Abstract
Introduction
Sexual dysfunction in men is common, and optimal treatment is complex. Although several systematic reviews concerning treatment approaches exist, a comprehensive overview without limitations concerning the population, interventions, or outcomes is lacking.
Aim
To conduct a “review of reviews” to compare the effectiveness of pharmacologic, non-pharmacologic, and combined interventions.
Methods
9 electronic databases, relevant journals, and reference lists up to July 2018 were searched. For each intervention, only the most recent and comprehensive meta-analysis or systematic review was included. The methodologic quality of the reviews was appraised using the Assessment of Multiple Systematic Reviews–2 tool.
Main Outcome Measure
Sexual functioning (via intravaginal ejaculatory latency time and international index of erectile function), sexual satisfaction, and adverse effects.
Results
30 systematic reviews were included. For premature ejaculation, several treatments, including oral pharmacotherapy (selective serotonin inhibitors, phosphodiesterase type 5 [PDE5] inhibitors, tricyclic antidepressants, and opioid analgesics), topical anesthetics, and combined drug and behavioral therapies demonstrated significant improvements of 1–5 minutes in the intravaginal ejaculatory latency time. Pharmacologic interventions (PDE5 inhibitors, penile injection, and testosterone), shockwave therapy, lifestyle modifications, and combined therapies (PDE5 inhibitors and psychological intervention) were effective in treating erectile dysfunction. Most pharmacologic therapies were associated with adverse effects.
Conclusions
There is suggestive evidence that pharmacologic interventions or combined therapies are more effective than non-pharmacologic interventions for treating sexual dysfunction in men; however, a range of treatment options should be presented to individual patients so they may consider the risks and benefits of treatments differently. Evidence related to behavioral and psychological interventions is insufficient compared with that related to drug trials, highlighting the necessity for larger and better randomized controlled trials. Ciocanel O, Power K, Eriksen A. Interventions to Treat Erectile Dysfunction and Premature Ejaculation: An Overview of Systematic Reviews. Sex Med 2019;7:251–269.
Key Words: Premature Ejaculation, Erectile Dysfunction, Systematic Review, Sexual Health
Introduction
Sexual dysfunction is a complex medical issue with biological, psychological, and social influences1, 2, 3 and is estimated to be highly prevalent in the global population. In men, the prevalence of any sexual problem has been estimated to be approximately 31%, and the incidence increases with advanced age.2, 4
According to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5), male sexual dysfunction can affect desire, arousal, and the ability to have an orgasm and includes 4 specific diagnoses: erectile disorder/dysfunction (ED), hypoactive sexual desire disorder (HSDD), delayed ejaculation, and premature ejaculation (PE).5 These can be lifelong (primary, defined as being present since the first sexual encounter), acquired (secondary, appearing later), situational (context dependent), or generalized (occurring in any situation).5
The most common sexual dysfunctions are ED and PE,6 which frequently coexist.7, 8 The latter is diagnosed by the presence of the following symptoms for ≥6 months, occurring in >75% of sexual encounters: (i) inability to attain or sustain a penile erection during sexual activity and (ii) a reduction in erectile rigidity.5 ED is estimated to affect approximately 52% of men aged 40–70 years,9 and it is believed that ≤322 million men worldwide will experience ED symptoms in 2025.10
Many definitions exist for PE; however, the International Society for Sexual Medicine (ISSM) defines the condition as a combination of (i) ejaculation within 1 minute after vaginal penetration in the case of lifelong PE or within 3 minutes in acquired PE; (ii) difficulty with delaying ejaculation, and (iii) a significant impact on the individual, including frustration, distress, or avoidance of sexual intimacy.11 Similarly, DSM-5 defines PE as recurrent ejaculation within 1 minute of penetration and before the person wishes it.5 The use of disparate definitions means that the prevalence of PE is difficult to estimate.5 Although epidemiologic studies have estimated the global incidence of PE to be 20%–30% across all age groups,12, 13, 14, 7 these rates may not meet the current diagnostic criteria for PE,11 and estimates according to the ISSM and DSM-5 criteria have been suggested to be approximately 4%.11
Sexual dysfunction can have a profound impact on an individual’s life and general well-being15 and has been associated with low self-esteem,16 mental health problems,17, 18, 19, 9 interpersonal and intimacy problems,16, 17, 18, 19 decreased sexual functioning and satisfaction18, and decreased quality of life.18, 20, 21 Therefore, it is important to identify and implement effective interventions. Current interventions include pharmacologic therapy, surgical treatments (penile prosthesis), topical therapy, hormonal treatment, psychosocial interventions (behavioral interventions, cognitive behavioral therapy, hypnosis, and mindfulness-based interventions), and complementary and alternative medicine (CAM) treatments (such as herbal treatments, yoga, and physical activity), with varying use and effectiveness.22, 23, 24, 25
Growing interest in sexual dysfunction and its treatment has resulted in a large number of studies, evident from the multitude of published systematic reviews and meta-analyses.26, 27, 28 However, although valuable, individual systematic reviews are often limited to a narrow field of research in terms of population, interventions, or outcomes. Although some reviews have focused on 1 type of intervention, such as behavioral interventions,12 bibliotherapy,29 or CAM,23 most reviews have targeted medications, with phosphodiesterase type 5 (PDE-5) inhibitors (sildenafil and tadalafil) and their effects in reducing the symptoms of ED attracting considerable attention.30, 31 Other reviews contain limitations, such as comparing drug and psychological interventions for sexual dysfunctions without determining all outcomes of clinical interest.26, 32 In addition, most reviews have targeted single disorders such as ED33 or PE.34 To the best of our knowledge, a succinct summary of the findings of these reviews across different interventions, including both pharmacologic and non-pharmacologic approaches, has not yet been published. An overview of reviews could summarize the evidence of a range of interventions, providing a more accessible resource for decision-makers than multiple systematic reviews.35, 36 This review of systematic reviews provides an overview of the comparative effectiveness of different pharmacologic, non-pharmacologic, and combined interventions currently used to treat ED and PE.
Materials and methods
The review protocol was registered a priori in the National Institute of Health Research International Prospective Register of Systematic Reviews (PROSPERO), registration number CRD42017077461. The protocol offers the full description of the method used. Only minor changes were made to the protocol during the review. This overview was carried out in accordance with PRISMA guidelines37 and other published overviews of reviews.38, 39, 40, 41, 42 The checklist is included in Supplementary Table 1.
Inclusion and Exclusion Criteria
Types of Reviews
This overview included peer-reviewed systematic reviews and meta-analyses published from inception up to July 2018. Given that randomized controlled trials (RCTs) are considered the “gold standard” for evaluating the effects of treatment approaches,43, 44 only reviews including RCTs and controlled clinical trials were included.
A review was considered systematic if the authors (i) performed a comprehensive literature search (ie, searched ≥2 databases and described their search strategy, search terms, or both) and (ii) appraised and described the quality of included studies. Specifically, the study was required to be classified as “Yes” for the following 2 items of the Assessment of Multiple Systematic Reviews tool (AMSTAR): “Was a comprehensive literature search performed?” and “Was the scientific quality of the included studies assessed and reported?”45 Previous overviews have used a similar approach for study selection.41, 42 These inclusion criteria ensured a minimum level of quality.
Population
The study population included males >18 years of age who were described by the review authors as suffering from sexual dysfunction (desire, arousal, orgasm, sexual satisfaction difficulties, or sexual pain) without severe medical or mental disorders. Several reviews have addressed interventions exclusively targeted to specific comorbid conditions such as cancer,46, 47 diabetes,48 hypertension,49 and spinal cord injury.50 These were excluded because of the large number of comorbidities studied and the possibility of interaction, as well as the fact that individuals with comorbidities may have unique characteristics, which could affect outcomes and confound interpretations of the efficacy of treatment approaches.
Intervention and Comparison
Interventions of any duration, intensity and frequency were included; including pharmacologic (PDE5-Is, surgery, or injections), psychosocial (“Stop-Start” or “Squeeze” techniques or sex therapy) and CAM therapies (exercise or red ginseng). Reviews of clinical trials comparing an intervention to a placebo, usual care, or another type of treatment were included.
Outcomes
Sexual function (for example, ejaculation latency), sexual satisfaction, and adverse events were reported as the primary or secondary outcomes in included reviews. Any assessment tools were permitted (for example, intravaginal ejaculatory latency time [IELT], Sexual Encounter Profile, and Global Assessment Questionnaire), because various tools are discussed in the literature.
Search Methods for Identification of Reviews
A comprehensive search strategy was performed, comprising an electronic search of 9 databases (Medline, PsychInfo, Embase, PubMed, DARE, CINAHL, ProQuest, PROSPERO, and Cochrane Library for Systematic Reviews). Searches were restricted to articles published in English from 1980 onward and were initially conducted in November 2017 and rerun (using Medline) before analysis in July 2018. The search strategy used broad search terms, key words, Medical Subject Headings, and filters for “systematic reviews” and “sexual function” (dysfunction, sexual satisfaction, vaginismus, dyspareunia, HSDD, erectile dysfunction, premature ejaculation, orgasmic disorder and sexual arousal disorder). The Medline search strategy is included in Appendix 1 and was appropriately modified to suit each database. Reference lists of selected reviews were also screened.
Data Collection and Analysis
Selection of Reviews
Searches were performed in 2 stages using a screening form. The first step was to screen all titles and abstracts and exclude as appropriate. This was performed by the lead author (O.C.), with a random of approximately 10% (in keeping with previous published overviews51) independently checked by a second reviewer (K.P.). We tested for inter-rater consistency, and there was 83.3% agreement. All discrepancies were resolved by discussion.
In the second stage, potentially relevant reviews were read in full and assessed against the inclusion criteria (Appendix 2) by the lead author (O.C.) and checked by a second reviewer (K.P.). Here, 100% consensus was reached with regard to inclusion.
For each intervention and outcome, we selected a single systematic review that offered the best available evidence. We used a spreadsheet to list the studies included in all identified reviews to explore whether any review included identical primary studies. If multiple systematic reviews met the eligibility criteria and evaluated the same studies, we first selected the 1 with the most complete presentation of results (ie, a meta-analysis), and then the 1 with the most recent search date. If >2 systematic reviews had similar search dates (<1 year apart), we selected the 1 with the largest number of studies. Before exclusion of the overlapping systematic reviews, we checked the spreadsheet including all primary studies to ensure that we did not miss any relevant RCTs. Any discrepancies were resolved by discussion. Eligible reviews were classified based on the type of sexual dysfunction and intervention approach.
