Abstract
Introduction
To reassess the prevalence of fallopian tube endometriosis (EM), and its associated clinicopathologic characteristics and risk factors.
Methods
Cross-sectional study was conducted from June 2016 to August 2017. Unpregnant premenopausal women who underwent unilateral or bilateral salpingectomy due to gynecologic diseases were recruited. Patient clinical data and fallopian tube specimens were collected. Hematoxylin-eosin (H&E) staining and CD10 immunohistochemistry were used to diagnose tubal EM.
Results
Tubal EM prevalence was 14.48% (161/1112, 95% confidence interval [CI] 12.41%–16.55%). Prevalence of tubal EM in patients with EM was 37.37% (95%CI 30.58%–44.17%) which is higher in patients without EM (9.52%, 95%CI 7.61%–11.42%) and even higher in those with multi-organ EM (43.94%, 95%CI 35.36%–52.52%). At unilateral or bilateral salpingectomy, tubal EM was more likely located in the left fallopian tube (52.17%) than the right one (40.37%) and presence of hydrosalpinx/ hematosalpinx increased in women with tubal EM than without tubal EM (43.47% versus 23.79%). With increasing severity of pelvic EM (r = 0.26, P<10−4) and adhesion (r = 0.25, P<10−4), the tubal EM prevalence also increased. Pathological examination found that tubal EM was more likely located in the mucosa of the proximal tube with significantly more surrounding inflammation and fibrotic lesions than the serosa/sub-serosa in the distal tube (r = 0.90, P<10−4). Multivariate analysis showed that abnormal uterine bleeding (AUB) (AOR = 3.10), previous EM surgery (AOR = 4.22) and tubal ligation (AOR = 2.33) were risk factors for tubal EM.
Conclusions
These data provide clinicians with important information that the prevalence of tubal EM among premenopausal unpregnant patients was higher than previous investigators, especially higher among women with EM diseases. Identifying its clinicopathologic characteristics and predictors may facilitate clinical decision making.
Abbreviations: EM, endometriosis; AM, adenomyosis; TEM, tubal endometriosis; OEC, ovarian endometriotic cyst; DIE, deep infiltrating EM; AUB, Abnormal uterine bleeding; IHC, immunohistochemistry; SD, standard deviation; ORs, Odds ratios; CIs, confidence intervals; IUD, intrauterine device
Keywords: Cross-sectional study, Tubal endometriosis, Endometriosis, Endometriotic diseases, Prevalence
1. Introduction
Human oviducts or fallopian tubes-a pair of slender and bending muscular tubes—play a major role in the reproductive activities, including sperm storage, sperm capacitation, and the acrosome reaction. Fallopian tubes are also critical to fertilization, cleavage, and transportation of gametes and morulae. Thus, the fallopian tube is vital for early embryonic formation in the spontaneous conception [1,2]. The presence of endometrial tissues in the fallopian tubes is pathologic and considered to be tubal endometriosis (EM) regardless of its pathogenesis [3]; and patients may be asymptomatic or present with chronic pelvic pain, infertility, and/or dyspareunia [2,3]. Based on previous studies, tubal EM could be classified into three pathologic types, the most common of which is the invasion of the tubal serosa or subserosa but not the smooth muscle by the endometrium, which might coexist with pelvic EM. In some cases, the endometrium can also invade the tubal mucosa, which is considered a distinct pathogenesis. Tubal stump EM after salpingectomy is the third type of tubal EM, where endometrium might localize in any of the tubal layers [4].
There are currently few studies in the literature that concern the prevalence of tubal EM, and the results vary widely. In a study conducted at the Peking Union Medical College Hospital, 6 cases were diagnosed with tubal EM among 150 patients with EM, exhibiting a prevalence of 4% [5]. In 1986, Jenkins et al. reported that among 182 cases of female infertility combined with EM as diagnosed by laparoscopy, 1.6% and 4.3% of patients had tubal EM in the right and left fallopian tubes, respectively [6]. Tubal EM in the mucosa of the isthmus or ampulla occurred in approximately 10% of cases according to the study by Clement and associates in 2007 [7]. Moreover, 14.3% of patients who underwent salpingectomy because of proximal tubal obstruction were diagnosed with mucosal tube EM [8].
There have been numerous studies published recently that described the increase in the prevalence of endometriotic diseases [7]. However, investigators paid little attention to tubal EM. Some studies were retrospective and contained only a case report or a small sample size. Visual diagnosis of tubal EM is difficult and pathologic examination is essential for correct diagnosis and treatment, so, a retrospective study might lead to increased false-negative bias. We therefore conducted a cross-sectional study to clarify the prevalence of tubal EM among women who underwent salpingectomy for gynecologic diseases. We performed pathological examinations to identify tubal EM for accurate diagnosis, and correlated tubal EM with other endometriotic diseases. Futhermore, we report the clinicopathologic features and risk factors of tubal EM to shed light on this class of diseases.
2. Material and methods
2.1. Study design and population
All the study participants were provided with informed consent prior to data collection and were informed of their right to refuse the interview and withdraw from the study at any time. All subjects were also assured of the confidentiality of their information.
