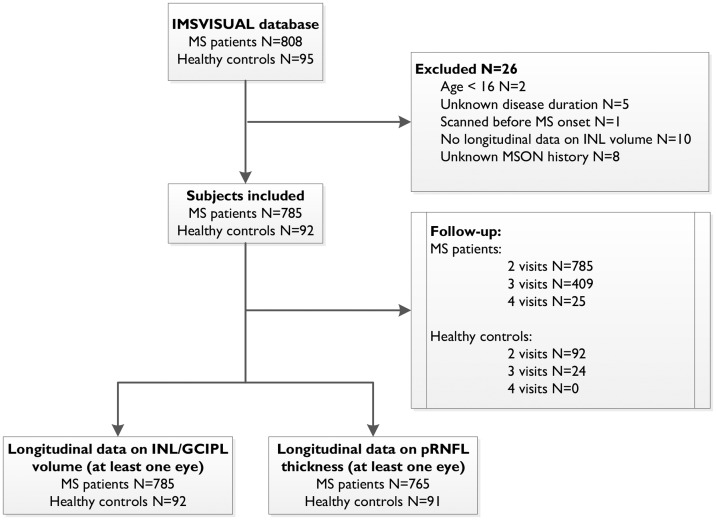
Data were collected between 2010 and 2017. MS patients were included if they were between ages 16 and 80 years, and had a diagnosis of clinically isolated syndrome or MS (including relapsing–remitting (RR), secondary progressive (SP) and primary progressive (PP) subtypes) according to the revised 2010 McDonald Criteria.12 HCs were included if they were between ages 18 and 80 years and had no history of any neurological disease or ophthalmologic reason for retinal pathology. Regarding the OCT assessments, individuals were included if they had at least two OCT measurements (baseline and at least one follow-up) with INL volume available for at least one eye (minimum follow-up period of six months). Patients were excluded if they had experienced symptomatic MSON within six months preceding the OCT assessment (baseline or follow-up), or if history of MSON was ambiguous or unknown. Inclusion and exclusion of individuals is shown in the flowchart in Figure 1.
Figure 1.
Flowchart of study design. Of the 903 individuals in the initial database, 785 patients and 92 healthy controls were included in this study. All participants had at least two visits, and a subset also had a third or fourth visit. Longitudinal data on inner nuclear layer (INL) and ganglion cell-inner plexiform layer (GCIPL) volume (at least two visits, minimum follow-up >6 months) was available for all included individuals and peripapillary retinal nerve fibre layer (pRNFL) for 765 patients and 91 healthy controls.
IMSVISUAL: International Multiple Sclerosis Visual System Consortium; MS: multiple sclerosis; MSON: multiple sclerosis optic neuritis.
OCT
Retinal OCT was performed at each centre by use of spectral-domain OCT with Spectralis (Heidelberg Engineering, Heidelberg, Germany, N = 10) or OCT-2000 (Topcon Corp, Itabashi, Japan, N = 1). Data on the INL and GCIPL volume (mm3) in the macular area were acquired using a macular volume scan centred on the fovea, using a 6 mm ring area. Data on global pRNFL thickness (µm) were obtained using a 12-degree ring scan (corresponding to a 3.4 mm diameter) manually placed around the optic disc. At each centre, automated segmentation of OCT scans and quality control (including the assessment whether eyes had signs of MMO) were performed.13,14 Importantly, the scanning device and protocols were kept identical for all longitudinal measurements within each centre.15
Clinical and ophthalmological outcome measures
Demographic data included data on sex, age at baseline and disease duration (from disease onset). Clinical data were collected longitudinally and included MS subtype, occurrence of relapses between visits, Expanded Disability Status Scale (EDSS) score, history of previous MSON and occurrence of new episodes of MSON between visits, presence of MMO and use of DMT. The assessment of history of symptomatic MSON (based on medical history, according to a standard protocol),16 EDSS score and data on clinical relapses were provided by the individual centres.
Importantly, given the longitudinal design of this study, we made a clear distinction between episodes of MSON before the study (referred to as ‘pre-study MSON’) and episodes of MSON during the follow-up of the study (referred to as ‘MSON during follow-up’).
EDSS assessment was performed by a certified examiner and in the absence of acute relapses. Disability progression was defined by an increase in EDSS score of 1.0 point in case EDSS score was less than 5.5 at baseline, or an increase of 0.5 if EDSS score was 5.5 or greater at baseline. This approach is consistent with previous IMSVISUAL collaborative projects.17
Statistical analyses
Annualised changes in retinal layer thickness or volume were calculated for every follow-up period. Subsequently, the annualised change scores were averaged over the complete observation period, resulting in one average annualised rate of change for every eye. All analyses were therefore performed on eye level, using generalised estimation equation (GEE) models with a correlation matrix structure that treats the eye measurements as exchangeable to adjust for intra-subject inter-eye dependency.15 All GEE models were additionally adjusted for relevant confounders (baseline OCT value, pre-study episodes of MSON, disease duration, use of DMT) as indicated. Figures showing longitudinal changes in retinal layer thickness were produced using relative annualised change scores (i.e. baseline was set as 100%).