Data Extraction and Management
1 author (O.C.) extracted data from the selected reviews, which were independently verified by a second reviewer (K.P.). A data extraction tool was developed and piloted before starting the review. The extracted data included the following: (i) general review of characteristics (such as country, year and author); (ii) clinical characteristics (such as age, diagnoses of participants, type of intervention and type of outcomes); (iii) methodologic characteristics (such as design of primary studies incorporated in the review, search strategy and methods for quality appraisal of primary studies); (iv) results (such as number of primary findings included and review findings); and (v) conclusions and recommendations for practice. The data extraction form is shown in Supplementary Table 2. We only extracted data on studies relevant to the review question from included reviews, as recommended by Pollock et al.52
Assessment of Methodologic Quality
2 authors (O.C. and K.P.) independently appraised the methodologic quality of the selected systematic reviews using A Measurement Tool to Assess the methodologic quality of Systematic Reviews (AMSTAR 2).45 Any discrepancies were discussed and resolved. Cohen’s Kappa statistic was used to assess inter-rater agreement, with a value <60% suggesting inadequate agreement among raters.53
AMSTSR 2, a revision of AMSTAR, includes 16 questions (10 items from the original AMSTAR tool) on domains such as “appropriateness of the literature search,” “adequacy of meta-analytical methods,” “consideration of risk of bias when discussing the findings of the review,” and “consideration of publication bias and heterogeneity across studies.” Unlike its predecessor, this tool does not assign an overall quality score, because these scores may not provide sufficient detail on specific critical weaknesses and can reduce the confidence in the review findings.45 The AMSTAR 2 checklist is presented in Supplementary Table 3.
Data Synthesis
Results are presented as a narrative synthesis, including summary tables to enhance the clarity of reporting.36 The findings were summarized based on each intervention and outcome measure. No additional statistical analyses were performed due to the high heterogeneity across reviews.
Results
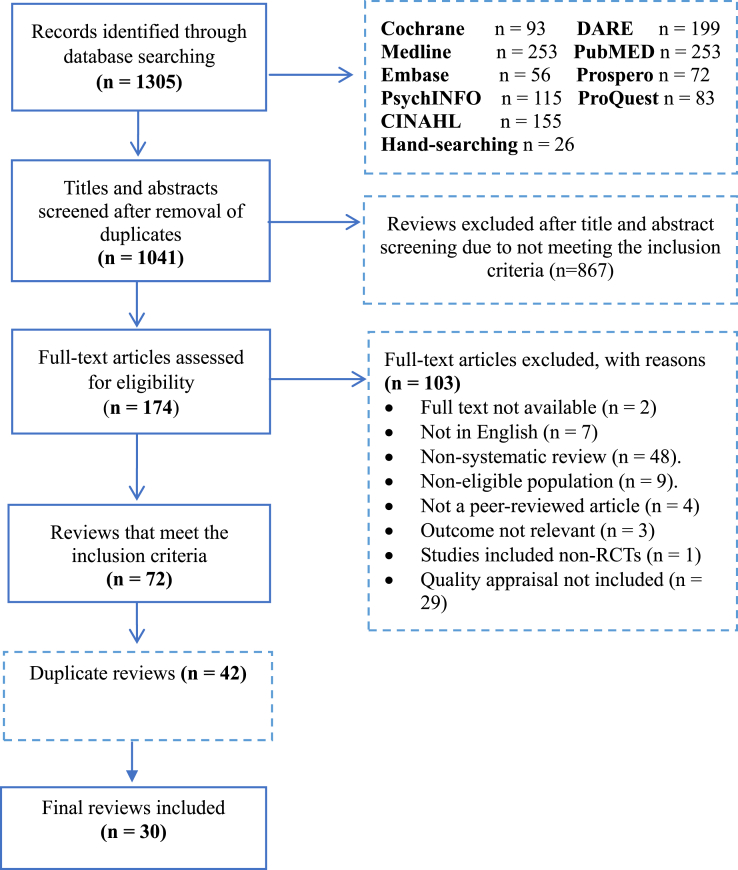
Figure 1 illustrates the flow diagram of the search process. A total of 30 systematic reviews were selected, of which 27 investigated single male sexual dysfunctions and 3 investigated mixed dysfunctions.
Figure 1.
PRISMA flow diagram.
Characteristics of Included Reviews
Table 1 outlines the characteristics of the included reviews. Details of the excluded reviews and the reasons for exclusion are presented in Supplementary Table 4. 28 reviews offered quantitative data with meta-analyses, and 2 provided descriptive data without pooled analyses. 3 reviews were published in the Cochrane Database of Systematic Reviews. All reviews were published between 1998 and 2017, with the majority (n = 24) published between 2010 and 2014. The reviews originated in the following countries: United Kingdom (n = 6), China (n = 6), Italy (n = 5), United States (n = 4), Brazil (n = 2), the Republic of Korea (n = 2), Switzerland (n = 2), Portugal (n = 1), South Africa (n = 1) and the Netherlands (n = 1).
Table 1.
Characteristics of included systematic reviews
| First author | Design | Date of search | Type of SD | No. of primary studies incl. in SR | No. of participants | Intervention/s | Comparison intervention | Outcomes for which data were reported (measurement instrument/s) |
|---|---|---|---|---|---|---|---|---|
| Pharmacological interventions | ||||||||
| Yuan28 | Syst & Meta | April 2012 | ED | 113 | 29,819 | PDE5-Is | Placebo | IIEF, GAQ |
| Cooper34 | Syst & Meta | August 2013 | PE | 27 | 1,588 | SSRIs, SNRIs, TCAs, alpha blockers | Waitlist, placebo or no treatment | IELT, ejaculation control, AEs |
| Castiglione54 | Syst & Meta | April 2015 | PE | 1 | 77 | Epelsiban | Placebo | IELT |
| Clavijo55 | Syst & Meta | March 2016 | ED | 7 | 602 | Shockwave Therapy | Sham therapy | IIEF-EFD |
| Corona56 | Syst & Meta | Nov 2015 | ED | 5 | 1,984 | Avanafil 100 & 200 mg | Placebo | SEP-3 |
| Corona57 | Syst & Meta | October 2016 | ED | 14 | 2,298 | Hormone therapy | Placebo | IIEF-EFD |
| De Hong58 | Syst & Meta | June 2014 | PE | 7 | 8,039 | Dapoxetine | Placebo | IELT, AEs |
| Du59 | Syst & Meta | March 2013 | ED | 3 | 374 | Mirodenafil 100 mg | Placebo | IIEF, SEP; GAQ, AEs |
| Fink60 | Syst & Meta | May 2002 | ED | 6 | 396 | Trazodone | Placebo | “Successful intercourse attempts”; AEs |
| Kostis61 | Syst & Meta | March 2013 | ED | 11 | 713 | Statins | Placebo and no medication | IIEF |
| Martyn St-James74 | Syst & Meta | August 2014 | PE | 4 | 721 | Tramadol; tramadol plus BT | Placebo | IELT, AEs |
| Martyn-St James75 | Syst & Meta | September 2015 | PE | 15 | 1,157 | PDE5-Is, PDE5-Is plus behavioral therapy | Placebo, SSRIs, “Squeeze” technique, Tramadol, Lidocaine | IELT, AEs |
| Martyn St-James67 | Syst & Meta | August 2014 | PE | 9 | 940 | Topical anesthetics | Placebo | IELT, AEs |
| Peixoto62 | Syst & Meta | August 2016 | ED, HSDD | 2 | 61 | Dehydroepiandrosterone | Placebo | IIEF, Sexual function (DISF) |
| Urciuoli68 | Syst & Meta | June 2003 | ED | 4 | 1,873 | Prostaglandin E1 | Placebo | At least one successful intercourse, AEs |
| Surgical procedure | ||||||||
| Yang69 | Syst& Meta | May 2016 | PE | 2 | 1,275 | Circumcision | No treatment | IELT, premature ejaculation |
| Complementary and alternative medicines | ||||||||
| Shin22 | Syst & Meta | December 2009 | ED | 1 | 50 | Maca (plant extract) | Placebo | IIEF-5 |
| Cooper34 | Syst & Meta | August 2013 | PE | 4 | 1,588 | Acupuncture and Chinese medicine | Waitlist, placebo or no treatment | IELT, control over ejaculation, AEs |
| Cui73 | Syst | March 2015 | ED | 3 | 183 | Acupuncture; Acupuncture plus psychological therapy | Sham therapy; Psychological therapy alone | IIEF-5 score; erection for penetration and intercourse |
| Ernst63 | Syst & Meta | 1997 | ED | 7 | 419 | Yohimbine | Placebo | Unclear |
| Jang64 | Syst & Meta | January 2008 | ED | 6 | 349 | Red ginseng | Placebo | IIEF, WSFQ |
| Schoonees71 | Syst & Meta | October 2010 | ED | 1 | 21 | Pycnogenol | Placebo | IIEF-5 score |
| Silva65 | Syst & Meta | July 2016 | ED | 7 | 487 | Physical activity and exercise | Usual care, drug treatment | IIEF |
| Xiong66 | Syst & Meta | June 2012 | ED | 21 | 2,253 | Chinese herbs; Chinese herbs plus drugs | Placebo, other Chinese herbs and WMT ∗ | Multiple |
| Behavioral and psychological interventions | ||||||||
| Cooper12 | Syst & Meta | August 2014 | PE | 10 | 521 | BT; BT plus drug treatment | Waitlist, drug treatment | IELT, sexual satisfaction, AEs |
| Melnik26 | Syst & Meta | 2007 | ED | 11 | 398 | Group psychotherapy and sex therapy; group psychotherapy plus drug treatment | Waitlist, injection, vacuum devices | “Persistence of erectile dysfunction” |
| Fruhauf27 | Syst & Meta | December 2008 | ED, PE | 8 | 447 | Psychological interventions | Waitlist | Symptom severity and satisfaction |
| van Lankveld29 | Syst & Meta | 1996 | Orgasm disorders, PE, ED | 12 | 397 | Psychological interventions (bibliotherapy) | Waitlist, no-treatment or placebo controlled | Unclear |
| Combined interventions | ||||||||
| Schmidt32 | Syst & Meta | January 2013 | ED | 8 | 562 | Psychological and PDE5-Is | PDE5-Is Psychological interventions |
IELT, Index of Sexual Satisfaction, DAS |
| Corona71 | Syst & Meta | July 2013 | ED | 5 | 894 | Testosterone therapy plus PDE5-Is | Placebo | IIEF-EF; IIEF-15 |
| Gupta72 | Syst & Meta | August 2010 | ED | 6 | 740 | Lifestyle modification and statins | Usual care | IIEF-5 score |
AE = adverse effect; AMSTAR = Assessment of Multiple Systematic Reviews tool; BT = behavioral therapy; DAS = Dyadic Adjustment Scale; DISF = Derogatis Interview for Sexual Function; ED = erectile dysfunction; HSDD = hypoactive sexual desire disorder; IIEF = International Index of Erectile Dysfunction; IIEF-EFD = International Index of Erectile Dysfunction erectile function domain; IELT = intravaginal ejaculatory latency time; GAQ = Global Assessment Questionnaire; PE = premature ejaculation; PDE5-I = phosphodiester type 5 inhibitor; SD = sexual dysfunction; SEP-3 = Sexual Encounter Profile Question 3; SR = systematic review; SSRI = selective serotonin reuptake inhibitor; SNRI = serotonin and norepinephrine reuptake inhibitor; TCA = tricyclic antidepressant; WMT = Western medicine therapy; WSFQ = Watts Sexual Function Questionnaire.