We conducted a cross-sectional study of a total of 1170 premenopausal women who underwent unilateral or bilateral salpingectomy in our department from June 2016 to August 2017 due to gynecologic diseases, of whom 1112 women were analyzed and 58 women with incomplete information were excluded. Pregnant women (ectopic or intrauterine pregnancy) and women who did not agree to participate in this study were excluded.
2.2. Outcome measures
Patient information, including preoperative baseline clinical characteristics (sociodemographic characteristics, reproductive history, gynecologic history, history of salpingectomy or ligation and contraception), indications for current gynecological surgery (pelvic EM, ovarian endometriotic cyst [OEC], deep infiltrating EM [DIE], uterine seromuscular EM, adenomyosis/adenomyoma [AM] and non-EM diseases) and intraoperative findings (degree of pelvic EM and adnexal adhesions, side of tubal EM and OEC) were collected. The evaluation of intraoperative findings was performed independently by two gynecologists. To analyze predictors for tubal EM, women were categorized to tubal EM group and Non tubal EM group according to the outcome of tubal EM. Preoperative baseline clinical data were used to identify risk factors.
Abnormal uterine bleeding (AUB) was diagnosed using the FIGO classification system [9]. The classification of pelvic EM (I, II, III, IV) and adnexal adhesions (0, 1, 2, 3) was according to the Revised American Society for Reproductive Medicine Classification of Endometriosis (r-AFS) [10] and the Hull and Rutherford (H&R) classifications [11].
The entire fallopian tube was collected for pathological examination. The diagnosis of tubal EM was confirmed by a final postoperative pathology study with hematoxylin and eosin (H&E) and CD10 immunohistochemistry (IHC) staining. Pathologic features of tubal EM were evaluated and classified in the same way as reported in previous studies (layers, portions and range) [12,13]. Based on the H&E slides, the degree of inflammatory infiltration could be assessed by the number of inflammatory cells either within or surrounding the endometrial lesions, and graded as extensive, moderate, mild, or absent [3]. The proliferation of fibrous connective tissues (fibroblasts and collagenous tissues surrounding the lesions), was also semi-quantified as absent, moderate, or extensive [3]. All assessments were performed independently by two experienced pathologists blinded to the source of the slides. Every diagnostic report of tubal EM was required to include the infiltrated layer, tubal segments, and the location (side) of the tubal EM.
2.3. Statistical methods
Data were analyzed with SAS software, version 9.2 (SAS 131 Institute, Inc., Cary, NC, USA). Prevalence of tubal EM outcomes in the study population was calculated with 95% confidence intervals (CIs) based on a binomial distribution. Proportions and means ± standard deviation (SD) were calculated. Differences between groups were detected using Pearson’s chi-square test (correction for continuity and the Fisher exact-probability test were used as appropriate) or Student’s t test. To predict the factors associated with tubal EM, multivariate logistic regression analysis was conducted by backward selection. Odds ratios (ORs) and their 95% CIs were calculated and adjusted for potential risk factors, including baseline socio-demographic characteristics (age, BMI), gynecologic history (AUB, previous EM-related surgery, intrauterine device (IUD) use, or previous tubal ligation). Correlation analysis was used to examine the associations between tubal EM and classification of pelvic EM or adnexal adhesions. To calculate the sample size, we conducted a pilot study and estimated the prevalence of tubal EM as 12%, δ as 2.0%, and CI as 0.95. The estimated sample size was therefore 1063 cases. Anticipating lost-to-follow up, an additional 10% was needed. Thus, 1170 women need to be included.
2.4. Ethical approval
The study protocol was approved by the Institutional Review Board of the International Peace Maternity and Child Health Hospital, Shanghai, China. The approval number is (GKLW) 2016-42.
3. Results
3.1. Risk factors of tubal EM
The age between tubal EM group and Non-tubal EM was 44.89 ± 6.00 (mean ± SD) and 45.90 ± 5.97 years, respectively (P=0.02). No difference was detected between groups for BMI, marital status, birthplace, educational attainment, individual annual income, smoking status, previous abortions, parity, cesarean section, pelvic inflammatory disease, and previous IUD use (Table 1). However, the prevalence of AUB, previous EM surgery, and previous tubal ligation among women with tubal EM was significantly higher compared with the Non-tubal EM group. Among 69 patients who underwent previous surgery for EM, 31 patients presented with tubal EM (44.93%), and 20 had multi-organ EM. Eighteen of 78 women (23.08%) who underwent ligation were diagnosed with tubal EM. Among these 18 women with post-ligation tubal EM, 14 cases with positive lesions showed involvement of the proximal mucosa and 4 cases of the distal serosa. Furthermore, 14 cases presented with Non-EM diseases, two had adenomyosis, and two had multi-organ EM. Multivariable analysis showed that AUB (AOR = 3.10; 95% CI, 2.06–4.66) (P<10−4), previous EM surgery (AOR = 4.22; 95% CI, 2.49–7.13) (P<10−4) and tubal ligation (AOR = 2.33; 95% CI, 1.30–4.18) (P<10-2) had higher rate of tubal EM.
Table 1.