Regarding the associations between annualised change in retinal thickness and the occurrence of relapses or disease progression, all eyes with a history of MSON were excluded. Short-term effects (clinical event and retinal change assessed within same follow-up period) as well as long-term effects (time-lag analyses, clinical event between baseline and first follow-up visit and change in retinal layer thickness between first and second follow-up visit) were investigated. Consequently, only individuals with at least three visits were included in these analyses. All analyses were adjusted for their respective baseline retinal layer thickness.
Correlations between the different layers were calculated with standardised regression coefficients in GEE models and are therefore also adjusted for inter-eye dependency. Statistical analyses were performed using SPSS V.22.0 (IBM Corp, Armonk, NY, USA) and Stata V.14.1 (StataCorp LP, College Station, TX, USA) with a two-sided statistical significance level of .05.
Results
Baseline
In total, 1570 eyes from 785 MS patients (68.3% female) and 184 eyes from 92 HCs (63.4% female) were included (Figure 1). MS patients had a median disease duration of 6.4 years (interquartile range (IQR) 1.9–15.0). The majority of patients (80.3%) had an RR disease course. More than half of all patients (N = 419, 53.4%) had never experienced a clinically confirmed MSON before baseline. Of all patients with a history of at least one confirmed episode of pre-study MSON (N = 366), 281 (77%) patients had unilateral MSON and 85 (23%) a history of MSON in both eyes (not necessarily simultaneously). MMO was present in 2.4% of patients (15/638) and in 1.4% (18/1275) of eyes. An overview of the baseline characteristics is shown in Table 1.
Table 1.
Baseline characteristics.
| All participantsN = 785 | Healthy controls(N = 92) | |
|---|---|---|
| Sex (female, N, %) | 536 (68.3%) | 59 (63.4%) |
| Age (y) | 41.0 (±12.6) | 43.4 (±11.5) |
| Disease duration (y, median (IQR)) | 6.4 (1.9–15.0) | |
| EDSS (median (IQR)) | 2.0 (1.0–3.0) | |
| Disease type | ||
| CIS | 45 (5.7%) | |
| RRMS | 630 (80.3%) | |
| SPMS | 74 (9.4%) | |
| PPMS | 36 (4.6%) | |
| MSON before baseline, N (%) | ||
| No previous MSON | 419 (53.4%) | |
| MSON | ||
| Unilateral MSON | 281 (35.8%) | |
| Bilateral MSON | 85 (10.8%) | |
| MMO before baseline (N = 638) | ||
| MMO– | 623 (97.6%) | |
| MMO+ | 15 (2.4%) | |
| Disease-modifying treatment at moment of baseline (N = 743) | ||
| None | 343 (46.2%) | |
| Interferon beta | 172 (23.2%) | |
| Glatiramer acetate | 72 (9.7%) | |
| Natalizumab | 61 (8.3%) | |
| Fingolimod | 53 (7.1%) | |
| Dimethyl fumarate | 20 (2.7%) | |
| Othera | 21 (2.8%) | |
CIS: clinically isolated syndrome; EDSS: Expanded Disability Status Scale; IQR: interquartile range, reported as 25th and 75th percentile; MMO: microcystic macular oedema; MSON: multiple sclerosis–related optic neuritis; PP: primary progressive; RR: relapsing remitting; SP: secondary progressive.
aRituximab, teriflunomide, azathioprine, mitoxantrone, cyclophosphamide, alemtuzumab and mycophenolate mofetil.
At baseline, MS patients showed significantly higher INL volume compared with HCs (difference of 0.02 mm3, p = .018) and lower GCIPL volume and pRNFL thickness (difference of –0.18 mm3 and –4.4 µm, respectively, p < .001 for both comparisons). Eyes with pre-study episodes of MSON showed a higher INL volume compared with eyes without (0.99 ± 0.08 mm3 and 0.97 ± 0.08 mm3, respectively, p = .001), whereas GCIPL volume and pRNFL thickness alike were lower in eyes with pre-study MSON compared with eyes without (Table 2).
Table 2.
Retinal layer thickness at baseline.
| All eyes N = 1570 |
MSON before
BL N = 451 |
No MSON before
BL N = 1119 |
HCs N = 184 |
p valuea MSON vs HC |
p valuea No MSON vs HCs |
p valuea No MSON vs MSON |
|
|---|---|---|---|---|---|---|---|
| INL (mm3) | 0.98 (0.08) | 0.99 (0.08) | 0.97 (0.08) | 0.96 (0.09) | .001 | .066 | .001 |
| GCIPL (mm3) | 1.79 (0.26) | 1.62 (0.25) | 1.86 (0.23) | 1.97 (0.19) | <.001 | <.001 | <.001 |
| pRNFL (µm) | 91.4 (15.8) | 81.4 (17.5) | 95.2 (13.2) | 95.8 (9.1) | <.001 | .106 | <.001 |
BL: baseline visit; HC: healthy controls; GCIPL: ganglion cell-inner plexiform layer; INL: inner nuclear layer; MSON: multiple sclerosis–related optic neuritis; pRNFL: peripapillary retinal nerve fibre layer.
aGeneralised estimation equation analyses, unadjusted.