Of the 340 individual studies included in this overview, 334 (98.2%) were RCTs, 4 (1.1%) were non-randomized or clinically-controlled trials, and 2 were quasi-RCTs (0.5%). The total number of participants across all studies was 61,226. 3 reviews included studies with <100 participants. Meta-analysis included 2–13 individual studies (median: 4 studies), with sample sizes from 49 to 6,206.
Characteristics of Interventions
19 of the included reviews focused solely on interventions for ED, and 8 addressed PE. Interventions were classified into different categories (Table 2). 17 reviews addressed pharmacologic interventions, including oral pharmacotherapy, topical therapies, penile injections, and surgical procedures; whereas 12 addressed non-medical approaches, including psychosocial interventions (psychological and behavioral interventions) and CAM and 9 addressed a combination of interventions. All included reviews explored comparisons of interventions with a placebo, no treatment, usual care, other interventions or dozens of different interventions.
Table 2.
Classification of interventions identified in the included systematic reviews
| Type of intervention | Examples | Type of SD∗ addressed | Number (%)† |
|---|---|---|---|
| Pharmacological interventions | 16 (55) | ||
| Oral pharmacotherapy | Phosphodiesterase type 5 inhibitors (eg, sildenafil, avanafil, vardenafil, tadalafil, mirodenafil, udenafil) | PE, ED | 13 (44) |
| Selective serotonin reuptake inhibitors eg, sertraline, paroxetine, fluoxetine, citalopram, dapoxetine) | PE | ||
| Serotonin-noradrenaline reuptake inhibitors (eg, duloxetine, venlafaxine) | PE | ||
| Alpha-blockers (eg, terazosin) | PE | ||
| Tricyclic antidepressants (eg, Clomipramine oral and nasal) | PE | ||
| Hormone therapy, testosterone | ED | ||
| Statins | ED | ||
| Topical treatment | Lidocaine gel, EMLA cream, TEMPE | PE | 1 (3.4) |
| Penile Injections | Alprostadil | ED | 1 (3.4) |
| Non-Pharmacologic interventions | 12 (41) | ||
| Shockwave therapy | ED | 1 (3.4) | |
| Psychosocial interventions | 8 (27.4) | ||
| Behavioral or sexual skills training | Sensate focus exercises, systematic desensitization, “Start-and-stop” or “squeeze” techniques | PE | 3 (10.3) |
| Psychological | Sex therapy, cognitive behavioral therapy, marital therapy, bibliotherapy | PE, ED | 4 (13.7) |
| Complementary and alternative therapies | 10 (34) | ||
| Acupuncture | Acupuncture | PE, ED | 2 (6.8) |
| Herbal medicine | Pycnogenol, red ginseng, maca, yohimbine, and Chinese herb formulae | PE, ED | 6 (20) |
| Lifestyle modification | Physical activity, diet, weight loss | ED | 2 (6.8) |
| Combined therapy | 9 (31) | ||
| Combination of drug therapies | PDE5-Is and hormone therapy | PE, ED | 1 (3.4) |
| Psychological and pharmacological | Group therapy and PDE5-Is | ED | 2 (6.8) |
| Behavioral and pharmacological | Behavioral and PDE5-Is/tramadol | PE | 3 (10.3) |
| Lifestyle modification and drugs | Diet/exercise and statins | ED | 1 (3.4) |
| Acupuncture and psychological | ED | 1 (3.4) | |
| Chinese herbs and drug treatment | Chinese herbs and simvastatin | ED | 1 (3.4) |
ED = erectile dysfunction; EMLA = eutectic mixture of local anesthetics; PDE5-I = phosphodiester type 5 inhibitor; PE = premature ejaculation; SD = sexual dysfunction; SR = systematic review; TEMPE = topical eutectic mixture for premature ejaculation.
Sexual dysfunction.
Number/percentage of reviews which addressed the respective interventions.
Methodologic Quality of Included Reviews
AMSTAR 2 assessments identified several methodologic weaknesses across all included reviews (see Appendix 3). More than half of the reviews did not refer to a protocol,22, 27, 28, 29, 32, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66 66.6% reviews did not explain the study designs for inclusion in the review,12, 28, 34, 54, 56, 57, 58, 59, 60, 61, 62, 64, 65, 66, 70, 71, 72 73.3% did not provide the list of excluded studies,27, 28, 29, 32, 55, 56, 57, 58, 59, 60, 61, 62, 63, 65, 66, 69, 71, 72, 73, 74, 75 and 83.3% of reviews did not clearly state the sources of funding for the included studies.12, 22, 26, 27, 28, 29, 34, 55, 56, 57, 58, 59, 60, 61, 62, 63, 65, 66, 67, 68, 69, 72, 73, 74, 75 Inter-rater agreement of the 2 authors was high (κ = 0.86).
Effects of Interventions for Premature Ejaculation
Effect sizes for IELT are presented in Table 3. Other outcomes listed in systematic reviews are discussed in the text.
Table 3.
Effects of interventions for premature ejaculation as reported in the included systematic reviews
| Treatment (follow-up period) | No. of studies (participants) | Effect size for IELT outcomes Mean Difference (95% CI) |
Significant between-group difference |
|---|---|---|---|
| Phosphodiesterase type 5 inhibitors | |||
| PDE5-Is vs placebo (postintervention)75 | 3 (231) | MD = 2.21 min (1.45–2.97) | Yes |
| PDE5-Is vs SSRIs (at end-point)75 | 6 (405) | MD = 0.33 min (−0.063 to 1.30) | No |
| PDE5-Is vs squeeze technique (at end-point)75 | 1 (120) | MD = 3.56 min (3.16–3.96) | Sildenafil, yes. |
| PDE5-Is vs lidocaine gel (4 weeks)75 | 1 (60) | MD = −0.83 min (−1.61 to −0.05) | Sildenafil, no |
| PDE5-Is vs tramadol (4 weeks)75 | 1(59) | MD = −2.04 (−2.87 to −1.21) | Sildenafil, no. |
| Short-acting selective serotonin reuptake inhibitors | |||
| Dapoxetine 30 or 60 mg vs placebo (postintervention)58 | 6 (6455) | MD = 1.39 (1.24–1.55) | Yes |
| Long-acting selective serotonin reuptake inhibitors | |||
| Citalopram vs placebo (2–12 weeks)34 | 4 (224) | MD = 3.13 (0.63–5.63) | Yes |
| Escitalopram vs placebo (at end-point)34 | 1 (30) | MD = 1.20 (0.79–1.61) | Yes |
| Fluoxetine vs placebo (3–12 weeks)34 | 6 (170) | MD = 2.41 (2.10–2.73) | Yes |
| Paroxetine vs placebo (6–12 weeks)34 | 2 (224) | MD = 5.34 (3.79–6.89) | Yes |
| Sertraline vs placebo (at end-point)34 | 5 (188) | MD = 2.72 (1.77–3.67) | Yes |
| Fluvoxamine vs placebo (6 weeks)34 | 1 (19) | MD = 0.01 (−0.71 to 0.73) | No |
| Selective oxytocin receptor antagonists | |||
| Epelsiban 150mg vs placebo (8 weeks)54 | 1 (49) | Epelsiban difference = 1.14 (P > .05) | No |
| Serotonin-norepinephrine reuptake inhibitors | |||
| Duloxetine vs placebo (12 weeks) (12 weeks)34 | 1 (20) | MD = 1.52 (0.08–2.24) | Yes |
| Venlafaxine vs placebo (postintervention)34 | 2 (115) | Not pooled; Venlafaxine not significantly better than placebo (P > .05) | No |
| Tricyclic antidepressants | |||
| Clomipramine oral vs placebo (at end-point)34 | 1 (72) | MD = 3.48 (0.97–5.99) | Yes |
| Clomipramine nasal vs placebo34 | 2 (73) | MD = 1.68 (1.06 to 2.29) | Yes |
| Alpha-blockers | |||
| Terazosin vs placebo (8 weeks)34,∗ | 1 (47) | Not pooled; Terazosin significantly better than placebo (P = .001) | Yes |
| Opioid analgesics | |||
| Tramadol vs placebo (at end point)74 | 4 (721) | MD = 1.24 (0.52–1.95) | Yes |
| Tramadol vs SSRI (paroxetine) (at end-point)74 | 1 (57) | MD = 2.74 (1.91–3.57) | Yes |
| Tramadol vs Lidocaine gel (at end-point)74 | 1 (60) | MD = 1.21 (0.23–2.19) | Yes |
| Topical anesthetics | |||
| EMLA cream vs placebo (>5 applications and 4–8 weeks)67 | 2 (49) | MD = 6.44 (6.01–6.87) | Yes |
| TEMPE spray vs placebo (12 weeks)67 | 2 (539) | MD = 2.12 (1.26–2.97) | Yes |
| Lidocaine gel vs placebo (at end point)67 | 1 (57) | MD = 3.29 min (2.60–3.98) | Yes |
| Lidocaine gel vs paroxetine (SSRI) (at end point)67 | 1 (56) | MD = 1.53 (0.76–2.30) | Yes |
| Surgical options | |||
| Circumcision vs control (18–24 months)69 | 1 (80) | OR = 2.80 (2.16–3.44) | Yes |
| Psychosocial (behavioral and psychological therapies) | |||
| BT (squeeze, stop-start) vs waiting list⁷ (postintervention)12 | 1 (36) | MD = 6.87 (5.10–8.64) | Yes |
| BT (functional-sexological) vs waiting list (postintervention)12 | 1 (36) | MD = 6.80 (5.04–8.56) | Yes |
| BT (stop-start with vibrating device) vs Waiting list (postintervention)12 | 1 (10) | MD = 0.35 (−2.26 to 2.96) | No |
| Internet-based therapy (sensate focus) vs waiting list control (postintervention)12 | 1 (42) | MD = −0.20 (−1.75 to 1.35) | No |
| BT (squeeze, sensate) vs paroxetine (postintervention)12 | 1 (80) | MD = −0.20 (−0.40 to −0.00) | No |
| BT (stop-start) vs citalopram (postintervention)12 | 1 (64) | MD = −3.55 (−3.88 to −3.22) | No |
| Sex therapy vs waiting list control (postintervention)27,† | 1 (53) | MD = 0.08 (−0.57 to 0.73) | No |
| Alternative and complimentary therapies | |||
| Acupuncture vs sham acupuncture (postintervention)34 | 1 (60) | Between group differences in post treatment scores, P < .05 | Yes |
| Acupuncture vs SSRI (citalopram) (postintervention)34‡∗ | |||
| 1 (111) | Between group differences in post-treatment scores, P < .05, favoring acupuncture | Yes | |
| Acupuncture vs SSRI (paroxetine) (postintervention)34 | |||
| 1 (60) | Between group differences in post treatment scores, P < .05, favoring paroxetine | No | |
| Chinese medicine vs treatment as usual (4 weeks)34 | 1 (68) | MD = 1.57 (1.11–2.03) | Yes |
| Chinese medicine vs fluoxetine (2 weeks)34 | 1 (76) | MD = −0.60 (−1.01 to 0.19) | No |
| Combined approaches | |||
| PDE5-Is plus SSRIs vs SSRIs alone (at end-point)75 | 6 (521) | MD = 1.52 (0.98–2.05) | Yes |
| PDE5-Is plus BT vs BT (6 weeks)34 | 1 (60) | MD = 1.81 (1.53–2.09) | Yes |
| Tramadol plus BT vs BT (at end point)74 | 1 (72) | MD = 1.65 (0.30–3.00) | Yes |
| BT plus paroxetine vs paroxetine (postintervention)12 | 1 (80) | MD = 0.40 (0.18–0.62) | Yes |
| BT plus citalopram vs citalopram (postintervention)12 | 1 (64) | MD = 0.46 (0.04–0.88) | Yes |
AEs = drug-related adverse effects; BT = behavioral therapies; EMLA = eutectic mixture of local anesthetics; IELT = intravaginal ejaculatory latency time; (“squeeze,” “stop-start,” or sensate focus techniques; stimulation device and pelvic floor; rehabilitation); PDE5-Is = phosphodiesterase 5 inhibitors; OR = odds ratio; SSRI = selective serotonin reuptake inhibitor; TEMPE = topical eutectic mixture for premature ejaculation.
