Abstract
Stroke is a serious worldwide medical condition that causes neurological function disability. Diffusional kurtosis imaging, which measures the non-Gaussianity of water diffusion, has been demonstrated to be a sensitive biomarker in many neuro-pathologies. This study explores the relationship between neural function recovery and transformation of the ischemic lesion and/or corticospinal tract during the sub-acute phase after stroke by using diffusional kurtosis imaging. We performed a prospective study of function recovery and K metrics of 43 patients with sub-acute ischemic stroke in the middle cerebral artery territory. The effect of rehabilitation treatment was evaluated using both the Fugl-Meyer motor function score and modified Barthel index score at post-treatment compared with admission, and patients were allocated to two groups: good and poor rehabilitation effect (GRE and PRE). Metrics of diffusional kurtosis imaging within ischemic lesion and along the corticospinal tract were acquired, respectively. All three relative axial diffusional kurtoses (rKas) along the corticospinal tract in the GRE group (n = 21) were significantly larger than those of the PRE group (n = 22), including rKa in the posterior limb of internal capsule, rKa in the cerebral peduncle, and rKa in the basal part of the pons (p = 0.014, 0.005, and 0.021, respectively). This multi-parametric magnetic resonance imaging study showed that diffusional kurtosis imaging has the potential to complement existing stroke imaging techniques and revealed its own advantages in elucidating the possible biophysical mechanism of functional restoration underlying ischemic stroke.
Keywords: Stroke, diffusional kurtosis imaging, K metrics, functional recovery, magnetic resonance imaging
Introduction
After focal cerebral ischemia, not only do nerve pathological changes occur in the cerebral infarction area, but, at the same time, descending fiber also exhibits a series of changes in structure and function, which is the potential primary basis for neurological dysfunction and now the target of many neural protective strategies1. The brain tissue damage and repair mechanism of nerve fibers has rarely been studied in previous research2. Therefore, we hope to provide new ideas for clinical rehabilitation treatment through the research of transformation of the ischemic lesion and closely linked corticospinal tract (CST) changes after stroke.
Exploring the functional magnetic resonance imaging (MRI) index, which can reflect the pathological changes in the descending fibers associated with nerve and motor function change, has important guiding significance for clinical practice3–5. Diffusion MRI (dMRI) is the most widely used neuroimaging technique for evaluating acute or sub-acute ischemic stroke assessment6. The index of conventional dMRI is based on the calculation of free water molecules obeying the dispersion of a Gaussian distribution model. Biological tissue is composed of many rooms characterized by heterogeneity, therefore, it cannot reflect the real diffuse movement of water molecules in the biological microenvironment. As the expansion of diffusion tensor imaging (DTI) technology, diffusional kurtosis imaging (DKI) can measure not only the traditional parameters based on DTI, such as fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (Da), and radial diffusivity (Dr), but also the specificity parameters, K metrics, which reflect the non-Gaussian dispersion degree of movement of water molecules7. Among these K parameters, the most representative are mean diffusional kurtosis (MK), axial diffusional kurtosis (Ka), radial diffusional kurtosis (Kr) and fractional anisotropy kurtosis (FAK)6–9.
In recent years, the region of interest (ROI) method to analyze DKI measurement has been reported in animal and clinical studies, especially in stroke cases, and has confirmed that K metrics can provide more abundant information than conventional dMRI6–10.
Other methods are being explored to improve characterization of ischemic brain injury; nevertheless, to the best our knowledge, little is known regarding the value of DKI parameters for ischemic stroke in clinical rehabilitation treatment. Therefore, the aim of the present study was to address this. We hypothesize that DKI parameters may serve as biomarkers that are more specific and/or sensitive to certain microstructural changes associated with pathological processes during ischemic stroke. As a consequence, DKI parameters have the potential to provide clues on the plausible biophysical mechanism of functional recovery underlying ischemic stroke.
Materials and Methods
Subjects
We performed a prospective study of clinical and imaging data on consecutive patients who were admitted to Huashan Hospital due to acute onset of neurological symptoms and subsequently diagnosed with acute/sub-acute ischemic stroke in the middle cerebral artery territory as the cause for neurological impairments according to Guidelines for Diagnosis and Treatment of Acute Ischemic Stroke in China 2014. The study protocol was approved by the institutional review board of Huashan Hospital and was conducted in accordance with the Declaration of Helsinki. The patients or their legal guardians provided informed consent.