Baseline clinical characteristics of tubal EM and Non tubal EM.
| Variables | Non Tubal EM (n=951) |
Tubal EM (n=161) |
P value | ||
|---|---|---|---|---|---|
| n a | % | n a | % | ||
| Socio-demographic characteristics | |||||
| Age (years) (mean±SD) | 45.90 ± 5.97 | 44.89 ± 6.00 | 0.02 | ||
| BMI (kg/m2) (mean±SD) | 23.01 ± 3.17 | 22.61 ± 2.99 | 0.09 | ||
| Marital status | |||||
| Married | 885 | 93.05 | 145 | 90.06 | 0.18 |
| Unmarried | 66 | 6.95 | 16 | 9.94 | |
| Birth place | |||||
| Shanghai | 625 | 65.72 | 111 | 68.94 | 0.42 |
| Out of Shanghai | 326 | 34.28 | 50 | 31.06 | |
| Educational attainment | |||||
| Primary school or lower | 223 | 23.45 | 37 | 22.98 | 0.99 |
| Middle school | 104 | 10.94 | 17 | 10.56 | |
| High school | 142 | 14.93 | 23 | 14.29 | |
| University or above | 482 | 50.68 | 84 | 52.17 | |
| Individual annual income (¥) | |||||
| <50,000 | 257 | 27.02 | 46 | 28.57 | 0.89 |
| 50,000-100,000 | 456 | 47.95 | 77 | 47.83 | |
| >100,000 | 238 | 25.03 | 38 | 23.60 | |
| Smoking status b | |||||
| Non-smoker | 890 | 93.58 | 151 | 93.79 | 0.99 |
| Occasional smoker | 48 | 5.05 | 8 | 4.97 | |
| Regular smoker | 13 | 1.37 | 2 | 1.24 | |
| Reproductive history | |||||
| Previous abortions (mean ± SD) | 1.05 ± 1.01 | 0.98 ± 0.81 | 0.38 | ||
| Parity (mean ± SD) | 1.13 ± 0.73 | 1.24 ± 0.78 | 0.28 | ||
| Cesarean section | 214 | 22.50 | 44 | 27.33 | 0.18 |
| Gynecologic history | |||||
| Abnormal uterine bleeding c | 476 | 50.05 | 126 | 78.26 | <10-3 |
| Previous confirmed endometriosis d | 38 | 2.52 | 31 | 14.29 | <10-3 |
| Pelvic inflammatory disease | 76 | 7.99 | 14 | 8.70 | 0.76 |
| Previous tubal ligation | 60 | 6.31 | 18 | 11.18 | 0.02 |
| Previous IUD use | 80 | 8.41 | 10 | 6.21 | 0.34 |
EM:endometriosis; SD:standard deviation; BMI:Body Mass Index.
The sum does not necessarily equal the sample size for all variables because of missing data.
Occasional smoker:cigarette smoking more than 4 times a week, but a day on average less than 1 cigarette. Regular smoker:cigarette smoking more than 1 cigarettes per day, continuous or accumulated 6 months.
Abnormal uterine bleeding was diagnosed using the FIGO classification system [9].
It was confirmed by previous operation pathology.
3.2. Prevalence of tubal EM
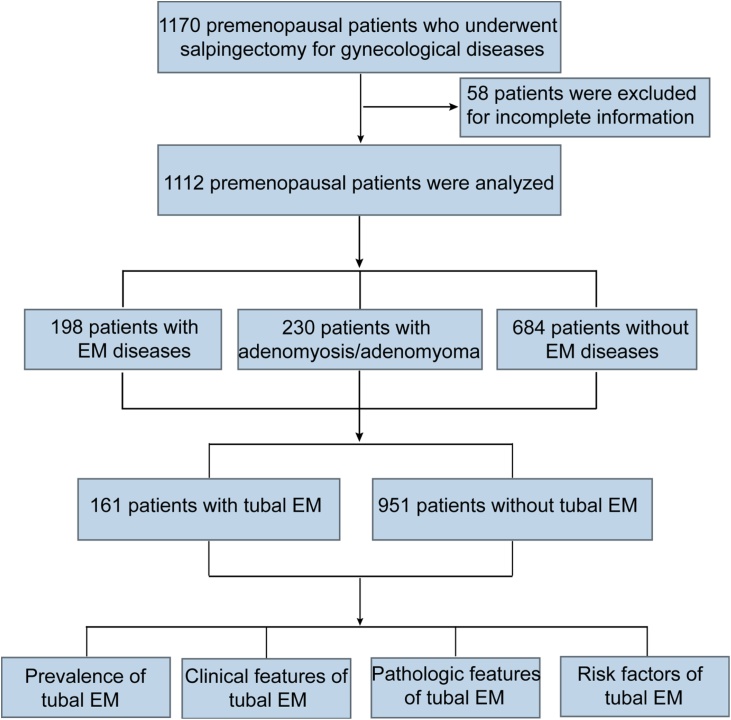
A total of 1170 premenopausal patients was enrolled in the present study, and of them, 58 were excluded due to incomplete information; thus 1112 premenopausal women were analyzed. Based on the surgical findings and pathologic results, 198 patients were diagnosed with EM diseases, 684 cases with non-EM diseases, and 230 women with adenomyosis/adenomyoma. A total of 161 patients were confirmed to have tubal EM (Fig. 1), and its prevalence among premenopausal women was 14.48% (95%CI 12.41%–16.55%).
