Abstract
It is important to investigate the clinical characteristics and identify the stroke mechanisms of patients with autoimmune disease-related stroke, which are necessary for early etiology diagnosis, accurate treatment and preventive strategies. In this article we retrospectively studied eight cases of acute ischemic stroke associated with autoimmune diseases, and without competing conventional stroke etiologies. The characteristics of stroke (clinical and radiological features), the laboratory tests especially serum D-dimer levels (as a marker of hypercoagulable state), and embolic signals on transcranial Doppler were evaluated for all eight patients. High-resolution magnetic resonance imaging (HRMRI), which can help to evaluate vasculitis was performed in four patients. The possible underlying mechanisms of these cases were discussed based on these manifestations. As a result, autoimmune diseases in our study included systemic lupus erythematosus (n=5), mixed connective tissue disease (n=1), central nervous system vasculitis (n=1), and Takayasu arteritis (n=1). All eight patients presented with acute infarction lesions in ≥2 vascular territories. Most patients presented with numerous small and medium infarction lesions located in the cortical and subcortical areas. Multiple stroke mechanisms were involved in these cases, including hypercoagulability (n=4), cardiac embolism (n=1) and vasculitis (n=3). Embolic signals could be detected on transcranial Doppler in all three stroke mechanisms. In conclusion, our study revealed the characteristics of autoimmune disease-related stroke. For patients with multiple acute cerebral infarcts within non-single arterial territories, autoimmune disease is an important etiology not to be neglected. Multiple stroke mechanisms were involved in these cases.
Keywords: Autoimmune disease, stroke, embolism, imaging, vasculitis
Introduction
It is usually difficult to identify the causes of stroke and up to one-quarter of patients with ischemic stroke have no probable cause found after standard workup1,2. Autoimmune disease is a rare cause of stroke. Several cases of ischemic stroke occurring in patients with autoimmune diseases have been reported. Patients with autoimmune disease such as rheumatoid arthritis and systemic lupus erythematosus (SLE) showed an excess risk of stroke over the general population. However, there are few systemic studies for evaluating autoimmune disease complicated with multiple acute ischemic stroke. It is important to investigate the clinical characteristics and identify the stroke mechanisms of these patients, which is necessary for early etiology diagnosis, accurate treatment and preventive strategies.
On the basis of existing experimental data, many laboratory and radiological examinations can help us to establish the mechanisms of stroke. The level of D-dimer has been used in many studies as a measure of hypercoagulability3. The detection of an embolic signal (ES) by transcranial Doppler (TCD) has been reported to have clinical significance, in that it clarifies cerebral embolism4 . However, the clinical implications of ES have been evaluated mainly in conventional stroke patients5 . There are few studies for evaluating ES in autoimmune disease complicated with stroke and the diagnosis of cerebral vasculitis by ES is difficult. High-resolution magnetic resonance imaging (HRMRI) is an emerging technique for characterizing intracranial arterial disease. It has been proven as an effective tool for identifying cerebral vasculitis6–8.
Our aim was to investigate the clinical, laboratory and radiological features of patients with autoimmune disease complicated with multiple acute ischemic stroke, and explore the possible stroke mechanisms.
Materials and Methods
Patients
Our study was approved by the Institutional Ethics Committees of the China-Japan Friendship Hospital. We retrospectively studied acute ischemic stroke patients with autoimmune diseases treated at the China-Japan Friendship Hospital (Beijing, China) from July 2013 to March 2017. The following patients were excluded from the study: (1) those who had not suffered focal symptoms or relevant acute infarction lesions; (2) those who had not undergone TCD monitoring for ES; (3) those who had infarctions due to conventional stroke etiologies; and (4) those who had incomplete workups for stroke etiology (either vascular or cardiologic studies). Finally, eight patients were finally included in this study.
Data Acquisition and Clinical Management
For all patients, the type of autoimmune disease, years from diagnosis of autoimmune disease to stroke, age, sex, and conventional stroke risk factors including hypertension, diabetes mellitus, hyperlipidemia, atrial fibrillation, ischemic heart disease, and tobacco consumption were collected. D-Dimer and erythrocyte sedimentation rate (ESR) levels were assessed at the time of hospitalization. All patients underwent extra- and intracranial Doppler and duplex sonography of cerebral arteries, computed tomography angiogram (CTA) or magnetic resonance angiogram (MRA), 24 h of electrocardiographic monitoring, transthoracic or transesophageal echocardiography (TEE). Stroke mechanisms were then identified by two experienced doctors.