Inclusion Criteria
Patients presenting with all of the following criteria were considered for study inclusion: (1) acute/sub-acute ischemic stroke in the middle cerebral artery territory (rather than lesion in the posterior limb of internal capsule, in accordance with the study aim); (2) 45 years old ≤ age ≤ 75 years old; (3) Mini-mental State Examination ≤24; (4) MRI obtained within two weeks of stroke onset; (5) the condition is stable, consciousness is clear, and the vital signs are stable.
Exclusion Criteria
Patients with one or more of the following conditions were excluded from this study: (1) history of brain tumors, brain trauma, brain parasitic diseases and other diseases which can cause cognitive dysfunction; (2) ischemic stroke in bilateral cerebral hemispheres, hemorrhagic stroke, posterior circulation infarct; (3) lesions < 1.00 mm in minimum diameter on diffusion weighted image (DWI); (4) serious speech, vision, hearing impairment or mental disorders affecting cognitive examinations; (5) cognitive disorders before the onset of this stroke, with use of drugs for cognitive impairment; (6) Beck Depression Inventory > 13 points; (7) history of alcohol and drug abuse; (8) combined with serious diseases such as heart, liver, kidney, endocrine system, and hematopoietic system; (9) pregnant and lactating women; (10) participating in other clinical trials that influence the evaluation of the results of this study.
Treatment Programs
A total of 43 sub-acute ischemic stroke patients admitted to Huashan Hospital between August 2015 and February 2016 were recruited. All patients received basic treatments and Chinese medicine rehabilitation treatments. In detail, basic treatments include medical treatment for primary disease, routine rehabilitation training, and health education. Chinese medicine rehabilitation treatments include cognitive rehabilitation training and acupuncture treatment.
Cognitive rehabilitation training program refers to the Clinical Guidelines for Stroke Management developed by the World Stroke Association in 2010 and the Guidelines for the Rehabilitation of Chinese Stroke developed by the Chinese Medical Association Neurology Branch in 2011. Different training was chosen for dysfunctional patients according to types of cognition. The duration and number of repetitions of each method of training are flexibly arranged based on the patient’s cognitive improvement. However, the general principle is flexible selection of various methods according to patients’ cognitive impairment, with repeated intensive training to maximize the improvement of cognitive function. The cognitive rehabilitation training needs to be performed after acupuncture. The acupuncture acu-points used are Baihui and God Court. The acu-point of Baihui is located at 7 in (18 cm) back from the middle of the hairline, that is, at the intersection of the centerline of the head and the line between the ears. The acu-point of God Court is located at 0.5 in (1.25 cm) straight in front of the hairline. Treatment time requirements: each treatment 30 min, once per day, five days of treatment per week, one course of treatment lasts four weeks, a total of 12 weeks of treatment. Follow-up period is 13–36 weeks.
The effect of rehabilitation treatment was evaluated using both the Fugl-Meyer motor function score and modified Barthel index score11,12. Good rehabilitation effect (GRE) was defined as an increase of 10 points in both Fugl-Meyer motor function score and modified Barthel index score at post-treatment follow-up compared with admission11,12. According to the Fugl-Meyer motor function score and modified Barthel index score at post-treatment follow-up versus admission, the subjects were allocated to two groups: the GRE group and the poor rehabilitation effect (PRE) group.
Imaging Acquisition
All patients underwent cranial DKI and conventional MRI (including T1 weighted imaging, T2 weighed imaging (T2WI), fluid attenuated inversion recovery, DWI, and apparent diffusion coefficient maps) using a 3.0 T whole-body scanner equipped with eight-channel head coil (Discovery MR750, GE Medical Systems, Milwaukee, WI, USA). DKI data were acquired using an echo planar imaging diffusion sequence with repetition time (TR)/echo time (TE) = 4700/100 ms, field of view (FOV) = 240 × 240 mm2, matrix = 128 × 128, number of excitations = 1, oblique axial slices (number/thickness/gap) 18/3.0 mm/1.5 mm, and 15 gradient encoding directions with three b values (0, 1000, 2000 s/mm2) for each direction in a total time of 4 min 12 s. T2WI images were acquired using a fast spin-echo sequence (TR/TE = 3480/110 ms, FOV = 240 × 240 mm2, matrix = 256 × 256, section thickness = 6 mm, gap = 2 mm). DWI images were acquired with the following acquisition parameters: TR/TE = 4800/74 ms, FOV = 240 × 240 mm2, matrix = 256 × 256, section thickness = 6 mm, no gap, b = 0 and 1000 s/mm2. Conventional axial MRI was performed from the vertex to the foramen magnum with 16 images per sequence. Time to MRI from stroke onset was 10.12 ± 2.08 days.
