Abstract
Objective
To explore the role of VEGF in attenuating endoplasmic reticulum stress in placental trophoblast cells.
Study design
Study was divided into following parts: 1. Serum Analysis of GRP78 and VEGF using sandwich ELISA. 2. Expression of VEGF and GRP78 in placentae by immunohistochemistry (IHC). 3. In Vitro experiments. Status of ER stress markers (GRP78, eIF2α, XBP1, ATF6 and CHOP) was assessed at various time points (8 h, 14 h, 24 h) when trophoblast cells were treated with varying concentration(s) of VEGF and also by adding recombinant VEGF at protein (Immunofluorescence, Western blot) and transcript levels (qRT-PCR).
Results
Increased GRP78 and decreased VEGF protein levels in sera and placentae of preeclamptic pregnant women and reduced expression of various ER stress markers at both transcript and protein levels was observed in trophoblast cells when they were exposed to recombinant VEGF thereby indicating positive role of VEGF in alleviating ER stress.
Conclusions
Reduced expression of ER stress markers in trophoblast cells against increased VEGF highlighted a new window to explore prospective drugs that can be designed to modulate the activities of various ER stress sensors in order to alleviate ER stress in pregnant women with preeclampsia.
Keywords: Preeclampsia, UPR, ER stress, VEGF
Introduction
Adequate angiogenesis and subsequent vascularization of a tissue is a vital step for cellular functions to satisfy energy requirements. Mammalian placentation requires extensive angiogenesis for the establishment of an appropriate vascular network to supply oxygen and nutrients to the growing fetus [1,2]. Vascular endothelial growth factor (VEGF) is believed to play a critical role in development of normal placental vasculature by binding to their receptors VEGFR-1 and VEGFR-2 [3]. The decrease in circulating VEGF and concomitant increase in its soluble receptor sVEGFR1 (sFlt-1) has been an established hallmark of preeclampsia (PE) [4,5]. The vascular transformation following invading foetal cells (extravillous cytotrophoblast) is incomplete in preeclamptic pregnancies unlike normal ones. There is ample evidence to prove that circulating VEGF is bound to the excess soluble fms-like tyrosine kinase- 1 (sFlt-1) that is produced by the preeclamptic placenta [6]. The stress which placenta experiences in PE has been variously categorized as oxidative stress, endoplasmic reticulum stress, and immunological stress [7]. Oxidative stress in preeclamptic placenta is induced due to hypoxia–reperfusion insult developed at maternal fetal interface, also activates endoplasmic reticulum (ER) stress [8,9]. Such stress disrupts homeostasis of cellular ambience and leads to the accumulation of unfolded or mis-folded proteins in the ER lumen. These alterations in homeostasis lead to activation of multiple signaling cascades comprising three transmembrane sensors, collectively known as unfolded protein response (UPR) [10]. The UPR ventures to restore ER function by attenuating protein translation and increasing folding capacity [11]. Failure of UPR to withstand such restoration of ER functions leads to individual ER stress pathways comprising PERK (PKR-like ER kinase), IRE1 (inositol-requiring protein 1) and ATF6 (activated transcription factor 6) arms [12].
Preeclampsia is a leading cause of maternal mortality and morbidity and accounts for nearly 5–8% of maternal deaths [13,14]. The prevalence and associated adverse outcomes are more so in developing (India) and under developed countries. Being an important angiogenic player, VEGF may influence multiple endpoints in pathophysiology of PE. Plenty of available evidence suggested protective role of VEGF in angiogenesis and cell survival, however, the role of VEGF in attenuation of ER stress in vitro has not been investigated so far. It is in this background, this study was planned to ascertain whether VEGF can minimize the ER stress in vitro.
Materials and methods
Study subjects
In this cross sectional, case control study, women with singleton pregnancy attending the antenatal clinic and the inpatient ward of the Department of Obstetrics and Gynaecology, All India Institute of Medical Sciences, New Delhi, India were screened. The preeclamptic women (PE, n = 30) after clinical diagnosis were enrolled as cases. Diagnosis of preeclampsia was based on ACOG guidelines (supplementary file). Maternal and gestational age matched, normotensive, non-proteinuric pregnant women (n = 30) were enrolled as controls. Pregnant women with chronic hypertension, chorioamnionitis, diabetes, renal disease, cardiac disease were excluded from the study. Institute Ethics Committee, AIIMS, New Delhi (IEC Ref.No.-RT37/28.09.2012) approved the study protocol and all the enrolled subjects gave their written informed consent before participation.
Serum of cases and controls was stored in aliquots at -80 °C for ELISA and cell culture experiments. Caesarean delivered placentae (5 PE and 5 normotensive) were used to analyze the protein expression of VEGF and GRP78 by Immunohistochemical staining (IHC).
ELISA
Sandwich ELISA was used to estimate the levels of VEGF and GRP78 in the serum of preeclamptic patients and controls (VEGF ELISA kit: R&D Systems Inc., Minneapolis, MN, U.S.A., GRP78 ELISA Kit: Enzo Life Sciences, Inc.).
Immunohistochemistry
Localization of VEGF and GRP78 in placentae was done by IHC. Paraffin tissue blocks were sectioned on microtome (Thermo Scientific™ HM 325) and were taken on Poly-L-lysine (Sigma) coated slides. IHC was performed using UltraVision™ Quanto Detection System HRP DAB by Thermo (TL-125-QHD). Primary antibodies against VEGF (PA5-16754; Thermo) at a dilution of 1:100 and GRP78 (ab181499; Abcam) at a dilution of 1:300 were used. Slides were observed under Nikon Eclipse Ti-S elements microscope using NiS-AR software. Other chemicals (analytical grade) were procured from Fischer Scientific.