Brain MRI Assessment and Analysis
Brain MRI examinations were performed in a 3 T scanner (Ingenia; Philips Healthcare, Best, The Netherlands) with a 15-channel phased-array head coil. The brain MRI scanning protocol included diffusion-weighted imaging (DWI), T1- and T2-weighted images, and T2-weighted fluid-attenuated inversion recovery (T2flair). The sizes and locations of all acute infarction lesions were noted. Lesions were considered ‘small’ when the largest axial diameter was <10 mm, ‘medium’ if 10–30 mm, and ‘large’ if >30 mm. Involved territories were arbitrarily assigned as (1) anterior/posterior circulation, or (2) unilateral/bilateral lesions9. Brain MRI was performed in seven patients. Comparison of two brain CT scans revealed new-onset infarction lesions in another one patient.
HRMRI was performed in three patients. Three-dimensional volumetric isotropic turbo spin echo acquisition (ACQ) images were obtained with the following parameters: repetition time/echo time = 1300 ms/36 ms, FOV = 140 × 200 × 135 mm3, matrix = 280 × 332 × 270, NSA = 2. ACQ voxel volume was 0.5 × 0.6 × 0.5 mm3. Reconstruction (REC) voxel volume was 0.5 × 0.5 × 0.5 mm3. The short axial cross-sections were constructed automatically with a 0.5-mm slice thickness.
TCD Monitoring Methods
TCD was used to monitor bilateral middle cerebral arteries (MCAs) using two 2 MHz probes with insonation depths of 40 to 60 mm for 30 minutes. All ESs were manually saved to a computer for review. ESs were identified by experienced doctors according to international standards10 (the author Li-Li Sun successfully passed the theoretical and practical examination in Neurosonology held by the Neurosonology Research Group of the World Federation of Neurology [WFN]).
Results
Clinical Characteristics and Laboratory Examinations
A total of eight patients were included in this study. Clinical characteristics and laboratory examinations are showed in Table 1. The median age was 40.5 years (30–58 years) and 87.5% were female. Autoimmune diseases in our study included SLE (n=5), mixed connective tissue disease (MCTD; n=1), central nervous system vasculitis (n=1), and Takayasu arteritis (n=1). The time from the diagnosis of the autoimmune disease to a stroke was 0–9 years; five of the eight patients presented stroke as the first manifestation of their autoimmune disease. All eight patients had neurological symptoms, including muscle weakness (n=3), dysarthria (n=1), facial numbness (n=2), paresthesia of limbs (n=2), somnolence (n=1), and a mental and behavior disorder (n=1). Overall, six of eight patients tested (75.0%) had elevated D-dimer values (0.56 to 5.62 mg/L; reference level ≤ 0.5 mg/L). Only two patients had hypertension, but with no atherosclerosis vasculopathy.
Table 1.
Clinical characteristics and laboratory examinations.
| Case | Type of autoimmune disease | Age (years)/sex |
Years from diagnosis of autoimmune disease to stroke | Symptoms | D-dimer (mg/L) | ESR (mm/h) |
Autoantibody | Conventional stroke risk factors |
|---|---|---|---|---|---|---|---|---|
| 1 | SLE | 42/F | 3 | Facial numbness; Paresthesia of four limbs | 0.56 | 25 | ds-DNA(+) Sm(+) SSA(+) APA(+) β2-GPI(+) |
HT |
| 2 | SLE | 51/F | First visit | Right side muscle weakness |
5.62 | 140 | ds-DNA(+) SSA(+) APA(+) β2-GPI(+) |
No |
| 3 | SLE | 37/M | 4 | Somnolence |
1.79 | 85 | ds-DNA(+) Sm(+) APA(+) β2-GPI(+) |
No |
| 4 | MCTD | 58/F | First visit | Right facial numbness | 1.97 | 22 | RF(+) anti-RNP(+) |
No |
| 5 | SLE | 47/F | First visit | Left side muscle weakness | 0.5 | 20 | ds-DNA(+) Sm(+) SSA(+) |
HT |
| 6 | SLE | 39/F | 9 | Right hand weakness; mental and behavior disorder | 0.19 | 44 | ds-DNA(+) Sm(+) |
No |
| 7 | CNS vasculitis | 30/F | First visit | paresthesia of four limbs | 1.54 | 60 | —— | No |
| 8 | Takayasu arteritis | 31/F | First visit | Dysarthria | 0.12 | 14 | —— | No |
CNS: central nervous system; F, female; HT: hypertension; M: male; MCTD: mixed connective tissue disease; SLE: systemic lupus erythematosus.