Fig. 1.
Flow chart of study participants.
As shown in Table 2, the prevalence of tubal EM was higher among the women with EM diseases (37.37%, 95%CI 30.58%–44.17%), compared to those without EM (9.52%, 95%CI 7.61%–11.42%) and AM (7.83%, 95%CI 4.33%–11.32%). In addition, the prevalence of tubal EM among women with pelvic multi-organ EM (43.94%, 95%CI 35.36%–52.52%) was significantly higher than that women with single-organ EM (24.24%, 95%CI 13.63%–34.86%). Peak prevalence occurred in cases with uterine seromuscular EM combined with DIE (84.21%, 95%CI 72.06%–96.36%), or combined with DIE and hydrosalpinx/ hematosalpinx (100%).
Table 2.
The prevalence of tubal EM among premenopausal women with indications for gynecological surgery.
| Indications for gynecological surgery | Total | Tubal EM |
|
|---|---|---|---|
| n (%) | 95% CI | ||
| Uterine fibroid | 308 | 26 (8.44) | [5.32, 11.56] |
| Ovarian simplex cyst/mullerian duct cyst | 52 | 2 (3.85) | [-1.56, 9.25] |
| Salpingitis infertility | 4 | 0 (0.00) | / |
| Hydrosalpinx | 134 | 26 (19.40) | [12.62, 26.19] |
| Gynecological malignant tumor | 178 a | 15 (8.43) b | [4.31, 12.55] |
| Elective tubal sterilization | 8 | 0 (0.00) | / |
| Adenomyosis/Adenomyomac | 230 | 18 (7.83) | [4.33, 11.32] |
| EM diseases | 198 | 74 (37.37) | [30.58, 44.17] |
| Single-organ EM | 66 | 16 (24.24) | [13.63, 34.86] |
| Pelvic EM | 12 | 2 (16.67) | [-8.07, 41.40] |
| OEC | 48 | 12 (25.00) | [12.29, 37.71] |
| Uterine seromuscular EM | 6 | 2 (33.33) | [-20.86, 87.53] |
| Multi-organ EMd | 132 | 58 (43.94) | [35.36, 52.52] |
| OEC + pelvic EM + DIE | 70 | 26 (37.14) | [25.54, 48.75] |
| OEC + hydrosalpinx | 44 | 20 (45.45) | [30.14, 60.77] |
| OEC + uterine seromuscular EM | 32 | 16 (50.00) | [31.68, 68.32] |
| Pelvic EM + hydrosalpinx | 48 | 24 (50.00) | [35.33, 64.67] |
| Uterine seromuscular EM + DIE | 38 | 32 (84.21) | [72.06, 96.36] |
| Uterine seromuscularEM + DIE + hydrosalpinx | 14 | 14 (100.00) | / |
CI:confidence interval; EM:endometriosis; EM diseases:endometriotic diseases; OEC:ovarian endometriotic cyst; DIE:Deep infiltrating endometriosis.
There are 188 women with malignant tumor. Among them, ten patients had concurrent endometriotic diseases: One patient with malignant ovarian neoplasms was counted as "gynecological malignant tumor"; Four cases with ovarian endometriotic cyst and combined cervical cancer were considered as "OEC"; Four patients and two patients with adenomyosis had cervical cancer and endometriotial cancer respectively, and they were all counted as "adenomyosis".
Six patients underwent ligation previously.
Women with adenomyosis/adenomyoma combined with EM diseases were all counted as EM diseases.
Women with multi-organ EM may be counted repeatedly, thus the sum of the number of patients in each subgroup was more than 132.
3.3. Clinical features of tubal EM
As for the distribution of OEC, tubal EM was more likely to be located on the left side (52.17%) than the right side (40.37%) (P < 0.05) (Table 3). Among 54 patients with OEC concurrent with tubal EM, 40.74% (22/54) patients exhibited left OEC, 29.63% (16/54) cases presented with right OEC, and another 29.63% (16/54) of women had bilateral OEC. Participants with left-side or right-side OEC exhibited a similar incidence of concurrent ipsilateral tubal EM (P > 0.05) (Table 3).
Table 3.
Distribution side of ovarian endometriotic cysts and tubal EM.
| Side of OEC |
||||||||
|---|---|---|---|---|---|---|---|---|
| Side of tubal EM | Tubal EM (N = 161) |
Left OEC (N = 70) |
Right OEC (N = 53) |
Bilateral OEC (N = 34) |
||||
| n | % b | n | % b | n | % b | n | % b | |
| Left tubal EM | 84a | 52.17 | 21 | 30.00 | 0 | 0.00 | 8 | 23.53 |
| Right tubal EM | 65 | 40.37 | 0 | 0.00 | 16 | 30.19 | 6 | 17.65 |
| Bilateral tubal EM | 12 | 7.45 | 1 | 1.43 | 0 | 0.00 | 2 | 5.88 |
| Total Tubal EM | 161 | 100.00 | 22 | 31.43 | 16 | 30.19 | 16 | 47.06 |
EM:endometriosis; OEC:ovarian endometriotic cyst.