Imaging Analysis
The acquired DKI data were post-processed in FuncTool on a GE Advanced Workstation 4.6 (GE Medical Systems, Milwaukee, WI, USA). The diffusion and kurtosis tensors were calculated on a voxel-by-voxel basis. Parametric maps for FA, MD, Da, Dr, MK, Ka, Kr and FAK were subsequently obtained.
Within the ischemic lesion, two ROIs (area = 5 mm2) were drawn manually. ROI1 was within the core region of the lesion, and ROI2 within the surrounding region of the lesion core. The lesion core was defined as the distinctly hyper-intense voxels on the T2WI images. Value of ROI was defined on the T2WI images, while the T2WI images provided anatomic reference, and the absolute values of FA, MD, Da, Dr, MK, Ka, Kr and FAK were measured. Moreover, three different manually drawn ROIs (area = 5 mm2) were placed in the posterior limb of internal capsule (PLIC), cerebral peduncle (CP), and the basal part of the pons (BPP) in ipsilateral hemisphere along the CST, respectively. Accordingly, three ROIs of the same size were located on a corresponding area of the normal-appearing contralateral hemisphere as controls. Value of ROI was defined on the FA images while using the T2WI images for anatomic reference, and the absolute values of FA, MD, Da, Dr, MK, Ka, Kr and FAK were measured.
For all ROIs, relative fractional anisotropy (rFA), relative mean diffusivity (rMD), relative axial diffusivity (rDa), relative radial diffusivity (rDr), relative mean diffusional kurtosis (rMK), relative axial diffusional kurtosis (rKa), relative radial diffusional kurtosis (rKr) and relative fractional anisotropy kurtosis (rFAK) were computed, as a previous study demonstrated that the absolute DKI values altered in the normal-appearing region of different brain regions and that calculating the relative DKI value can reduce the instability of the absolute DKI value13. The relative values of the eight diffusion metrics within the ischemic lesion were calculated using the formula: (D2 – D1)/D1, where D2 and D1 denote the diffusion metric within the surrounding region of the lesion core and the lesion core, respectively. Similarly, the relative values of the eight diffusion metrics along the CST from ipsilateral to normal were calculated using the formula: (Di – Dc)/Dc, where Di and Dc denote the diffusion metric in the ipsilateral and the contralateral hemisphere, respectively.
The diagnosis of ischemic lesions and image analysis were judged based on single-blind studies by two radiologists. A third radiologist was consulted when there were different opinions of the two primary radiologists.
Statistical Analysis
Statistical analysis was performed using SPSS 19.0 software (SPSS, Chicago, IL, USA). Results were expressed as the mean ± standard deviation for quantitative variables and as proportions for categorical findings. Patients were dichotomized into two groups: GRE and PRE. Mann–Whitney U test and paired t-test were used to compare radiological and clinical differences between these two groups. The differences between the ROIs of all relative dMRI parameters were tested using paired t-tests. A two-tailed value of p < 0.05 was considered statistically significant.
Results
Demographic Data
The final cohort comprised 43 sub-acute ischemic stroke patients: mean age was 60.52 ± 10.61 years and 20 (47%) were women. Mean admission Fugl-Meyer motor function score was 33.28 ± 12.76 and the post-treatment one was 46.86 ± 10.12. Similarly, mean admission modified Barthel index score was 73.44 ± 19.16 and the post-treatment one was 80.78 ± 14.39. Patients were allocated to the GRE group (n = 21) and the PRE group (n = 22) according to these two functional scores at post-treatment versus admission (Table 1). There was no statistically significant difference between the patients in the GRE and PRE groups in terms of gender, age, arterial hypertension, hyperlipidemia, diabetes, coronary heart disease, atrial fibrillation, C-reactive protein increase, history of myocardial infarction, family history of stroke, admission National Institutes of Health Stroke Scale score, and infarct volume.
Table 1.
