Abstract
Objective
To evaluate outcomes of fluorinated corticosteroids, with or without other medications, for treatment of congenital heart block in-utero.
Study design
A search was conducted through MEDLINE, EMBASE, WEB OF SCIENCE and SCOPUS from inception to October 2017. Only comparative studies are considered eligible. Outcomes include fetal death, downgrade of heart block, neonatal death, need for neonatal pacing, fetal and maternal complications. Random effects model was used.
Results
Out of 923 articles, 12 studies were eligible. Compared to no treatment, there was no significant difference in incidence of fetal death (OR 1.10, 95%CI 0.65–1.84), neonatal death (OR 0.98, 95%CI 0.41–2.33), or need for pacing (OR 1.46, 95%CI 0.78–2.74). Heart block downgrade was significantly higher in treatment group (9.48%vs.1.76%, OR 3.27, 95%CI 1.23–8.71).
Conclusion
antenatal fluorinated corticosteroids do not improve fetal/neonatal morbidity or mortality of congenital heart block and are associated with higher incidence of fetal and maternal complications.
Keywords: Congenital heart disease, Fetal diagnosis, Fetal therapy, Prenatal treatment
Introduction
Congenital heart block (CHB) is a rare fatal condition that may eventually lead to fetal demise, neonatal death, or permanent pacemaker implantation [1]. Incidence of CHB is approximately 1 in 20,000–30,000 live births [2]. CHB may occur in a structurally normal heart (isolated CHB) as a complication of maternal autoimmune disease or in fetuses with congenital heart defects (complex CHB) [3].
The incidence of CHB is 2% among women with Ro-positive antibodies without previously affected offspring, 15–20% among women with Ro-positive antibodies and with previously affected offspring, and 5% among women with mixed connective tissue and/or Sjögren Syndrome [[4], [5], [6]]. Among fetuses exposed to anti-Ro antibodies, 17.5% may be complicated by fetal demise, and 70% would eventually need permanent pacemaker implantation in the 10 years of life [7]. Permanent pacemaker implantation has been considered the only intervention that improves survival rate among neonates with CHB [8,9].
Prenatal diagnosis of CHB can be achieved early in the second trimester, either incidentally during intermittent auscultation or during anatomical survey ultrasound, and can be confirmed by fetal echocardiogram with Doppler techniques to determine level of heart block and verify any underlying major structural heart lesions. Therefore, several studies investigated a possible role of immediate post-diagnosis fetal therapy to improve fetal and neonatal outcomes of CHB. Treatment options include fluorinated and non-fluorinated corticosteroids, immunoglobulins or combined treatment. The aim of treatment is to reverse or downgrade CHB and to prevent intrauterine progression of the disease which can be manifested as hydrops fetalis, pericardial effusion, cardiomegaly, which impacts overall survival rate and lines of treatment [10].
In this study, the aim is to summarize the effect of fetal treatment of CHB with fluorinated corticosteroids, with or without other medications, on fetal and neonatal survival rates and the need for permanent pacemaker implantation. We also aimed to evaluate potential maternal and fetal complications associated with prenatal treatment.
Materials and methods
Literature search
The authors conducted a literature search for studies that assessed maternal, fetal and neonatal outcomes among pregnant women whose fetuses were diagnosed with congenital heart block during pregnancy. Studies that compared the use of fluorinated corticosteroids with or without other medications in comparison to no treatment during pregnancy. The search covered MEDLINE, EMBASE (with online Ovid interface), WEB OF SCIENCE and SCOPUS and was done in collaboration with an expert librarian. Studies conducted from the date of database inception to October 2017 were included. We used the following search terms: "Treatment" OR "management" AND "fetal" OR "congenital" OR "in utero" AND "heart block" OR "aterioventricular block". Search was set to filter out conference papers and review articles. In addition, manual search on additional references was achieved by reviewing references of articles retrieved by initial search. The detailed search strategy is appended (Appendix I). The risk of bias was assessed using the Newcastle-Ottawa Scale (NOS) [11]
Eligibility criteria and study selection
After conduction of literature search, 2 reviewers performed independent screening of titles and abstracts to exclude irrelevant studies. After exclusion of irrelevant studies, we reviewed the full text of the remaining studies for final selection of eligible articles. Minor discrepancies in data were adjudicated by consensus among reviewers. Comparative studies that address obstetric and neonatal outcomes among pregnant women who and who did not receive fluorinated corticosteroids (with or without other medications) to manage congenital heart block. Case reports, case series, and single arm studies were not included. However, neither language nor sample size was considered for exclusion.
