Abstract
Introduction
The aim of this study was to evaluate the oncologic outcome of women with stage I ovarian endometrioid carcinoma (EC) who underwent fertility-sparing surgery (FSS).
Materials and nethods
Between 1986 and 2017, a total of 3227 patients with epithelial ovarian carcinoma were retrospectively evaluated based on a central pathological review and search of the medical records from multiple institutions. We identified 24 and 54 patients with stage I EC who underwent FSS and conventional radical surgery (CRS), respectively. Inverse probability of treatment weighting (IPTW)–adjusted Kaplan-Meier and Cox regression analyses were employed to compare OS between the two groups.
Results
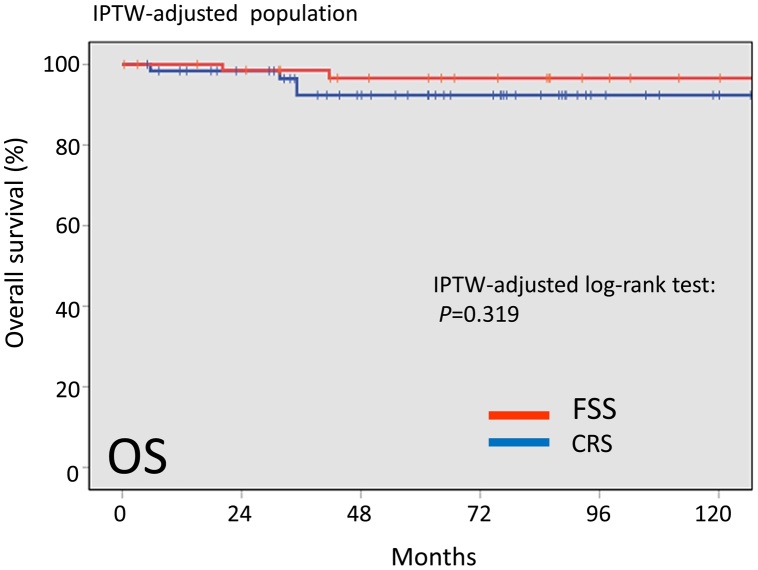
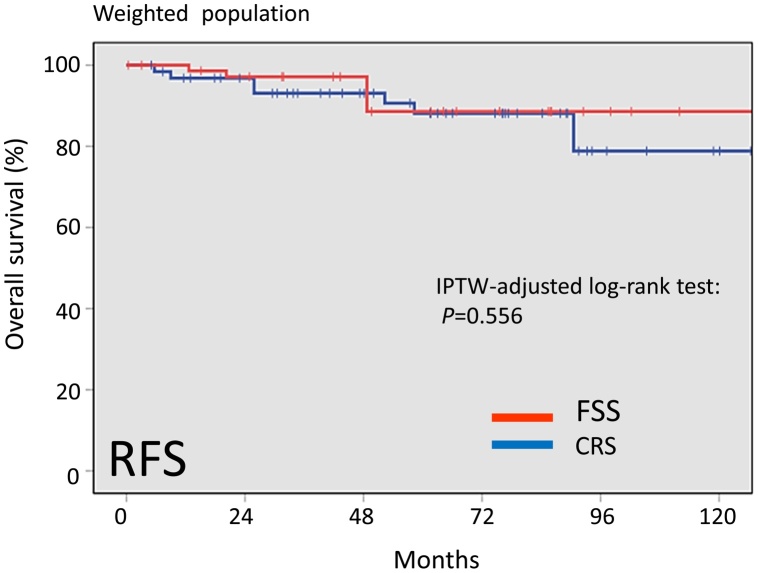
With follow-up of a total of 78 patients, 9 patients (11.5%) developed recurrence. In addition, 5 patients (6.4%) died of the disease. Recurrence was noted in 3 (10.7%) patients in the FSS group and 6 (11.1%) patients in the CRS group. Death was noted in 2 (8.3%) patients in the FSS group and 3 (5.6%) patients in the CRS group. In the original cohort, there was no significant difference in overall survival (OS) or recurrence-free survival (RFS) between the FSS and RS groups {Log-rank: OS (P = 0.630), RFS (P = 0.757)}. In the IPTW-adjusted cohort, the 5-year OS rates were 96.6 and 92.4% in patients with FSS and CRS, respectively (P = 0.319). Furthermore, the 5-year RFS rate was 88.6% for the FSS group and 88.1% for the CRS group (Log-rank: P = 0.556).
Conclusions
Young women with stage I EC undergoing FSS showed a relatively satisfactory prognosis, equal to those receiving CRC.
Abbreviations: EOC, epithelial ovarian carcinoma; EC, endometrioid carcinoma; FSS, fertility-sparing surgery; CRS, conventional radical surgery; FIGO, the federation of gynecology and obstetrics; OS, overall survival; RFS, recurrence-free survival; PS, propensity score; IPTW, inverse probability of treatment weighting
Keywords: Ovarian endometrioid carcinoma, Fertility-sparing surgery, Oncologic outcome, Propensity score, Inverse probability of treatment weighting
1. Introduction
Epithelial ovarian carcinoma (EOC) is the leading cause of mortality among malignancies of the female reproductive organ [1]. Based on the recent Cancer Statistics, 295,414 patients were newly diagnosed, and 184,799 died of this tumor worldwide [2]. Since EOC commonly remains asymptomatic, known as a “silent killer”, in clinical practice, the majority of patients show widespread peritoneal metastases at the initial diagnosis [3]. According to previous reports, 3–17% of all EOCs occur in women of reproductive age [[4], [5], [6], [7], [8], [9]]. Young patients with EOC may strongly desire to preserve their fertility and anxious about the clinical outcome. The standard operation for EOC patients is conventional radical surgery (CRS), including hysterectomy, bilateral salpingo-oophorectomy, and omentectomy, with surgical staging. Fertility-sparing surgery (FSS) has been frequently conducted in young women with encapsulated/well-differentiated EOC as well as germ cell and borderline tumors, aiming to conserve the endocrine and reproductive functions. However, the relevant evidence on selecting FSS has been too limited to accurately estimate the risk of recurrence.