Change over time in INL, GCIPL and pRNFL thickness and the effect of MSON
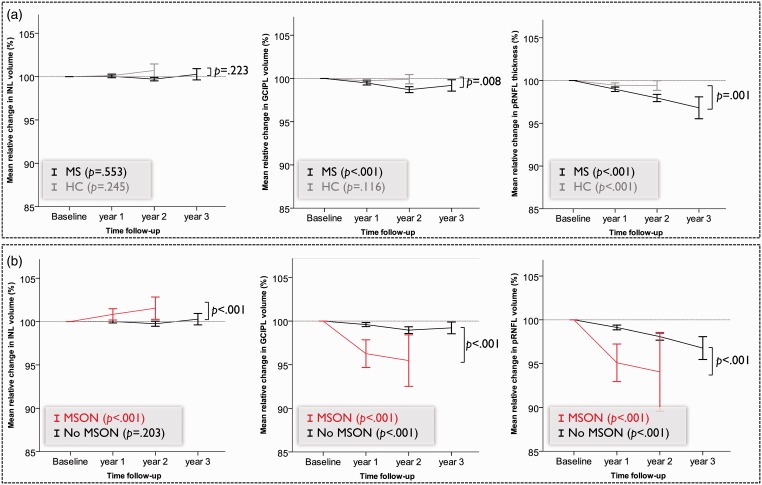
The median follow-up duration was 2.1 years (range, 0.5 to 5.2 years) for MS patients and 2.0 years (range, 0.6 to 4.6 years) for HCs. When all eyes of MS patients were analysed together, the INL showed a non-significant average annualised rate of change of –0.0003 mm3 (p = .553, Figure 2(a)). HCs also showed no significant change (annualised rate of change 0.006 mm3, p = .245).
Figure 2.
Relative change in retinal layer thickness with 95% confidence interval (based on generalised estimation equation model) for (a) all multiple sclerosis (MS) and healthy control (HC) eyes and (b) stratified by multiple sclerosis optic neuritis (MSON).
GCIPL: ganglion cell-inner plexiform layer; INL: inner nuclear layer; pRNFL: peripapillary retinal nerve fibre layer.
Regarding the effect of MSON, there was a clear difference between pre-study MSON (i.e. before the baseline OCT assessment) and MSON occurring during the follow-up period. Pre-study MSON did not affect the rate of change in INL thickness significantly. Eyes with and without pre-study MSON showed similar rates of change of the INL (β = 0.001, p = .219). In contrast, any episode of MSON during the observation period strongly affected INL volume. In eyes with MSON during follow-up (N = 61/1562), INL volume showed a significant annualised increase of 0.01 mm3 (p < .001). In contrast, in eyes without MSON during the observation period, no significant annualised change in INL was observed (β = –0.001 mm3, p = .203, Figure 2(b)). Exclusion of patients with a progressive disease type, or adjustments for use of DMT, disease duration or participating centre, did not change these results (data not shown).
The annualised rate of change for GCIPL in MS patients was –0.012 mm3 (p < .001), which was significantly more than observed in HCs (–0.004 mm3, p = 0.116, p value for comparison .008). The pRNFL showed significantly more thinning in MS patients (–0.97 µm, p < .001) compared with HCs (–0.42 µm, p < .001, p value for comparison .001, Figure 2(a)). For both layers, eyes with episodes of MSON during the follow-up period showed significantly more thinning than unaffected eyes (Figure 2(b)).
Short- and long-term effects of clinical disease activity on retinal layer thickness
Table 3 demonstrates the effects of new episodes of MSON during follow-up, other clinical relapses and disease progression on annualised change in INL and GCIPL volume and pRNFL thickness. The short-term (clinical event and retinal change assessed within the same follow-up period, Table 3(a)) as well as the long-term (time-lag analyses, clinical event between t0 and t1 and change in retinal layer thickness between t1 and t2, Table 3(b)) effects are reported. The median duration of t0–t1 was 1.1 year (IQR 1.0–1.9) and for t1–t2 the median duration was 1.0 year (IQR 1.0–1.7).
Table 3.
(a) Short- and (b) long-term effects of MSON, clinical relapses (other than MSON) and disability progression on annualised change in INL and GCIPL volume and pRNFL thickness.