Radiological Characteristics, ESs and Stroke Mechanisms
According to the MRI, CTA, and TCD, the stroke mechanisms in these eight cases were identified into hypercoagulability (n=4), cardiac embolism (n=1) and vasculitis (n=3).
The radiological characteristics of the eight cases are depicted in Table 2. All these eight patients presented acute infarction lesions in ≥2 vascular territories and numerous small or medium infarction lesions located in the cortical and subcortical areas. The infarction lesions were located in the bihemispheric territories (n=5), or bihemispheric territories and posterior circulation (n=3). MRA and/or CTA were also normal in the other four patients (Cases 1–4), and combined with the elevation of D-dimer, the stroke mechanism was considered as hypercoagulability (Fig 1). In one patient (Case 5), the stroke mechanism was attributed to a cardiac embolism because of cardiac valvular vegetations on TEE, and normal D-dimer levels and normal arterial examinations (Fig 2). There were three patients (Cases 6–8) that showed bilateral anterior circulation vasculopathy on CTA and/or MRA which were regarded as vasculitis. HRMRI in one of the three patients showed circumferential vessel wall thickening and enhancement of bilateral MCAs (Fig 3).
Table 2.
Radiological characteristics and embolic signal characteristics.
| Case | Lesions of cerebral infarction | Vasculopathy | HRMRI | UCG | ES (vascular/number) |
Days from stroke to TCD monitoring | Stroke mechanism | ||
|---|---|---|---|---|---|---|---|---|---|
| locations | sizes | number | |||||||
| 1 | Bilateral anterior circulations+posterior
circulation; cortical+ subcortical |
Small | 7 | Normal | ND | Normal | LMCA/27; RMCA/11 | 28 | Hypercoagulability |
| 2 | Bilateral anterior circulations; subcortical |
medium | 4 | Normal | ND | Normal | LMCA/8; RMCA/5 | 5 | Hypercoagulability |
| 3 | Bilateral anterior circulations+posterior
circulation; cortical+ subcortical |
Small+medium | 12 | Normal | Normal | Normal | 0 | 30 | Hypercoagulability |
| 4 | Bilateral anterior circulations+posterior
circulation; Cortical+ subcortical |
Small | >20 | Normal | Normal | Normal | LMCA/7; RMCA/5 | 19 | Hypercoagulability |
| 5 | Bilateral anterior circulations; cortical+ subcortical |
Small | 16 | Normal | ND | mitral valve vegetation | LMCA/0; RMCA/1 | 25 | Cardiac embolism |
| 6 | Bilateral anterior circulations; cortical+ Subcortical+basal ganglion |
Small+medium+large | >20 | Occlusion of bilateral ICAs, bilateral MCAs, and left ACA | ND | Normal | 0 | 30 | Vasculitis |
| 7 | Bilateral anterior circulations; cortical+ subcortical |
Small | >20 | Stenosis of siphon segment of left ICA, bilateral MCAs | Circumferential vessel wall thickening and enhancement of bilateral anterior circulations | Normal | LMCA/12; RMCA/15 | 2 | Vasculitis |
| 8 | Bilateral anterior circulations; subcortical |
Small | 13 | Circumferential vessel wall thickening of bilateral CCAs | ND | Normal | LMCA/2; RMCA/4 | 25 | Vasculitis |
ACA: anterior cerebral artery; CCA: common carotid artery; ICA: internal carotid artery; LMCA: left MCA; MCA: middle cerebral artery; ND: not done; RMCA: right MCA; UCG: ultrasonic cardiogram.