In particular, according to histologic criteria, there are various subtypes in EOC with differences in the molecular background, biological hallmarks, and susceptibility to chemotherapeutic agents. Endometrioid carcinoma (EC) comprises 13% of all histologic subtype EOC [10]. EC is a relatively common histology in women with early-stage EOC undergoing FSS [11,12]. In general, individuals with EC displayed comparatively higher chemosensitivity, leading to a favorable oncologic outcome than other types of EOC [13,14]. However, confining to young patients with this tumor, the validity and safety of applying FSS has yet unelucidated.
In the current study, after a central pathological review and detailed search of the medical records from multiple institutions, we investigated the impact of FSS on long-term clinical outcomes of young women with early-stage EC who received FSS in comparison with those undergoing CRS using the IPTW technique.
2. Materials and methods
2.1. Patient enrollment
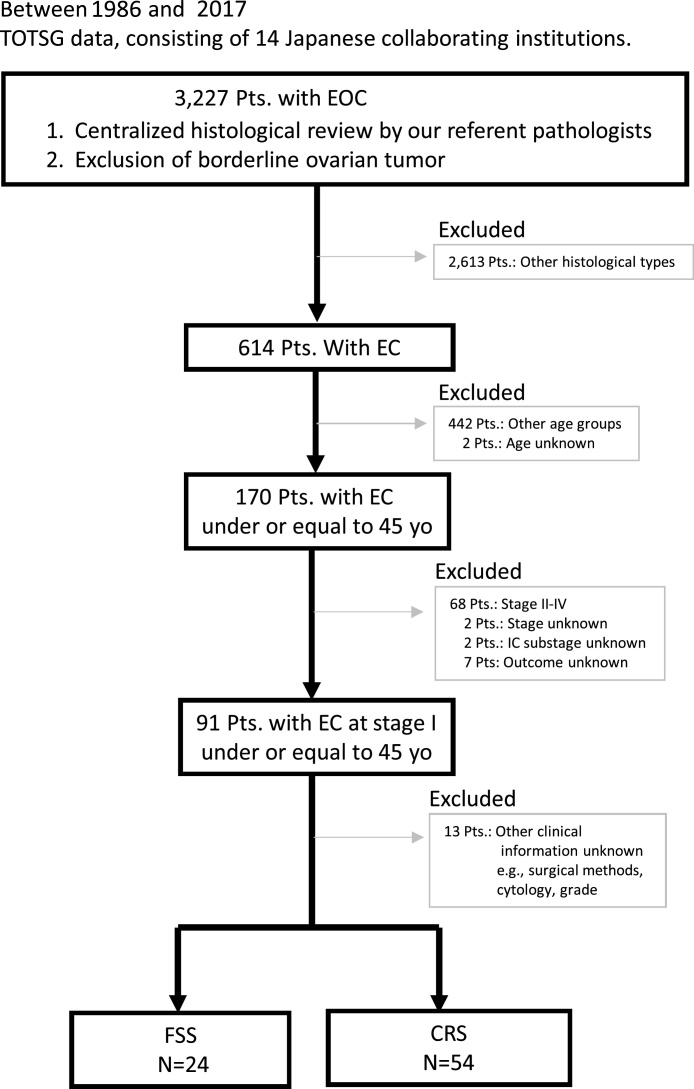
Between 1986 and 2017, patients with malignant ovarian tumors were registered and accumulated by the Tokai Ovarian Tumor Study Group (TOTSG), consisting of 14 collaborating institutions [15]. All histological slides were reviewed by two expert pathologists with no knowledge of the patients’ clinical data under a central pathological review system. Initially, a total of 3,227 patients with EOC were retrospectively evaluated. Of these, we finally identified 24 and 54 patients with stage I EC who underwent FSS and conventional radical surgery (CRS), respectively (Fig.1). Eligible cases included: 1) aged under 45 years old at the time of the initial diagnosis, 2) histologically confirmed stage I EC, 3) underwent initial surgery and periodic follow-up; and 4) women for whom there was sufficient information about the first-line chemotherapy, and date of recurrence or death. The histological cell types were evaluated based on the criteria of the World Health Organization (WHO) staging system [16]. The clinical stage was assigned according to the International Federation of Gynecology and Obstetrics (FIGO) [17]. This study was approved by the ethics committee of Nagoya University.
Fig. 1.
Flowchart.
2.2. Treatments
FSS was defined as the surgery, including at least preservation of the uterus and contralateral ovary. The therapeutic principles in patients who underwent the FSS were as follows: 1) Patients had strongly desired to conserve fertility, 2) In a preoperative counseling session, these women were informed of the possible risks and benefits of FSS, and signed a consent form, 3) Conservation of the uterus and contralateral ovary and fallopian tube with at least full peritoneal staging, 4) Systematic retroperitoneal lymphadenectomy, wedge resection of the remaining ovary, and omentectomy, were optional. If retroperitoneal lymphadenectomy was omitted, the absence of an enlarged lymph node more than 1 cm in diameter was confirmed by preoperative imaging; if present, palpable nodes were appropriately sampled. Furthermore, the standard treatments in patients who underwent the CRS were as follows: 1) conventional radical surgery, including, principally hysterectomy and bilateral salpingo-oophorectomy with complete staging surgery. Complete staging surgery was defined as peritoneal staging and lymph node evaluation. Peritoneal staging included peritoneal exploration, cytology, biopsy, and/or omentectomy or omental biopsy. Lymph node evaluation involved one of the following: 1) lymph node dissection, 2) lymph node sampling, or 3) palpation and removal of enlarged lymph nodes.
