Introduction
Merkel cell carcinoma (MCC) is a rare, aggressive malignancy derived from cutaneous neuroendocrine cells perceiving light touch.1 MCC usually presents in elderly patients on sun-exposed areas, most commonly of the head and neck.2 Merkel cell polyomavirus (MCPyV) is directly involved in the pathogenesis of 80% of MCCs in which clonal integration results in expression of 2 key antigenic oncoproteins.3 MCPyV-negative tumors harbor many ultraviolet signature mutations with high levels of infiltrating T lymphocytes and programmed death ligand 1 (PD-L1) expression.4 MCC is thus an attractive target for immunotherapy because virus-positive tumors express foreign oncoproteins, and virus-negative tumors carry ultraviolet signature mutations providing non–self-epitopes for immune recognition.
Historically, metastatic MCC was treated with chemotherapy. Although objective response rates (ORR) exceeded 50%, median progression-free survival (PFS) was approximately 3 months with no clear overall survival benefit.5 Recent phase II trials of immune checkpoint blockade (ICB) in advanced MCC targeting PD-1 with pembrolizumab or nivolumab or PD-L1 with avelumab have shown ORR of 65% in treatment-naïve patients and 40% following chemotherapy with PFS substantially superior to chemotherapy. However, fewer than 20% of patients achieve complete responses (CR), and the largest trial showed 12 months PFS of 30%.6 In March 2017, US Food and Drug Administration (FDA) approval of avelumab for advanced MCC marked the dawn of a new era of treatment for this highly immune responsive malignancy.
Talimogene laherparepvec (TVEC) is a genetically modified herpes simplex 1 virus that encodes human granulocyte-macrophage colony-stimulating factor to enhance dendritic cell antigen presentation. Intratumoral injection directly lyses malignant cells and alters the microenvironment favoring induction of systemic antitumor immunity.7 TVEC received an indication for advanced melanoma in October 2015, making it the first FDA-approved oncolytic viral immunotherapy. Here we describe 4 consecutive patients treated with TVEC for regionally advanced MCC that ultimately achieved durable complete responses to therapy all ongoing for up to greater than 27 months following TVEC initiation with minimal toxicity. We have previously reported on cases 1 and 28 but have additional treatment and follow-up information.
Overview of cases
Four elderly white men underwent wide local excision of primary MCC arising in the head or neck (Table I) obtaining negative margins where anatomically possible. Risk factors for development of MCC included advanced age, prolonged statin use in patient 3, and immunosuppression for Crohn's disease in patient 4.9 Serology from patients 1 and 2 was negative for antibody against MCPyV oncoprotein, although negative predictive value is low. All patients experienced multifocal, regional recurrence within 8 months of initial resection. TVEC therapy was selected based on the immunogenicity of MCC, absence of detectable distant metastases, significant cardiac comorbidity in patients 1 and 2 limiting their tolerance of potentially more toxic treatment, and concern that ICB could exacerbate underlying autoimmune conditions in patients 3 and 4 with Parkinson and Crohn's disease, respectively. Furthermore, avelumab was not FDA approved for MCC until March 2017, after TVEC initiation in patients 1 and 2. TVEC was administered according to the standard dose and schedule for melanoma into all injectable tumors. This treatment consists of an initial dose of 1 to 4 mL of 106 pfu/mL followed 3 weeks later by doses of 1 to 4 mL of 108 pfu/mL at 2-week intervals.
Table I.
TVEC treatment outcomes
| Patient | Age (yr) | No. of TVEC injected lesions | Best overall response | Durable response (Y/N)∗ | PFS (mo)† | OS (mo)‡ |
|---|---|---|---|---|---|---|
| 1 | 87 | 8 | CR | Y | 24+ | 24+ |
| 2 | 77 | 4 | CR | Y | 19 | 28+ |
| 3 | 81 | 2 | CR | Y | 10+ | 10+ |
| 4 | 76 | 3 | CR | Y | 13+ | 13+ |
| Median | — | — | — | 16+ | 18.5+ |
RECIST 1.1 response persisting for at least 6 months.
PFS calculated from first dose of TVEC.
Overall survival calculated from first dose of TVEC.