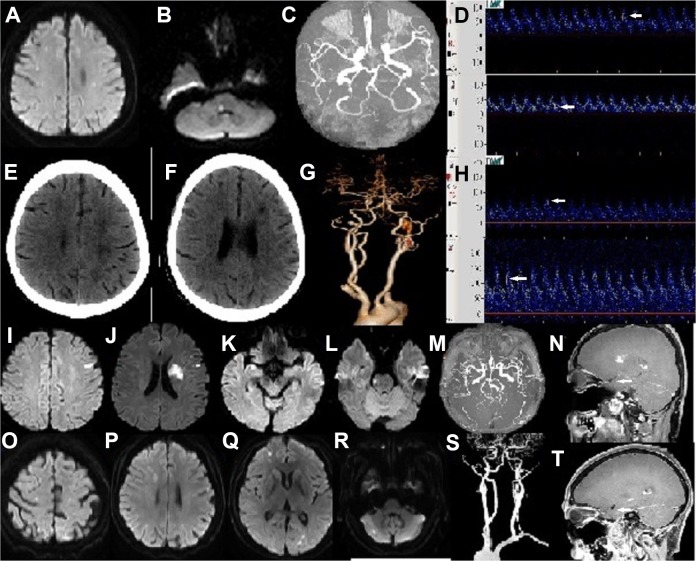
Fig 1.
Patients with the hypercoagulability stroke mechanism (Patients1–4). Patient 1. 42/F/SLE. (A, B) Diffusion-weighted images showed multiple acute infarcts in the bilateral cerebral hemispheres and pons; (C) MR angiography showed normal cerebral arteries; (D) White arrows showed ESs in the LMCA and RMCA. Patient 2. 51/F/SLE. (E, F) Brain CT showed multiple infarcts in bilateral cerebral hemispheres; (G) CT angiography showed normal cerebral arteries; (H) White arrows showed ESs in the LMCA and RMCA. Patient 3. 37/M/SLE. (I, J, K, L) Diffusion-weighted images showed multiple acute infarcts in the bilateral cerebral hemispheres and pons; (M) MR angiography showed normal cerebral arteries; (N) HRMRI (sagittal view) showed normal MCA without circumferential vessel wall thickening or enhancement (white arrow). Patient 4. 58/F/MCTD. (O, P, Q, R) Diffusion-weighted images showed multiple acute infarcts in the bilateral cerebral hemispheres and left cerebellum; (S) CT angiography showed normal cerebral arteries; (T) HRMRI (sagittal view) showed normal MCA without circumferential vessel wall thickening or enhancement (white arrow).
CT: computed tomography; ES: embolic signal; F: female; HRMRI: high-resolution magnetic resonance imaging; LMCA: left middle cerebral artery; M, male; MCTD: mixed connective tissue disease; RMCA: right middle cerebral artery; SLE: systemic lupus erythematosus.
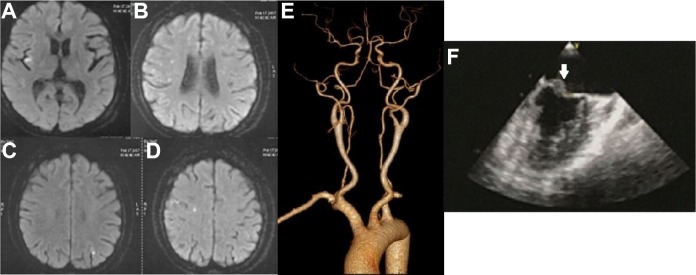
Fig 2.
Patient 5, with a cardiac embolism stroke mechanism. Patient 5. 47/F/SLE. (A, B, C, D) Diffusion-weighted images showed multiple acute infarcts in the bilateral cerebral hemispheres; (E) CT angiography showed normal cerebral arteries; (F) White arrow showed mitral valve vegetation on echocardiography.
CT: computed tomography; F: female; SLE, systemic lupus erythematosus.