Of all stage I patients, 60 were treated postoperatively with 3–6 cycles of adjuvant platinum-based chemotherapy. A total of 18 patients (23.1%) did not receive adjuvant platinum-based chemotherapy due to meeting the criterion of omission (stage IA/grade 1–2), or an individual decision of each institution. Details of the chemotherapy regimen in each period were described previously [18].
2.3. Follow-up and analysis
At the end of treatment, all patients underwent a strict follow-up, consisting of clinical checkups such as a pelvic examination, ultrasonographic scan, CA125 evaluation, and periodic radiologic imaging. Radiologic recurrence was defined as tumor recurrence based on computed tomography (CT), and/or magnetic resonance imaging (MRI), and/or PET (Positron emission tomography), and/or ultrasound, and clinical recurrence was defined as the development of ascites, elevated CA125, or a clinically palpable mass according to the Gynecologic Cancer InterGroup (GCIG) criteria in principle [19]. Overall survival (OS) was defined as the time between the date of surgery and that of the last follow-up or death from any cause. Recurrence-free survival (RFS) was defined as the time interval between the date of surgery and that of recurrence or the last follow-up. The distributions of clinicopathologic events were evaluated using the Chi-square tests. To balance the patient and tumor characteristics between FSS and CRS groups, propensity score (PS) weighting was performed. The rationale and methods underlying the use of PS were previously described [20]. PS was estimated by multivariate logistic regression models for the probability of FSS adjusting for age, FIGO substage, tumor grade, preoperative CA125 value, ascites volume, cytology of ascites, and presence or absence of chemotherapy. In the subsequent survival analysis model, the patients were weighted according to the inverse probability of receiving the treatment that the subject underwent [21]. With this method, each patient was weighted by the inverse probability of being in the FSS versus CRS group, with the goal of balancing observed characteristics between the two groups. i.e. In addition, we used Kernel density plots to depict the pre-and post-IPTW adjustment distribution of PS in each treatment group. Within the original and IPTW-adjusted cohorts, survival curves were generated using Kaplan-Meier methods. A Cox proportional hazards regression model was used to examine associations between the type of surgery (FSS vs. CRS) and OS/RFS. All statistical analyses were performed with SPSS Ver. 26 (IBM Japan, Tokyo) and JMP Pro Ver.10.0 (SAS Institute Japan). A P-value of < 0.05 was considered significant.
3. Results
3.1. Patients’ characteristics
A total of 78 patients with stage I EC were entered into this study. The characteristics of patients in the FSS and CRS groups are summarized in Table 1. The cohort included 24 women (30.7%) who underwent FSS and 54 women (69.3%) receiving CRS. The median (SD) age of those who received FSS was 36 (19–44) years. Patients who underwent FSS were significantly younger than were those who received CRS (P < 0.0001). The median follow-up duration of all patients was 65.3 months. The median (SD) follow-up of women in FSS and CRS groups were 63.7 (38.2) and 66.2 (43.4) months, respectively. There were no difference in follow-up duration between the two groups (P = 0.4245). Among patients in the FSS group, 9 (37.5%) had IA disease, and 15 (62.5%) had IC disease. Regarding the distribution of the substage, grade, preoperative CA125 value, volume of ascites, ascites cytology, and rate of chemotherapy, there was no difference between the two groups.
Table 1.
Patients' characteristics.
| CRS |
FSS |
P-value* | ||||
|---|---|---|---|---|---|---|
| Total | N | % | N | % | ||
| Total | 54 | 24 | ||||
| Age (median/range) | 43 (32-45) | 36 (19-44) | <0.0001 | |||
| FIGO stage | 0.689 | |||||
| IA-IB | 31 | 22 | 40.7 | 9 | 37.5 | |
| IB | 0 | 0.0 | 0 | 0.0 | ||
| IC1 | 34 | 22 | 40.7 | 12 | 50.0 | |
| IC2/IC3 | 13 | 10 | 18.5 | 3 | 12.5 | |
| Grade | 0.797 | |||||
| G1/G2 | 74 | 51 | 94.4 | 23 | 95.8 | |
| G3 | 4 | 3 | 5.6 | 1 | 4.2 | |
| CA125 | 0.602 | |||||
| ≤35 U/mL | 26 | 19 | 7 | 29.2 | ||
| >35 U/mL | 52 | 35 | 19.0 | 17 | 70.8 | |
| Ascites volume | 0.554 | |||||
| ≤100 m L | 69 | 47 | 87.0 | 22 | 91.7 | |
| >100 m L | 9 | 7 | 13.0 | 2 | 8.3 | |
| Ascites cytology | 0.329 | |||||
| Negative | 54 | 45 | 83.3 | 9 | 37.5 | |
| Positive | 11 | 9 | 16.7 | 2 | 8.3 | |
| Chemotherapy | 0.394 | |||||
| No | 18 | 11 | 20.4 | 7 | 29.2 | |
| Yes | 60 | 43 | 79.6 | 17 | 70.8 | |
CRS: conventional radical surgery, FSS: fertility-sparing surgery, FIGO: Internatinal Federation of Gynecology and Obstetrics.