In-Vitro assays
The effect of VEGF on ER stress was analysed in an in-vitro set up using BeWo cells (human choriocarcinoma cell line; ATCC). The cell line was maintained in Ham’s F 12 medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml Streptomycin. Cells were passaged with 0.025% trypsin and 0.01% EDTA. Four experimental groups were set up depending on various treatments given to BeWo cells. BeWo cells of group 1 were exposed to sera from preeclamptic pregnant women who had lower levels of VEGF (125–150 pg/ml). The sera from five different preeclamptic mothers with VEGF concentration ranging (125–150 pg/ml) were selected and pooled. The BeWo cells were then treated with these sera in Ham’s F-12 incomplete medium for 8 h, 14 h, 24 h respectively. In group 2, recombinant VEGF (250 pg/ml) was added to the sera from preeclamptic mothers and was then used to treat BeWo cells. Tunicamycin (2.5–5 μg/ml for 5 h) treated cells served as positive control and were marked as group 3. Tunicamycin is known to block the initial step of glycoprotein biosynthesis in the ER, causing accumulation of unfolded glycoproteins in the ER, leading to ER stress. The cells of group 4 did not receive any treatment. Following the above mentioned treatments, stimulation of ER stress markers (GRP78, eIF2α, XBP1, ATF6 and CHOP) was assessed at various time points (8 h, 14 h, 24 h) at protein level (IF, Western blot) and transcript level (qRT-PCR).
Immunofluorescence (IF)
BeWo cells were given various treatments as mentioned above for 8 h, 14 h and 24 h. The cells were then fixed in 4% paraformaldehyde for 15 min at room temperature followed by permeabilization with PBS + 0.1% Triton X-100. Nonspecific blocking was carried out using 5% normal goat serum in PBS and Triton X. The cells were incubated in primary antibodies GRP78 (ab21685, 1:1000), eIF2α (ab5369, 1:200), XBP1 (ab37152, 1:200), ATF6 (ab83504, 1:1000) and DDIT3/CHOP (ab27539 1:500) for 12 h at 4℃. Cells were washed with PBSTx before incubating with secondary antibody at 1:500 dilution for 1 h at room temperature in dark. After washing, they were mounted in flouroshield mounting media with DAPI on the slide and observed under the fluorescence microscope (Nikon Eclipse Ti-S elements using NiS-AR software).
Immunoblot blot
Cells were lysed in SDS-PAGE sample buffer [10% SDS, 60 mM Tris-HCl (pH 6.8), 10% glycerol, 0.001% bromophenol blue, 0.33% mercaptoethanol] and boiled for 5 min. The lysates were analyzed by immunoblotting. The Nitrocellulose membrane was incubated with the following primary antibodies i.e. GRP78 (ab21685, 1:1000), eIF2α (ab5369, 1:200), anti XBP1 (ab37152, 1:200) ATF6 (ab83504, 1:1000), CHOP (ab27539 1:500) for 12 h at 4℃. The blots were then incubated in secondary antibody (HRP conjugated) for 2 h and visualized using DAB, Tetrahydrochloride and H2O2. β-actin was used as protein loading control. For densitometric analysis, the blots were scanned in a gel documentation system, using Quantity 1 software (Bio-Rad, Hercules, CA, USA).
qRT-PCR (quantitative real time-polymerase chain reaction)
RNA isolation was done using Ambion, Invitrogen kit. The quality of RNA was examined by denaturing gel, visualized by ethidium bromide (EtBr) stain under UV and quantity was measured on micro-volume UV/Visible Spectrophotometer (Thermo Fisher Scientific- NanoDrop TM 2000). c-DNA synthesis was done using Thermo revert aid H-minus reverse transcriptase kit. Quality of cDNA was checked on 0.8% agarose gel visualized by ethidium bromide (EtBr) stain under UV, its quantity was measured on UV spectrophotometer and was subsequently used for qRT-PCR (CFX96 Touch™ Real-Time PCR Detection System). qRT- PCR reactions were carried out in 20 μl volume, including SYBR Green (Thermo), forward, reverse primer (Sigma), cDNA (template) and nuclease free water. Relative quantification cycles of gene of interest (ΔCq) was calculated by ΔCq = Cq (target) - Cq (reference). Relative mRNA expression was calculated subsequently by 2−ΔCq. Primers were designed by NCBI and confirmed by In silico PCR (Table 1). β actin and GAPDH were used as house-keeping genes.
Table 1.
Primers: Designed by NCBI.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| GRP78 | 5´-TGTTCAACCAATTATCAGCAAACTC - 3´ | 5´ - TTCTGCTGTATCCTCTTCACCAGT - 3´ |
| eIF2α | 5´ - AAGCATGCAGTCTCAGACCC - 3´ | 5´- GTGGGGTCAAGCGCCTATTA - 3´ |
| XBP1 | 5´ - TGGCCGGGTCTGCTGAGTCCG- 3´ | 5´ - ATCCATGGGGAGATGTTCTGG- 3´ |
| ATF6 | 5´ - CCACTAGTAGTATCAGCAGGAACTC- 3´ | 5´ - CCTTCTGCGGATGGCTTCAA- 3´ |
| CHOP | 5´ - AGAACCAGGAAACGGAAACAGA - 3´ | 5´ - TCTCCTTCATGCGCTGCTTT - 3´ |
| GAPDH | 5´ - AGCCGAGCCACATC - 3´ | 5´ - TGAGGCTGTTGTCATACTTCTC - 3´ |
| β-Actin | 5´ - GAGCACAGAGCCTCGCCTTT - 3´ | 5´ - TCATCATCCATGGTGAGCTGG - 3´ |
Statistical analysis
Data was analyzed by Graph Pad Prism 7. Relative quantification cycles of gene of interest (ΔCt) was calculated by ΔCt = Ct (target) - Ct (reference). Relative mRNA expression with respect to internal control gene (β actin and GAPDH) was calculated by 2−ΔCt. Average level of the variable between the two groups was compared by paired t-test and Wilcoxon signed rank test. ANOVA test with Bonferroni correction and Kruskal Wallis with Dunn’s test were used for comparing more than two groups. p value<0.05 was considered statistically significant.