Group Comparison of Good and Poor Rehabilitation Effects.
| GRE group n = 21 |
PRE group n = 22 |
t | p | |
|---|---|---|---|---|
| Age, years | 58.9 ± 14.11 | 61.1 ± 11.2 | 0.258 | 0.085 |
| Women | 10 (48) | 10 (45) | 0.572 | |
| Arterial hypertension | 14 (67) | 16 (73) | 0.208 | |
| Hyperlipidemia | 4 (19) | 3 (14) | 0.933 | |
| Diabetes | 6 (29) | 9 (41) | 0.265 | |
| Coronary heart disease | 5 (24) | 4 (18) | 0.367 | |
| Atrial fibrillation | 1 (5) | 1 (5) | 1 | |
| C-reactive protein increase | 7 (33) | 8 (36) | 0.661 | |
| History of myocardial infarction | 1 (5) | 0 | 1 | |
| Family history of stroke | 4 (19) | 5 (23) | 0.427 | |
| Admission NIHSS score | 11.5 ± 2.1 | 11.4 ± 3.1 | 0.103 | 0.731 |
| Infarct volume, mm3 | 22.6 ± 3.9 | 26.8 ± 4.1 | 0.669 | 0.122 |
Age, admission NIHSS score and infarct volume are expressed as mean ± SD. All other data are expressed as number (percentage).
GRE: good rehabilitation effect; PRE: poor rehabilitation effect; NIHSS: National Institutes of Health Stroke Scale.
Parameters Comparison on DTI and DKI within Ischemic Lesion
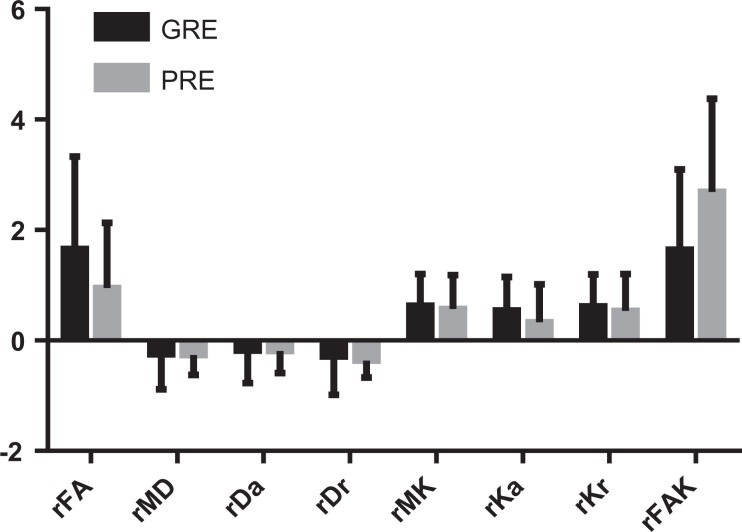
The sub-acute unilateral ischemic lesion was characterized by a prolonged T2. The DTI and DKI maps within the ischemic lesion are provided in Fig. 1. There was no statistically significant difference between the GRE and PRE groups in terms of rFA, rMD, rDa, rDr, rMK, rKa, rKr, and rFAK within the ischemic lesion (Fig. 2).