3.2. Oncologic outcome using the original cohort
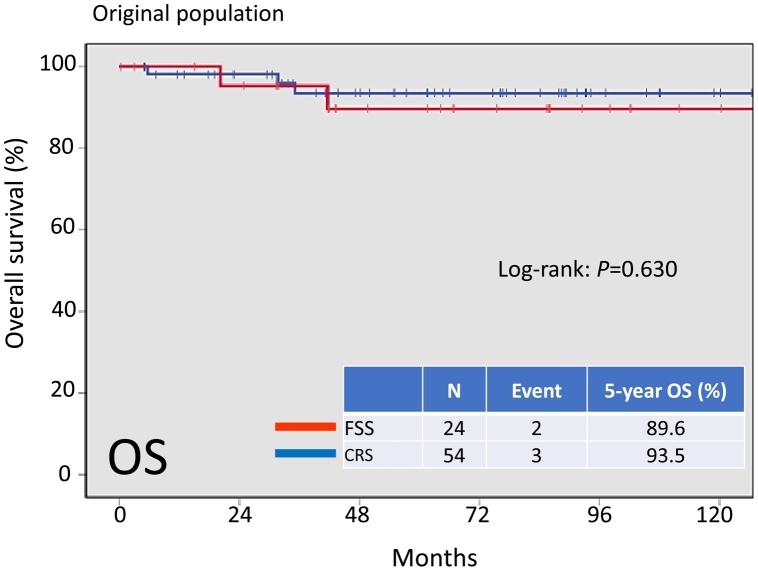
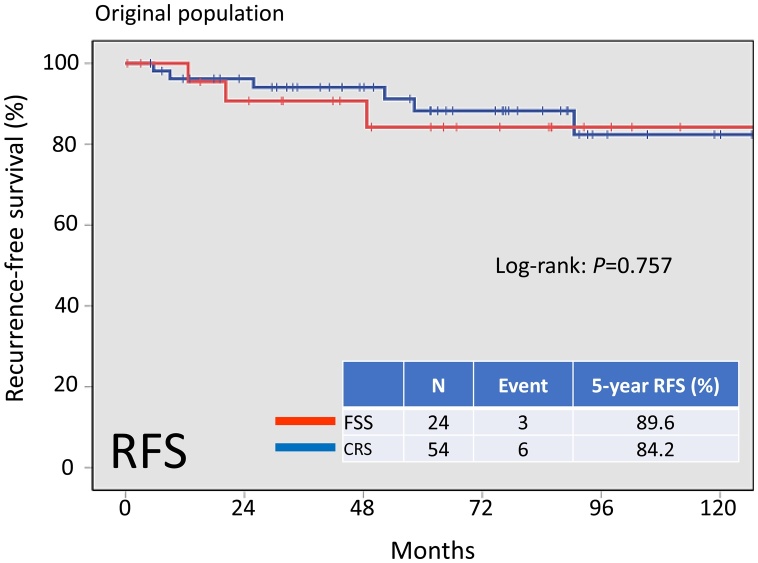
On follow-up of a total of 78 patients, 9 (11.5%) developed recurrence. In addition, 5 patients (6.4%) died of the disease. Recurrent disease was noted in 3 (10.7%) patients in the FSS group and 6 (11.1%) patients in the CRS group. Death occurred in 2 (8.3%) patients in the FSS group and 3 (5.6%) patients in the CRS group. There was no significant difference in OS among these groups (Fig.2, log-rank: P = 0.630). In addition, the 5-year RFS rate of all patients in the FSS group was 89.6%, compared with 84.2% in the CRS group B. On Kaplan-Meier analysis, the difference in RFS among these groups was also non-significant (Fig.3, log-rank: P = 0.757).
Fig. 2.
Kaplan-Meier-estimated overall survival (OS) on stratifying by the surgical type {FSS (N = 24) vs. CRS (N = 54)}. The original cohort.
Fig. 3.
Kaplan-Meier-estimated recurrence-free survival (RFS) on stratifying by the surgical type {FSS (N = 24) vs. CRS (N = 54)}. The original cohort.
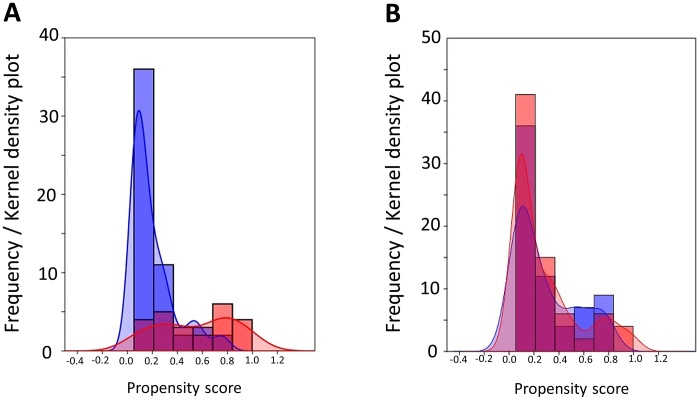
Calculation of PS was then performed for each patient based on seven clinicopathologic variables: age, substage, grade, volume of ascites, CA125 value, cytology, and presence or absence of chemotherapy. The weighted baseline characteristics of eligible patients, stratified according to the performance of FSS vs. CRS, are presented in Table 2. After IPTW adjustment, all conditioning variables were well-balanced. Fig. 4 demonstrates the Kernel density plots to display the distribution of PS before and after IPTW adjustment in each treatment cohort. The distributions of PS in both groups were similar after IPTW adjustment, suggesting that those confounders are well-balanced across the two subgroups.
Table 2.