Tubal EM is more likely to be located in left fallopian tube than right one (Left tubal EM compared with Right tubal EM) (P < 0.05).
The percentage was calculated using the formula n/N.
The prevalence of hydrosalpinx/hematosalpinx diagnosed during operation was 43.48% (70/161) among women with tubal EM, significantly higher than that women without tubal EM (13.25%, 126/951) (P<10−4). Among patients with tubal EM and hydrosalpinx/hematosalpinx, 62.86% cases exhibited positive lesions located in the mucosal layer, 25.71% in the serosa, 8.57% co-located in mucosal and serosal layers, and 2.86% in the seromuscular layer. Furthermore, 78% of patients suffered from prolonged menstruation and ovulatory bleeding; however, we could not analyze the prevalence of infertility as those patients did not desire fertility.
With increasing severity of pelvic EM, the prevalence of tubal EM also increased (I, 0%; II, 26.09%; III, 35.00%; IV, 39.62%) (r = 0.26, P<10−4). The prevalence also increased with the Hull and Rutherford score of adnexal adhesion (0, 7.67%; I, 23.88%; II, 28.96%; III, 27.27%) (r = 0.25, P<10−4).
3.4. Pathologic features of tubal EM
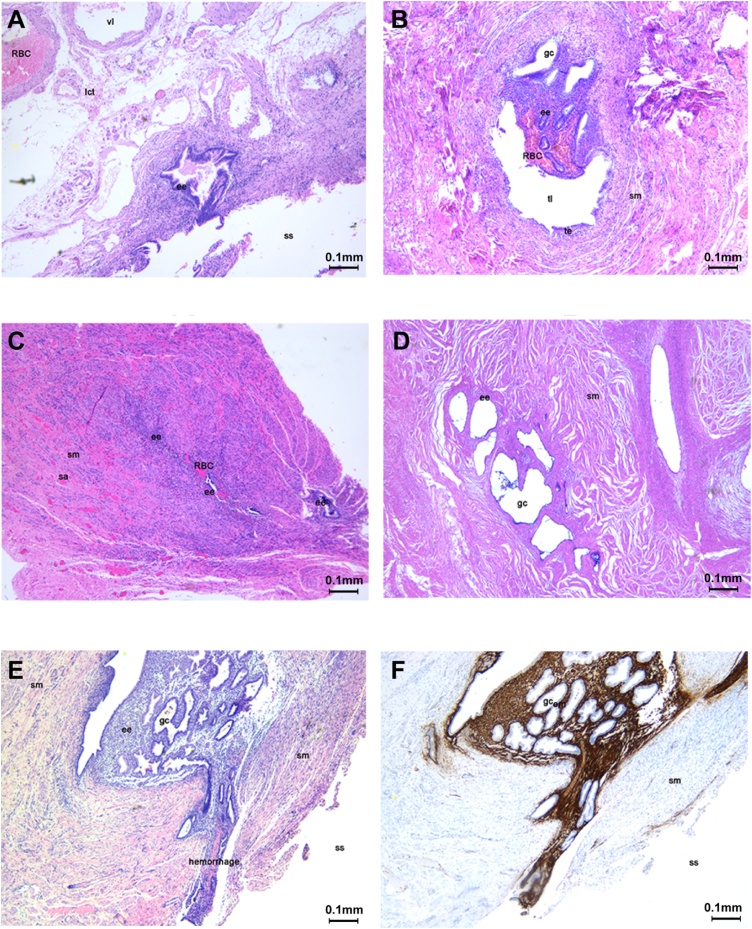
Fig. 2 illustrates the histopathologic types of tubal EM. The ectopic endometrium was located in the subserosa (A), mucosa (B) and muscular layer (C). As the diseases progresses, the morphology of the fallopian tube was damaged, and the tubal lumen disappeared (D). The ectopic endometrium located in the tubal serosa also infiltrated the subserosa layer (E) and CD10 decorating endometrial stromal cells helped confirm the diagnosis (Fig. 2F).
Fig. 2.
Histopathologic types of tubal EM.
RBC = red blood cell, vl = vessel lumen, lct = loose connective tissue, ee = ectopic endometrium, ss = serous surface, sm = smooth muscle, tl = tubal lumen, te = tubal endometrium, sa = small artery, gc = glandular cavity.
The ectopic endometrium was located in the subserosa and surrounded by loose connective tissue and small arteries (A). Half of the tubal mucosa was replaced by endometrium that grew into the tubal lumen accompanied by bleeding in the endometrial stroma (B). The ectopic endometrium was located in the thick muscular layer of the tubal isthmus and showed bleeding (C). Several glandular cavities were formed by the ectopic endometrium; and the endometrium was atrophic and surrounded by interstitial fibrosis. The morphology of the fallopian tube was damaged, and the tubal lumen disappeared (D). The ectopic endometrium located in the tubal serosa also infiltrated the subserosa layer (E). The immunohistochemical staining of CD10 positive expression in endometrial stromal cells confirmed the endometriotic nature of the tissue (F).(scale bar: 0.1 mm).