Results
The clinical parameters of the 60 pregnant women were analyzed and are represented in Table 2.
Table 2.
Clinical Characteristics of Preeclamptic and normotensive, non proteinuric pregnant women (controls).
| Study Groups | |||
|---|---|---|---|
| Clinical characteristics | Preeclampsia (n = 30) | Normotensive, Non proteinuric (Control) (n = 30) | Statistical significance (p value)* |
| Systolic blood pressure (mmHg) | 158.9 ± 11.88 | 117.8 ± 7.34 | p < 0.0001 |
| Diastolic blood pressure (mmHg) | 101.43 ± 8.39 | 74.2 ± 6.39 | p < 0.0001 |
| Body Mass Index | 27.83 ± 5.61 | 23.67 ± 3.59 | p < 0.0001 |
| Protein (g/day) | 4.9 ± 1.6 | 0.7 ± 0.2 | p < 0.0001 |
| Placenta (g) | 411 ± 121 | 464.72 ± 55.50 | p = 0.0087 |
n = number of subjects, Data presented as mean ± SD, Paired t test, *statistical significance, p < 0.05.
VEGF and GRP78 levels in sera and placentae of preeclamptic pregnant mothers
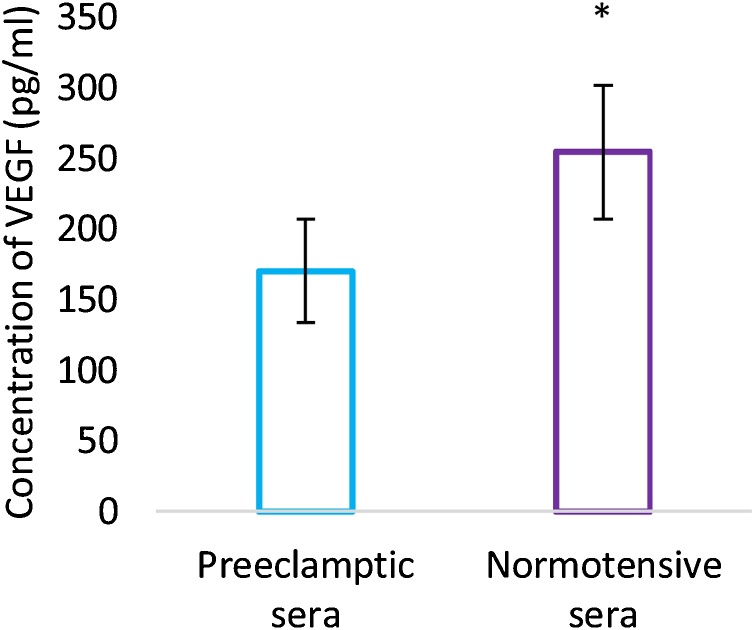
Circulating levels of VEGF measured in the maternal sera were reduced in preeclamptic pregnant women (170.53 + 36.55 pg/ml) as compared to maternal and gestational age matched controls (254.61 + 47.39 pg/ml (p < 0.0001) (Fig. 1). On the contrary, GRP78 levels were found to be increased in sera of preeclamptic pregnant women (1,103,260 + 104,270 pg/ml) in comparison to control group (1,018,610 + 125,510 pg/ml) and the difference was statistically significant. (p = 0.012) (Fig. 2).
Fig. 1.
Maternal serum levels of VEGF. Values are represented as Mean ± SD. Error bars represent standard deviation. *Statistical significance, p < 0.05.
Fig. 2.
Maternal serum levels of GRP78. Values are represented as Mean ± SD. Error bars represent standard deviation. *Statistical significance, p < 0.05.
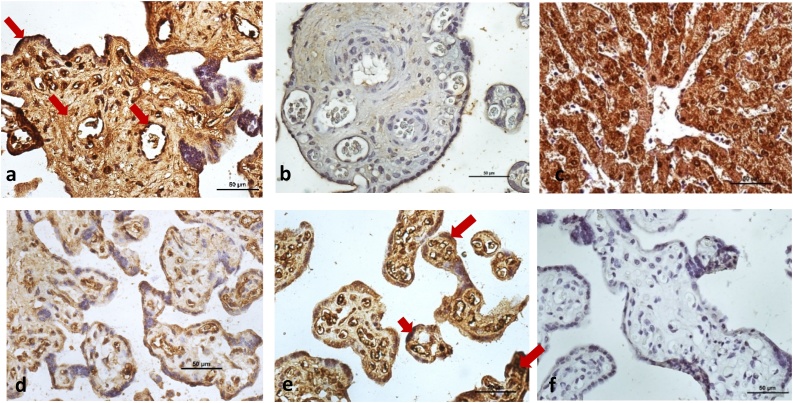
Interestingly the levels of the two proteins measured in the placentae of the same mothers corroborated with our results of the circulating VEGF and GRP78 levels in sera. The preeclamptic placentae revealed enhanced GRP78 and weaker VEGF expression of protein. Immunohistochemistry staining demonstrated stronger expression of GRP78 in syncytiotrophoblast and variable pattern (from weak to moderate) was seen around the walls of blood vessels and stromal cells. However, weaker expression of VEGF was observed in syncytiotrophoblast, endothelial cells and stromal cells in preeclamptic placentae (Fig. 3) as compared to the normotensive controls.
Fig. 3.