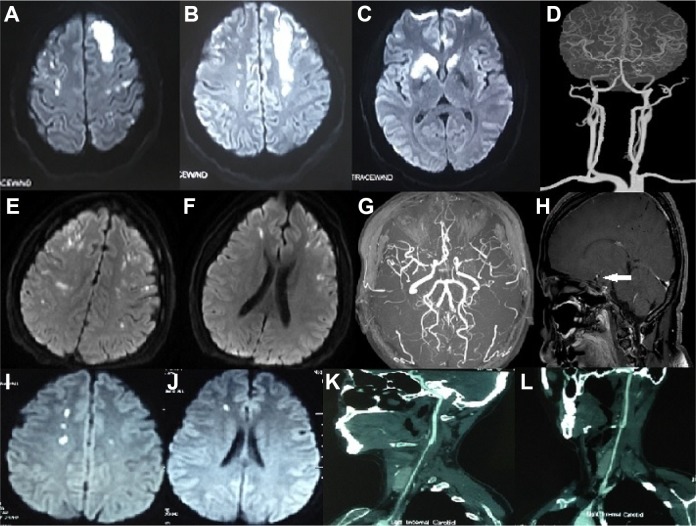
Fig 3.
Patients with a vasculitis stroke mechanism (Patients 6–8). Patient 6. 39/F/SLE. (A, B, C) Diffusion-weighted images showed multiple acute infarcts in the bilateral cerebral hemispheres; (D) CT angiography showed occlusion of the bilateral ICAs, bilateral MCAs, and left ACA. Patient 7. 30/F/CNS vasculitis. (E, F) Diffusion-weighted images showed multiple acute infarcts in the bilateral cerebral hemispheres; (G) MR angiography showed stenosis of the siphon segment of the left ICA, and bilateral MCAs; (H) HRMRI (sagittal view) showed circumferential vessel wall thickening and enhancement of MCA (white arrow). Patient 8. 31/F/Takayasu arteritis. (I, J) Diffusion-weighted images showed multiple acute infarcts in the bilateral cerebral hemispheres; (K, L) CT angiography showed circumferential vessel wall thickening of bilateral CCAs.
ACA: anterior cerebral artery; CCA: common carotid artery; CNS: central nervous system; CT: computed tomography; F: female; ICA: internal carotid artery; MCA: middle cerebral artery; MR: magnetic resonance; SLE: systemic lupus erythematosus.
ESs were observed in six (75.0%) patients with various mechanisms (Table 2). The time from stroke to TCD monitoring of these patients was 2–28 days. The time from stroke to TCD monitoring of another two patients without ES was 30 days.
Discussion
Autoimmune disease is an important etiology for stroke11,12, especially for young female patients. In our study, five patients even presented with stroke as the first manifestation. The types of autoimmune diseases in our study included SLE, MCTD, central nervous system vasculitis, and Takayasu arteritis. In addition, cerebral infarction occurred in patients with other autoimmune diseases, such as Churg–Strauss syndrome and rheumatoid arthritis13–16.
The patients included in our study mostly presented multiple, disseminated small and medium infarction lesions involving multiple arterial territories, located in the cortical and subcortical areas. This is in line with the TCD monitoring results, showing a high prevalence of ESs detected in bilateral MCAs. Infarction lesions involving multiple vascular supply territories were mostly attributed to atrial fibrillation, hematological diseases, or cancer17. However, autoimmune disease receives little attention. What is more, in our study, we found that large lesions were less common in autoimmune disease-related stroke than cancer-related stroke or atrial fibrillation-related stroke9,18,19. Only one patient (Case 6) with mechanism vasculitis had large infarction lesions in the basal ganglion. Since hypercoagulability was not the unique mechanism, the number of infarction lesions was not significantly correlated with D-dimer levels.
We found that the stroke mechanisms in our study included hypercoagulability, cardiac embolism and vasculitis. The level of D-dimer, which is a direct laboratory measure of activated coagulation, has been used in many previous studies as a measure of hypercoagulability3. D-dimer is the smallest fibrinolysis-specific degradation product found in the circulation. The D-dimer is very sensitive to intravascular thrombus and may be markedly elevated in disseminated intravascular coagulation20. In four patients (Cases 1–4), stroke mechanisms were attributed to hypercoagulability with elevated D-dimer levels and normal arterial, cardiac examinations. Recent studies have revealed that inflammation may change the hemostatic balance in a thrombogenic direction. The immune system and coagulations system are linked, with many molecular components being important for both systems21,22. Antiphospholipid syndrome is an important cause of hypercoagulability. In our study, three patients with antiphospholipid syndrome all presented multiple, disseminated small and medium infarction lesions involved multiple arterial territories.