Fetal death, downgrade of heart block, development of hydrops, average intrauterine fetal heart rate, neonatal death, and the need for neonatal pacing present our primary outcomes. Secondary outcomes were fetal and maternal complications that could be potentially related to treatment e.g. oligohydramnios, intrauterine growth restriction (IUGR). These outcomes were analyzed separately. However, average intrauterine fetal heart was not included in final analysis because documentation was missing in the majority of selected studies.
Data abstraction
A standardized form was designed for abstraction of data from selected studies. The form consists of study authors, study origin, type of study, time frame during which the study was conducted, sample size, gestational age at diagnosis, maternal and fetal risk factors, selection criteria of study population, study arms, type of medications used,duration of intervention and studied outcomes. The form also included primary and secondary outcomes as listed above.
Data analysis
Binary outcomes were expressed as odds ratios (OR) and 95% confidence interval (CI). Due to anticipated heterogeneity, pooling of results was performed using random-effect model [12].
Heterogeneity was evaluated using I squared statistic. I squared value over 50% is consistent with substantial heterogeneity [13]. Review Manager (RevMan) Version 5.3 was used to conduct statistical analysis for this review (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) [14].
Results
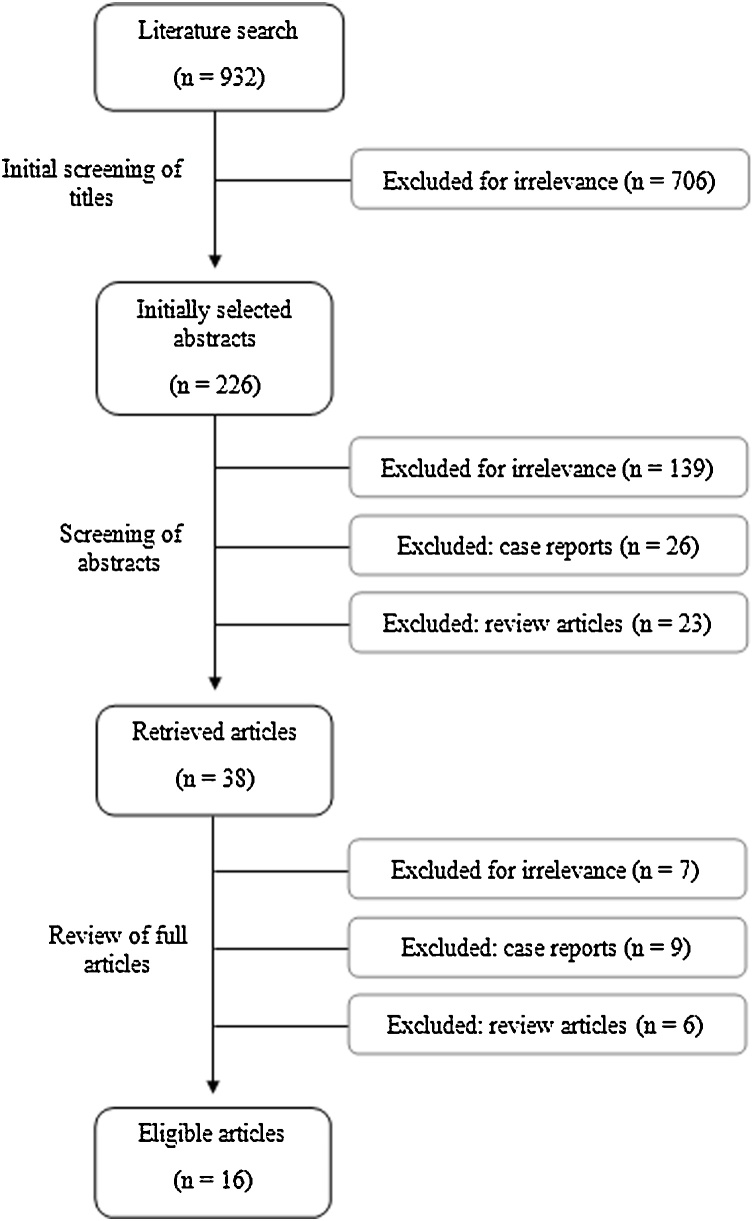
Database search yielded 923 articles. Preliminary screening of titles allowed exclusion of 706 articles for irrelevance. Reviewing abstracts of the remaining 217 articles, 87 were excluded for irrelevance, 26 were case reports, 23 were review articles. Full texts of the remaining 38 articles were retrieved. Of those 38 articles, 16 articles meet our inclusion criteria (Fig. 1). Summary of study demographics and study design is illustrated in Table 1, Table 2 [7,[15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29]]. Appendix II summarized risk of bias of included studies.