Immunolocalization of GRP78 in human placentae from Preeclamptic (Fig. 3a) and Normotensive pregnant women (Fig. 3b). GRP78 was strongly localized in syncytiotrophoblast and weak to moderate around wall of blood vessels and stromal cells (arrows; Fig. 3a). Immunohistochemical staining of VEGF in human placentae from Preeclamptic (Fig. 3d) and Normotensive pregnant women (Fig. 3e). VEGF was mainly localized in syncytiotrophoblast, endothelial cells and stromal cells (arrows; Fig. 3e). Human liver tissue was used as positive control (Fig. 3c). Negative control (Fig. 3f). Nuclei were counterstained by hematoxylin. All panels are in the same magnification. Bar = 50 μm.
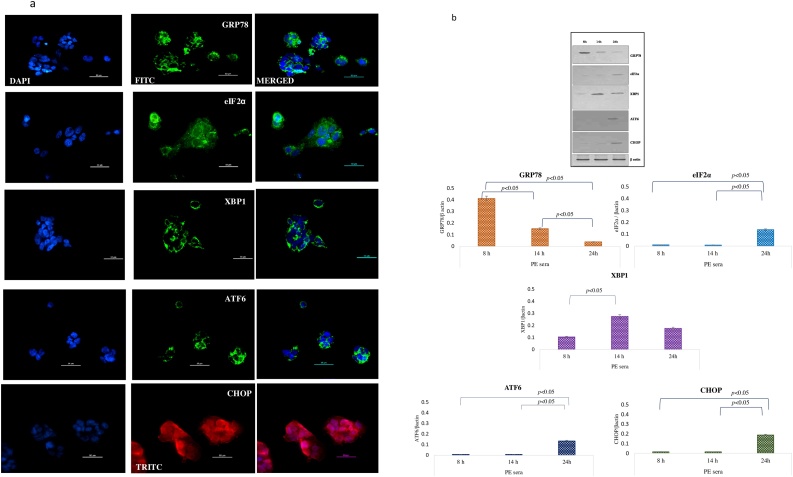
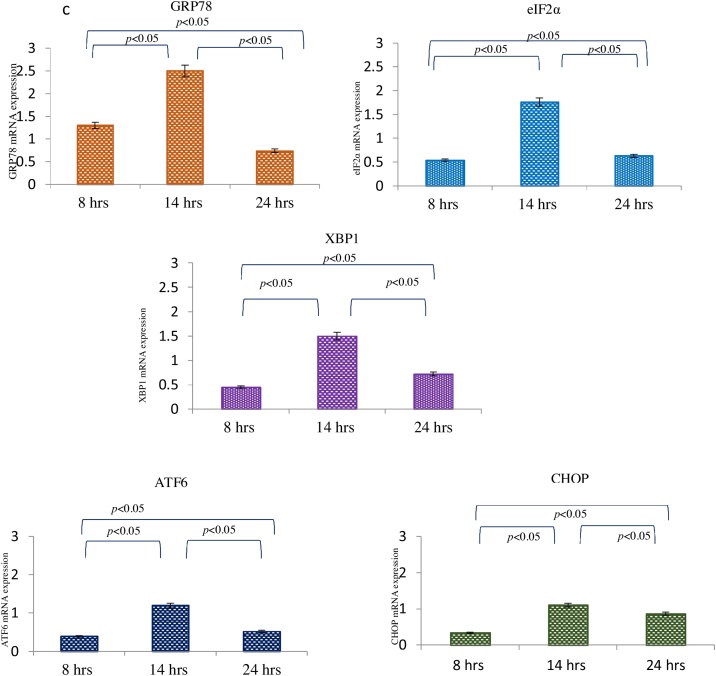
BeWo cells treated with pre-eclamptic sera undergo endoplasmic reticulum stress
Bewo cells were treated with sera isolated from pre-eclamptic mothers for 3 different timepoints. To assess the ER stress on the cells post treatment, various ER stress markers like GRP78, eIF2α, XBP1, ATF6 and CHOP as molecular readouts were analyzed by immunofluorescence and immuoblotting. The protein levels of GRP78 were found to be higher at 8 h as compared to 14 and 24 h. The higher intensity of eIF2α and ATF6 was observed at 14 h by IF whereas its expression in immunoblot could be observed at 24 h [Fig. 4a, b]. XBP-1 levels were raised at 14 h in both IF and western blot experiments. However, CHOP could be only induced after 24 h of treatment in IF as well as immunoblot. mRNA levels of GRP78, eIF2α, XBP1, ATF6 and CHOP were found upregulated at 14 h as compared to 8 h and 24 h [Fig. 4c]. In summary the markers of ER stress were unregulated both at the protein and transcript levels post treatment with sera from PE mothers.
Fig. 4.
a Immunolocalization of ER stress markers in BeWo cells following treatment with PE sera. The GRP78 was seen at 8 h, eIF2α, XBP1 and ATF6 at 14 h and CHOP expression at 24 h. b Representative images of immunoblot showing ER stress markers in BeWo cells following treatment with PE sera. β-Actin was used as protein loading control. The Bar diagrams represent the normalized values of the markers. Results are representative of 7 independent experiments. Data presented as mean ± SD. Statistical analysis was done using one way ANOVA with Bonferroni’s post hoc test. c Comparison of relative mRNA expression of ER stress markers in BeWo cells following treatment with PE sera at 8, 14 and 24 h. GAPDH was used as positive control. Data presented as mean ± SD. One way ANOVA test with Bonferroni correction was applied (p values indicated on graph itself).