Standardized difference of independent variables.
| Variable | Non-adjusted |
IPTW-adjusted |
||||
|---|---|---|---|---|---|---|
| CRS (%) | FSS (%) | Stand. Diff | CRS (%) | FSS (%) | Stand. Diff | |
| Age (mean, SD) | 41.7 (3.28) | 34.9 (6.78) | 1.276 | 40.2 (4.27) | 40.0 (5.55) | 0.040 |
| FIGO stage | 0.168 | 0.253 | ||||
| IA-IB-IC1 | 81.4 | 87.5 | 83.8 | 92.0 | ||
| IC2/IC3 | 18.6 | 12.5 | 13.2 | 8.0 | ||
| Grade | 0.064 | 0.041 | ||||
| G1/G2 | 94.4 | 95.8 | 95.5 | 94.6 | ||
| G3 | 5.6 | 5.2 | 4.5 | 5.4 | ||
| CA125 | 0.128 | 0.155 | ||||
| ≤35 U/mL | 35.1 | 29.1 | 29.4 | 22.6 | ||
| >35 U/mL | 64.9 | 70.9 | 70.6 | 77.4 | ||
| Ascites volume | 0.149 | 0.192 | ||||
| ≤100 m L | 87.0 | 91.6 | 88.2 | 81.3 | ||
| >100 m L | 13.00 | 8.4 | 11.8 | 18.7 | ||
| Ascites cytology | 0.260 | 0.263 | ||||
| Negative | 83.3 | 91.6 | 85.2 | 93.3 | ||
| Positive | 16.7 | 8.4 | 14.8 | 6.7 | ||
| Chemotherapy | 0.205 | 0.051 | ||||
| No | 20.3 | 29.1 | 20.5 | 22.6 | ||
| Yes | 79.7 | 70.6 | 79.5 | 77.4 | ||
FIGO: Internatinal Federation of Gynecology and Obstetrics.
Fig. 4.
Frequency and Kernel density plots to depict the pre- (A) and post- (B) IPTW adjustment distribution of the propensity score in each treatment group.
In the IPTW-adjusted cohort, the 5-year overall survival rates were 96.6 and 92.4% in patients with FSS and CRS, respectively (Fig.5). The difference was also non-significant between the two surgical groups (Log-rank: P = 0.319). In addition, the 5-year RFS rate was 88.6% for the FSS group and 88.1% for the CRS group (Log-rank: P = 0.556) (Fig.6). Thus, after IPTW adjustment, FSS and OS maintained similar trends with the full dataset.
Fig. 5.
Kaplan-Meier-estimated OS on stratifying by the surgical type {FSS vs. CRS}. The IPTW-adjusted cohort.
Fig. 6.
Kaplan-Meier-estimated RFS on stratifying by the surgical type {FSS vs. CRS}. The IPTW-adjusted cohort.
3.3. Multivariable cox hazard model
To eliminate selection bias from a number of clinicopathologic factors as thoroughly as possible, we finally performed multivariate analyses of OS and RFS. The surgery (FSS vs. CRS), age (≤50 vs. > 50), FIGO stage (IA-IC1 vs. IC2-3), and preoperative CA125 value (≤ 35U/mL vs. > 35 U/mL) were entered into the multivariate analyses (Table 2). In these analyses, only the substage retained its significance for OS (IC2-3/IA-IC1, HR: 7.227, 95% CI: 1.198–43.611, P = 0.031) and RFS (IC2-3/IA-IC1, HR: 4.395, 95% CI: 1.167–16.551, P = 0.029). Moreover, in another Cox multivariable model selecting the surgery and PS-rank as variables, the performance of FSS was not a significant prognostic indicator for OS or RFS (OS: P = 0.660, RFS: P = 0.892) (Table 3). Furthermore, regardless of IPTW adjustment or non-adjustment for multiple confounders, the performance of FSS itself was not a significant predictor of the risk of mortality or recurrence {IPTW-adjusted: OS: HR (95% CI): 0.303 (0.047–1.951), P = 0.209, RFS: HR (95% CI): 0.633 (0.172–2.334), P = 0.492} (Table 4).
Table 3.
Cox multivarible analyses.
| Variable | OS | RFS | ||||
|---|---|---|---|---|---|---|
| HR | 95.0% CI | P-value | HR | 95.0% CI | P-value | |
| Model 1 (multivariable adjusted) | ||||||
| Age | 0.931 | 0.774–1.120 | 0.446 | 0.974 | 0.844–1.124 | 0.718 |
| Surgery (FSS vs. conventional) | 0.762 | 0.071–8.164 | 0.822 | 1.043 | 0.154–7.073 | 0.965 |
| Sub-stage (IA-IC1 vs. IC2/3) | 7.227 | 1.198–43.611 | 0.031 | 4.395 | 1.167–16.551 | 0.029 |
| Preoperative CA125 value (< 35 U/mL vs. ≥ 35 U/mL) | 0.500 | 0.052–4.814 | 0.549 | 0.798 | 0.189–3.368 | 0.758 |
| Model 2 (PS-rank adjusted) | ||||||
| Surgery (FSS vs. conventional) | 0.549 | 0.038–7.945 | 0.660 | 0.871 | 0.118–6.430 | 0.892 |
HR: hazard ratio, 95%CI: 95% confidence interval, OS: overall survival, RFS: recurrence-free survival, PS: propensity score, FSS: fertility-sparing surgery.
Table 4.
