Introduction
Approximately 4 million cases of nonmelanoma skin cancer affecting 2.5 million individuals were diagnosed in the United States in 2012, with nearly half being cutaneous squamous cell carcinoma (CSCC).1 Risk factors for the development of CSCC include ultraviolet exposure, older age, and immunosuppression.2 There are no standard guidelines to manage advanced or recurrent CSCC. Various systems have been devised to predict which patients are at high risk of recurrence or metastasis, based on clinical or pathologic characteristics, including the American Joint Committee on Cancer and the Brigham and Women's Hospital staging systems. Management of these cases typically requires multidisciplinary care involving Mohs micrographic surgery, surgical oncology, medical oncology, and radiation oncology.
The programmed cell death protein 1 (PD-1) receptor, found on the membranes of T cells, binds to the programmed death ligands 1 and 2 (PD-L1 and PD-L2), which are highly expressed in squamous cell cancers.3 The PD-1/PD-L1 interaction results in deactivation of T cells and thereby reduces anticancer immune surveillance. Increased PD-1 and PD-L1 expression levels have been associated with high-risk CSCC, perineural invasion, and organ transplant–associated CSCC compared with noncancerous skin specimens.4 A recent study examining the PD-1 inhibitor cemiplimab in unresectable and metastatic CSCC yielded response rates of 50% and 47%, respectively, with durable responses exceeding 6 months in more than 50% of patients.5 Subsequently, the US Food and Drug Administration approved cemiplimab for the treatment of unresectable and metastatic CSCC.
Although PD-1 inhibition is now the standard of care for metastatic CSCC, its use in combination with other treatment modalities in the definitive setting is not yet well described. Here, we present the case of a patient with a diagnosis of locally advanced CSCC who showed complete response to concurrent radiation therapy and PD-1 inhibition.
Clinical case
A 65-year-old white man with a history of hypertension and a family history of melanoma presented in January 2016 with a nonhealing lesion of the scalp measuring 4.0 × 3.5 cm. The patient reported a history of extensive ultraviolet radiation exposure but no history of immunosuppression or hematologic malignancy. Biopsy showed an invasive, infiltrative, moderately to poorly differentiated CSCC with involvement of the biopsy sample edges and associated scar; cytokeratin 5/6 and p40 staining results were positive (Fig 1). The patient was advised to have Mohs surgery; however, he declined surgery and elected to use herbal supplementation instead. Over the next year, the lesion progressed in size to 8.0 × 7.5 × 2.0 cm with purulent and bloody discharge (Fig 2).
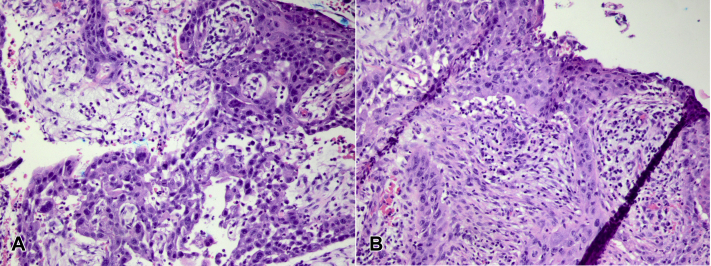
Fig 1.
A and B, Hematoxylin-eosin–stained sections show poorly differentiated carcinoma arranged in sheets and associated with scarring. The poorly differentiated areas stain with cytokeratin 5/6 and p40 immunohistochemical stains, which confirm a squamous origin.
Fig 2.
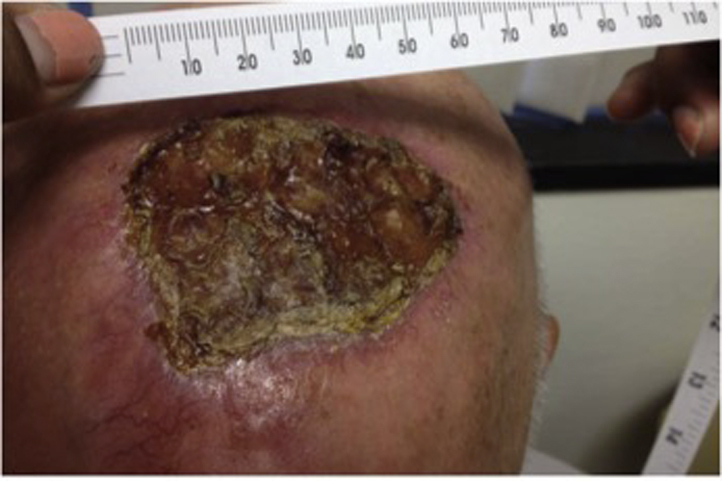
The patient's lesion in June 2017, 3 weeks after completing radiation therapy 66 Gy in 33 fractions and receiving 2 cycles of pembrolizumab.