Fig. 1.
Study selection flow chart.
Table 1.
Demographics of population of included studies.
| Authors | Study origin | Study type | Time frame | Sample size | Gestational age at diagnosis | Maternal/fetal risk factors |
|---|---|---|---|---|---|---|
| Buyon et al. (1995) | New York, USA | Retrospective study | 1985-1993 | 72 | 16 to 40 weeks of gestation. In 4 (5.6%) pregnancies, diagnosis time was not reported |
|
| Shinohara et al. (1999) | Osaka, Japan | Retrospective study | 1979-1996 | 15 | 20-21 weeks of gestation |
|
| Saleeb et al. (1999) | New York, USA | Retrospective study | 1983-1998 | 50 | 21.6 weeks and 24.2 weeks of gestation for the treated and untreated groups, respectively |
|
| Jaeggi et al. (2004) | Ontario, Canada | Retrospective study | 1990-2003 | 37 | 27 ± 6.5 weeks of gestation (1990–1996), 24.7 ± 3.7 5 weeks (1997-2003) |
|
| Lopes et al. (2008) | São Paulo, Brazil | Retrospective study | 1988-2006 | 57 | 29 (18–40) weeks of gestation |
|
| Fesslova et al. (2009) | Milan, Italy | Retrospective study | 1992-2004 | 28 | 25 (19 to 32) weeks of gestation |
|
| Jaeggi et al. (2010) | Ontario, Canada | Prospective study | 2000-2008 | 34 | 22.5 weeks (19-39 weeks) | None reported |
| Trucco et al. (2011) | Ontario, Canada | Retrospective study | 1998-2009 | 20 | 23 weeks (range 18 to 38 weeks) |
|
| Eliasson et al. (2011) | 27 centers in Europe and 1 in Brazil | Retrospective study | 2000-2007 | 175 | 24.3 ± 4.3 weeks for all cases, 23.4 ± 2.9 weeks for steroid treated, and 24.9 ± 4.9 weeks for the untreated group |
|
| Izmirly et al. (2011) | New York, USA | Retrospective study | 1963-2010 | 21 | 24.8 weeks (for deceased cases) and 26.9 weeks (for survived cases) | None reported |
| Miyoshi et al. (2012) | Suita, Japan | Questionnaire study | 2002-2008 | 77 | 24 ± 3.2 weeks for intervention group and 28 ± 5.7 weeks for non-intervention group |
|
| Perin et al. (2014) | Granada, Spain | Retrospective multicenter study | 2008-2010 | 19 | 23.5 week of gestation |
|
| Levesque et al. (2015) | Paris, France | Retrospective study | 1976-2014 | 202 | Median gestational age at time of diagnosis was 23 weeks of gestation |
|
| Kuleva et al. (2015) | Paris, France | Retrospective study | 2002-2012 | 39 | 22 – 23 weeks of gestation on average |
|
| Izmirly et al. (2016) | New York, USA | Retrospective study | 1972-2013 | 156 | 22.1 ± 2.8 weeks for intervention group and 22.8 ± 3.1 weeks for the non-intervention group |
|
| Van den Berg et al. (2016) | Utrecht, The Netherlands | Retrospective study | 2003-2013 | 56 | Mean gestational age was 23.4 ± 5 weeks of gestation |
|
Table 2.