In Case 5, the stroke mechanism was attributed to a cardiac embolism identified by cardiac valvular vegetations displayed on TEE (Fig 2), and with normal D-dimer levels and normal arterial examinations. Cardiac valvular disease of this patient presented as non-bacterial thrombotic endocarditis (NBTE), a rare condition characterized by non-infective inflammatory and/or thrombotic vegetations on the heart valve leaflets. NBTE can be seen in autoimmune diseases such as SLE, antiphospholipid syndrome, and rheumatoid arthritis23,24. Previous studies showed that NBTE in SLE was associated with a higher risk for embolic stroke and suggested that NBTE might be a source of cerebral emboli24,25.
In another three patients, the stroke mechanism was attributed to vasculitis. Case 8 was diagnosed as Takayasu arteritis. Carotid ultrasound of this patient revealed circumferential vessel wall thickening of the bilateral common carotid arteries (CCAs). Another two patients presented with Moyamoya syndromes on CTA. It has been reported that large-vessel stroke due to Moyamoya syndrome presented as a rare manifestation of SLE26,27. Cerebral vasculitis in the setting of lupus may lead to large cerebral vessel occlusions and lead to Moyamoya syndrome. HRMRI of
Case 7 showed circumferential vessel wall thickening and enhancement of bilateral anterior circulations, which confirmed the diagnosis of central nervous system vasculitis (Fig 3). Recent studies have proven HRMRI as an effective tool for identifying cerebral vasculitis28.
Since ES has clinical significance, in that it clarifies cerebral embolism, ES can help to distinguish cerebral infarction from other complications of autoimmune diseases. ESs were detected in 5.7% of unselected stroke patients and in almost 50% of cancer-related stroke patients in the previous studies4,29. However, the frequency and the number of ESs detected in autoimmune disease-related stroke in our study are much higher. The time from stroke to TCD monitoring of ES-positive patients was 2–28 days, while ES was seldom positive after 1 week in conventional stroke patients. More interestingly, we found that ES was detected not only in hypercoagulability and cardiac embolism patients, but also in vasculitis patients.
Conclusion
Autoimmune disease is an important etiology and should not be neglected for stroke, especially in young patients in the absence of conventional stroke risk factors. Our study revealed the characteristics of autoimmune disease-related stroke, which were distinct from those of conventional stroke. These patients mostly presented multiple, disseminated small and medium infarction lesions involving multiple arterial territories, located in the cortical and subcortical areas. We found that multiple stroke mechanisms were involved in these patients, including hypercoagulability, cardiac embolism and vasculitis. For these patients, we suggest complete workups for the identification of stroke etiology, which is necessary for further accurate treatment and preventive strategies.
Limitations
First, the number of cases enrolled in our study was limited, but the examination data of these eight cases were comprehensive. TCD microembolic monitoring and HRMRI were done in our study, which were not included in the other studies. More patients will be enrolled in our further study. Second, we will further explore the unique treatment and prognosis evaluation strategies of patients with autoimmune diseases compared with traditional cerebral infarction patients. In our study, corticosteroid and immunosuppression, together with antiplatelet and statin therapy were effective for these patients, and most patients recovered more quickly than traditional cerebral infarction patients.
Supplemental Material
Supplemental_Figures for Clinical Manifestations and Mechanisms of Autoimmune Disease-Related Multiple Cerebral Infarcts by Li-Li Sun, Wen-Xiong Tang, Min Tian, Lu Zhang and Zun-Jing Liu in Cell Transplantation
Footnotes
Ethical Approval: This study was approved by the Institutional Ethics Committees of China-Japan Friendship Hospital, Beijing, China (No. 2017-65).
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the Institutional Ethics Committees of the China-Japan Friendship Hospital, Beijing, China (No. 2017-65) and their approved protocols.
Statement of Informed Consent: Verbal informed consent was obtained from the patients for their anonymized information to be published in this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Grant support or other sources of funding: supported by National Natural Science Foundation of China (No. 31771045) and the China-Japan Friendship Hospital Youth Science and Technology Project (No. 2015-2-QN-36).