| (a) | β (95% CI) short term | p valuea |
| MSON (N = 26 eyes) vs no MSON (N = 1039 eyes) | ||
| INL | 0.01 (0.006 to 0.020) | <.001 |
| GCIPL | –0.13 (–0.18 to –0.08) | <.001 |
| pRNFL | –7.61(–10.8 to –4.3) | <.001 |
| Relapse (N = 214 eyes) vs no relapse (N = 789 eyes) | ||
| INL | 0.000 (–0.004 to 0.004) | .868 |
| GCIPL | –0.10 (–0.18 to –0.002) | .012 |
| pRNFL | –0.54 (–1.14 to 0.07) | .082 |
| Progression (N = 223 eyes) vs no progression (N = 673 eyes) | ||
| INL | 0.001 (–0.004 to 0.005) | .774 |
| GCIPL | 0.001 (–0.006 to 0.008) | .764 |
| pRNFL | –0.13 (–0.66 to 0.41) | .646 |
| (b) | β (95% CI) long term (time-lag model) | p valuea |
| MSON (N = 11 eyes) vs no MSON (N = 581 eyes) | ||
| INL | –0.006 (–0.026 to 0.013) | .535 |
| GCIPL | 0.023 (–0.065 to 0.112) | .604 |
| pRNFL | –1.124 (–3.78 to 1.53) | .406 |
| Relapse (N = 148 eyes) vs no relapse (N = 440 eyes) | ||
| INL | 0.005 (0.001 to 0.01) | .025 |
| GCIPL | –0.005 (–0.015 to 0.005) | .307 |
| pRNFL | –0.40 (–1.57 to 0.77) | .501 |
| Progression (N = 97 eyes) vs no progression (N = 409 eyes) | ||
| INL | 0.001 (–0.004 to 0.007) | .609 |
| GCIPL | –0.006 (–0.02 to 0.006) | .329 |
| pRNFL | –0.65 (–0.69 to 1.99) | .342 |
β = regression coefficient; CI = confidence interval; HC: healthy controls; GCIPL: ganglion cell-inner plexiform layer; INL: inner nuclear layer; MSON: multiple sclerosis–related optic neuritis; pRNFL: peripapillary retinal nerve fibre layer.
aGeneralised estimation equation model adjusted for inter-eye dependency and baseline retinal thickness.
Clinical episodes of MSON during follow-up demonstrated only a short-term effect on INL (thickening) and GCIPL and pRNFL (thinning). In the time-lag analyses investigating the long-term effects, these effects disappeared. Exclusion of patients with a progressive disease course did not change the statistical findings.
Clinical relapses (other than MSON) during follow-up were present in 24.4% of patients. The occurrence of clinical relapses during the first follow-up was not related to change in INL within the same period (median 1.1 years from baseline, β = 0.000, 95% confidence interval (CI) –0.004 to 0.004, p = .868) but was significantly associated with an increase in INL volume in the subsequent follow-up (median 2.2 years from baseline, β = 0.005, 95% CI 0.001 to 0.01, p = .025). This effect was similar when only patients with a relapsing disease course were included (N = 508 eyes, β = 0.005 (95% CI 0.00 to 0.01, p = .049)). For GCIPL volume and pRNFL thickness, this effect was more pronounced in the short term (i.e. relapse and retinal volume change within the same follow-up period, Table 3(a)).
Disability progression was observed in 17.2% (during the entire follow-up period). Annualised change in INL volume was independent of disability progression both in the short and long term. Likewise, disability progression was not significantly associated with annualised changes in GCIPL or pRNFL (Table 3(a) and 3(b)).
Effect of MMO, disease type and DMT on retinal changes
In the 1.4% of eyes with MMO before or during the study (18/1275 eyes), the INL volume at the last visit was 0.07 mm3 higher compared with eyes without MMO (p = .006, adjusted for new episodes of MSON). Likewise, the average annualised rate of change of INL volume was significantly higher in eyes with MMO compared with eyes without (β = 0.01, p = .011, adjusted for baseline INL and episodes of MSON during follow-up), showing a significant annualised increase over time in MMO eyes (0.01 ± 0.02 mm3), but no change in eyes without (–0.0002 ± 0.02 mm3).
Just more than half the patients (53.8%) used DMT during the study. Although the annualised change in INL volume was not influenced by use of DMT, the absolute INL volume was significantly higher in patients using fingolimod compared with RRMS patients who did not use any DMT, independent of history of pre-study MSON and MMO, EDSS at baseline and disease duration (difference 0.03 mm3, p = .004). Other therapies did not show significant differences in INL volume.
Interrelationship between layers
All analyses regarding the interrelationships between the layers demonstrated effect modification by presence of a new episode of MSON during follow-up and are therefore stratified. In eyes with MSON during follow-up, an increase in INL volume was related to a decrease in GCIPL volume (standardised β = –0.42, p = .006, black line in Supplementary Figure 1(a)) and to a lesser extent (although not statistically significant) to a decrease in pRNFL (standardised β = –0.15, p = .148, black line in Supplementary Figure 1(b)). In eyes without new MSON, no significant association with change in INL volume was observed (grey lines in Supplementary Figures 1(a) and (b)). In contrast, GCIPL and pRNFL show positive correlations both in eyes with new MSON (standardised β = 0.41, p < .001) and without MSON (standardised β = 0.26, p = .004, see Supplementary Figure 1(c)).
Discussion
This longitudinal, multi-centre study demonstrates that thickening of the INL as measured with spectral-domain OCT reflects adjacent inflammation of the optic nerve. Besides this association with local inflammation, the INL seems to reflect some degree of global disease activity, as the occurrence of clinical relapses in any functional system was significantly associated with a subsequent increase in INL volume. However, this effect was relatively small (difference of 0.005 mm3) and should be interpreted with caution, as the sensitivity might be limited.