Study design of included studies.
| Authors | Eligibility criteria | Comparison groups | Type of intervention | Duration of intervention | Study outcomes |
|---|---|---|---|---|---|
| Buyon et al. (1995) | Women with positive anti-SSA/Ro and/or SSB/La antibodies whose fetuses were diagnosed with congenital heart block | 45 pregnancies received no treatment, 8 pregnancies received prednisone only, and 19 pregnancies received fluorinated steroids |
|
From the time of diagnosis to the time of delivery. |
|
| Shinohara et al. (1999) | Positive maternal serum for anti-Ro/SSA antibodies. | 11 fetuses received no treatment and 4 fetuses received corticosteroid therapy. | 15–20 mg of prednisolone per day or betamethasone | After 16 weeks’ gestation (as a prophylaxis) till delivery |
|
| Saleeb et al. (1999) | Positive maternal serum for antibodies for 52/60-kd SSA/Ro, and/or 48-kd SSB/La RNPs during or within 1 year of pregnancy, and isolated heart block diagnosed in-utero before 5 weeks of birth | 22 fetuses received no treatment in-utero compared to 28 fetuses that were exposed to fluorinated steroids. | Trans-placental treatment with fluorinated steroids (dexamethasone 4–9 mg/day or betamethasone 12–24 mg/week) | Treatment started within three weeks of diagnosis of heart block and for 3–19 weeks (for dexamethasone) or > 6 weeks (for betamethasone) |
|
| Jaeggi et al. (2004) | Isolated congenital atrioventricular block diagnosed by M-mode or Doppler echocardiography. |
|
|
For the time of diagnosis till delivery |
|
| Lopes et al. (2008) | Isolated fetal heart block diagnosed via standard echocardiography by a fetal cardiologist | 46 fetuses received no treatment compared to 11 fetuses who received trans-placental therapy. | Trans-placental therapy for 11 (19.5%) fetuses (dexamethasone (4 or 8 mg/d for 2 weeks, followed by 4 mg/d maintained for the duration of the pregnancy) (3.5%), Steroid and sympathomimetic for 4(7%) and sympathomimetic only for 6(9%). |
From the time of diagnosis for the duration of the pregnancy. |
|
| Fesslova et al. (2009) | Diagnosis of isolated heart block via echocardiography by a cardiologist. | 7 fetuses with isolated heart block unexposed to any treatment compared to 21 fetuses treated with dexamethasone and/or sympathomimetics in-utero |
Dexamethasone alone (18 cases) at 4 mg per day, combined with salbutamol (2 cases) or isoproterenol (1 case) |
Treatment was started within 2 weeks of presentation until time of delivery |
|
| Jaeggi et al. (2010) | Positive maternal anti-Ro and-La antibodies by ELISA. | Six fetuses were not exposed to any treatment in utero compared to 28 fetuses treated with dexamethasone and intravenous immunoglobulins. | Maternal dexamethasone (4 or 8 mg/day for 2 weeks, followed by 4 mg/day) then (2 mg/day) and intravenous immunoglobulins 70 gram every 2 to 3 weeks. | Starting from the time of diagnosis till the third trimester |