Table 4 describes the pathologic features of tubal EM. Regarding endometrial infiltration of different portions of the fallopian tube, ectopic focus involvement in proximal regions of the tube were comparable to those of the distal tube. With respect to different layers of tubal tissues, ectopic lesions were commonly involved in the mucosa (54.66%) and serosa (32.30%). Furthermore, there was an association between ectopic endometrial tissue that involved different portions and layers of the tubes (r = 0.90, P<10−4). Greater than 80% of the proximal tubal lesions were located in the mucosa, while 53.85% of distal tube ectopic endometrium was located in serosa. Massive inflammatory reactions and extensive proliferation of fibroblasts and collagenous tissues surrounding the lesions were more common in serosal lesions than in mucosal EM (28.85% versus 11.36% and 34.62% versus 23.86%, respectively).
Table 4.
Pathologic features of tubal EM.
| Layers |
||||||
|---|---|---|---|---|---|---|
| Pathologic features | Total | Mucosa | Myosalpinx | Serosa | Mucosa + serosa | P valuec |
| n (%)a | n (%) | n (%) | n (%) | n (%) | ||
| Total | 161 (100.00) | 88 (54.66) | 10 (6.21) | 52 (32.30) | 11 (6.83) | |
| Portions of tubal EM | ||||||
| Proximal tubes | 78 (48.45) | 64 (82.05)b | 4 (5.13)b | 8 (10.26)b | 2 (2.56)b | <10−3 |
| Distal tubes | 78 (48.45) | 24 (30.77)b | 6 (7.69)b | 42 (53.85)b | 6 (7.69)b | |
| Proximal & distal tubes | 5 (3.10) | 0 (0.00)b | 0 (0.00)b | 2 (40.00)b | 3 (60.00)b | |
| Local inflammation | ||||||
| Mild or absent | 105 (65.22) | 60 (68.18) | 8 (80.00) | 30 (57.69) | 7 (63.64) | <10−3 |
| Moderate | 29 (18.01) | 18 (20.46) | 2 (20.00) | 7 (13.46) | 2 (18.18) | |
| Extensive | 27 (16.77) | 10 (11.36) | 0 (0.00) | 15 (28.85) | 2 (18.18) | |
| Fibrosis | ||||||
| Absent | 68 (42.23) | 45 (51.14) | 2 (20.00) | 16 (30.76) | 5 (45.46) | <10−3 |
| Moderate | 51 (31.68) | 22 (25.00) | 8 (80.00) | 18 (34.62) | 3 (27.27) | |
| Extensive | 42 (26.09) | 21 (23.86) | 0 (0.00) | 18 (34.62) | 3 (27.27) | |
The percentage of different portions of tubal EM (on the base of total 161).
The percentage of different layers among different portions.
Pearson’s chi-square test or Fisher's Exact Test.
4. Discussion
The risk factors identified in our study may facilitate patient risk prediction. Consistent with prior studies [4,14,15], we found previous ligation was closely correlated to tubal EM. Tubal EM could be found in 20–50% of the residual tubes following ligation or post salpingectomy [14], and might be associated with salpingitis isthmica nodosa. The lesion is analogous to uterine AM, consisting of endometrial glands and stroma extending from the endosalpinx into the myosalpinx and frequently to the serosal surface [4]. AUB and previous EM surgery were also risk factors. And converting knowledge on these risk factors into effective decisions may be one meaningful area of explore.
In the study, the prevalence of tubal EM among premenopausal unpregnant patients with gynecologic diseases was 14.48%(95%CI 12.41%–16.55%), distinctly higher than that reported by previous investigators [[4], [5], [6], [7]], especially higher among women with EM diseases (37.37%, 95%CI 30.58%–44.17%) compared to those with Non-EM (9.52%, 95%CI 7.61%–11.42%) or AM (7.83%, 95%CI 4.33%–11.32%), and even higher in those with multi-organ EM (43.94%, 95%CI 35.36%–52.52%) compared to single-organ EM (24.24%, 95%CI 13.63%–34.86%). During operation, we found that tubal EM was more likely to invade the left fallopian tube (52.17%) rather than the right tube (40.37%) (P < 0.05). And, women with tubal EM were more likely to suffer from hydrosalpinx/hematosalpinx, as the prevalence of hydrosalpinx/hematosalpinx diagnosed during operation was 43.48% among women with tubal EM, significantly higher than that women without tubal EM (13.25%, 126/951) (P<10−4). Moreover, the prevalence of tubal EM increased with advanced degree of pelvic EM (I, 0%; II, 26.09%; III, 35.00%; IV, 39.62%) (r = 0.26, P<10−4) and adnexal adhesions (0, 7.67%; I, 23.88%; II, 28.96%; III, 27.27%) (r = 0.25, P<10−4). Pathological examination showed the ectopic lesions were most commonly located in the mucosa of the proximal tube (>80%), but tended to be in the serosa/subserosa of the distal tube (53.85%). Furthermore, significantly more surrounding inflammation and fibrotic lesions was observed with tubal EM of the serosa than in the mucosal type (28.85% versus 11.36% and 34.62% versus 23.86%, respectively).