Case 1
Complete resection of a right cheek MCC was followed by adjuvant radiotherapy. Biopsy confirmed regional recurrence 7 months later with 3 dermal nodules, and positron emission tomography/computed tomography (PET/CT) found a 9-mm hypermetabolic cutaneous nodule in the right cheek but no evidence of nodal or hematogenous metastases. Within 3 weeks, he had 8 palpable dermal metastases up to 1.4 cm widely distributed over the right side of the face from the orbital rim to the jaw. With the patient's consent, TVEC was administered on 4 occasions into all detectable metastases with toxicity limited to mild fatigue. Nine weeks after treatment initiation, he had a complete clinical response.8 Serial physical examinations and PET/CTs at 3- to 4-month intervals have found no recurrence for greater than 24 months since initiating TVEC therapy.
Case 2
An apical scalp MCC recurred 1 month after complete resection with palpable left postauricular and level IIA/B cervical lymphadenopathy up to 3 cm with PET standardized uptake values (SUVs) of 9.3 and 11.0, respectively, and no distant metastases. With the patient's consent, TVEC was administered into all palpable metastases on 8 occasions with toxicity limited to mild fatigue and transient nausea. Palpable, injectable disease resolved within 16 weeks, and a neck CT found a partial response by RECIST 1.1 criteria with 46% reduction in index lesions with new central necrosis. PET/CT 4 months later showed resolution of hypermetabolism and further reduction of index lesions by 62% from baseline.8
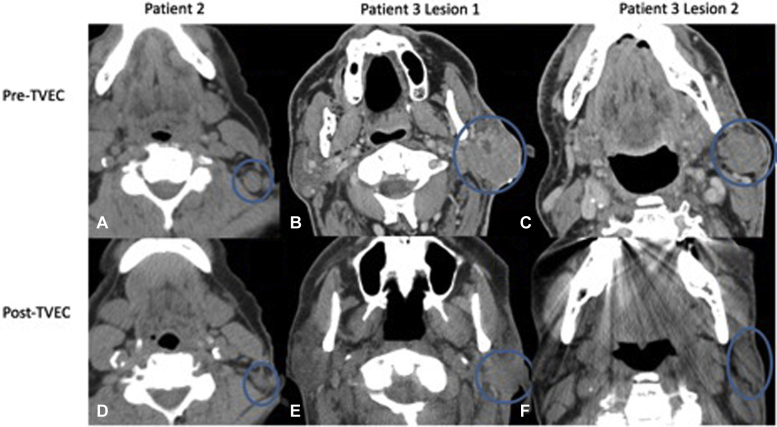
Fifteen months after TVEC discontinuation, the patient had a newly palpable, more anterior, left cervical node of 1.6 cm with maximum SUV of 10.7 (Fig 1). Needle biopsy confirmed MCC, and TVEC dosing was resumed. PET/CT 3 weeks after the seventh TVEC dose found shrinkage of the injected node to 1.1 cm with maximum SUV of 2.3 (Fig 1). TVEC was discontinued, and repeat PET/CT in April showed radiographic CR, which is ongoing more than 28 months after initiation of TVEC therapy.
Fig 1.
Top row represents pretreatment images including a PET/CT from patient 2 depicting the hypermetabolic left cervical node in (A) and a neck CT from patient 3 showing the left parotid and postauricular nodules (B and C, respectively). Bottom row provides comparable images soon after completing TVEC therapy from patient 2 (D) and patient 3 (E and F).