Measured ejaculation control.
Measured symptom severity and sexual satisfaction.
Measured Chinese Index of Sexual Function for PE.
Pharmacologic Interventions Vs Placebo
The vast majority of pharmacologic interventions for PE were reported to significantly increase IELT. Evidence of effectiveness was found for the following pharmacologic intervention approaches, in order of decreasing effect size: paroxetine (a selected serotonin reuptake inhibitors [SSRI]), oral clomipramine (a tricyclic antidepressant [TCA]), lidocaine gel (topical anesthetic), citalopram (SSRI), sertraline (SSRI), fluoxetine (SSRI), PDE5-Is, topical eutectic mixture for PE (topical anesthetic), nasal clomipramine nasal (TCA), dapoxetine (SSRI), and tramadol (opioid analgesic). The overall evidence for PDE5-Is, paroxetine, sertraline and nasal and oral clomipramine is limited, and most meta-analyses included a small number (72–231) of participants.34, 67, 75 Dapoxetine and tramadol are currently the only available drugs for which efficacy has been tested in large, well-designed and described clinical trials. Both lead to a substantial increase in IELT compared with placebo.58, 74
A review of escitalopram (a long-acting SSRI) included 1 study (30 participants), which found a significant improvement in IELT compared with placebo.34 The same review also found a significant increase in IELT scores for duloxetine (a serotonin and norepinephrine reuptake inhibitor [SNRI]) and a topical anesthetic (eutectic mixture of local anesthetics). Nevertheless, the small number of participants may indicate significant risk of bias in terms of overestimating the effect sizes. No evidence of improved IELT scores was found for fluvoxamine.34
Evidence from one study indicates that venlafaxine is not effective for IELT.34 Evidence for no effectiveness was shown for oral epelsiban at a dose of 150 mg or 50 mg.54 Only terazosin was reported to be effective for improving PE compared with the placebo. Examination of the evidence showed no significant difference between PDE5-Is and SSRIs (Table 3)75; lidocaine gel was significantly more effective than sildenafil75 and paroxetine67; tramadol was more effective than paroxetine,74 lidocaine gel,74 and sildenafil.75
Effect of Dosage of Pharmacological Interventions
Effect estimates for pharmacologic interventions for PE varied depending on the specific agent and dosage. For instance, 60 mg dapoxetine was more effective than 30 mg (5 studies, 3,346 participants, mean difference [MD] = 0.39, CI: 0.23, 0.56)58 and tramadol 100 mg was more effective than 50 mg (1 study, 100 participants, MD = 13.06, CI: 12.23, 13.79, P < .00001) or 25 mg (1 study, 100 participants, MD = 23.32, CI: 22.59, 24.05, P < .0001).74
Surgical Options
One systematic review presented a surgical option (Table 3).69 Circumcision was not associated with decreased or increased prevalence of PE (2 studies, 1,275 participants, odds ratio [OR] = 0.61, 0.28 to 1.34). Although improvements in IELT were reported in the circumcised group, the authors concluded that circumcision does not help to improve ejaculation and should not be offered to patients with PE.
Non-Pharmacologic Interventions
Non-pharmacologic interventions were compared with waiting list control, usual care, and alternative treatments in 3 reviews.12, 27, 34 Behavioral therapies included physical techniques, and the evidence was mixed.12 2 studies involving functional-sexologic therapy and “squeeze,” “stop-start,” and sensate focus techniques showed significant improvement in IELT compared with waitlist control. 2 other studies (including stimulation device and web-based sensate focus) found no differences in IELT compared with the control. The overall evidence for behavioral therapies is limited, owing to the inclusion of a small number of studies with few participants (10–80).12
Alternative therapies included acupuncture and Chinese medicine and were found to be effective in the included systematic review.34 Acupuncture was described to be effective at improving IELT compared with sham acupuncture (although this was based on 1 study including a small sample size of 60 participants). Similarly, Chinese medicine was stated to be more effective than treatment as usual in improving IELT outcomes. The evidence relating to psychological interventions is limited, with 1 review showing no evidence for effectiveness in improving IELT over waitlist control.27
Direct comparisons of behavioral therapy vs drug treatment presented mixed results, mostly either favoring drug treatment or showing no significant difference. For example, behavioral therapy was not effective compared with paroxetine, citalopram,12 or sildenafil.75
Direct comparisons of acupuncture with drug therapy showed conflicting results. 1 study found acupuncture to be more effective for PE (measured with the Chinese Index of Sexual Function for PE, CIPE) than citalopram, while another trial using paroxetine found the opposite.34
Combined Therapies
Combined approaches, including various drug treatments or combined behavioral and drug treatments, were reported to significantly improve IELT outcomes. For example, a combination of sildenafil with sertraline was reported to be more effective than SSRI monotherapy.75 Sildenafil combined with behavioral therapy is better than behavioral therapy alone,34 as is tramadol plus behavioral therapy.74
Adverse Effects
Adverse events were reported for all pharmacologic interventions. Topical anesthetics were associated with significantly increased risks of side effects, including loss of sensitivity (men and women), loss of erection, and irritation (men and women, after application for >20 minutes).67 Furthermore, PDE5-Is were associated with increased risk of flushing, headache, and palpitations,75 and dapoxetine was associated with nausea, dizziness, headache, diarrhea, and insomnia, although the reviewers reported these as mild and tolerable. Dapoxetine 60 mg was associated with more adverse events than dapoxetine 30 mg.58 Long-acting SSRIs have been linked with headache, decreased libido, nausea, dry mouth, diarrhea, dizziness, insomnia, and drowsiness.34 Tramadol was reported to be linked with several tolerable adverse events, such as somnolence, pruritus, ED, nausea, somnolence, headache, dry mouth, dizziness, vomiting, and constipation; addiction was not reported.74 Adverse effects of SNRIs included nausea and dry mouth, whereas TCAs were associated with local irritation in cases of nasal administration.34 Last, although data for α-blockers were not well reported, reported adverse events included headache and drowsiness.34 No significant adverse effects were reported for behavioral interventions and alternative therapies, although safety data were limited.34, 67
Other Outcomes
Other outcomes reported in the systematic reviews included ejaculation control and sexual satisfaction. Behavioral therapies,12 SSRIs (citalopram,34 paroxetine,34 and dapoxetine58) PDE5-Is,75 and tramadol74 have been reported to be superior to placebo or waiting list control in improving sexual satisfaction. With regard to ejaculation control, sertraline,34 dapoxetine,58 terazosin,34 and behavioral therapies12 significantly increased control compared with a placebo or waitlist control.
Effects of Interventions for Erectile Dysfunction
Effects are interventions for ED are presented in Table 4. General comparisons were difficult due the different outcomes and tools of the reviews. Measures of symptoms of ED included the use of validated instruments; the majority of reviews used the International Index of Erectile Function subdomains,76 although the Global Assessment Questionnaire and Sexual Encounter Profile, as well as other non-validated measures (for example, “At least 1 successful intercourse” and “Erection sufficient for penetration and intercourse”) were also presented.
Table 4.