The inconsistency in the prevalence of tubal EM between the present study and previous studies might be attributed to the following three reasons: first, the lack of awareness about tubal EM, second, the participants enrolled in our study were those with benign gynecological disease, third, the prevalence of tubal EM has increased with the ever rising incidence of EM recently. The most common form of EM is involved in the tubal serosa or subserosa. Most cases were associated with EM elsewhere in the pelvis, which can be considered an extension of pelvic EM to the peritoneal surface of the fallopian tubes [14,16]. The current study showed that with respect to serosal or seromuscular tube EM, 2/3 of cases were associated with EM elsewhere in the pelvis, which was significantly higher than that for the mucosal tube EM (1/3). The study also showed that a higher prevalence of tubal EM in the EM group especially higher among women with multi-organ EM diseases indicated that aside from tubal EM can be thought an extension of pelvic EM, on the other hand, it might be a specific type of EM with unique pathogenesis. Therefore, it is of great importance to explore tubal EM deeper in the further researches.
In addition, mucosal tube EM may be different from the other types of tubal EM. Presently, there is no consensus as to whether mucosal tube EM can be treated as a physiologic phenomenon. Whether the ectopic endometrial tissue is developmental, metaplastic, or a result of the direct or embolic spread of eutopic endometrium or replacement for tubal epithelium is yet to be clarified [3,17,18]. Lesions located in the mucosa of the tubal isthmus have been described as “endometrial colonization” to stress the different nature of this lesion compared to typical EM [3]. However, if the endometrium grows into the tubal cavity and leads to blockage, endometrial tissue within the menstrual flow might seed in the proximal end of the oviduct, leading to a vicious cycle and ultimately resulting in tubal EM. The ectopic endometrium may result in a variety of lesions, including endometrial-type polyps, tubal adenomyosis (analogous to salpingitis isthmica nodosa), and intraluminal endometriosis with occlusion of one or both tubal lumens; the latter accounting for about 10–15% of proximal tubal occlusion and tube-related infertility [[17], [18], [19], [20], [21]]. We reported in our previous studies that AM and EM without tubal EM did not affect tubal function. However, tubal EM resulted in a lower ciliary-beat frequency, lower ciliated-cell percentage, weaker muscular contraction amplitude, and lower contraction frequency [22]. These results showed that ectopic endometrium involving the fallopian tube may be one of the primary pathologic factors affecting the tubal epithelium and muscle and tubal transport functions.
Hormonal and immune factors conspire to modulate local inflammatory microenvironment and promote two cardinal symptoms, pain and infertility. Inflammation is considered a characteristic element of endometriosis, and tubal EM is no exception. In synchrony with menstruation, the ectopic lesions also bleed and proliferate periodically. Over time, an aberrant inflammatory reaction results in morphologic damage to the fallopian tubes [22]. Other classes of biochemical factors secreted by endometriotic lesions or neighboring peritoneal cells could also contribute to the adhesions that classically surround the implants and adjoining pelvic organs. As the disease progresses, hydrosalpinx/hematosalpinx may develop from fibrosis and scar formation. Furthermore, we found that inflammatory reactions and fibrotic lesions were more common with EM in the tubal serosa than in the mucosa. These factors might play an important role in the pathogenesis of tubal EM and explain the incidence of tubal EM among women with pelvic EM diseases, which was significantly higher than in those women without EM diseases; and tubal EM increased concomitantly with the increase in EM classification or Hull and Rutherford score.
However, the present study also has several limitations. First, women with gynecologic diseases who required salpingectomy were included in the current study, which could lead to selection bias. Obtaining the prevalence of tubal EM in the general population is difficult as its diagnosis depends pathology examination and is difficult to recognize visually. Our results might not be generalized to other women of reproductive age. The diagnosis of tubal EM in tubal pregnancy is also challenging because the ectopic gestational sac often damages the oviduct severely. Thus, the prevalence of tubal EM among women with tubal pregnancy is unknown. Additionally, a majority of the subjects recruited to the present study were over 40 years of age and had no desire for fertility. Consequently, the relationship between tubal EM and tubal infertility need yet to be elucidated. Questions requiring more investigations include whether all types of tubal EM influence tubal functions; how different kinds of tubal EM affect the internal tubal environment, tubal muscular motility, tubal ciliary movement, and reproductive function; which type of tubal EM leads to tubal fibrosis and has a maximal impact on tubal function; and how to recover reproductive function once damaged by tubal EM.
5. Conclusions
The prevalence of tubal EM among premenopause patients with gynecologic diseases (except for pregnancy) and required salpingectomy was distinctly higher than that reported previously. And, women with multi-organ EM diseases were also more likely to present with tubal EM compared to those with single-organ EM or without EM diseases. Thus, this study contributed new insights to the prevalence of tubal EM and we need to raise our awareness about tubal EM. And, further studies are warranted to elucidate the tubal function of various types of tubal EM.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Funding information
This work was supported by a grant from the Shanghai Jiao Tong University School of Medicine (grant number YG2016ZD07).