ORCID iD: Li-Li Sun  https://orcid.org/0000-0001-7394-6954
https://orcid.org/0000-0001-7394-6954
Zun-Jing Liu  https://orcid.org/0000-0002-7962-8217
https://orcid.org/0000-0002-7962-8217
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Saver JL. Cryptogenic Stroke. N Engl J Med. 2016;374(21):2065–2074. [DOI] [PubMed] [Google Scholar]
- 2. Jauch EC, Barreto AD, Broderick JP, Char DM, Cucchiara BL, Devlin TG, Haddock AJ, Hicks WJ, Hiestand BC, Jickling GC, June J, Liebeskind DS, Lowenkopf TJ, Miller JB, O’Neill J, Schoonover TL, Sharp FR, Peacock WF. Biomarkers of acute stroke etiology (BASE) study methodology. Transl Stroke Res. 2017;8(5):424–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Righini M, Perrier A, De Moerloose P, Bounameaux H. D-Dimer for venous thromboembolism diagnosis: 20 years later. J Thromb Haemost. 2008;6(7):1059–1071. [DOI] [PubMed] [Google Scholar]
- 4. Poppert H, Sadikovic S, Sander K, Wolf O, Sander D. Embolic signals in unselected stroke patients: prevalence and diagnostic benefit. Stroke. 2006;37(8):2039–2043. [DOI] [PubMed] [Google Scholar]
- 5. Markus HS, Droste DW, Kaps M, Larrue V, Lees KR, Siebler M, Ringelstein EB. Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis evaluated using Doppler embolic signal detection: the Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis (CARESS) trial. Circulation. 2005;111(17):2233–2240. [DOI] [PubMed] [Google Scholar]
- 6. Küker W, Gaertner S, Nagele T, Dopfer C, Schoning M, Fiehler J, Rothwell PM, Herrlinger U. Vessel wall contrast enhancement: a diagnostic sign of cerebral vasculitis. Cerebrovasc Dis. 2008;26(1):23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Obusez EC, Hui F, Hajj-Ali RA, Cerejo R, Calabrese LH, Hammad T, Jones SE. High-resolution MRI vessel wall imaging: spatial and temporal patterns of reversible cerebral vasoconstriction syndrome and central nervous system vasculitis. AJNR Am J Neuroradiol. 2014;35(8):1527–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mandell DM, Matouk CC, Farb RI, Krings T, Agid R, terBrugge K, Willinsky RA, Swartz RH, Silver FL, Mikulis DJ. Vessel wall MRI to differentiate between reversible cerebral vasoconstriction syndrome and central nervous system vasculitis: preliminary results. Stroke. 2012;43(3):860–862. [DOI] [PubMed] [Google Scholar]
- 9. Kwon HM, Kang BS, Yoon BW. Stroke as the first manifestation of concealed cancer. J Neurol Sci. 2007;258(1–2):80–83. [DOI] [PubMed] [Google Scholar]
- 10. Consensus Committee of the Ninth International Cerebral Hemodynamic Symposium. Basic identification criteria of Doppler microembolic signals. Stroke. 1995;26(6):1123. [PubMed] [Google Scholar]
- 11. Sacco S, Casalena A, Gallucci M, Carolei A. Showered cortical infarctions and brain atrophy in Churg–Strauss syndrome. Eur Neurol. 2011;65(2):112. [DOI] [PubMed] [Google Scholar]
- 12. TKanda K, Sato A, Abe D, Nishijima S, Ishigami T. The unique coexistence of anti-ss-a/ro antibodies in a neonate with symptomatic ischemic stroke. Pediatr Neurol. 2016;62:47–50. [DOI] [PubMed] [Google Scholar]
- 13. Kang DW, Kim DE, Yoon BW, Seo JW, Roh JK. Delayed diagnosis: recurrent cerebral infarction associated with Churg–Strauss syndrome. Cerebrovasc Dis. 2001;12(3):280–281. [DOI] [PubMed] [Google Scholar]
- 14. Cheng MJ, Huang PH, Liao PW, Chen JT, Chiang TR. Multiple cerebral and cerebellar infarcts as the first clinical manifestation in a patient with Churg–Strauss syndrome: case report and literature review. Acta Neurol Taiwan. 2012;21(4):169–175. [PubMed] [Google Scholar]