Effects of interventions for erectile dysfunction as reported in the included systematic reviews
| Treatment (follow-up period) | Number of studies (participants) | Outcome | Effect size (95% CI) | Significant between-group difference |
|---|---|---|---|---|
| Phosphodiesterase type 5 inhibitor | ||||
| Tadalafil vs placebo (unclear)28 | 8 (1,877) | IIEF-EF | MD = 8.07 (7.18–8.96) | Yes |
| Vardenafil vs placebo (unclear)28 | 6 (1,151) | IIEF-EF | MD = 7.05 (5.60–8.50) | Yes |
| Sildenafil vs placebo (unclear)28 | 12 (3,404) | IIEF-EF | MD = 6.03 (5.38–6.68) | Yes |
| Udenafil vs placebo (unclear)28 | 5 (1,620) | IIEF-EF | MD = 5.92 (4.58–7.26) | Yes |
| Mirodenafil vs placebo (12 weeks)59 | 3 (364) | IIEF-EF | MD = 8.13 (6.64–9.61) | Yes |
| Avanafil (100 mg) vs placebo (8–12 weeks)56 | 5 (1,984) | IIEF-EF | MD = 3.92 (2.68–5.15) | Yes |
| Avanafil (200 mg) vs placebo (8–12 weeks)56 | 5 (1,984) | IIEF-EF | MD = 4.92 (3.66–6.19) | Yes |
| Selective serotonin antagonist inhibitors | ||||
| Trazodone vs placebo (post intervention) (4–13 weeks)60 | 6 (396) | “Positive treatment response” | RR = 1.6 (0.8–3.3) | No |
| Statins | ||||
| Statins vs placebo/no medication (12–104 weeks)72 | 11 (713) | IIEF-5 | MD = 3.4 (1.7–5.00) | Yes |
| Hormone therapy | ||||
| Testosterone vs placebo (12–156 weeks)57 | 13 (1,806) | IIEF-EFD | MD = 2.31 (1.41–3.22) | Yes |
| DHEA vs placebo (24 weeks)62,∗ | 1 (40) | IIEF-5 | Not pooled. The study showed significant differences between the groups. | Yes |
| Penile injections | ||||
| Intraurethral alprostadil (prostaglandin E1 vs placebo (postintervention)68 | 2 (1,101) | “At least one successful intercourse” | OR = 7.22 (5.68–9.19) | Yes |
| Prostaglandin E1 vs sex therapy (12–26 weeks)26 | 2 (86) | “Persistence of erectile dysfunction” | RR = 1.11 (0.62–1.97) | No |
| Shockwave therapy | ||||
| Shockwave therapy vs sham (postintervention) (13–56 weeks)55 | 7 (602) | IIEF-EF | MD = 4.17 (−0.50 to 8.83) | Yes |
| Complementary and alternative medicine | ||||
| Acupuncture vs sham therapy (unclear)73 | 2 (81) | IIEF-5 | Not pooled. 1 study showed significant effect (RR = 7.53 [1.13–50.00]) whereas another study was not significant (RR = 1.40 [0.67–2.91]) | Unclear |
| Physical activity vs usual care (8–104 weeks)65 | 7 (483) | IIEF | MD = 3.88 (2.35–5.41) | Yes |
| Lifestyle intervention vs usual care (12–104 weeks)72 | 4 (597) | IIEF-5 | MD = 2.40 (1.19–3.61) | Yes |
| Pycnogenol vs placebo (16 weeks)3,70,† | 1 (21) | IIEF-5 | MD = 6.00 (3.33 to 8.67) | Yes |
| Red ginseng vs placebo (unclear)64 | 6 (349) | Improvement of erectile dysfunction | RR = 2.38 (1.72 to 3.29) | Yes |
| Maca vs placebo (12 weeks)22 | 1 (50) | IIEF-5 | Not pooled. One trial reported significant differences between the groups | Yes |
| Yohimbine vs placebo (unclear)63,‡ | 7 (419) | Unclear | OR = 3.85 (2.22 to 6.67) | Yes |
| Chinese herb formula vs placebo (12 weeks)66 | 1 (200) | Total clinical effective rate | RR = 4.19 (2.85 to 6.17) | Yes |
| Chinese herb formula vs Simvastatin (12 weeks)66 | 4 (322) | IIEF-5 | Not pooled. Individual studies showed positive results. MD = 4.10 (3.62 to 4.58); MD = 2.87 (1.66 to 4.08) | Yes |
| Psychological therapies | ||||
| Psychological vs waiting list control (postintervention)27,§ | 7 (235) | Symptom severity | d = 0.53 (−0.08 to 1.14) | No |
| Sex therapy vs waiting list control (postintervention)27 | 3 (115) | Symptom severity | d = 0.21 (−0.31 to 0.74) | Yes |
| Marital therapy vs waiting list control (postintervention)27 | 1 (16) | Symptom severity | d = 0.17 (−2.61 to 2.94) | Unclear |
| Educational intervention vs waiting list control (postintervention)27 | 1 (20) | Symptom severity | d = −0.04 (−2.81 to 2.73) | Unclear |
| Group psychotherapy vs waiting list (26 weeks)26 | 5 (100) | “Persistence of erectile dysfunction” | RR = 0.40 (0.17 to 0.98) | Yes |
| Psychological interventions vs PDE5-Is (postintervention)32 | 3 (207) | Erectile dysfunction symptoms | d = −0.28 (−1.19 to 0.64) | No |
| Sex therapy vs intracavernosal injection (unclear)26 | 1 (29) | “Persistence of erectile dysfunction” | RR = 0.49 (0.14 to 1.67) | No |
| Sex therapy vs vacuum devices (6 weeks)26 | 1 (45) | Response to treatment | RR = 0.40 (0.14 to 1.14) | No |
| Combined therapies | ||||
| PDE5-Is plus hormone therapy vs PDE5-Is (12 weeks)71 | 5 (894) | IIEF | MD = 0.49 (−0.16 to 1.15) | No |
| Statins plus lifestyle modification vs usual care (12–104 weeks)72 | 6 (740) | IIEF-5 | MD = 2.66 (1.86 to 3.47) | Yes |
| Acupuncture plus psychological therapy vs psychological therapy (4 weeks)73 | 1 (102) | “Erection sufficient for intercourse” | RR = 1.99 (1.38 to 2.55) | Yes |
| Chinese herb formula plus simvastatin vs simvastatin (unclear)66 | 1 (63) | IIEF-5 | MD = 4.52 (4.08 to 4.96) | Yes |
| Psychological plus PDE5-Is vs PDE5-Is (postintervention)32 | 7 (550) | Erectile dysfunction | d = 0.45 (0.03 to 0.89) | Yes |
| Psychological plus PDE5-Is vs PI (postintervention)32 | 4 (312) | Erectile dysfunction symptoms | d = 0.67 (0.24 to 1.58) | Yes |
| Group therapy plus sildenafil citrate vs sildenafil (26 weeks)63 | 2 (71) | “Persistence of erectile dysfunction” | RR = 0.46 (0.24 to 0.88) | Yes |
d = Cohen’s d, effect size indicating the standardized difference between 2 means; DHEA = Dehydroepiandrosterone; IIFF-EF = International Index of Erectile Function-Erectile Function; IIEF-5 = International Index of Erectile Dysfunction; EFD = erectile function domain; MD = mean difference; OR = odds ratio; PDE5-Is = phosphodiesterase type 5 inhibitors; PI = psychological intervention; RR = risk ratio.
Sexual hormones or neurosteroids.
Pine bark extract.
Derived from the bark of a West African evergreen tree.
Psychological therapies: sex therapy, marital therapy, educational intervention, other psychotherapy.
Pharmacologic Interventions Vs Placebo
The vast majority of pharmacologic interventions for ED, including oral drugs and penile injections, resulted in significant improvement of symptoms compared with placebo, no treatment, or usual care, with effect sizes ranging from 0.30–1.19. Evidence of improvement for the following pharmacologic interventions was found in order of effect sizes: penile injection (alprostadil),68 mirodenafil, tadalafil, sildenafil, vardenafil, udenafil, trazadone60 (a serotonin antagonist and reuptake inhibitor), avanafil (200 mg), testosterone therapy, avanafil (100 mg), and statins. Alprostadil had the largest effect size,68 and trazodone had a moderate effect based on 6 studies.60 Testosterone therapy and PDE5-Is are the most well-studied interventions, with large and well-designed trials.28, 56, 57, 59, 71 Among the different classes of PDE5-Is, mirodenafil had the largest effect size followed by tadalafil, sildenafil, vardenafil, udenafil and avanafil. Although 1 review investigating hormone therapy (dehydroepiandrosterone) did not provide an effect size, the findings from 1 study, including 40 participants, reported significant improvement in terms of intercourse satisfaction, erectile function, and orgasmic function.62
Non-Pharmacologic Interventions
Non-pharmacologic interventions for ED were investigated in 11 reviews and included psychological and CAM interventions (physical activity, lifestyle changes, acupuncture, red ginseng, maca, pycnogenol, yohimbine, and Chinese medicine) compared with placebo, sham therapy, waiting list control, usual care, and alternative treatments. Shockwave therapy and physical activity are currently the only non-pharmacologic interventions for which efficacy has been confirmed in large, well-designed clinical trials. Pooled analyses of shockwave therapy indicated a statistically significant improvement in erectile function when compared with sham therapy.55 Similarly, physical activity was reported to have a significant effect in improving erectile functioning.65 Other combined lifestyle interventions (such as diet, physical activity, and weight loss) were also reported to be effective in treating ED symptoms.72
Psychological therapies for ED included Masters and Johnson’s sex therapy, marital therapy, group psychotherapy, and educational therapy.26, 27, 32 Evidence of improvement was mixed. Overall, psychological therapies were not found to significantly reduce symptoms,27 although sex therapy was more effective compared with a waiting list control group. No differences in terms of ED symptoms were identified between psychotherapy approaches vs local injection and vacuum devices.33 Analysis of the efficacy of other psychological interventions (educational interventions and marital therapy) was inconclusive, although only 2 studies with very small sample sizes were included and reported either negative results or small effect sizes.27
Overall, the evidence for psychological therapies was limited, and most meta-analyses included only a small number (16–235) of participants (Table 4). Comparisons of alternative therapies, such as acupuncture, red ginseng, yohimbine, and Chinese herbs, revealed conflicting results. Acupuncture was reported to have significant effects for ED in 1 study, whereas a second study showed no significant differences to sham therapy73; furthermore, although yohimbine,63 red ginseng,64 and Chinese herbs66 have been demonstrated to have a positive effect, the evidence was based on a small number of studies and small number of participants in each case (Table 4). Pycnogenol was found to cause a significant increase in erectile function compared with the placebo.70
Combined Therapies
Combined approaches were reported to significantly improve erectile function. For example, a combination of statins with lifestyle modification (such as diet, weight loss and physical activity) reduced ED symptoms relative to usual care.72 Acupuncture combined with psychological interventions was also reported to be better than psychological therapy alone.73 In addition, psychological interventions combined with PDE5-Is were reported to be better than PDE5-Is or psychological interventions alone.27 The combination of PDE5-Is with hormone therapy was not better than PDE5-Is alone, based on 5 studies; however, this was the only combined approach that failed to produce positive effects on ED.71
Adverse Effects
Most of the reviews that investigated pharmacologic interventions reported on adverse events. No safety data were reported for treatment with statins.61, 72 or testosterone therapy.57 The PDE5-Is (sildenafil, tadalafil, vardenafil, avanafil, udenafil, and mirodenafil) were associated with increased risk of flushing, headache and dyspepsia compared with placebos, although these symptoms were generally found to be mild. The safety of different PDE5 agents was similar, although tadalafil caused a higher incidence of myalgia compared with sildenafil.28, 59 No differences in the incidence of severe adverse events were observed between avanafil 100–200 mg.71 Although the data for trazodone were not well reported, adverse events included dry mouth, sedation, dizziness and fatigue.60 Alprostadil was associated with a higher incidence of penile pain, urethral trauma, and dizziness.68
Only 2 of the reviews that investigated non-pharmacologic interventions reported on adverse events. No safety data were reported for treatment with shockwave therapy,55 acupuncture,73 or pycnogenol.70 Reporting on adverse events for red ginseng was limited, although the events were reported to be mild and included headache, insomnia, gastric upset, and constipation.64 Some adverse events caused by Chinese herb formulas included dry mouth, loss of appetite, constipation, and diarrhea.66 Yohimbine was found to be well tolerated, with only minor and reversible adverse effects (rash, hypertension, dizziness, anxiety, increased frequency of urination, chills, headache, and gastrointestinal disturbances).63
Discussion
To our knowledge, this is the first published overview of reviews of pharmacologic and non-pharmacologic interventions for ED and PE. This overview identified 30 reviews reporting on >40 treatment options. For patients with PE, evidence from 8 reviews suggested that pharmacologic and combined therapies demonstrated the largest effect sizes. Pharmacologic treatments improved the time to ejaculation by 1–5 minutes and included SSRIs (paroxetine, citalopram, sertraline, fluoxetine, and dapoxetine), TCAs (oral and nasal clomipramine), topical anesthetics (lidocaine gel and topical eutectic mixture for PE), PDE5-Is, and opioid analgesics (tramadol). However, these drugs were associated with adverse effects. These findings support clinical guidelines that recommend that pharmacologic options for PE should include “on-demand” dapoxetine, topical anesthetic agents, or tramadol, or daily use of clomipramine or long-acting SSRIs.6
There was limited evidence of the efficacy of non-pharmacologic interventions on PE compared with drug treatments. Non-significant effects of such interventions for PE must be interpreted with caution, because low numbers of studies and small sample sizes increase the uncertainty of results and reduce the power to detect a significant effect, possibly leading to misclassification of interventions.