These findings build on previous findings from other studies, demonstrating the relationship between thickening of the INL and physical disability9 and disease activity.10 Saidha et al.10 reported that INL/OPL thickening at baseline was predictive of clinical relapses, new T2 and contrast-enhancing lesions on magnetic resonance imaging and disability progression during follow-up. In contrast, in the present study we did not observe any predictive value of INL thickening on clinical relapses or disability progression. Our data demonstrated an association between INL volume and clinical relapses only in the time-lag model, in which clinical relapses preceded INL thickening. This would suggest that INL thickening occurred subsequent to inflammatory disease activity. A predictive effect of baseline INL volume on the occurrence of relapses or disability progression, as described by Saidha and colleagues,10 was not observed in the present study.
When thickening of the INL was first described, it was directly linked to the presence of MMO.9 Although MMO is present in MS and is related to increased disability,9,10,18 it is not specific for MS and may vary over time in individual patients.19,20 Importantly, we have previously demonstrated that MMO was transient in 84% of cases.19 In the present study, MMO was present in 2.4% of patients, which is consistent with previous findings.9–11,21 In the present study we observed increased INL volume in eyes with MMO, which is also consistent with existing literature.1 Moreover, eyes with MMO also showed a significant increase in INL volume over time. Nevertheless, the findings of the present study did not change when MMO eyes were excluded, suggesting that thickening of the INL can occur in the absence of visually detectable MMO.
The underlying mechanism responsible for thickening of the INL remains unknown. The findings of this study would imply that previously suggested mechanisms such as inflammation-related dynamic fluid shifts and Müller cell dysfunction are more likely than other non-inflammatory mechanisms such as traction and retrograde trans-synaptic degeneration.21,22 Dynamic retinal layer volume changes can be explained by fluid shifts due to a combination of osmotic and hydrostatic gradients, the retinal glymphatic system.23–26 The INL is embedded between the superficial vascular plexus and the deep capillary plexus, which can be clearly visualised on OCT angiography.24 Typically, fluid reaches the retina through the internal limiting membrane and both plexuses, whereas both plexuses and Müller cells can absorb the interstitial fluid. In case of inflammation, diffusion of fluid from the retinal blood vessels increases, leading to an increase in INL volume. Another suggested mechanism is pathology of Müller cells, which would impair the absorption of interstitial fluid, also resulting in increased INL volume. Other suggested non-inflammatory mechanisms such as traction or retrograde trans-synaptic degeneration are also plausible but less well supported by our data, given the clear and direct increase in INL volume following MSON. One approach to further elucidate the underlying mechanism would be the investigation of the retinal vessels using OCT angiography.
Previously, we and others have described the limited susceptibility of the INL to retrograde degeneration caused by MSON.1 This is in line with our current findings, in which the pRNFL and GCIPL clearly showed significant thinning in eyes with previous episodes of MSON, whereas for the INL no thinning but rather an increase in volume was observed. The opposing effects of local inflammation on the INL on the one hand and pRNFL/GCIPL on the other are clearly demonstrated by the negative correlation between the layers. This further substantiates the potential of pRNFL/GCIPL as a measure for neurodegeneration, whereas INL volume may be a valuable parameter for reflecting inflammatory activity.
A recent study by Knier et al. reported that effective treatment with DMT in patients with MS is associated with a sustained reduction in INL volume in the absence of MSON, and they suggested that INL volume may be a response marker for successful treatment of inflammation.11 Building on these findings, we investigated the effect of DMT on INL volume changes. Although the absolute INL volume was significantly higher in patients using fingolimod compared with RRMS patients who did not use any DMT, which corroborates previous findings on retinal effects of this drug,27,28 the annualised change in INL volume was not influenced by use or type of DMT. However, it should be noted that DMT data were available only at baseline for the majority of patients, and that data on the exact duration of treatment or previous DMTs were not available. This lack of detailed information did not permit a thorough investigation of direct effects of DMT or replication of previous results.
Another limitation of the study was that the data did not permit the determination of how acute new episodes of MSON were. A systematic assessment of the early time course of acute MSON would be extremely valuable, and there will need to be a consensus as to what will be defined as onset of an acute episode of MSON. Furthermore, disease activity was recorded only by clinical relapse activity. Data on radiological disease activity (new T2 and/or gadolinium-enhancing lesions during follow-up) were not available. Therefore, no conclusions could be made regarding the relationship of INL volume and radiological disease activity. A common limitation of multi-centre studies is the difference in methodology among the participating centres.29,30 The OCT device and software were the same for all centres (Spectralis) but one (Topcon). The data on retinal layer thickness of this particular centre were not significantly different from the other centres’, and additional adjustment for a potential centre effect did not change any of the results (data not shown).
In summary, our data demonstrate that an increase in INL volume is strongly associated with inflammation of the optic nerve, and to a lesser degree with other clinical relapses. Therefore, INL volume may be a valuable parameter for capturing inflammatory disease activity and may be considered as an outcome measure for MS and MSON treatment trials.