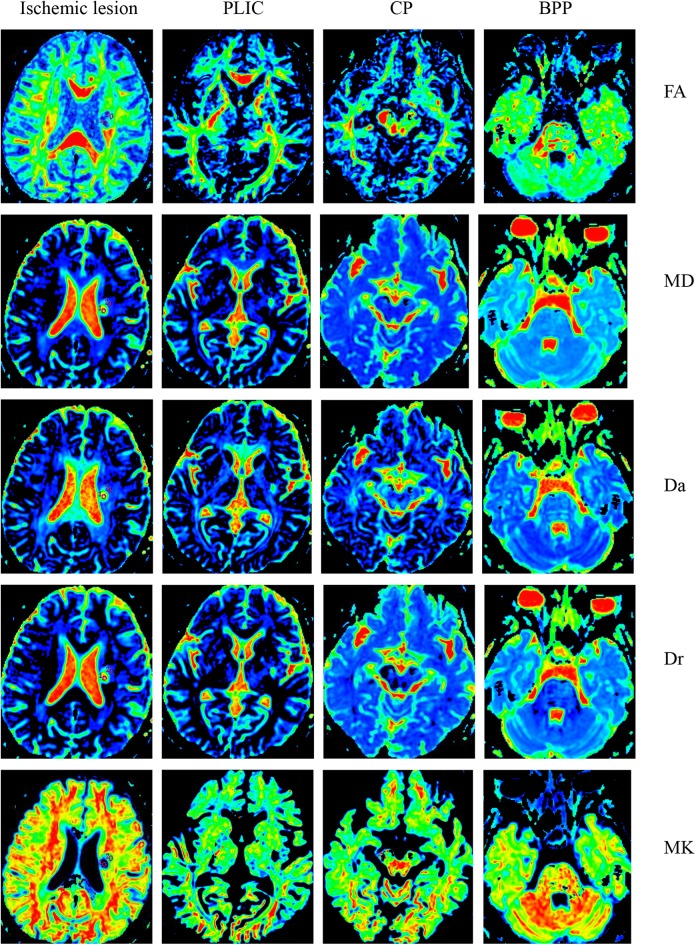
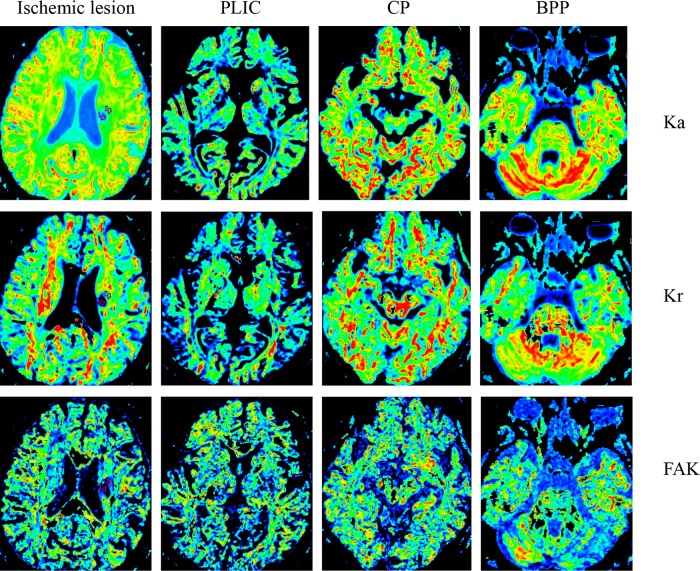
Figure 1.
The FA, MD, Da, Dr, MK, Ka, Kr and FAK maps within the ischemic lesion and along the corticospinal tract of a sub-acute ischemic stoke patient.
PLIC: posterior limb of internal capsule; CP: cerebral peduncle; BPP: basal part of the pons; FA: fractional anisotropy; MD; mean diffusivity; Da: axial diffusivity; Dr: radial diffusivity; MK: mean diffusional kurtosis; Ka: axial diffusional kurtosis; Kr: radial diffusional kurtosis; FAK: fractional anisotropy kurtosis.
Figure 2.
Parameters (rFA, rMD, rDa, rDr, rMK, rKa, rKr and rFAK) within the ischemic lesion of the sub-acute ischemic stroke. There was no statistically significant difference between the GRE and PRE groups in terms of rFA, rMD, rDa, rDr, rMK, rKa, rKr and rFAK.
rFA: relative fractional anisotropy; rMD: relative mean diffusivity; rDa: relative axial diffusivity; rDr: relative radial diffusivity; rMK: relative mean diffusional kurtosis; rKa: relative axial diffusional kurtosis; rKr: relative radial diffusional kurtosis; rFAK: relative fractional anisotropy kurtosis; GRE: good rehabilitation effect; PRE: poor rehabilitation effect.