Presenting a range of treatment options to individuals might be a useful approach, because some may favor a behavioral option, whereas others may prefer a pharmacologic approach. Combinations of psychological and pharmacologic options may be beneficial when there is a strong relationship or psychosocial problem.11
Most of the reviews included an assessment of IELT or another measure of ejaculatory latency, with only a few reporting other outcomes such as sexual satisfaction and anxiety. It is important that more primary studies aim to measure non-ILET outcomes, as emphasized in the recently updated ISSM definition of PE, which includes the negative personal consequences.11
For patients with ED, evidence from 22 reviews suggested that pharmacologic therapy (PDE5-Is, penile injection, and testosterone), shockwave therapy, lifestyle modification, and combined therapies (PDE5-Is and psychological intervention) demonstrated the largest effect sizes for improving ED. After consideration of the possible adverse effects, benefits, and the quality of the evidence base, it is recommended that health care professionals should consider the prescription of PDE5-Is for treating ED, because they have been demonstrated to be effective in improving sexual intercourse. The number of primary studies appraising the efficacy of PDE5-Is is large compared with those investigating other areas of ED treatment. Although these drugs are generally safe and well tolerated with no major differences in their safety profiles, some patients may have contraindications to PDE5-Is or may not tolerate their side effects, which include headache, dizziness, and vision changes. Furthermore, some may not respond to this therapy (11%–44% of patients are estimated to be non-responders to PDE5 monotherapy).77 Patients may have different treatment preferences depending on their perceptions of the treatments’ attributes (such as efficacy, side effects, and ease of administration), supporting the suggestion that a range of treatments should be offered. Where medication is contraindicated or oral drugs are ineffective, intervention choices include intracavernous injections, lifestyle modification, and shockwave therapy. Indeed, the current evidence supports the statistically significant reduction in ED symptoms achieved by penile injection, which suggests that this treatment approach should be considered for ED. However, penile injections are associated with significant adverse effects such as penile pain, dizziness, and urethral trauma.26, 66
Shockwave therapy and lifestyle modifications were reported to have minimal or no adverse effects and should therefore be recommended for individuals with ED. Notably, the effect sizes for some of these interventions were similar to those of pharmacologic interventions. For example, comprehensive lifestyle modifications (combined diet, weight loss, and physical activity) as well as single modifications (physical activity) were associated with substantial improvements in ED symptoms.
The potential benefits of lifestyle modifications may be particularly relevant for people with ED and specific comorbid conditions such as diabetes, hypertension, or cardiovascular or metabolic disorders,78, 79, 80 because they may also improve cardiovascular, metabolic, and overall health.75, 81 These findings support clinical guidelines that recommend that addressing reversible causes or lifestyle changes should be the first consideration in the treatment of ED. Lifestyle changes should also accompany pharmacotherapy or psychological therapy.6
Strengths and Limitations of This Review
The methodology of this study ensured that a large number of databases were searched and duplication was avoided, and that the quality of methodologies was assessed. In addition, specific inclusion criteria ensured that only good-quality systematic reviews were included.
However, some limitations of our review should be noted. First, we could only present the evidence base of interventions that were within the included systematic reviews. Clinical trials may have been carried out for some treatment approaches that have not yet been evaluated in systematic reviews.
Second, it is possible that some recent primary studies may not have been incorporated in the reviews and are therefore not included in our overview. Although the search dates were relatively recent, some RCTs for specific treatment approaches may have been missed.
Third, overviews of reviews depend on the findings from other investigators in relation to their inclusion criteria and meta-analytic methods. Although we identified few issues with methodologies, it is possible that the investigators missed some relevant trials.
Fourth, the quality and quantity of evidence were a limitation of this overview. The quality of evidence was generally low, and most of the studies were judged to be at high or unclear risk of bias because of inadequate blinding. Furthermore, some systematic reviews included only a few studies with small sample sizes. These factors may have a considerable impact on the present effect sizes and credibility of our review.
Fifth, the characteristics of participants, interventions, and findings are not described in detail within our review, which may present a major limitation regarding conclusions. Our findings suggest that practitioners need to familiarize themselves with the interventions reported to make decisions regarding treatment for male sexual dysfunction.
Sixth, our review excluded interventions exclusively targeting individuals with specific comorbid conditions such as cancer, spinal cord injury, or hypertension. The efficacy of a particular intervention may vary among sub-groups, and the generalization of findings in populations with comorbid conditions remains unknown. Furthermore, the included trials were conducted in a number of countries worldwide. Consequent differences in study populations, cultural attitudes toward sexual dysfunctions, definitions of PE and ED, and inconsistencies in the collection of data, reporting of outcomes, and acceptability of treatments may mean that our findings are not representative for all individuals.
Last, factors such as ease of administration, reversibility, cost and mode of action, and accessibility of interventions were not considered, although they may influence treatment selection. All of these factors must be addressed in future studies involving longer follow-up periods.
Implications for Practice
Our findings will provide clinicians and patients with up-to-date information regarding the most effective treatment approaches for male sexual dysfunction that have been evaluated in robust systematic reviews. Evidence of positive outcomes was reported in systematic reviews of many of the interventions. For patients with a preference for non-pharmacologic interventions, clinicians can present several therapies that have been shown to have similar effects to drugs. Lifestyle modification, for example, is a well-supported approach for treatment of ED. Other psychological or complementary interventions can also be effective, although the evidence base is less reliable. Most complementary therapies such as acupuncture and Chinese medicine are either not effective or have small effect sizes, suggesting that clinicians should avoid their use. Nevertheless, the treatment chosen by patients will be influenced by factors such as safety and effectiveness, as well as patient and partner expectations and preferences. Therefore, providing individuals with a range of options may be the best approach for treating male sexual dysfunction. This recommendation supports the principles of evidence-based realistic medicine, which uses relevant scientific evidence base along with clinical judgement and patients’ values and preferences.82
Implications for Research
Our findings highlight some areas for future research. Interventions for which no systematic reviews were identified (for example, surgical implants, yoga, mindfulness-based interventions) require further investigation, possibly including more primary trials. Until a systematic review is conducted, it is difficult to comment on their usefulness.
Based on systematic reviews, more primary studies are needed—particularly with regard to PE—for vacuum devices, behavioral interventions (“stop-start” and sensate focus techniques), psychological interventions (cognitive behavioral therapy, couples counseling, mindfulness-based interventions), CAM (red ginseng, acupuncture, Chinese medicine), and combination therapy. Given the quality of the literature on these interventions, better-quality research is needed, including stronger study designs, clearer descriptions of the interventions, and consistency in the collection and reporting of outcomes for ED and PE (using validated instruments). In addition, to ensure consistency of outcome data and enable meta-analyses, future research needs to recruit men who meet the ISSM and DSM-5 definitions of PE and ED. Furthermore, the design of future RCTs should consider “head-to-head” comparisons of various combinations of treatments, including physical and counseling approaches for PE, and pharmacological and behavioral interventions for both PE and ED. More research into the effectiveness of interventions on other sexual dysfunctions, such as HSDD, low libido, or delayed ejaculation, is also needed.
Conclusions
The findings of this overview could lead to significant benefits for patients, clinicians, researchers, and health service commissioners. Most importantly, this overview will provide a solid framework to guide discussion regarding the best available treatment option for erectile dysfunction and premature ejaculation. Taken together, evidence for positive outcomes exists for many of the interventions investigated in systematic reviews of male sexual dysfunction. Pharmacologic interventions or combined therapies appear to be more effective than non-pharmacologic interventions; however, the evidence relating to behavioral and psychological interventions is insufficient compared with drug trials, which underlines the necessity for larger and better RCTs.