Cox hazard model with IPTW.
| Variable | OS |
RFS |
||||
|---|---|---|---|---|---|---|
| HR | 95.0% CI | P-value | HR | 95.0% CI | P-value | |
| Model 1 (non-adjusted with IPTW) | ||||||
| Surgery (FSS vs CRS) | 0.433 | 0.079–2.365 | 0.334 | 0.719 | 0.230–2.245 | 0.57 |
| Model 2 (multivariable#1 adjusted with IPTW) | ||||||
| Surgery (FSS vs CRS) | 0.303 | 0.047–1.951 | 0.209 | 0.633 | 0.172–2.334 | 0.492 |
HR: hazard ratio, 95%CI: 95% confidence interval, OS: overall survival, RFS: recurrence-free survival, FSS: fertility-sparing surgery, #1: #2: surgery, age, substage, grade, CA125 value, ascites cytology, and chemotherapy.
4. Discussion
According to microscopic morphologic features, there are four dominant histologic subtypes of EOC: serous, clear-cell, mucinous, and endometrioid carcinomas. In spite of the fact that all EOC patients are treated similarly, biological hallmarks and clinical behaviors are different from each other [22]. Thus, we should comprehensively verify the validity of FSS based on each histological type. We previously used histology-type-specific data to compare young patients with early-stage clear-cell and mucinous carcinomas who underwent FSS and CRS. In both histological types, we could not show any prognostic difference between the two patient groups with or without FSS [23,24]. The validity of FSS in patients with EC is unknown. As well as clear-cell carcinoma, EC is known as a representative histologic subtype of endometriosis-associated ovarian carcinoma. Based on an earlier review regarding endometriosis-associated ovarian carcinoma, the most and second most common pathological type were clear-cell carcinoma (35% / 390 cases) and EC (27% / 648 cases) [25]. Moreover, in another comprehensive analysis of 13 retrospective studies, endometriosis was associated with a significantly higher risk of EC {169 /1,220 cases (13.9%) vs. 818 /13,226 controls (6.2%)}, as well as clear-cell carcinoma. Generally, EC is commonly diagnosed in the post-menopausal generation, and is infrequent in patients of reproductive age. Nevertheless, considering the higher rate of comorbidity of endometriosis in this age demographic, the incidence of EC in young women is a critical issue based on not only the possibility of the loss of fertility / endocrine functions, but also its life-threatening nature. In the current study, recurrent disease was noted in 3 (10.7%) patients in the FSS group and 6 (11.1%) patients in the CRS group. Table 5 shows eight representative series reported on the recurrence rate after FSS in patients with stage I EC [9,11,12,[26], [27], [28], [29], [30]]. The total recurrence rate of those studies was 11.9% (16/134). These data are consistent with our current data. Furthermore, Jiang, et al reported that the recurrence rates of women receiving FSS and CRS were 18.2 (2/11) and 18.8% (3/16), respectively [26]. This result suggests that the recurrence rates of the two groups were similar, which is also in accordance with our present data. Particularly in the current study, in comparison of patients with stage I EC belonging to the original FSS and CRS group, the prognostic difference was not significant, suggesting that performing FSS itself was not a critical factor for those patients with EC. According to the largest comparison analysis by Fruscio, et al, including 1031 patients (242 with FSS and 789 with CRS), multivariate analysis did not show that the performance of FSS itself led to a negative impact neither on relapse-free interval (RFI) nor on cancer-specific survival (CSS) [RFI (FSS vs. CRS): HR (95% CI) = 0.82 (0.41–1.65), CSS (FSS vs. CRS): HR (95% CI) = 0.85 (0.27–2.64)]. This study also included 258 patients with EC (55: FSS, 203: CRS) [31]. Our study was based on retrospective data with a limited patient number, and so we cannot suggest that women with stage I EC have an acceptable oncologic outcome. We hope that the current results are verified by other researchers in the future.
Table 5.
Representative series reported on the recurrence rate after FSS in patients with stage I EC.
| Report | Year | Total, N | Recurrence, N | Recurrence, % |
|---|---|---|---|---|
| Zanetta | 1997 | 13 | 1 | 7.7 |
| Schilder | 2002 | 10 | 1 | 10.0 |
| Park | 2008 | 8 | 1 | 12.5 |
| Kwon | 2009 | 2 | 0 | 0.0 |
| Satoh | 2010 | 27 | 5 | 18.5 |
| Kashima | 2013 | 3 | 0 | 0.0 |
| Fruscio | 2013 | 60 | 6 | 10.0 |
| Jiang | 2017 | 11 | 2 | 18.2 |
| Total | 134 | 16 | 11.9 |
EC: endometrioid carcinoma.
In general, we are more likely to adopt FSS for women with a more favorable clinicopathologic background. Namely, we can easily expect that patients with favorable factors, including an encapsulated, well-differentiated, non-clear-cell histological type will tend to undergo FSS. Indeed, even if there was a non-significant difference in oncologic outcomes, a number of clinicopathological profiles were inconsistent between the two cohorts. Accordingly, if FSS itself has a negative impact on patient survival, it is possible for us to observe an equal prognostic tendency of individuals who belonged to the two cohorts. Thus, through our current abovementioned results, we cannot conclude the safety of FSS. In our subsequent work, the IPTW method was employed to adjust for different baseline clinicopathologic backgrounds, one of the major limitations of our previous observational studies. As expected, patients in the IPTW-adjusted cohort who underwent FSS showed a generally similar oncologic outcome, compared with those who received CRS. Furthermore, adjusted multivariable Cox regression analyses with the IPTW technique demonstrated that the influence of FSS on both OS and RFS was non-significant. To our knowledge, this is the first study to conduct the prognostic comparison analyses involving patients who received FSS with those who received CRS using the IPTW model. Although it is difficult to make a definite conclusion with regard to the impact of FSS from this work, it is worthwhile to continuously investigate the appropriateness of this conservative surgery because it could be an acceptable option in EC patients at an early stage who are of reproductive age.