Case 3
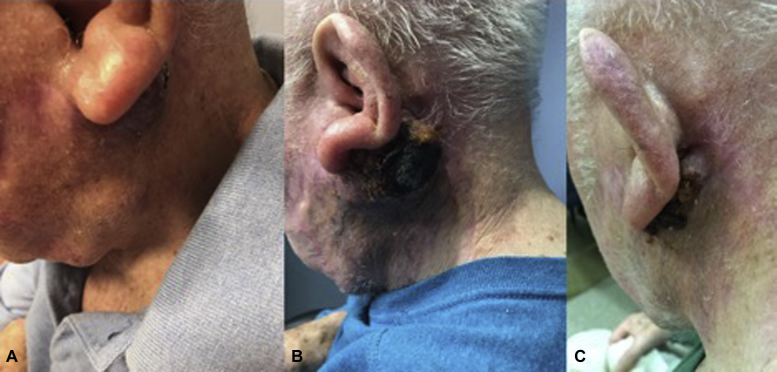
A left cheek MCC recurred 5 months after complete resection with neck CT showing bulky left parotid and left postauricular nodules 4.1 and 2.8 cm, respectively, with no distant metastases by chest and body CT (Fig 1). Biopsy confirmed surgically incurable MCC. With the patient's consent, TVEC was initiated, and the injected nodules transiently enlarged after the first 3 doses consistent with pseudoprogression but subsequently improved (Fig 2). After the seventh dose, a neck CT found the left parotid nodule had decreased to 2.9 cm and the postauricular nodule to 1.7 cm. An eighth, final dose of TVEC was administered followed 7 weeks later by biopsy of residual fullness in the left parotid region, which found fibroconnective tissue and plasma cells with no malignancy. Neck CT found resolution of the postauricular nodule to linear scarring (Fig 1). Toxicity consisted of mild, transient flu-like symptoms. Physical examinations and serial CT scans at 3-month intervals have shown no recurrence for more than 10 months after TVEC initiation.
Fig 2.
Photographs from patient 3 obtained immediately before TVEC initiation (A), with pseudoprogression after the third dose (B), and after dose 6 of 8 with marked improvement (C).
Case 4
A patient with Crohn's disease treated with infliximab converted to vedolizumab, a gut-selective anti-inflammatory agent, had a right anterior neck MCC. Six months after wide local incision, MCC recurred with 2 palpable, dermal nodules along the scar. CT scans of the chest and body found no distant spread. Before resection could be performed, a third palpable metastasis developed in the right neck. TVEC was initiated with the patient's consent into 3 tumor nodules measuring up to 1.2 cm. After 5 doses, a clinical CR was achieved. Toxicity was minimal with no activation of Crohn's disease, and the patient has maintained a CR with serial physical examinations and PET/CTs for more than 13 months since TVEC initiation.
Discussion
ICB of PD-1/PD-L1 interactions is the most effective approved therapy for surgically incurable MCC. However, CRs are rare, and most patients progress in less than a year. Here we report promising results with TVEC in 4 consecutive patients with recurrent regionally advanced MCC. Durable CRs were achieved in all 4 patients with a median PFS of greater than 16 months and no serious adverse events (Table I). In addition to complete regional response of injected tumors, TVEC has also prevented outgrowth of distant metastases in these high-risk individuals.
Our observations parallel the clinical trial experience with TVEC in melanoma showing greatest efficacy in stage IIIB/C patients with regionally advanced disease.10 Interim analysis of a randomized phase II trial of TVEC neoadjuvant treatment plus surgery versus surgery alone for resectable stage IIIB to IVM1a melanoma found a pathologic CR rate of 21% and an improved R0 resection rate from 41% to 56%.11 Phase II trials are underway examining TVEC monotherapy in surgically incurable MCC, basal cell carcinoma, and squamous cell skin cancer and TVEC with or without radiotherapy for regionally advanced MCC or melanoma (Table II). Until data are available from these prospective studies, our observations provide compelling evidence that TVEC has important clinical activity in this rare malignancy and suggests a potential role for TVEC in marginally resectable MCC worthy of prospective evaluation.
Table II.
Current trials of TVEC in cutaneous malignancies
| Treatment trial design | Cancer types | Indication | Clinical Trials.gov Registry no. |
|---|---|---|---|
| TVEC monotherapy | Phase II MCC, SCC, and BCC |
Locally advanced | NCT03458117 |
| TVEC ± radiotherapy | Phase II MCC and melanoma |
Locally advanced | NCT02819843 |
| TVEC + nivolumab | Phase II MCC, SCC, and BCC |
Locally advanced or widely metastatic | NCT02978625 |
| TVEC + pembrolizumab | Phase Ib/III melanoma |
Locally advanced or widely metastatic | NCT02263508 |
BCC, Basal cell carcinoma; SCC, squamous cell carcinoma.