Statement of authorship
Category 1
-
(a)Conception and Design
- Oana Ciocanel; Kevin Power; Ann Eriksen
-
(b)Acquisition of Data
- Oana Ciocanel
-
(c)Analysis and Interpretation of Data
- Oana Ciocanel
Category 2
-
(a)Drafting the Article
- Oana Ciocanel
-
(b)Revising It for Intellectual Content
- Oana Ciocanel; Kevin Power; Ann Eriksen
Category 3
-
(a)Final Approval of the Completed Article
- Oana Ciocanel; Kevin Power; Ann Eriksen
Footnotes
Conflicts of Interest: The authors report no conflicts of interest.
Funding: None.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.esxm.2019.06.001.
Supplementary Data
References
- 1.Berman J.R. Physiology of female sexual function and dysfunction. Int J Impot Res. 2005;17:S44–S51. doi: 10.1038/sj.ijir.3901428. [DOI] [PubMed] [Google Scholar]
- 2.Lewis R.W., Fugl-Meyer K.S., Bosch R. Epidemiology/risk factors of sexual dysfunction. J Sex Med. 2004;1:35–39. doi: 10.1111/j.1743-6109.2004.10106.x. [DOI] [PubMed] [Google Scholar]
- 3.Thomas H.N., Thurston R.C. A biopsychosocial approach to women’s sexual function and dysfunction at midlife: A narrative review. Maturitas. 2016;87:49–60. doi: 10.1016/j.maturitas.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ernst E., Pittler M.H., Wider B. 2nd ed. Elsevier Mosby; Edinburgh: 2006. The desktop guide to complementary and alternative medicine. [Google Scholar]
- 5.American Psychiatric Association . 5th ed. American Psychiatric Association; Washington, DC: 2013. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- 6.Hatzimouratidis K., Amar E., Eardley I. Guidelines on male sexual dysfunctions: Erectile dysfunction and premature ejaculation. Eur Urol. 2010;57:804–814. doi: 10.1016/j.eururo.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 7.Laumann E.O., Paik A., Rosen R.C. Sexual dysfunction in the United States: Prevalence and predictors. J Am Med Assoc. 1999;281:537–544. doi: 10.1001/jama.281.6.537. [DOI] [PubMed] [Google Scholar]
- 8.Jannini E.A., Lombardo F., Lenzi A. Correlation between ejaculatory and erectile dysfunction. Int J Androl. 2005;28:40–45. doi: 10.1111/j.1365-2605.2005.00593.x. [DOI] [PubMed] [Google Scholar]
- 9.Feldman H.A., Goldstein I., Hatzichristou D.G. Impotence and its medical and psychosocial correlates: Results of the Massachusetts Male Aging Study. J Urol. 1994;151:54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- 10.Ayta I.A., McKinlay J.B., Krane R.J. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int. 1999;84:50–56. doi: 10.1046/j.1464-410x.1999.00142.x. [DOI] [PubMed] [Google Scholar]
- 11.Althof S.E., McMahon C.G., Waldinger M.D. An update of the International Society of Sexual Medicine's guidelines for the diagnosis and treatment of premature ejaculation (PE) J Sex Med. 2014;11:1392–1422. doi: 10.1111/jsm.12504. [DOI] [PubMed] [Google Scholar]
- 12.Cooper K., James M.S., Kaltenthaler E. Behavioral therapies for management of premature ejaculation: A systematic review. Sex Med. 2015;3:174–188. doi: 10.1002/sm2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laumann E.O., Nicolosi A., Glasser D.B. Sexual problems among women and men aged 40–80 y: Prevalence and correlates identified in the Global Study of Sexual Attitudes and Behaviors. Int J Impot Res. 2005;17:39–57. doi: 10.1038/sj.ijir.3901250. [DOI] [PubMed] [Google Scholar]
- 14.Porst H., Montorsi F., Rosen R.C. The Premature Ejaculation Prevalence and Attitudes (PEPA) survey: Prevalence, comorbidities, and professional help-seeking. Eur Urol. 2013;51:816–823. doi: 10.1016/j.eururo.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Christensen B.S., Gronbaek M., Osler M. Associations between physical and mental health problems and sexual dysfunctions in sexually active Danes. J Sex Med. 2011;8:1890–1902. doi: 10.1111/j.1743-6109.2010.02145.x. [DOI] [PubMed] [Google Scholar]
- 16.Tan H.M., Tong S.F., Ho C.C. Men’s health: Sexual dysfunction, physical, and psychological health—Is there a link? J Sex Med. 2012;9:663–671. doi: 10.1111/j.1743-6109.2011.02582.x. [DOI] [PubMed] [Google Scholar]
- 17.Rowland D., Perelman M., Althof S. Self-reported premature ejaculation and aspects of sexual functioning and satisfaction. J Sex Med. 2004;1:225–232. doi: 10.1111/j.1743-6109.2004.04033.x. [DOI] [PubMed] [Google Scholar]
- 18.Rowland D.L., Patrick D.L., Rothman M. The psychological burden of premature ejaculation. J Urol. 2007;177:1065–1070. doi: 10.1016/j.juro.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 19.Symonds T., Roblin D., Hart K. How does premature ejaculation impact a man’s life? J Sex Marital Ther. 2003;29:361–370. doi: 10.1080/00926230390224738. [DOI] [PubMed] [Google Scholar]
- 20.McMahon C.G., Althof S., Waldinger M.D. An evidence-based definition of lifelong premature ejaculation: Report of the International Society for Sexual Medicine Ad Hoc Committee for the Definition of Premature Ejaculation. BJU Int. 2008;102:338–350. doi: 10.1111/j.1464-410X.2008.07755.x. [DOI] [PubMed] [Google Scholar]
- 21.Althof S.E., Abdo C., Dean J. International Society for Sexual Medicine's Guidelines for the diagnosis and treatment of premature ejaculation. J Sex Med. 2010;7:2947–2969. doi: 10.1111/j.1743-6109.2010.01975.x. [DOI] [PubMed] [Google Scholar]
- 22.Shin B., Lee M., Yang E. Maca (L. meyenii) for improving sexual function: A systematic review. BMC Complement Altern Med. 2010;10:44. doi: 10.1186/1472-6882-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ernst E., Posadzki P., Lee M.S. Complementary and alternative medicine (CAM) for sexual dysfunction and erectile dysfunction in older men and women: An overview of systematic review. Maturitas. 2011;70:37–41. doi: 10.1016/j.maturitas.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Bella A.J., Lee J.C., Carrier S. CUA practice guidelines for erectile dysfunction. Can Urol Assoc. 2015;9:23–29. doi: 10.5489/cuaj.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waldinger M.D. History of Premature Ejaculation. In: Jannini E., McMahon C.G., Waldinger M.D., editors. Premature ejaculation. From etiology to diagnosis and treatment. Springer-Verlag; Milan: 2013. pp. 5–24. [Google Scholar]
- 26.Melnik T., Soares B., Nasello A.G. Psychosocial interventions for erectile dysfunction. Cochrane Database Syst Rev. 2007;2:CD004825. doi: 10.1002/14651858.CD004825.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fruhauf S., Gerger H., Schmidt H.M. Efficacy of psychological interventions for sexual dysfunction: A systematic review and meta-analysis. Arch Sex Behav. 2013;42:915–933. doi: 10.1007/s10508-012-0062-0. [DOI] [PubMed] [Google Scholar]
- 28.Yuan J., Zhang R., Yang Z. Comparative effectiveness and safety of oral phosphodiesterase type 5 inhibitors for erectile dysfunction: A systematic review and network meta-analysis. Eur Urol. 2013;63:902–912. doi: 10.1016/j.eururo.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 29.van Lankveld J.J.D.M. Bibliotherapy in the treatment of sexual dysfunctions: A meta-analysis. J Consul Clin Psych. 1998;66:702–708. doi: 10.1037//0022-006x.66.4.702. [DOI] [PubMed] [Google Scholar]
- 30.Burls A., Gold L., Clark W. Systematic review of randomised controlled trials of sildenafil (Viagra) in the treatment of male erectile dysfunction. Br J Gen Pract. 2001;51:1004–1012. [PMC free article] [PubMed] [Google Scholar]
- 31.Gong B., Ma M., Xie W. Direct comparison of tadalafil with sildenafil for the treatment of erectile dysfunction: A systematic review and meta-analysis. Int Urol Nephrol. 2017;49:1731–1740. doi: 10.1007/s11255-017-1644-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt H.M., Munder T., Gerger H. Combination of psychological intervention and phosphodiesterase-5 inhibitors for erectile dysfunction: A narrative review and meta-analysis. J Sex Med. 2014;11:1376–1391. doi: 10.1111/jsm.12520. [DOI] [PubMed] [Google Scholar]
- 33.Melnik T., Soares B., Nasello A.G. The effectiveness of psychological interventions for the treatment of erectile dysfunction: Systematic review and meta-analysis, including comparisons to sildenafil treatment, intracavernosal injection, and vacuum devices. J Sex Med. 2008;5:2562–2574. doi: 10.1111/j.1743-6109.2008.00872.x. [DOI] [PubMed] [Google Scholar]
- 34.Cooper K., Martyn-St James M., Kaltenthaler E. Interventions to treat premature ejaculation: a systematic review short report. Health Technol Assess. 2015;19:1–180. doi: 10.3310/hta19210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Higgins J.P.T., Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration2011. 2011. https://handbook.cochrane.org Available at:
- 36.Smith V., Devane D., Begley C. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med Res Method. 2011;11:15. doi: 10.1186/1471-2288-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moher D., Altman D.G., Liberati A. PRISMA statement. Epidemiology. 2011;22:128. doi: 10.1097/EDE.0b013e3181fe7825. [DOI] [PubMed] [Google Scholar]
- 38.Dyer S.M., Harrison S.L., Laver K. An overview of systematic reviews of pharmacological and non-pharmacological interventions for the treatment of behavioral and psychological symptoms of dementia. Int Psychogeriatr. 2018;30:295–309. doi: 10.1017/S1041610217002344. [DOI] [PubMed] [Google Scholar]