This study includes several limitations. Firstly, the present investigation was fundamentally retrospective, in which various confounders relevant to the therapeutic decision were not as strictly balanced as in an RCT. Secondly, it is possible that there was an unknown confounder affecting the reliability of the estimated PS. Thirdly, the constitution of the study subjects may have been influenced by referral bias owing to long-term multicenter analysis. Lastly, although the obstetric outcome is as important as oncological safety for patients receiving FSS, unfortunately, we did not present accurate data on reproductive outcome. In contrast, the greatest strength of this study was the performance of central pathological review by expert pathologists for gynecologic malignancy.
In summary, the main finding of this study was that, even with a uniform histological type, EC patients who underwent FSS demonstrated an acceptable prognosis equal to those receiving CRS. This information will be beneficial for patients and physicians to share risk-and-benefit data before selecting this surgery. From now on, concerning the patient background and specificity of this surgery, it may not be practical to conduct an RCT. We understand that the current results may be merely hypothesis-generating. However, they have prompted us to conduct future research based on a prospective, larger-scale patient registry system including all early-stage ovarian malignancies, such as patients with other histological types that have received FSS.
Funding information
This study was supported by JSPS (Japan Society for the Promotion of Science) KAKENHI Grants-in-Aid for Scientific Research: Grant Numbers 17H04338 and 16K15704.
Author contribution statement
H. Kajiyama: Data analysis and interpretation, Writing manuscript, M. Yoshihara, S. Tamauchi, S. Suzuki, and N. Yoshikawa: Data collection, F. Kikkawa: supervising and funding
Acknowledgements
We sincerely thank members of the Tokai Ovarian Tumor Study Group for collaborating in data collection. We sincerely thank Dr. Tetsuro Nagasaka (Department of Healthcare Administration, Nagoya University Graduate School of Medicine) for collaborating in central pathological review.
References
- 1.Greenlee R.T., Hill-Harmon M.B., Murray T., Thun M. Cancer statistics. CA Cancer J Clin. 2001;51:15–36. doi: 10.3322/canjclin.51.1.15. 2001. [DOI] [PubMed] [Google Scholar]
- 2.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018 doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Eisenkop S.M., Friedman R.L., Wang H.J. Complete cytoreductive surgery is feasible and maximizes survival in patients with advanced epithelial ovarian cancer: a prospective study. Gynecol Oncol. 1998;69:103–108. doi: 10.1006/gyno.1998.4955. [DOI] [PubMed] [Google Scholar]
- 4.Smedley H., Sikora K. Age as a prognostic factor in epithelial ovarian carcinoma. Br J Obstet Gynaecol. 1985;92:839–842. doi: 10.1111/j.1471-0528.1985.tb03056.x. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez M., Nguyen H.N., Averette H.E., Steren A.J., Penalver M.A., Harrison T. National survey of ovarian carcinoma XII. Epithelial ovarian malignancies in women less than or equal to 25 years of age. Cancer. 1994;73:1245–1250. doi: 10.1002/1097-0142(19940215)73:4<1245::aid-cncr2820730419>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 6.Swenerton K.D., Hislop T.G., Spinelli J., LeRiche J.C., Yang N., Boyes D.A. Ovarian carcinoma: a multivariate analysis of prognostic factors. Obstet Gynecol. 1985;65:264–270. [PubMed] [Google Scholar]
- 7.Duska L.R., Chang Y.C., Flynn C.E., Chen A.H., Goodman A., Fuller A.F. Epithelial ovarian carcinoma in the reproductive age group. Cancer. 1999;85:2623–2629. doi: 10.1002/(sici)1097-0142(19990615)85:12<2623::aid-cncr19>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 8.Plaxe S.C., Braly P.S., Freddo J.L., McClay E., Kirmani S., Howell S.B. Profiles of women age 30-39 and age less than 30 with epithelial ovarian cancer. Obstet Gynecol. 1993;81:651–654. [PubMed] [Google Scholar]
- 9.Schilder J.M., Thompson A.M., DePriest P.D., Ueland F.R., Cibull M.L., Kryscio R.J. Outcome of reproductive age women with stage IA or IC invasive epithelial ovarian cancer treated with fertility-sparing therapy. Gynecol Oncol. 2002;87:1–7. doi: 10.1006/gyno.2002.6805. [DOI] [PubMed] [Google Scholar]
- 10.Chan J.K., Teoh D., Hu J.M., Shin J.Y., Osann K., Kapp D.S. Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers. Gynecol Oncol. 2008;109:370–376. doi: 10.1016/j.ygyno.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Fruscio R., Corso S., Ceppi L., Garavaglia D., Garbi A., Floriani I. Conservative management of early-stage epithelial ovarian cancer: results of a large retrospective series. Ann Oncol. 2013;24:138–144. doi: 10.1093/annonc/mds241. [DOI] [PubMed] [Google Scholar]
- 12.Satoh T., Hatae M., Watanabe Y., Yaegashi N., Ishiko O., Kodama S. Outcomes of fertility-sparing surgery for stage I epithelial ovarian cancer: a proposal for patient selection. J Clin Oncol. 2010;28:1727–1732. doi: 10.1200/JCO.2009.24.8617. [DOI] [PubMed] [Google Scholar]