Supplementation of re-VEGF to PE sera alleviated the induction of ER stress in BeWo cells
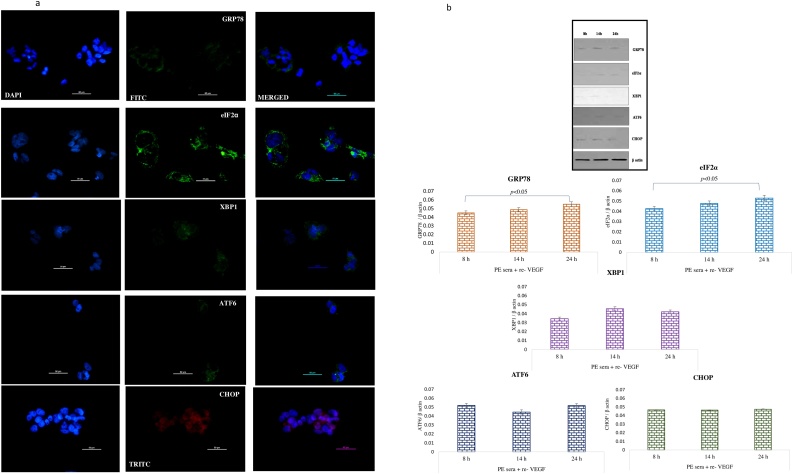
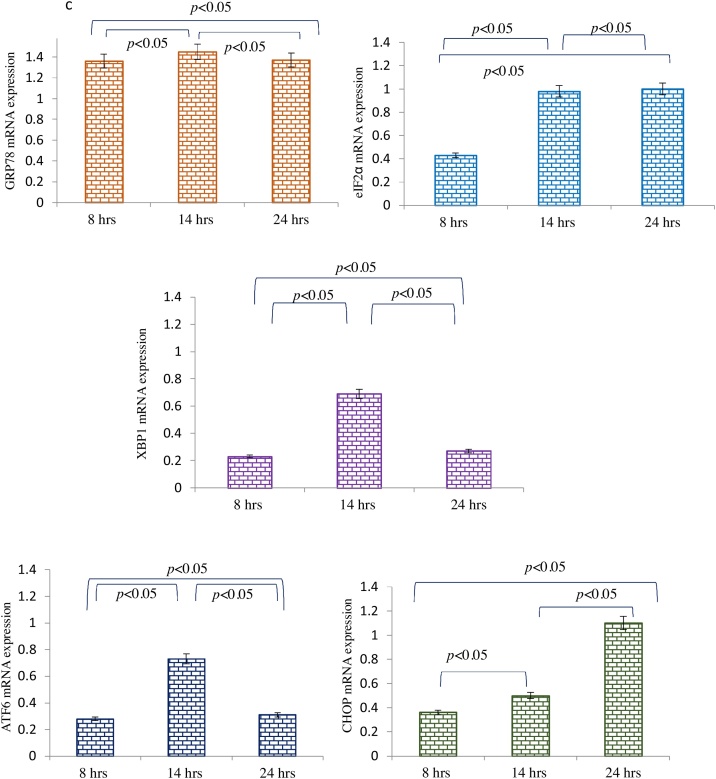
Recombinant VEGF exogenously added to the sera from preeclamptic mothers before treating the BeWo cells mitigated the PE sera effect of ER stress. Immunofluorescence staining revealed that expressions of GRP 78, eIF2α, XBP1, ATF6 and CHOP were reduced significantly at 8, 14 and 24 h [Fig. 5a]. Similarly, immunoblot data also demonstrated very low expression of GRP78, eIF2α and CHOP at 8 h and 14 h [Fig. 5b]. Interestingly, the mRNA levels of the tested genes were consistent with that of protein expression [Fig. 5c].
Fig. 5.
a Immunolocalisation of ER stress markers in BeWo cells following treatment with PE sera supplemented with re-VEGF. The GRP78 was seen at 8 h, eIF2α, XBP1 and ATF6 at 14 h and CHOP expression at 24 h. b Representative images of immunoblot showing ER stress markers in BeWo cells following treatment with PE sera supplemented with re- VEGF. β-Actin was used as protein loading control. The Bar diagrams represent the normalized values of the markers. Results are representative of 7 independent experiments. Data presented as mean ± SD. Statistical analysis was done using one way ANOVA with Bonferroni’s post hoc test. c Comparison of relative mRNA expression of ER stress markers in BeWo cells following treatment with PE sera supplemented with re- VEGF at 8, 14 and 24 h. GAPDH was used as positive control. Data presented as mean ± SD. One way ANOVA test with Bonferroni correction was applied (p values indicated on graph itself).
Tunicamycin treated cells showed strong expression of all the ER stress markers [S1a-c]
Tunicamycin is a known inducer of ER stress. Addition of Tunicamycin to BeWo cells worked as a positive control. It generated an intense expression of all the ER stress markers assayed both by IF and immunoblots (supplementary file, S1 a–c), thus activating all three arms of the UPR pathway. Immunoblot data revealed higher expressions of GRP 78 and eIF2α at 14 h and 24 h as compared to 8 h. XBP1, ATF6 and CHOP expressions was found to be dose dependent. [Figure S1b]. The transcriptional activation of GRP78, eIF2α, XBP1 and ATF6 mRNAs was observed by 14 h. CHOP mRNA levels were found maximum at 24 h [supplementary file, Figure S1c].
The expression of ER stress markers was very feeble when BeWo cells did not receive any treatment [Figure S2a-c]
No expression of XBP1, ATF6 and CHOP, whereas mild expression of GRP78 at 8 h and eIF2α at 14 h was observed in IF. [Figure S2a]. Western blot analysis also revealed weaker intensity of most of the markers at all the time points. However, mild expression of GRP78 and eIF2α was seen at 14 h. [Figure S2b]. Weak mRNA levels could be detected only in GRP78, eIF2α, XBP1 and ATF6 at variable time periods. However, CHOP expression was negligible till 24 h. [supplementary file, Figure S2c].