- 15. Ghaeni L, Siebert E, Ostendorf F, Endres M, Reuter U. Multiple cerebral infarctions in a patient with Churg–Strauss syndrome. J Neurol. 2010;257(4):678–680. [DOI] [PubMed] [Google Scholar]
- 16. Ohta K, Tanaka M, Funaki M, Sakauchi M, Suzuki N. Multiple cerebral infarction associated with cerebral vasculitis in rheumatoid arthritis. Rinsho Shinkeigaku. 1998;38(5):423–429. [PubMed] [Google Scholar]
- 17. Finelli PF, Nouh A. Three-territory DWI acute infarcts: diagnostic value in cancer-associated hypercoagulation stroke (Trousseau syndrome). Am J Neuroradiol. 2016;37(11):2033–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ishikawa M, Nakayama K, Ishibashi T, Sato E, Nakamura K, Katagiri H, Kyo S. Case series of cerebral infarction with Trousseau’s syndrome associated with malignant gynecological tumors. Mol Clin Oncol. 2016;5(1):138–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim BJ, Sohn H, Sun BJ, Song JK, Kang DW, Kim JS, Kwon SU. Imaging characteristics of ischemic strokes related to patent foramen ovale. Stroke. 2013;44(12):3350–3356. [DOI] [PubMed] [Google Scholar]
- 20. Olson JD. D-dimer: an overview of hemostasis and fibrinolysis, assays, and clinical applications. Adv Clin Chem. 2015;69:1–46. [DOI] [PubMed] [Google Scholar]
- 21. Zöller B, Li X, Sundquist J, Sundquist K. Autoimmune diseases and venous thromboembolism: a review of the literature. Am J Cardiovasc Dis. 2012;2(3):171–183. [PMC free article] [PubMed] [Google Scholar]
- 22. Yao Z, Wang L, Wu X, Zhao L, Chi C, Guo L, Tong D, Yang X, Dong Z, Deng R, Novakovic VA, Thatte HS, Bi Y, Tian Y, Shi J, Zhou J, Kou J, Hu S. Enhanced procoagulant activity on blood cells after acute ischemic stroke. Transl Stroke Res. 2017;8(1):83–91. [DOI] [PubMed] [Google Scholar]
- 23. Choi JH, Park JE, Kim JY, Kang T. Non-bacterial thrombotic endocarditis in a patient with rheumatoid arthritis. Korean Circ J. 2016;46(3):425–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Malvar B, Almeida FM, Rebocho L, Moniz JC, Azevedo F. Cerebral embolism from Libman-Sacks endocarditis. BMJ Case Rep. 2011;2011:bcr0420114071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roldan CA, Sibbitt WL, Jr, Qualls CR, Jung RE, Greene ER, Gasparovic CM, Hayek RA, Charlton GA, Crookston K. Libman-sacks endocarditis and embolic cerebrovascular disease. JACC Cardiovasc Imaging. 2013;6(9):973–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhou G, An Z, Gokhale S. Moyamoya syndrome as an unusual presenting manifestation of systemic lupus erythematosus in a young woman. Med Princ Pract. 2014;23(3):279–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang R, Xu Y, Lv R, Chen J. Systemic lupus erythematosus associated with Moyamoya syndrome: a case report and literature review. Lupus. 2013;22(6):629–633. [DOI] [PubMed] [Google Scholar]
- 28. Mossa-Basha M, Hwang WD, De Havenon A, Hippe D, Balu N, Becker KJ, Tirschwell DT, Hatsukami T, Anzai Y, Yuan C. Multicontrast high-resolution vessel wall magnetic resonance imaging and its value in differentiating intracranial vasculopathic processes. Stroke. 2015;46(6):1567–1573. [DOI] [PubMed] [Google Scholar]
- 29. Bang OY, Seok JM, Kim SG, Hong JM, Kim HY, Lee J, Chung PW, Park KY, Kim GM, Chung CS, Lee KH. Ischemic stroke and cancer: stroke severely impacts cancer patients, while cancer increases the number of strokes. J Clin Neurol. 2011;7(2):53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental_Figures for Clinical Manifestations and Mechanisms of Autoimmune Disease-Related Multiple Cerebral Infarcts by Li-Li Sun, Wen-Xiong Tang, Min Tian, Lu Zhang and Zun-Jing Liu in Cell Transplantation