Supplemental Material
Supplemental Material for Retinal inner nuclear layer volume reflects inflammatory disease activity in multiple sclerosis; a longitudinal OCT study by Lisanne J Balk, Danko Coric Benjamin Knier Hanna G Zimmermann Raed Behbehani Raed Alroughani Elena H Martinez-Lapiscina Alexander U Brandt Bernardo Sánchez-Dalmau Angela Vidal-Jordana Philipp Albrecht, Valeria Koska, Joachim Havla Marco Pisa Rachel C Nolan Letizia LeocaniFriedemann Paul, Orhan Xavier Montalban Aktas , Laura J Balcer Pablo Villoslada Olivier OutteryckThomas Korn, Axel Petzold and on behalf of the IMSVISUAL consortium in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Acknowledgements
LJ Balk contributed to the conception and design of the study, acquisition and analysis of data, and drafting of the manuscript. DC contributed to the acquisition of data and drafting of the manuscript. BK, HGZ, RB, RA, EHML, AUB, BSD, AVJ, PA, VK, JH, MP, RCN, LL, FP, OA, XM, LJ Balcer, PV, OO and TK contributed to the acquisition and preparation of data and critically revised the manuscript. AP contributed to the conception and design of the study, analysis of data, and drafting of the manuscript. All authors have given final approval of the submitted version of the manuscript.
Contributor Information
Danko Coric, Department of Neurology, Amsterdam UMC, Vrije Universiteit Amsterdam, the Netherlands.
Benjamin Knier, Department of Neurology, Klinikum rechts der Isar, Technische Universität München, Germany.
Hanna G Zimmermann, Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, NeuroCure Clinical Research Center, Germany.
Raed Behbehani, Al-Bahar Ophthalmology Centre, Ibn Sina Hospital, Kuwait.
Raed Alroughani, Division of Neurology, Department of Medicine, Amiri Hospital, Kuwait.
Elena H Martinez-Lapiscina, IDIBAPS – Hospital Clinic, University of Barcelona, Spain.
Alexander U Brandt, Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, NeuroCure Clinical Research Center, Germany.
Bernardo Sánchez-Dalmau, IDIBAPS – Hospital Clinic, University of Barcelona, Spain.
Angela Vidal-Jordana, Multiple Sclerosis Centre of Catalonia, Neurology-Neuroinmunology Unit, Vall d’Hebron Hospital, Spain.
Joachim Havla, Institute of Clinical Neuroimmunology, Ludwig-Maximilians Universitaet Muenchen, Germany.
Marco Pisa, Vita-Salute San Raffaele University, Italy.
Rachel C Nolan, New York University School of Medicine, USA.
Letizia Leocani, Vita-Salute San Raffaele University, Italy.
Orhan Aktas, Department of Neurology, Medical Faculty, Heinrich Heine University, Germany.
Laura J Balcer, New York University School of Medicine, USA.
Pablo Villoslada, IDIBAPS – Hospital Clinic, University of Barcelona, Spain.
Olivier Outteryck, Université Lille (Inserm U1171), France.
Conflict of interests
L.J. Balk has received an institutional research grant from Teva and is a researcher for the OCTIMS study, an observational study for validating OCT as a marker for MS sponsored by Novartis.
D. Coric has nothing to declare.
B. Knier has received research grants from Novartis and travel expenses from Novartis, Merck and Bayer.
H.G. Zimmermann has received a research grant from Novartis and speaking fees from Teva.
R. Behbehani has nothing to declare.
R. Alroughani has received honoraria as a speaker and for serving on scientific advisory boards for Bayer, Biogen, GSK, Merck, Novartis, Sanofi-Genzyme and Roche; and research grants from Biogen, Novartis and Sanofi-Genzyme.
E.H. Martinez-Lapiscina reports personal fees from Biogen, Genzyme, and Novartis; travel reimbursement from Roche; grants from Instituto de Salud Carlos III – (CM13/00150; MV15/00012; JR16/0006; MV17/00021; PI17/01228), Fundació Marató TV3 (20142030), GMSI (2016), Fundaciò Cellex Barcelona, Sanofi-Genzyme, Novartis, Sanofi Genzyme and Roche, outside the submitted work; is a member of the working group of IMSVISUAL; and is a researcher for the OCTIMS study.
A.U. Brandt is named as inventor on several patent applications describing serum biomarkers in MS, motor function assessments using perceptive visual computing, and methods for analysing retinal images. He is cofounder of shareholder of the companies Motognosis and Nocturne, the latter of which has an interest in using OCT for MS diagnosis.
B. Sánchez-Dalmau has received compensation for consulting services or speaking honoraria from Novartis and received research grants from Sanofi-Aventis; and is a researcher for the OCTIMS study.
A. Vidal-Jordana receives support for Juan Rodés contracts (JR16/00024) from Fondo de Investigaciones Sanitarias, Instituto de Salud Carlos III, Spain, and has received compensation for consulting services or speaking honoraria from Novartis, Roche and Sanofi-Aventis.
P. Albrecht reports grants and non-financial support from Biogen; grants, personal fees and non-financial support from Allergan; personal fees and non-financial support from Bayer; personal fees and non-financial support from Merck; grants, personal fees and non-financial support from Merz Pharmaceuticals; grants, personal fees and non-financial support from Novartis; grants, personal fees and non-financial support from Roche; grants, personal fees and non-financial support from Teva; and grants, personal fees and non-financial support from Ipsen, outside the submitted work.