ER stress was reduced when BeWo cells were exposed to PE sera supplemented by recombinant VEGF as compared to the cells which were treated with only PE sera [Figure S3a-j]
We compared the normalized protein values of various ER stress markers between the first two groups i.e. PE sera and PE sera along with recombinant VEGF (PE + re-VEGF) treated BeWo cells. At 8 and 14 h, the GRP78 and XBP1 expression were significantly higher in PE group (p < 0.0001) (Figure S3 a and c), whereas by 24 h, the significantly enhanced expression could also be seen in other markers i.e. eIF2α, XBP1, ATF6 and CHOP in this group. (Figure S3 a–e). Significantly increased mRNA expression at 8 h was observed in eIF2α, XBP1 and ATF6 markers in the BeWo cells treated with sera from PE women. Interestingly the upregulation of the other two markers i.e. GRP78 and CHOP in addition to eIF2α, XBP1 and ATF6 could be seen only after 14 h in the above mentioned group (p < 0.0001) (Figure S3g-i).
Discussion
The human placenta throughout pregnancy period undergoes high levels of both angiogenesis and vasculogenesis [15]. VEGF promotes neovascularization and stabilizes endothelial cells in mature blood vessels, essential for maintaining endothelial cell homeostasis and therefore, is a key survival factor for vascular endothelium [[16], [17], [18], [19]]. Reduced levels of VEGF would affect the endothelial homeostasis and may cause disordered placental development. VEGF has also been shown to participate in the regulation of trophoblast cell survival, proliferation, migration and endovascular differentiation [20]. However, the role of VEGF in influencing the ER stress in trophoblast has not been reported in the literature. Hence, we theorized/speculated that trophoblast cells may undergo ER stress on exposure to reduced concentrations of VEGF. We used BeWo cells as representative of placental trophoblast cells and treated them with sera from preeclamptic pregnant women having varying concentrations (125–150 pg/ml) of endogenous VEGF. Protein and mRNA expressions of different ER stress markers were analyzed thereafter in the treated BeWo cells. In the present study, we observed that the sera from preeclamptic women had lower concentration of VEGF and higher concentrations of GRP78 as compared to controls. Similar results for both were also seen in term placentae from preeclamptic women. These results are consistent with the previous studies [[21], [22], [23], [24]] and indicate towards the contribution of endoplasmic reticulum stress in pathophysiology of PE [25]. The lower amounts of VEGF in sera of PE pregnant women has been attributed to presence of increased amount of soluble form of its receptor sVEGFR-1(sFlt-1), acting as a decoy receptor which traps the VEGF and reduces the bioavailability of free VEGF [26]. Interplay of multiple other factors like nitric oxide, hypoxia, oxidative stress, LCPUFA (Long Chain Polyunsaturated Fatty acids) have been shown to influence the levels of VEGF and coordinate placental angiogenesis. LCPUFA (omega 3 and 6) have recently been shown to play numerous roles during placentation as well as fetal growth and assist trophoblast in their angiogenic mission [27]. High doses of n-3 PUFA have inhibitory effects on angiogenesis. It can suppress endothelial proliferation, migration and VEGF production in many cell types including tumors [28]. Interestingly, on the contrary, one of the n-3 PUFA i.e. DHA (Docosahexaenoic acid) has been shown to stimulate angiogenesis in trophoblast cells which is beneficial during early placentation [29]. These paradoxical findings need further experimentations to provide better understanding of the underlying mechanisms. We reported stronger expression of GRP78 in the syncytiotrophoblast of PE placentae. This may be due to the extensive secretory activity of the syncytiotrophoblast cells in placentae [30]. We only observed GRP78 expression as dissociation of GRP78 (central regulator of UPR) from distinct trans-membrane sensors (PERK, IRE1 and ATF6) is the hallmark of UPR activation, signaling and ER stress pathway subsequently [31]. With these outcomes, we speculated that the increased levels of GRP78 in PE patients might be one of the several reasons for its earlier dissociation from trans-membrane sensors and its subsequent release in the maternal serum that could have induced ER stress following signaling through individual ER stress arms.
Following this, in-vitro assays were planned to examine if treatment of BeWo cells (placental trophoblast cells) with sera from PE patients (group 1) could upregulate ER stress. Signaling response of all ER stress markers (GRP78, eIF2α, XBP1, ATF6 and CHOP) was examined at different time points (8 h, 14 h and 24 h). When BeWo cells were treated with sera having reduced concentration of VEGF (PE sera: 125–150 pg/ml), the expression of GRP78 was found maximum at 8 h and started declining thereafter. Thus, indicating its earlier dissociation from ER stress sensors. On the other hand, when BeWo cells were exposed to PE sera supplemented with recombinant VEGF (group 2) i.e. higher concentration of VEGF, expression of GRP78 was found to be significantly reduced in group 2 as compared to group 1 (PE sera treated) cells at all the time points. Thus, pattern of GRP78 expression at both mRNA and protein levels observed in group 2 indicates towards an important role which may be played by VEGF in attenuating the ER stress. Activation of PERK results in phosphorylation of eIF2 α, blocking protein translation and reducing the protein burden within ER. In the present study, expression of eIF2α was found maximum at 24 h in BeWo cells treated with reduced VEGF concentration (group 1: PE sera treated) indicating the delayed attenuation time of PERK arm which is consistent with the study carried out by Walter et al. in 2007 where they observed eIF2α signal till 30 h [32] whereas increased VEGF exposure reduced the eIF2α expression. Our study demonstrated that although all three branches (PERK, IRE1 