The case was discussed by the multidisciplinary tumor board and staged as American Joint Committee on Cancer (eighth edition) T2N0M0 and Brigham and Women's Hospital staging T2b with multiple high-risk features: (1) tumor of greater than 10 mm in size on the scalp, (2) poorly differentiated histology, and (3) depth greater than 6 mm. PD-L1 expression was positive 2+, 8%. The consensus recommendation was surgical resection; however, the patient again declined and was referred to the radiation oncology department for definitive treatment. Computed tomography of the head showed involvement of the galea aponeurotica and subgaleal space but no evidence of direct calvarial involvement or lymphadenopathy. In April 2017, radiation therapy was initiated with the plan to deliver 66 Gy in 33 2-Gy fractions (Fig 3). Given the high risk of recurrence and metastases, PD-1 inhibition therapy with pembrolizumab was started in May 2017 at a dose of 200 mg intravenously every 3 weeks. Radiation therapy was completed in June 2017, shortly after pembrolizumab was started.
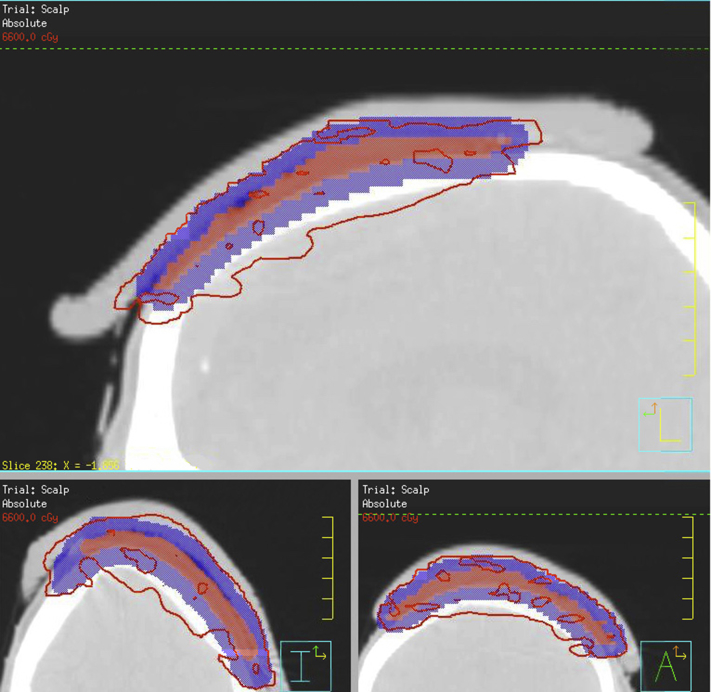
Fig 3.
Radiation plan. The red line shows the 66-Gy isodose line. The red colorwash shows the clinical tumor volume (gross disease +2-cm lateral margin). The blue colorwash shows the planning tumor volume, expanded 5 mm from the clinical tumor volume.
In August 2017, after 5 cycles of pembrolizumab, the patient noted granulation tissue and less drainage from the tumor. In November 2017, after 8 cycles, the lesion measured 3.0 × 3.0 cm, with a repeat computed tomography scan showing no evidence of metastases. After 16 cycles, the patient showed complete regression of the tumor in May 2018 (Fig 4). After nearly 19 months of therapy, PD-1 inhibition was discontinued, given that the patient had no evidence of disease. Throughout treatment, he remained largely free of adverse effects, experiencing only minor grade 1 fatigue and rash, which both resolved without intervention.
Fig 4.
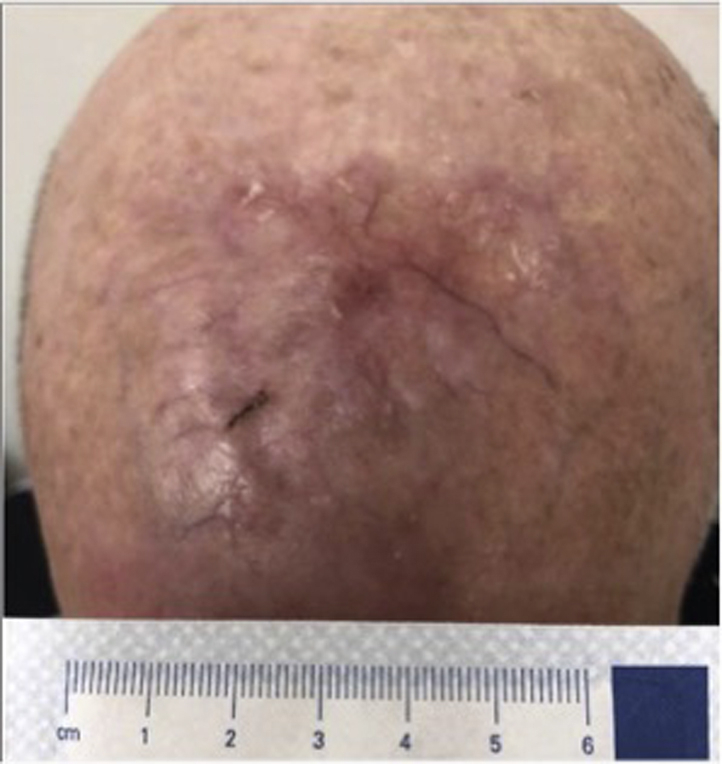
Complete regression of the patient's lesion in May 2018, after radiation and 16 cycles of pembrolizumab.