Parameters Comparison on DTI and DKI along the CST
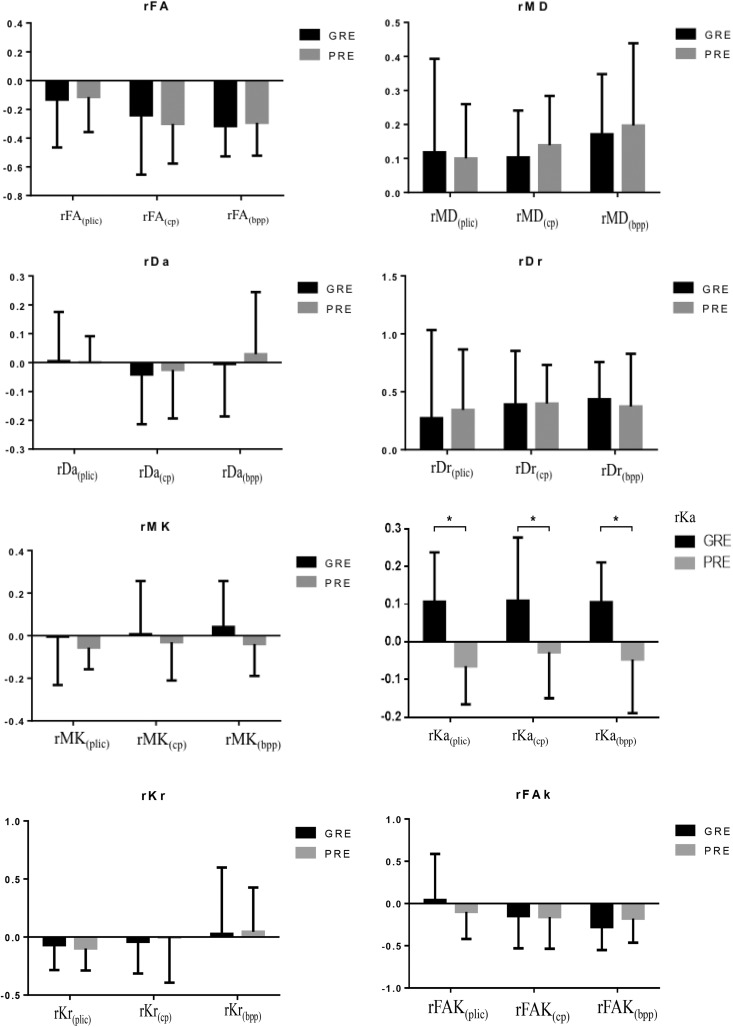
Fig. 1 shows the FA, MD, Da, Dr, MK, Ka, Kr and FAK maps along the CST. Three ROIs were located on the PLIC, CP, and BPP in the ipsilateral hemisphere, respectively. The relative DTI DKI parameters along the CST of the GRE and PRE groups are provided in Fig. 3. All three rKas (rKaPLIC, rKaCP, and rKaBPP) in the GRE group are significantly larger than those of the PRE group (p = 0.014, 0.005 and 0.021, respectively). However, the rFAs, rMDs, rDas, rDrs, rMKs, rKrs, and rFAKs along the CSTs of the two groups did not show statistically significant differences.
Figure 3.
The relative parameters (rFA, rMD, rDa, rDr, rMK, rKa, rKr and rFAK) along the corticospinal tract of the sub-acute ischemic stroke. Statistically significant differences between groups are shown.
*p < 0.05.
rFA: relative fractional anisotropy; rMD: relative mean diffusivity; rDa: relative axial diffusivity; rDr: relative radial diffusivity; rMK: relative mean diffusional kurtosis; rKa: relative axial diffusional kurtosis; rKr: relative radial diffusional kurtosis; rFAK: relative fractional anisotropy kurtosis; PLIC: posterior limb of internal capsule; CP: cerebral peduncle; BPP: basal part of the pons; GRE: good rehabilitation effect; PRE: poor rehabilitation effect.
Discussion
In this study, we applied the DKI parameters to assess changes of the ischemic lesion and CST related to sensorimotor recovery during the sub-acute phase after stroke. Our findings suggest that K metrics are not only more sensitive to sub-acute ischemic stroke changes than conventional diffusion metrics, but also have their own advantages in elucidating the possible biophysical mechanism of functional restoration underlying ischemic stroke.
The most important result of this study is the large synchronous increase of rKa value (including rKaplic, rKacp, and rKabpp), which is the value of diffusion kurtosis parallel to the principal diffusion tensor eigenvector, from the ipsilateral hemisphere to the contralateral hemisphere along the CST for the GRE group. This result is consistent with previous data in the field, that diffusion in ischemic stroke is more non-Gaussian, with this effect being much more pronounced in the axial direction than in the radial direction14. A large change in the intra-axonal diffusivity could increase Ka by increasing the diffusional heterogeneity in the axial direction. However, a change in the intra-axonal diffusivity would not necessarily change the radial diffusivity of the axonal compartment. On the one hand, ischemia can cause axonal varicosities and these bead-like swellings could act as diffusion dead-space microdomains, thereby lowering the along-axis diffusivity. On the other hand, ischemia could alter the endoplasmic reticulum (the principal intra-axonal diffusion barriers) in such a manner as to decrease the intra-axonal diffusivity and this alteration could conceivably be a change in geometrical arrangement, perhaps caused by cytoskeletal breakdown, a decrease in permeability, or an accumulation of unfolded proteins within the lumen of the endoplasmic reticulum14. Based on the above-mentioned pathological changes underlying ischemic stroke, there was significant increase in Ka, which was attributed to a sharp decrease in the axial diffusivity for the axonal compartment, but little change in the radial axonal diffusivity, which was forced to be very low by the axonal membranes and hence insensitive to the intra-axonal diffusivity.