and ATF6) of UPR were activated upon induction of ER stress yet the behavior of individual signaling pathways varied markedly with time after the onset of stress. Out of the two isoforms (IRE1α and IRE1β) of IRE1 in mammalian genome, the dissociation of IRE1α from GRP78 due to an elevated level of unfolded proteins in the ER leads to activation of IRE1α (XBP1) [[33], [34], [35], [36]]. Expressions of XBP1, ATF6 and CHOP were also upregulated when cells were treated with lower amounts of VEGF in sera, which reduced on exposing them to higher amounts of VEGF concentration. However, XBP1 was found maximum at 14 h, and ATF6 as well as CHOP at 24 h in both groups. Since long time, ATF6 has been considered to fulfill merely adaptive functions during ER stress, and its sole intersection with ER stress-induced apoptosis consist of a possible role in regulation of CHOP expression [37]. Our finding is consistent with the results of Karali et al. in which they established that activation of ATF6 and PERK contributes to the survival effect of VEGF on endothelial cells (ECs) by positively regulating mTORC2-mediated phosphorylation of AKT on Ser473, which is required for full activity of AKT [38]. If the various UPR-induced mechanisms fail to alleviate ER stress, both the intrinsic and extrinsic pathways for apoptosis can become activated. CHOP (CCAAT-enhancer-binding protein homologous protein) plays a convergent role in the UPR and it has been identified as one of the most important mediators of ER stress-induced apoptosis [39]. An important evidence has come to light to prove that the major pro-apoptotic consequence of eIF2α phosphorylation is the upregulation of transcription factor CHOP, mediated by ATF4 [40]. Thus, markers of ER stress were upregulated though at different time points when BeWo cells were treated with lower VEGF concentration (PE sera) and their expressions were reduced when the VEGF amount was increased by supplementing the sera with recombinant VEGF. In the present study we confirmed that higher VEGF levels can alleviate the ER stress in BeWo cells. The experiments need to be repeated on the primary trophoblast cells and also the placental explants which are one of the most appropriate representatives of the in-vivo conditions as it maintains the tissue integrity.
Conclusions and perspectives
The VEGF exclusively plays its role in angiogenesis and progression of placental vasculature. Segregation and degeneration of affected tissue may be driven if stimulus is stronger than the adaptive capacity of UPR associated ER stress pathways. The therapeutic potential of VEGF can be comprehended using modulation of ER stress sensors like eIF2α and ATF6. Further decoding of the interplay between sensors of UPR and vascular factors (angiogenic and anti-angiogenic) will open a prospective window which would provide both valuable and fundamental therapeutic insights. Thus, our study can serve as an experimental template to explore unacquainted therapeutic dispensation of preeclampsia.
Authors contributions
SM and RD conceived and designed the study. SM did all the experiments. PA and VY assisted in experiments. NB provided the clinical inputs and critical suggestions. SD assisted in statistical analysis. SM and RD wrote first draft. MKD gave a hand in manuscript editing. KL, NR, SKG, SS and PA assisted in compilation of the final draft. *We sincerely thank SK Pallavi for her contribution in proofreading of this manuscript.
Declarations of interest
None.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.eurox.2019.100070.
Contributor Information
Sankat Mochan, Email: aiims79@gmail.com.
Renu Dhingra, Email: renudhingraaiims@gmail.com.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Ferris T.F. Pregnancy, preeclampsia, and the endothelial cell. N Engl J Med. 1991;325:1439–1440. doi: 10.1056/NEJM199111143252009. [DOI] [PubMed] [Google Scholar]
- 2.Roberts J.M., Taylor R.N., Musci T.J., Rodgers G.M., Hubel C.A., McLaughlin M.K. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161:1200–1204. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 3.Young B.C., Levine R.J., Karumanchi S.A. Pathogenesis of preeclampsia. Annu Rev Pathol. 2010;5:173–192. doi: 10.1146/annurev-pathol-121808-102149. [DOI] [PubMed] [Google Scholar]
- 4.Levine R.J., Maynard S.E., Qian C., Lim K.H., England L.J., Yu K.F. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 5.Tannetta D., Sargent I. Placental Disease and the Maternal Syndrome of Preeclampsia: Missing Links? Curr Hypertens Rep. 2013;15(6):590–599. doi: 10.1007/s11906-013-0395-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maynard S.E., Min J.Y., Merchan J., Lim K.H., Li J., Mondal S. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts J.M., Bell M.J. If we know so much about preeclampsia, why haven’t we cured the disease? J Reprod Immunol. 2013;99:1–9. doi: 10.1016/j.jri.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yung H.W., Korolchuk S., Tolkovsky A.M. Endoplasmic reticulum stress exacerbates ischemia–reperfusion-induced apoptosis through attenuation of Akt protein synthesis in human choriocarcinoma cells. FASEB J. 2007;21:872–884. doi: 10.1096/fj.06-6054com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yung H.W., Calabrese S., Hynx D., Hemmings B.A., Cetin I., Charnock-Jones D.S. Evidence of placental translation inhibition and endoplasmic reticulum stress in the etiology of human intrauterine growth restriction. Am J Pathol. 2008;173(2):451–462. doi: 10.2353/ajpath.2008.071193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jelkman W. Pitfalls in the measurement of circulating vascular endothelial growth factor. Clin Chem. 2001;47:617–623. [PubMed] [Google Scholar]