Discussion
CSCC is a highly immunogenic malignancy, as evidenced by its increased incidence among immunosuppressed and elderly patients. PD-1 inhibition has shown efficacy in advanced and metastatic CSCC, further supporting the importance of immune surveillance in this malignancy. However, PD-1 inhibition works in only approximately 47% to 50% of patients.5 Therefore, current research aims at improving outcomes to PD-1 inhibition in CSCC and other tumors.
Radiation may provide a synergistic benefit in combination with PD-1 inhibition for patients with CSCC, improving local and distant tumor control in a T-cell–dependent manner.6, 7 In addition to inducing apoptotic cell death, radiation therapy modulates class I major histocompatibility complex and novel antigen expression by tumor cells, generates type I interferon signaling, and enhances the diversity of T-cell receptors on tumor-infiltrating lymphocytes.6, 8, 9 This has led to further interest in the possibility of the abscopal effect, whereby radiation may further stimulate CD8+ T-cell–mediated immune responses to tumors outside the field of radiation.7, 10 Further studies should explore how to combine radiation therapy with PD-1 inhibition in CSCC, including the dosage and timing of radiation. Biomarkers are also needed to determine which patients will benefit from this approach.
Management of advanced or recurrent CSCC remains clinically challenging because of the variability in clinical presentations and the lack of standard management guidelines. In this case report, we describe a patient with advanced CSCC who had a complete response to treatment using concurrent radiation therapy with PD-1 inhibition. This case report shows that this combination can be performed safely and potentially with improved efficacy as well.
Footnotes
Funding sources: None.
Conflicts of interest: Dr In has served on the advisory boards of Bristol Meyers Squibb and Boehringer Ingelheim and as a speaker for Merck.
References
- 1.Rogers H.W., Weinstock M.A., Feldman S.R., Coldiron B.M. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151(10):1081–1086. doi: 10.1001/jamadermatol.2015.1187. [DOI] [PubMed] [Google Scholar]
- 2.Que S.K.T., Zwald F.O., Schmults C.D. Cutaneous squamous cell carcinoma: incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol. 2018;78(2):237–247. doi: 10.1016/j.jaad.2017.08.059. [DOI] [PubMed] [Google Scholar]
- 3.Malm I.-J., Bruno T.C., Fu J. Expression profile and in vitro blockade of programmed death-1 in human papillomavirus-negative head and neck squamous cell carcinoma. Head Neck. 2015;37(8):1088–1095. doi: 10.1002/hed.23706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevenson M.L., Wang C.Q.F., Abikhair M. Expression of programmed cell death ligand in cutaneous squamous cell carcinoma and treatment of locally advanced disease with pembrolizumab. JAMA Dermatol. 2017;153(4):299–303. doi: 10.1001/jamadermatol.2016.5118. [DOI] [PubMed] [Google Scholar]
- 5.Migden M.R., Rischin D., Schmults C.D. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379(4):341–351. doi: 10.1056/NEJMoa1805131. [DOI] [PubMed] [Google Scholar]
- 6.Twyman-Saint Victor C., Rech A.J., Maity A. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dovedi S.J., Cheadle E.J., Popple A.L. Fractionated radiation therapy stimulates antitumor immunity mediated by both resident and infiltrating polyclonal T-cell populations when combined with PD-1 blockade. Clin Cancer Res. 2017;23(18):5514–5526. doi: 10.1158/1078-0432.CCR-16-1673. [DOI] [PubMed] [Google Scholar]
- 8.Reits E.A., Hodge J.W., Herberts C.A. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203(5):1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnette B.C., Liang H., Lee Y. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res. 2011;71(7):2488–2496. doi: 10.1158/0008-5472.CAN-10-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park S.S., Dong H., Liu X. PD-1 Restrains radiotherapy-induced abscopal effect. Cancer Immunol Res. 2015;3(6):610–619. doi: 10.1158/2326-6066.CIR-14-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]