Acute or sub-acute ischemic stroke leading to CST Wallerian degeneration may result in swelling or beading of down-stream axons and dendrites. Previous studies have assessed the correlations between DTI and DKI-derived measures of CST integrity and functional motor impairment in ischemic stroke12,15,16. In agreement with these previous studies, our work demonstrates that DKI-derived diffusion metrics can detect microstructural changes of the CST associated with sub-acute ischemic stroke. More importantly, these DKI metrics may ultimately prove to be powerful adjuncts to clinical assessment in triaging patients who may benefit from neuro-rehabilitation treatment being as stroke rehabilitative treatments are resource-intensive and patient selection is critical.
Despite the importance of studying hyper-acute/acute ischemic stroke, it is also critical to develop useful techniques for studying patients during the sub-acute phase. Given that recovery of sensorimotor function is likely mediated by the neuroplasticity of remaining intact tissue17,18, measurement of the neuroplasticity could potentially assist in the planning of rehabilitative intervention. We believe that in vivo monitoring of the neuroplastic event in ischemic brain may be aided by using DKI.
There is one uncertainty that should be taken into consideration when interpreting the results. This study suggests that the decrease in the axial diffusivity for the axonal compartment in the GRE group is sharper than in the PRE group. Maybe future studies benefiting from more advanced techniques will be able to answer this question.
The current study has several limitations that should be taken into consideration. The first is that the number of patients was small, and thus, the data varied widely. The second limitation is that we could not follow the imaging findings for long enough to observe the time course of diffusion kurtosis after ischemic stroke. Thus, how strongly the kurtosis changes depending on the time from symptom onset is not clear. The third limitation is that we cannot provide pathological experimental evidence about the relationship between functional recovery and neuroanatomic reorganization. Further studies need to be conducted in this field. Finally, DKI is less demanding regarding imaging time, hardware requirements, and post-processing effort compared with 3D q-space imaging, yet DKI contrasts could be modulated by using high angular resolution acquisition, higher b-value, and variable diffusion times. Maybe improved angular resolution could improve DKI parameter estimation.
Conclusions
In conclusion, we detected transformation along the CST by using DKI, whose connectivity corresponded with the recovery of sensorimotor function after ischemic stroke. The current study documented that DKI has some advantages over conventional DTI because of its sensitivity to tissue heterogeneity. The next major logical steps for translation include: exploring different methods to improve DKI contrasts, translating to clinical applications by optimizing scan parameters, and applying DKI to evaluate novel therapeutic strategies. These will be discussed in our subsequent study.
Acknowledgement
CLi, C La, and XZ contributed equally to this work.
Footnotes
Ethical Approval: This study was approved by the institutional review board of Huashan Hospital.
Statement of Human Rights: The study was conducted in accordance with the Declaration of Helsinki.
Statement of Informed Consent: All participants or their legal guardians provided written informed consent.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work was supported by the grant from National Natural Science Foundation of China (Nos. 81771788 and 81371521).