V. Koska has nothing to declare.
J. Havla has received speaker honoraria, travel expenses, and personal compensations from Merck, Roche, Santhera, Biogen, Bayer Healthcare, Sanofi Genzyme and Novartis Pharma.
M. Pisa has nothing to declare.
R.C. Nolan has nothing to declare.
L. Leocani reports grants, personal fees and non-financial support from Merck; personal fees and non-financial support from Roche; personal fees from Teva; grants, personal fees and non-financial support from Biogen; non-financial support from Almirall; and grants and non-financial support from Novartis, outside the submitted work.
F. Paul served on the steering committee for the Novartis OCTIMS study and MedImmune; has received speaker honoraria and travel funding from Bayer, Novartis, Biogen Idec, Teva, Sanofi-Aventis/Genzyme, Merck Serono, Alexion, Chugai and MedImmune; is an academic editor for PLoS One; is an associate editor for Neurology: Neuroimmunology & Neuroinflammation; has consulted for Sanofi Genzyme, Biogen Idec and MedImmune; and has received research support from Bayer, Novartis, Biogen Idec, Teva, Sanofi-Aventis/Genzyme, Alexion, Merck Serono, German Research Council, Werth Stiftung of the City of Cologne, German Ministry of Education and Research (BMBF Competence Network Multiple Sclerosis) and Arthur Arnstein Stiftung Berlin.
O. Aktas has received grants from the German Research Foundation (DFG), Eugène Devic European Network (EU-FP7), German Ministry of Education and Research, and the Schaufler Foundation; honoraria for lectures from Almirall, Novartis, Bayer, Genzyme, Teva, Merck Serono, Biogen, Roche and Medimmune; and received travel/accommodation/meeting expenses from Novartis, Bayer Schering and Merck Serono.
X. Montalban has received personal compensation for activities with Biogen, Celgene, Sanofi Genzyme, Merck, Novartis, Roche and Teva Pharmaceuticals.
L.J. Balcer has nothing to declare.
P. Villoslada is currently an employee of Genentech; this work was conducted before this affiliation and independently of the company. He holds stocks in Bionure Inc, Spire Bioventures, Mintlabs and Health Engineering; is academic editor of Multiple Sclerosis and Demyelinating Diseases; and is on the executive committee of the European Association of Systems Medicine.
O. Outteryck reports grant for research from Novartis; grants and personal fees from Biogen-Idec; and funding for travel from Biogen-Idec, Genzyme-Sanofi, Merck-Serono, Novartis and Teva Pharmaceutical Industries, outside the submitted work.
T. Korn reports compensation for travel expenses from Merck and speaker honoraria from Novartis.
A. Petzold reports that the VUmc Multiple Sclerosis Center Amsterdam participated in the OCTIMS study and the PASSOS study, which were sponsored by Novartis, and the centre has received research support for OCT projects from the Dutch Multiple Sclerosis Society. AP’s research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Moorfields Eye Hospital National Health Service (NHS) Foundation Trust and University College London Institute of Ophthalmology. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Petzold A, Balcer LJ, Calabresi PA, et al. Retinal layer segmentation in multiple sclerosis: A systematic review and meta-analysis. Lancet Neurol 2017; 16: 797–812. [DOI] [PubMed] [Google Scholar]
- 2.Saidha S, Al-Louzi O, Ratchford JN, et al. Optical coherence tomography reflects brain atrophy in multiple sclerosis: A four-year study. Ann Neurol 2015; 78: 801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dorr J, Wernecke KD, Bock M, et al. Association of retinal and macular damage with brain atrophy in multiple sclerosis. PLoS One 2011; 6: e18132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gordon-Lipkin E, Chodkowski B, Reich DS, et al. Retinal nerve fiber layer is associated with brain atrophy in multiple sclerosis. Neurology 2007; 69: 1603–1609. [DOI] [PubMed] [Google Scholar]
- 5.Sepulcre J, Murie-Fernandez M, Salinas-Alaman A, et al. Diagnostic accuracy of retinal abnormalities in predicting disease activity in MS. Neurology 2007; 68: 1488–1494. [DOI] [PubMed] [Google Scholar]
- 6.Balk LJ, Steenwijk MD, Tewarie P, et al. Bidirectional trans-synaptic axonal degeneration in the visual pathway in multiple sclerosis. J Neurol Neurosurg Psychiatry 2015; 86: 419–424. [DOI] [PubMed] [Google Scholar]
- 7.Green AJ, McQuaid S, Hauser SL, et al. Ocular pathology in multiple sclerosis: Retinal atrophy and inflammation irrespective of disease duration. Brain 2010; 133: 1591–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balk LJ, Twisk JW, Steenwijk MD, et al. A dam for retrograde axonal degeneration in multiple sclerosis? J Neurol Neurosurg Psychiatry 2014; 85: 782–789. [DOI] [PubMed] [Google Scholar]