References
- 1.Daftary S., Chakravarti S. 3rd edn. Elsevier; 2011. Manual of obstetrics; pp. 1–16. ISBN 9788131225561. [Google Scholar]
- 2.Wang C., Liu Y., Chang C., Wu S., Gao J., Zhang Y. Human fallopian tube proteome shows high coverage of mesenchymal stem cells associated proteins. Biosci Rep. 2016;36 doi: 10.1042/BSR20150220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tao X., Xie Y., Wang L., Gu W., Yu X., Zhou X. The expression of Cox-2, NF-κB, and VEGF in ectopic endometrial tissues with fallopian tubes suggests different etiologies. Int J Gynecol Pathol. 2014;33:411–417. doi: 10.1097/PGP.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 4.Irving Julie A., Clement Philip B. Blausteinâ’s pathology of the female genital tract. 6th edn. Springer Verlag; New York: 2011. Diseases of the peritoneum’; pp. 625–678. [Google Scholar]
- 5.Lian Z.J., Lin Q.Z. 2nd ed. People’s Medical Publishing House; Beijing: 1996. Gynecological oncology; p. 763. [Google Scholar]
- 6.Jenkins S., Olive D.L., Haney A.F. Endometriosis: pathogenetic implications of the anatomic distribution. Obstet Gynecol. 1986;67:335–338. [PubMed] [Google Scholar]
- 7.Clement P.B. The pathology of endometriosis: a survey of the many faces of a common disease emphasizing diagnostic pitfalls and unusual and newly appreciated aspects. Adv Anat Pathol. 2007;14:241–260. doi: 10.1097/PAP.0b013e3180ca7d7b. [DOI] [PubMed] [Google Scholar]
- 8.Fortier K.J., Haney A.F. The pathologic spectrum of uterotubal junction obstruction. Obstet Gynecol. 1985;65:93–98. [PubMed] [Google Scholar]
- 9.Munro M.G., Critchley H.O., Broder M.S., Fraser I.S., FIGO Working Group on Menstrual Disorders System FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynecol Obstet. 2011;113:3–13. doi: 10.1016/j.ijgo.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 10.American Society for Reproductive Medicine Revised American Society for Reproductive Medicine classification of endometriosis. Fertil Steril. 1996;67(5):817–821. doi: 10.1016/s0015-0282(97)81391-x. 1997. [DOI] [PubMed] [Google Scholar]
- 11.Rutherford A.J., Jenkins J.M. Hull and Rutherford classification of infertility. Hum Fertil (Camb) 2002;5(Suppl. 1):S41–S45. doi: 10.1080/1464727022000199911. [DOI] [PubMed] [Google Scholar]
- 12.Kurman Robert J., Ronnett Brigitte M. sixth edition. Springer; 2019. Lora Hedrick Ellenson. Blaustein’s pathology of the female genital tract. [Google Scholar]
- 13.Groisman G.M., Meir A. CD10 is helpful in detecting occult or inconspicuous endometrial stromal cells in cases of presumptive endometriosis. Arch Pathol Lab Med. 2003;127:1003–1006. doi: 10.5858/2003-127-1003-CIHIDO. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X.M., Shi Y.F., Lin J. Analysis of 21 cases of tubal endometriosis. Prog Obstet Gynecol. 2002;11:265–267. [Google Scholar]
- 15.Maker A.P.1, Keersmaekers G.H., Vanderheyden J.S., Hänsch C. Development of endosalpingoblastosis and tuboperitoneal fistulas following tubal sterilization: relation with uterine adenomyosis. Eur J Obstet Gynecol Reprod Biol. 1993;52:187–191. doi: 10.1016/0028-2243(93)90070-s. [DOI] [PubMed] [Google Scholar]
- 16.Sheldon R.S., Wilson R.B., Dockerty M.B. Serosal endometriosis of the fallopian tubes. Am J Obstet Gynec. 1967;99:882–884. doi: 10.1016/0002-9378(67)90406-1. [DOI] [PubMed] [Google Scholar]
- 17.Lisa J.R., Gioia J.D., Rubin J.C. Observations on othe interstitial portion of the fallopian tube. Surg Gynecol Obstet. 1954;99:159–169. [PubMed] [Google Scholar]
- 18.Rubin I.C., Lisa J.R., Trinidad S. Further observations on ectopic endometrium of the fallopian tube. Surg Gynecol Obstet. 1956;103:469–474. [PubMed] [Google Scholar]
- 19.Fortier K.J., Haney A.F. The pathologic spectrum of uterotubal junction obstruction. Obstet Gynecol. 1985;65:93–98. [PubMed] [Google Scholar]
- 20.Zhang D., Zeng Y., Chen X. Pathological findings of proximal tubal occlusive infertility. Zhonghua Fu Chan Ke Za Zhi. 1995;30:352–355. [PubMed] [Google Scholar]
- 21.Kissler S., Hamscho N., Zangos S., Gätje R., Müller A., Rody A. Diminished pregnancy rates in endometriosis due to impaired uterotubal transport assessed by hysterosalpingoscintigraphy. BJOG. 2005;112:1391–1396. doi: 10.1111/j.1471-0528.2005.00676.x. [DOI] [PubMed] [Google Scholar]
- 22.Xia W., Zhang D., Ouyang J., Liang Y., Zhang H., Huang Z. Effects of pelvic endometriosis and adenomyosis on ciliary beat frequency and muscular contractions in the human fallopian tube. Reprod Biol Endocrinol. 2018;16:48. doi: 10.1186/s12958-018-0361-y. [DOI] [PMC free article] [PubMed] [Google Scholar]