- 13.Geyer J.T., Lopez-Garcia M.A., Sanchez-Estevez C., Sarrio D., Moreno-Bueno G., Franceschetti I. Pathogenetic pathways in ovarian endometrioid adenocarcinoma: a molecular study of 29 cases. Am J Surg Pathol. 2009;33:1157–1163. doi: 10.1097/PAS.0b013e3181a902e1. [DOI] [PubMed] [Google Scholar]
- 14.Soyama H., Miyamoto M., Takano M., Iwahashi H., Kato K., Sakamoto T. A Pathological Study Using 2014 WHO Criteria Reveals Poor Prognosis of Grade 3 Ovarian Endometrioid Carcinomas. In Vivo (Brooklyn) 2018;32:597–602. doi: 10.21873/invivo.112281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kajiyama H., Suzuki S., Yoshihara M., Nishino K., Yoshikawa N., Utsumi F. The possible existence of occult metastasis in patients with ovarian clear-cell carcinoma who underwent complete resection without any residual tumours. Oncotarget. 2018;9:6298–6307. doi: 10.18632/oncotarget.23921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen V.W., Ruiz B., Killeen J.L., Cote T.R., Wu X.C., Correa C.N. Pathology and classification of ovarian tumors. Cancer. 2003;97:2631–2642. doi: 10.1002/cncr.11345. [DOI] [PubMed] [Google Scholar]
- 17.Zeppernick F., Meinhold-Heerlein I. The new FIGO staging system for ovarian, fallopian tube, and primary peritoneal cancer. Arch Gynecol Obstet. 2014;290:839–842. doi: 10.1007/s00404-014-3364-8. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki S., Kajiyama H., Shibata K., Ino K., Nawa A., Sakakibara K. Is there any association between retroperitoneal lymphadenectomy and survival benefit in ovarian clear cell carcinoma patients? Ann Oncol. 2008;19:1284–1287. doi: 10.1093/annonc/mdn059. [DOI] [PubMed] [Google Scholar]
- 19.Rustin G.J., Vergote I., Eisenhauer E., Pujade-Lauraine E., Quinn M., Thigpen T. Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1.1 and CA 125 agreed by the gynecological cancer intergroup (GCIG) Int J Gynecol Cancer. 2011;21:419–423. doi: 10.1097/IGC.0b013e3182070f17. [DOI] [PubMed] [Google Scholar]
- 20.Joffe M.M., Rosenbaum P.R. Invited commentary: propensity scores. Am J Epidemiol. 1999;150:327–333. doi: 10.1093/oxfordjournals.aje.a010011. [DOI] [PubMed] [Google Scholar]
- 21.Austin P.C., Stuart E.A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–3679. doi: 10.1002/sim.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salgado-Ceballos I., Rios J., Perez-Montiel D., Gallardo L., Barquet-Munoz S., Salcedo-Hernandez R. Is lymphadenectomy necessary in mucinous ovarian cancer? A single institution experience. Int J Surg. 2017;41:1–5. doi: 10.1016/j.ijsu.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 23.Kajiyama H., Shibata K., Mizuno M., Hosono S., Kawai M., Nagasaka T. Fertility-sparing surgery in patients with clear-cell carcinoma of the ovary: is it possible? Hum Reprod. 2011;26:3297–3302. doi: 10.1093/humrep/der342. [DOI] [PubMed] [Google Scholar]
- 24.Kajiyama H., Shibata K., Mizuno M., Nawa A., Mizuno K., Matsuzawa K. Fertility-sparing surgery in young women with mucinous adenocarcinoma of the ovary. Gynecol Oncol. 2011;122:334–338. doi: 10.1016/j.ygyno.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 25.Somigliana E., Vigano P., Parazzini F., Stoppelli S., Giambattista E., Vercellini P. Association between endometriosis and cancer: a comprehensive review and a critical analysis of clinical and epidemiological evidence. Gynecol Oncol. 2006;101:331–341. doi: 10.1016/j.ygyno.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 26.Jiang X., Yang J., Yu M., Xie W., Cao D., Wu M. Oncofertility in patients with stage I epithelial ovarian cancer: fertility-sparing surgery in young women of reproductive age. World J Surg Oncol. 2017;15:154. doi: 10.1186/s12957-017-1222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kashima K., Yahata T., Fujita K., Tanaka K. Outcomes of fertility-sparing surgery for women of reproductive age with FIGO stage IC epithelial ovarian cancer. Int J Gynaecol Obstet. 2013;121:53–55. doi: 10.1016/j.ijgo.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 28.Kwon Y.S., Hahn H.S., Kim T.J., Lee I.H., Lim K.T., Lee K.H. Fertility preservation in patients with early epithelial ovarian cancer. J Gynecol Oncol. 2009;20:44–47. doi: 10.3802/jgo.2009.20.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park J.Y., Kim D.Y., Suh D.S., Kim J.H., Kim Y.M., Kim Y.T. Outcomes of fertility-sparing surgery for invasive epithelial ovarian cancer: oncologic safety and reproductive outcomes. Gynecol Oncol. 2008;110:345–353. doi: 10.1016/j.ygyno.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 30.Zanetta G., Chiari S., Rota S., Bratina G., Maneo A., Torri V. Conservative surgery for stage I ovarian carcinoma in women of childbearing age. Br J Obstet Gynaecol. 1997;104:1030–1035. doi: 10.1111/j.1471-0528.1997.tb12062.x. [DOI] [PubMed] [Google Scholar]
- 31.Fruscio R., Ceppi L., Corso S., Galli F., Dell’Anna T., Dell’Orto F. Long-term results of fertility-sparing treatment compared with standard radical surgery for early-stage epithelial ovarian cancer. Br J Cancer. 2016;115:641–648. doi: 10.1038/bjc.2016.254. [DOI] [PMC free article] [PubMed] [Google Scholar]