Although single-agent TVEC has limited utility in stage IV melanoma with lung or visceral metastases, combining it with ipilimumab CTLA-4 ICB appeared to enhance the ORR and PFS compared with monotherapy with either agent.12 Similarly, the phase Ib portion of a phase Ib/III trial combining TVEC with pembrolizumab PD-1 ICB in advanced melanoma reported objective responses in 62% of patients with a CR rate of 33%, superior to either single agent.13 Based on these observations, a basket trial is evaluating the combination of TVEC with nivolumab in advanced or refractory nonmelanoma skin cancers including MCC (Table II).
The landscape of available cancer immunotherapeutics is evolving so rapidly that prospective evaluation of monotherapies and combinations in a malignancy as rare as MCC may not allow timely access to treatment. The combination of retrospective case series as provided here and extrapolation from similar, immune-responsive, more prevalent skin malignancies like melanoma may allow clinical implementation of important treatment advances years before prospective data can be obtained.
Footnotes
Funding sources: None.
Conflicts of interest: Dr Conry is a member of the speaker Bureau for Amgen, Inc. The rest of the authors have no conflicts to disclose.
References
- 1.Tothill R., Estall V., Rischin D. Merkel cell carcinoma: emerging biology, current approaches, and future directions. Am Soc Clin Oncol Educ Book. 2015:e519–e526. doi: 10.14694/EdBook_AM.2015.35.e519. [DOI] [PubMed] [Google Scholar]
- 2.Albores-Saavedra J., Batich K., Chable-Montero F., Sagy N., Schwartz A.M., Henson D.E. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol. 2010;37:20–27. doi: 10.1111/j.1600-0560.2009.01370.x. [DOI] [PubMed] [Google Scholar]
- 3.Feng H., Shuda M., Chang Y., Moore P.S. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319(5866):1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goh G., Walradt T., Markarov V. Mutational landscape of MCPyV-positive and MCPyV-negative Merkel cell carcinomas with implications for immunotherapy. Oncotarget. 2016;7:3403–3415. doi: 10.18632/oncotarget.6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palla A.R., Doll D. Immunotherapy in Merkel cell carcinoma: role of Avelumab. Immunotargets Ther. 2018;7:15–19. doi: 10.2147/ITT.S135639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan I.S., Bhatia S., Kaufman H.L., Lipson E.J. Immunotherapy for Merkel cell carcinoma: a turning point in patient care. J Immunother Cancer. 2018;6:23. doi: 10.1186/s40425-018-0335-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conry R.M., Westbrook B., McKee S., Norwood T.G. Talimogene laherparepvec: first in class oncolytic virotherapy. Hum Vacc Immunother. 2018;14(4):839–846. doi: 10.1080/21645515.2017.1412896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blackmon J.T., Dhawan R., Viator T.M., Terry N.L., Conry R.M. Talimogene laherparepvec for regionally advanced Merkel cell carcinoma: a report of 2 cases. JAAD Case Rep. 2017;3(3):185–189. doi: 10.1016/j.jdcr.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mascitelli L., Goldstein M.R. Do the immunosuppressive effects of statins increase Merkel cell carcinoma risk? Int J Dermatol. 2014;53:e406–e409. doi: 10.1111/ijd.12443. [DOI] [PubMed] [Google Scholar]
- 10.Harrington K.J., Andtbacka R.H., Collichio F. Efficacy and safety of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor in patients with stage IIIB/C and IVM1a melanoma: subanalysis of the Phase III OPTiM trial. Oncotargets Ther. 2016;9:7081–7093. doi: 10.2147/OTT.S115245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andtbacka R.H.I., Dummer R., Gyorki D. Interim analysis of a randomized, open-label phase 2 study of talimogene laherparepvec (T-VEC) neoadjuvant treatment (neotx) plus surgery (surgx) vs surgx for resectable stage IIIB-IVM1a melanoma (MEL) J Clin Oncol. 2018;36 (suppl; abstr 9508) [Google Scholar]
- 12.Chesney J., Puzanov I., Collichio F. Randomized, open-label phase II study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J Clin Oncol. 2018;36(17):1658–1667. doi: 10.1200/JCO.2017.73.7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ribas A., Dummer R., Puzanov I. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-pd-1 immunotherapy. Cell. 2017;170(6):1109–1119.e10. doi: 10.1016/j.cell.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]