- 11.Maynard S.E., Min J.Y., Merchan J., Lim K.H., Li J., Mondal S. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Claudio H. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 13.Svein R., Ebbing C., Irgens L.M. Predicting preeclampsia from a history of preterm birth. PLoS One. 2017;12(7) doi: 10.1371/journal.pone.0181016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pennington K.A., Schlitt J.M., Jackson D.L., Schulz L.C., Schust D.J. Preeclampsia: multiple approaches for a multifactorial disease. Dis Model Mech. 2012;5(1):9–18. doi: 10.1242/dmm.008516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cerdeira A.S., Karumanchi S.A. Angiogenic factors in preeclampsia and related disorders. Cold Spring Harb Perspect Med. 2012;2(11) doi: 10.1101/cshperspect.a006585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez R.R., Cherfils S., Escobar M., Yoo J.H., Rueda B.R. Leptin signaling promotes the growth of mammary tumors and increases the expression of vascular endothelial growth factor (VEGF) and its receptor type two (VEGF-R2) J Biol Chem. 2006;281(36):26320–26328. doi: 10.1074/jbc.M601991200. [DOI] [PubMed] [Google Scholar]
- 17.Hood J.D., Meininger C.J., Ziche M., Granger H.J. VEGF upregulates ecNOS message, protein, and NO production in human endothelial cells. Am J Physiol. 1998;274:H1054–H1058. doi: 10.1152/ajpheart.1998.274.3.H1054. [DOI] [PubMed] [Google Scholar]
- 18.Jelkman W. Pitfalls in the measurement of circulation endothelial growth factor. Clin Chem. 2001;47:617–623. [PubMed] [Google Scholar]
- 19.Chaiworapongsa T., Romero R., Kim Y.M. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of preeclampsia. J Matern Fetal Neonatal Med. 2005;17:3–18. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- 20.Lala P.K., Nandi P. Mechanisms of trophoblast migration, endometrial angiogenesis in preeclampsia: the role of decorin. Cell Adh Migr. 2016;10(1-2):111–125. doi: 10.1080/19336918.2015.1106669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torry D.S., Torry R.J. Angiogenesis and the expression of vascular endothelial growth factor in endometrium and placenta. Am J Reprod Immunol. 1997;37:21–29. doi: 10.1111/j.1600-0897.1997.tb00189.x. [DOI] [PubMed] [Google Scholar]
- 22.Maynard S.E., Min J.Y., Merchan J., Lim K.H., Li J., Mondal S. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyall F., Young A., Boswell F., Kingdom J.C.P., Greer I.A. Placental expression of vascular endothelial growth factor in Placentae from pregnancies complicated by pre-eclampsia and intrauterine growth restriction does not support placental hypoxia at delivery. Placenta. 1997;18:269–276. doi: 10.1016/s0143-4004(97)80061-6. [DOI] [PubMed] [Google Scholar]
- 24.Chung J.Y., Song Y., Wang Y., Magness R.R., Zheng J. Differential expression of VEGF, EG-VEGF, and VEGF receptors in human placentas from normal and pre-eclamptic pregnancies. J Clin Endocrinol Metab. 2004;89(5):2484–2490. doi: 10.1210/jc.2003-031580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burton G.J., Yung H.W., Cindrova-Davies T., Charnock-Jones D.S. Placental Endoplasmic Reticulum Stress and Oxidative Stress in the Pathophysiology of Unexplained Intrauterine Growth Restriction and Early Onset Preeclampsia. Placenta. 2009;30:43–48. doi: 10.1016/j.placenta.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burton G.J., Yung H.W. Endoplasmic reticulum stress in the pathogenesis of early onset preeclampsia. Pregnancy Hypertens. 2011;1:72–78. doi: 10.1016/j.preghy.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shibuya M. Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis a crucial target for Anti- and pro-angiogenic therapies. Genes Cancer. 2011;2(12):1097–1105. doi: 10.1177/1947601911423031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burchakov D.I., Kuznetsova I.V., Uspenskaya Y.B. Omega-3 long-chain polyunsaturated fatty acids and preeclampsia: trials say “No,” but is it the final word? Nutrients. 2017;9(12):1364. doi: 10.3390/nu9121364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhuang W., Wang G., Li L., Lin G., Deng Z.J. Omega-3 polyunsaturated fatty acids reduce vascular endothelial growth factor production and suppress endothelial wound repair. Cardiovasc Transl Res. 2013;6(2):287–293. doi: 10.1007/s12265-012-9409-0. [DOI] [PubMed] [Google Scholar]
- 30.Duttaroy A.K., Basak S. CRC Press; 2015. Human placental trophoblasts: impact of maternal nutrition; p. 426. [Google Scholar]
- 31.Corazzari M., Gagliardi M., Fimia G.M., Piacentini M. Endoplasmic reticulum stress, unfolded protein response, and cancer cell fate. Front Oncol. 2017;7:78. doi: 10.3389/fonc.2017.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ron D., Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 33.Woehlbier U., Hetz C. Modulating stress responses by the UPRosome: a matter of life and death. Trends Biochem Sci. 2011;36:329–337. doi: 10.1016/j.tibs.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Walter P., Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 35.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 36.Hetz C., Glimcher L.H. Fine-tuning of the unfolded protein response: assembling the IRE1α interactome. Mol Cell. 2009;35:551–561. doi: 10.1016/j.molcel.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshida H. ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol Cell Biol. 2000;20:6755–6767. doi: 10.1128/mcb.20.18.6755-6767.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karali E., Bellou S., Stellas D., Klinakis A., Murphy C., Fotsis T. VEGF Signals through ATF6 and PERK to promote endothelial cell survival and angiogenesis in the absence of ER stress. Mol Cell. 2014;54(4):559–572. doi: 10.1016/j.molcel.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 39.Sano R., Hou Y.C., Hedvat M., Correa R.G., Reed J.C. Endoplasmic reticulum protein BI-1 regulates Ca2+ -mediated bioenergetics to promote autophagy. Genes Dev. 2012;26:1041–1054. doi: 10.1101/gad.184325.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rozpedek W., Pytel D., Mucha B., Leszczynska H., Alan Diehl J., Majsterek I. The role of the PERK/eIF2α/ATF4/CHOP signaling pathway in tumor progression during endoplasmic reticulum stress. Curr Mol Med. 2016;16(6):533–544. doi: 10.2174/1566524016666160523143937. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.