References
- 1. Bjartmar C, Yin X, Trapp BD. Axonal pathology in myelin disorders. J Neurocytol. 1999;28:383–395. [DOI] [PubMed] [Google Scholar]
- 2. Otero-Ortega L, Gutiérrez-Fernández M, Ramos-Cejudo J, Rodríguez-Frutos B, Fuentes B, Sobrino T, Navarro Hernanz T, Campos F, Antonio López J, Cerdán S, J Vázquez J, Díez-Tejedor E. White matter injury restoration after stem cell administration in subcortical ischemic stroke. Stem Cell Res Ther. 2015;6:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meng N, Shi S, Su Y. Proton magnetic resonance spectroscopy as a diagnostic biomarker in mild cognitive impairment following stroke in acute phase. Neuroreport. 2016;27:559–563. [DOI] [PubMed] [Google Scholar]
- 4. Jang SH, Yi JH, Choi BY, Chang CH, Jung YJ, Lee HD, Yeo SS. Changes of the corticospinal tract in the unaffected hemisphere in stroke patients: a diffusion tensor imaging study. Somatosens Mot Res. 2016;33:1–7. [DOI] [PubMed] [Google Scholar]
- 5. Sibon I, Tourdias T, Felix S, Asselineau J, Bracoud L, Vivot A, Rouanet F, Renou P, Orgogozo JM, Dousset V. Magnetisation transfer parameters and stroke outcome. J Clin Neurosci. 2015;22:1012–1017. [DOI] [PubMed] [Google Scholar]
- 6. Nie X, Hamlett ED, Granholm AC, Hui ES, Helpern JA, Jensen JH, Boger HA, Collins HR, Falangola MF. Evidence of altered age-related brain cytoarchitecture in mouse models of down syndrome: a diffusional kurtosis imaging study. Magn Reson Imaging. 2015;33:437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jiang R, Jiang J, Zhao L, Zhang JX, Zhang S, Yao YH, Yang SQ, Shi JJ, Shen NX, Su CL, Zhang L, Zhu WZ. Diffusion kurtosis imaging can efficiently assess the glioma grade and cellular proliferation. Oncotarget. 2015;6:42380–42393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ito K, Sasaki M, Ohtsuka C, Yokosawa S, Harada T, Uwano I, Yamashita F, Higuchi S, Terayama Y. Differentiation among parkinsonisms using quantitative diffusion kurtosis imaging. Neuroreport. 2015;26:267–272. [DOI] [PubMed] [Google Scholar]
- 9. Zhang XX, Yin LK, Hao XZ, Tian JQ, Li CC, Feng XY, Yang YM. Imaging the transformation of ipsilateral internal capsule following focal cerebral ischemia in rat by diffusion kurtosis imaging. J Stroke Cerebrovasc Dis. 2017;26:42–48. [DOI] [PubMed] [Google Scholar]
- 10. Sun PZ, Wang Y, Mandeville E, Chan ST, Lo EH, Ji XM. Validation of fast diffusion kurtosis MRI for imaging acute ischemia in a rodent model of stroke. NMR Biomed. 2014;27(11):1413–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang Y, Zhao Q, Zhang Y, Wu Q, Jiang X, Cheng G. Effect of mirror therapy on recovery of stroke survivors: a systematic review and network meta-analysis. Neuroscience. 2018;390:318–336. [DOI] [PubMed] [Google Scholar]
- 12. Spampinato MV, Chan C, Jensen JH, Helpern JA, Bonilha L, Kautz SA, Nietert PJ, Feng W. Diffusional kurtosis imaging and motor outcome in acute ischemic stroke. AJNR Am J Neuroradiol. 2017;38:1328–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guo YL, Li SJ, Zhang ZP, Shen ZW, Zhang GS, Yan G, Wang YT, Rao HB, Zheng WB, Wu RH. Parameters of diffusional kurtosis imaging for the diagnosis of acute cerebral infarction in different brain regions. Exp Ther Med. 2016;12:933–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jensen JH, Falangola MF, Hu CX, Tabesh A, Rapalino O, Lo C, Helpern JA. Preliminary observations of increased diffusional kurtosis in human brain following recent cerebral infarction. NMR Biomed. 2011;24:452–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Doughty C, Wang J, Feng W, Hackney D, Pani E, Schlaug G. Detection and predictive value of fractional anisotropy changes of the corticospinal tract in the acute phase of a stroke. Stroke. 2016;47:1520–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Feng W, Wang J, Chhatbar PY, Doughty C, Landsittel D, Lioutas VA, Kautz SA, Schlaug G. Cortico-spinal tract lesion load: an imaging biomarker for stroke motor outcomes. Ann Neurol. 2015;78:860–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wiersma AM, Fouad K, Winship IR. Enhancing spinal plasticity amplifies the benefits of rehabilitative training and improves recovery from stroke. J Neurosci. 2017;37:10983–10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murphy TH. Two-photon imaging of neuronal structural plasticity in mice during and after ischemia. Cold Spring Harb Protoc. 2015;2015:548–557. [DOI] [PubMed] [Google Scholar]