- 9.Gelfand JM, Nolan R, Schwartz DM, et al. Microcystic macular oedema in multiple sclerosis is associated with disease severity. Brain 2012; 135: 1786–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saidha S, Sotirchos ES, Ibrahim MA, et al. Microcystic macular oedema, thickness of the inner nuclear layer of the retina, and disease characteristics in multiple sclerosis: A retrospective study. Lancet Neurol 2012; 11: 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knier B, Schmidt P, Aly L, et al. Retinal inner nuclear layer volume reflects response to immunotherapy in multiple sclerosis. Brain 2016; 139: 2855–2863. [DOI] [PubMed] [Google Scholar]
- 12.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schippling S, Balk LJ, Costello F, et al. Quality control for retinal OCT in multiple sclerosis: Validation of the OSCAR-IB criteria. Mult Scler 2015; 21: 163–170. [DOI] [PubMed] [Google Scholar]
- 14.Tewarie P, Balk L, Costello F, et al. The OSCAR-IB consensus criteria for retinal OCT quality assessment. PLoS One 2012; 7: e34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cruz-Herranz A, Balk LJ, Oberwahrenbrock T, et al. The APOSTEL recommendations for reporting quantitative optical coherence tomography studies. Neurology 2016; 86: 2303–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petzold A, Wattjes MP, Costello F, et al. The investigation of acute optic neuritis: A review and proposed protocol. Nat Rev Neurol 2014; 10: 447–458. [DOI] [PubMed] [Google Scholar]
- 17.Martinez-Lapiscina EH, Arnow S, Wilson JA, et al. Retinal thickness measured with optical coherence tomography and risk of disability worsening in multiple sclerosis: A cohort study. Lancet Neurol 2016; 15: 574–584. [DOI] [PubMed] [Google Scholar]
- 18.Kaufhold F, Zimmermann H, Schneider E, et al. Optic neuritis is associated with inner nuclear layer thickening and microcystic macular edema independently of multiple sclerosis. PLoS One 2013; 8: e71145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burggraaff MC, Trieu J, de Vries-Knoppert WA, et al. The clinical spectrum of microcystic macular edema. Invest Ophthalmol Vis Sci 2014; 55: 952–961. [DOI] [PubMed] [Google Scholar]
- 20.Brandt AU, Oberwahrenbrock T, Kadas EM, et al. Dynamic formation of macular microcysts independent of vitreous traction changes. Neurology 2014; 83: 73–77. [DOI] [PubMed] [Google Scholar]
- 21.Balk L, Killestein J, Polman C, et al. Microcystic macular oedema confirmed, but not specific for multiple sclerosis. Brain 2012; 135(pt 12): e226; author reply e227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lujan BJ, Horton JC. Microcysts in the inner nuclear layer from optic atrophy are caused by retrograde trans-synaptic degeneration combined with vitreous traction on the retinal surface. Brain 2013; 136: e260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petzold A. Retinal glymphatic system: An explanation for transient retinal layer volume changes? Brain 2016; 139: 2816–2819. [DOI] [PubMed] [Google Scholar]
- 24.Spaide RF. Retinal vascular cystoid macular edema: Review and new theory. Retina 2016; 36: 1823–1842. [DOI] [PubMed] [Google Scholar]
- 25.Wostyn P, De Groot V, Van Dam D, et al. Age-related macular degeneration, glaucoma and Alzheimer’s disease: Amyloidogenic diseases with the same glymphatic background? Cell Mol Life Sci 2016; 73: 4299–4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Denniston AK, Keane PA, Aojula A, et al. The ocular glymphatic system and idiopathic intracranial hypertension: Author response to “Hypodense Holes and the Ocular Glymphatic System”. Invest Ophthalmol Vis Sci 2017; 58: 1134–1136. [DOI] [PubMed] [Google Scholar]
- 27.Nolan R, Gelfand JM, Green AJ. Fingolimod treatment in multiple sclerosis leads to increased macular volume. Neurology 2013; 80: 139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dinkin M, Paul F. Higher macular volume in patients with MS receiving fingolimod: Positive outcome or side effect? Neurology 2013; 80: 128–129. [DOI] [PubMed] [Google Scholar]
- 29.Warner CV, Syc SB, Stankiewicz AM, et al. The impact of utilizing different optical coherence tomography devices for clinical purposes and in multiple sclerosis trials. PLoS One 2011; 6: e22947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coric D, Petzold A, Uitdehaag BMJ, et al. P1084 Updates in OCT segmentation software influence longitudinal assessment of retinal atrophy. Mult Scler 2017; 23(3 suppl): 563–564. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Retinal inner nuclear layer volume reflects inflammatory disease activity in multiple sclerosis; a longitudinal OCT study by Lisanne J Balk, Danko Coric Benjamin Knier Hanna G Zimmermann Raed Behbehani Raed Alroughani Elena H Martinez-Lapiscina Alexander U Brandt Bernardo Sánchez-Dalmau Angela Vidal-Jordana Philipp Albrecht, Valeria Koska, Joachim Havla Marco Pisa Rachel C Nolan Letizia LeocaniFriedemann Paul, Orhan Xavier Montalban Aktas , Laura J Balcer Pablo Villoslada Olivier OutteryckThomas Korn, Axel Petzold and on behalf of the IMSVISUAL consortium in Multiple Sclerosis Journal – Experimental, Translational and Clinical