- 39.Laver K., Dyer S., Whitehead C. Interventions to delay functional decline in people with dementia: a systematic review of systematic reviews. BMJ Open. 2016;6:e010767. doi: 10.1136/bmjopen-2015-010767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perry R., Leach V., Davies P. An overview of systematic reviews of complementary and alternative therapies for fibromyalgia using both AMSTAR and ROBIS as quality assessment tools. Syst Rev. 2017;6:97. doi: 10.1186/s13643-017-0487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mickan S., Tilson J.K., Atherton H. Evidence of effectiveness of health care professionals using handheld computers: A scoping review of systematic reviews. J Med Internet Res. 2013;15:e212. doi: 10.2196/jmir.2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bobrovitz N., Heneghan C., Onakpoya I. Medications that reduce emergency hospital admissions: an overview of systematic reviews and prioritisation of treatments. BMC Med. 2018;16:115. doi: 10.1186/s12916-018-1104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riegelman R. Lippincott Williams & Wilkins; Philadelphia, PA: 2000. Studying a study and testing a test: How to read the medical evidence. [Google Scholar]
- 44.Nallamothu B., Hayward R.A., Bates E.R. Beyond the randomized clinical trial: The role of effectiveness studies in evaluating cardiovascular therapies. Circulation. 2008;118:1294e303. doi: 10.1161/CIRCULATIONAHA.107.703579. [DOI] [PubMed] [Google Scholar]
- 45.Shea B.J., Reeves B.C., Wells G. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miles C.L., Candy B., Jones L. Interventions for sexual dysfunction following treatment for cancer. Cochrane Database Syst Rev. 2007;17:CD005540. doi: 10.1002/14651858.CD005540.pub2. [DOI] [PubMed] [Google Scholar]
- 47.Carter J., Lacchetti C., Andersen B.L. Interventions to address sexual problems in people with cancer: American Society of Clinical Oncology Clinical Practice Guideline Adaption of Cancer Care Ontario Guideline. J Clin Oncol. 2018;36:492–511. doi: 10.1200/JCO.2017.75.8995. [DOI] [PubMed] [Google Scholar]
- 48.Balhara Y.P., Sarkar S., Gupta R. Phosphodiesterase-5 inhibitors for erectile dysfunction in patients with diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Indian J Endocrinol Metab. 2015;19:451–461. doi: 10.4103/2230-8210.159023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shabsigh R., Duval S., Shah M. Efficacy of vardenafil for the treatment of erectile dysfunction in men with hypertension: A meta-analysis of clinical trial data. Curr Med Res Opin. 2007;23:2453–2460. doi: 10.1185/030079907X219616. [DOI] [PubMed] [Google Scholar]
- 50.Garcia-Perdomo H.A., Echeverria-Garcia F., Tobias A. Effectiveness of phosphodiesterase 5 inhibitors in the treatment of erectile dysfunction in patients with spinal cord trauma: systematic review and meta-analysis. Urol Int. 2017;98:198–204. doi: 10.1159/000448290. [DOI] [PubMed] [Google Scholar]
- 51.Thomson K., Bambra C., McNamara C. The effects of public health policies on population health and inequalities in European welfare states: protocol for an umbrella review. Syst Rev. 2016;5:57. doi: 10.1186/s13643-016-0235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pollock M., Fernandes R.M., Becker L.A. What guidance is available for researchers conducting overviews of reviews of healthcare interventions? A scoping review and qualitative meta summary. Syst Rev. 2016;5:190. doi: 10.1186/s13643-016-0367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McHugh M.L. Interrater reliability: The Kappa statistic. Biochemia Medica. 2012;22(3):276–282. [PMC free article] [PubMed] [Google Scholar]
- 54.Castiglione F., Albersen M., Hedlund P. Current pharmacological management of premature ejaculation. A systematic review and meta-analysis. Eur Urol. 2016;69:904–916. doi: 10.1016/j.eururo.2015.12.028. [DOI] [PubMed] [Google Scholar]
- 55.Clavijo R.I., Kohn T.P., Kohn J.R. Effects of low-intensity extracorporeal shockwave therapy of erectile dysfunction: A systematic review and meta-analysis. J Sex Med. 2017;14:27–35. doi: 10.1016/j.jsxm.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 56.Corona G., Rastrelli G., Burri A. The safety and efficacy of Avanafil, a new 2(nd) generation PDE5i: comprehensive review and meta-analysis. Expert Opin Drug Saf. 2016;15:237–247. doi: 10.1517/14740338.2016.1130126. [DOI] [PubMed] [Google Scholar]
- 57.Corona G., Rastrelli G., Morgentaler A. Meta-analysis of results of testosterone therapy on sexual function based on International Index of Erectile function scores. Eur Urol. 2017;72:1000–1011. doi: 10.1016/j.eururo.2017.03.032. [DOI] [PubMed] [Google Scholar]
- 58.De Hong C., Ren L.L., Yu H. The role of dapoxetine hydrochloride on-demand for the treatment of men with premature ejaculation. Sci Rep. 2014;4:7269. doi: 10.1038/srep07269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Du W., Li J., Fan N. Efficacy and safety of mirodenafil for patients with erectile dysfunction: a meta-analysis of three multicenter, randomised, double-blind, placebo-controlled trials. Aging Male. 2014;17:107–111. doi: 10.3109/13685538.2013.858114. [DOI] [PubMed] [Google Scholar]
- 60.Fink H.A., MacDonald R., Rutks I.R. Trazodone for erectile dysfunction: A systematic review and meta-analysis. BJU Int. 2003;92:441–446. doi: 10.1046/j.1464-410x.2003.04358.x. [DOI] [PubMed] [Google Scholar]
- 61.Kostis J.B., Dobrzynski J.M. The effect of statins on erectile dysfunction: A meta-analysis of randomized trials. J Sex Med. 2014;11:1626–1635. doi: 10.1111/jsm.12521. [DOI] [PubMed] [Google Scholar]
- 62.Peixoto C., Carrilho C.G., Barrros J.A. The effects of dehydroepiandrosterone on sexual function: A systematic review. Climacteric. 2017;20:129–137. doi: 10.1080/13697137.2017.1279141. [DOI] [PubMed] [Google Scholar]
- 63.Ernst E., Pittler M.H. Yohimbine for erectile dysfunction: a systematic review and meta-analysis of randomised clinical trials. J Urol. 1998;159:433–436. doi: 10.1016/s0022-5347(01)63942-9. [DOI] [PubMed] [Google Scholar]
- 64.Jang D.J., Lee M.S., Shin B.C. Red ginseng for treating erectile dysfunction: A systematic review. Br J Clin Pharmacol. 2008;66:444–450. doi: 10.1111/j.1365-2125.2008.03236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Silva A.B., Sousa N., Azevedo L.F. Physical activity and exercise for erectile dysfunction: A systematic review and meta-analysis. Br J Sports Med. 2017;51:1419–1424. doi: 10.1136/bjsports-2016-096418. [DOI] [PubMed] [Google Scholar]
- 66.Xiong G., Li B., Wang K., Li H. Chinese herb formulae for treatment of erectile dysfunction: A systematic review of randomised controlled clinical trials. Andrologia. 2014;46:201–223. doi: 10.1111/and.12074. [DOI] [PubMed] [Google Scholar]
- 67.Martyn St., James M., Cooper K., Ren K. Topical anaesthetics for premature ejaculation: a systematic review and meta-analysis. Sex Health. 2016;13:114–123. doi: 10.1071/SH15042. [DOI] [PubMed] [Google Scholar]
- 68.Urciuoli R., Cantisani T.A., Carlini M. Prostaglandin E1 for treatment of erectile dysfunction. Cochrane Database Syst Rev. 2004;2:CD00178. doi: 10.1002/14651858.CD001784.pub2. [DOI] [PubMed] [Google Scholar]
- 69.Yang Y., Wang X., Bai Y. Circumcision does not have effect on premature ejaculation: A systematic review and meta-analysis. Andrologia. 2018;50:e12851. doi: 10.1111/and.12851. [DOI] [PubMed] [Google Scholar]
- 70.Schoonees A., Visser J., Musekiwa A. Pycnogenol for the treatment of chronic disorders. Cochrane Database Syst Rev. 2012;15:CD008294. doi: 10.1002/14651858.CD008294.pub4. [DOI] [PubMed] [Google Scholar]
- 71.Corona G., Isidori A.M., Buvat J. Testosterone supplementation and sexual function: A meta-analysis study. J Sex Med. 2014;11:1577–1592. doi: 10.1111/jsm.12536. [DOI] [PubMed] [Google Scholar]
- 72.Gupta B., Murad M.H., Clifton M.M. The effect of lifestyle modification and cardiovascular risk factor reduction on erectile dysfunction: A systematic review and meta-analysis. Arch Intern Med. 2011;171:1797–1803. doi: 10.1001/archinternmed.2011.440. [DOI] [PubMed] [Google Scholar]
- 73.Cui X., Zhou J., Qin Z. Acupuncture for erectile dysfunction: A systematic review. Biomed Res Int. 2016:2171923. doi: 10.1155/2016/2171923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martyn-St James M., Cooper K., Kaltenthaler E. Tramadol for premature ejaculation: A systematic review and meta-analysis. BMC Urol. 2015;15:6. doi: 10.1186/1471-2490-15-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martyn-St James M., Cooper K., Ren S. Phosphodiesterase-5 inhibitors for premature ejaculation: A systematic review and meta-analysis. Eur Urol Focus. 2017;3:119–129. doi: 10.1016/j.euf.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rosen R.C., Riley A., Wagner G. The International index of erectile dysfunction (IIEF): A multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–830. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 77.Dhir R.R., Hao-Cheng L., Canfield S.E. Combination therapy for erectile dysfunction: An update review. Asian J Androl. 2011;13:382–390. doi: 10.1038/aja.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moyad M.A., Barada J.H., Lue T.F. Prevention and treatment of erectile dysfunction using lifestyle changes and dietary supplements: What works and what is worthless, part I. Urol Clin North Am. 2004;31:249–257. doi: 10.1016/j.ucl.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 79.Moyad M.A., Barada J.H., Lue T.F. Prevention and treatment of erectile dysfunction using lifestyle changes and dietary supplements: What works and what is worthless, part II Urol. Clin North Am. 2004;31:259–273. doi: 10.1016/j.ucl.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 80.Derby C.A., Mohr B.A., Goldstein I. Modifiable risk factors and erectile dysfunction: Can lifestyle changes modify risk? Urology. 2000;56:302–306. doi: 10.1016/s0090-4295(00)00614-2. [DOI] [PubMed] [Google Scholar]
- 81.Esposito K., Giugliano F., Di P.C. Effect of lifestyle changes on erectile dysfunction in obese men: A randomized controlled trial. JAMA. 2004;291:2978–2984. doi: 10.1001/jama.291.24.2978. [DOI] [PubMed] [Google Scholar]
- 82.Calderwood C. Realistic medicine: Chief Medical Officer’s Annual Report 2014–15. http://www.gov.scot/Resource/0049/00492520.pdf Available from.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.