|
| Trucco et al. (2011) |
|
three fetuses were not exposed to any treatment in utero compared to 17 fetuses treated with dexamethasone only or plus intravenous immunoglobulins and/or beta-sympathomimetic. | Dexamethasone only (4-8 mg/day) for 4 (%20) mothers. Dexamethasone (4-8 mg/day) plus intravenous immunoglobulins (1 g/dose) for 4 (20%) mothers. Dexamethasone (4-8 mg/day) plus beta-sympathomimetic for 4 (20%) mothers. Dexamethasone (3-16 mg/day), beta-sympathomimetic plus intravenous immunoglobulins (1 g/dose) for 5 (25%) mothers. |
From diagnosis till delivery and during neonatal period |
|
| Eliasson et al. (2011) |
|
108 untreated fetuses compared to 67 fetuses (38%) treated fetuses with trans-placental steroids |
|
Treatment started from a median of 10 weeks (1–21 weeks) till delivery. |
|
| Izmirly et al. (2011) |
|
8 fetuses with second degree heart block were not treated in utero versus 13 fetuses treated with fluorinated steroid | In utero treatment with dexamethasone | From diagnosis till delivery |
|
| Miyoshi et al. (2012) | Diagnosis of fetal atrioventricular block with structurally normal hearts | 31 fetuses (23 with complete heart block and 8 with second degree hear block (untreated) compared to 46 fetuses (38 with complete heart block and 8 second degree) that did not receive treatment in utero | Trans-placental Beta-sympathomimetic and/or a steroid (dose was not specified) | From the time of detection till delivery |
|
| Roy et al. (2014) |
|
No comparison groups in terms of treatment | Intrauterine treatment with dexamethasone 4 mg/day | Treatment started at 25 weeks till delivery |
|
| Perin et al. (2014) | Diagnosis of fetal bradycardia | Nine cases who did not receive any medication compared to 10 cases treated with steroids and beta-stimulants. | Trans-placental dexamethasone (administered in doses of 4 mg every 24 hours; a loading dose of 6-8 mg/day was administered in 3 cases). Two cases were treated with beta-stimulants. |
Treatment continued for an average of 5 weeks (ranged from 2 to 12 weeks) |
|
| Levesque et al. (2015) | Inclusion:
|
One hundred twenty three fetuses who were not exposed to trans-placental corticosteroids compared to 79 fetuses exposed to fluorinated steroids |
Intrauterine treatment with fluorinated steroids with median initial dose of 2 mg-10 mg/d that was progressively tapered | A median of 56 days (10 to 126 days). |
|
| Kuleva et al. (2015) |
|
Twenty two fetuses not exposed to fluorinated steroids compared to 17 fetuses treated with fluorinated steroids in utero | Maternal administration of dexamethasone (4 mg/day) or betamethasone (4–8 mg/day. | Treatment started around the mid-gestation till delivery. |
|
| Izmirly et al. (2016) | Inclusion:
|
Seventy one fetuses were treated with fluorinated steroids compared to 85 fetuses not exposed to fluorinated steroids | Dexamethasone was given with an average daily dose of 2.8 ± 1.8 mg daily (range: 2–8 mg/day) | Treatment started within the first week of diagnosis of isolated block detection till delivery |
|
| Van den Berg et al. (2016) | Inclusion:
|
Forty two fetuses did not receive any medication compared to 14 fetuses treated with dexamethasone in-utero | Intrauterine dexamethasone treatment with median initial dose of 2-16 mg/day. | From time of diagnosis till delivery. |
|
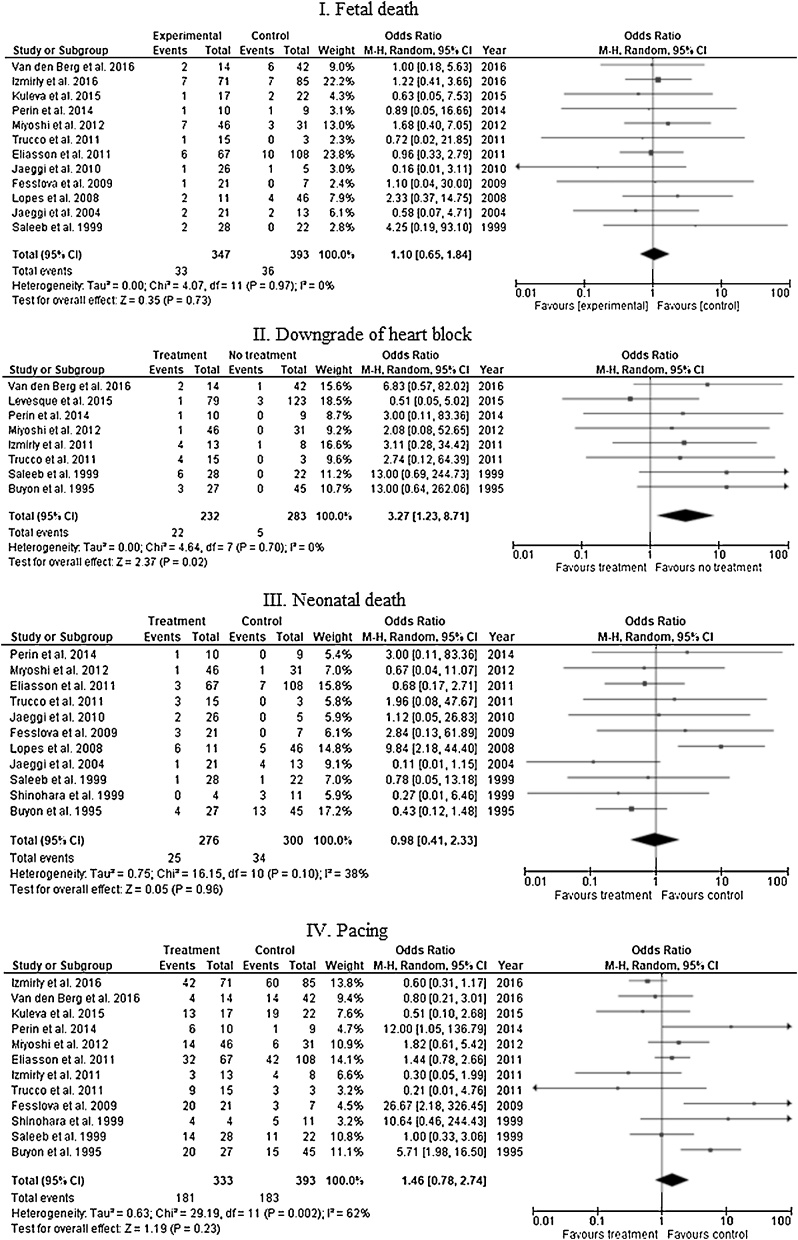
Comparing fetuses diagnosed with CHB who were treated with fluorinated steroids with or without other treatment options to fetuses who were not exposed to any treatment, 12 studies reported the rate of fetal death. The rate of fetal death among exposed group was 9.5% (33/347) compared to 9.16% (36/393) in the non-exposed group, the difference was not statistically significant (OR 1.10, 95% CI 0.65–1.84), I square value is 0%. Downgrading of CHB was reported in 8 studies. The incidence of downgrading was reported in 9.5% (22/232) versus 1.8% (5/283) in treated and non-treated groups, respectively. The difference was statistically significant (OR 3.27, 95% CI 1.23–8.71) with I square value of 0%. There was no significant difference in the incidence of neonatal death between both group as reported in 11 studies (9.1% [25/276] in the treated group versus 11.3% [34 / 300] in the untreated group, OR 0.98, 95% CI 0.41–2.33). I square value is 38%. Also, twelve studies assessed the need for pacing among treated and untreated fetuses, which was reported in 54.35% of the treated group (181/333) versus 46.56% (183/393) of the untreated group (OR 1.46, 95% CI 0.78–2.74). Data on this outcome yielded substantial heterogeneity (I square value is 62%) (Fig. 2).
Fig. 2.
Fetal and neonatal outcomes of treatment versus no-treatment among fetuses with congenital heart block.
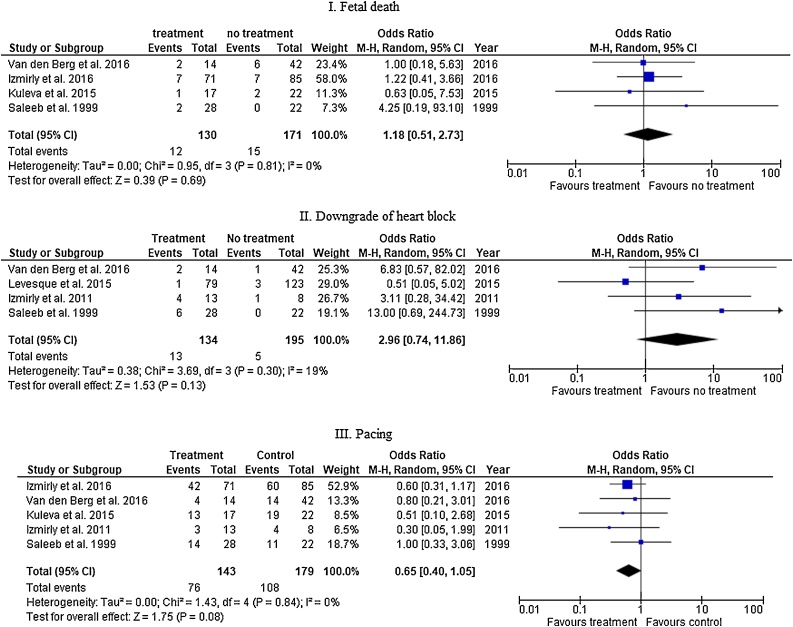
Subgroup analysis of studies was conducted for studies that compared steroids only to no treatment. In 4 studies, fetal death was 9.2% (12/130) in the treated group and 8.8% (15/171) in the untreated group (OR 1.18, 95% CI 0.51 – 2.73, I square value is 0%). CHB downgrading was documented in 4 studies. The incidence was 9.7% (13/134) and 2.6% (5/195) among treated and untreated groups, respectively (OR 2.96, 95% CI 0.74 – 11.86, I square value is 19%). Five studies compared the rate of pacing among treated and untreated groups (53.1% [76 / 143], 60.3% [108 / 179], respectively, OR 0.65, 95% CI = 0.40–1.05, I square value is 0% (Fig. 3).
Fig. 3.
Fetal and neonatal outcomes of fluorinated steroids only versus no-treatment among fetuses with congenital heart block.
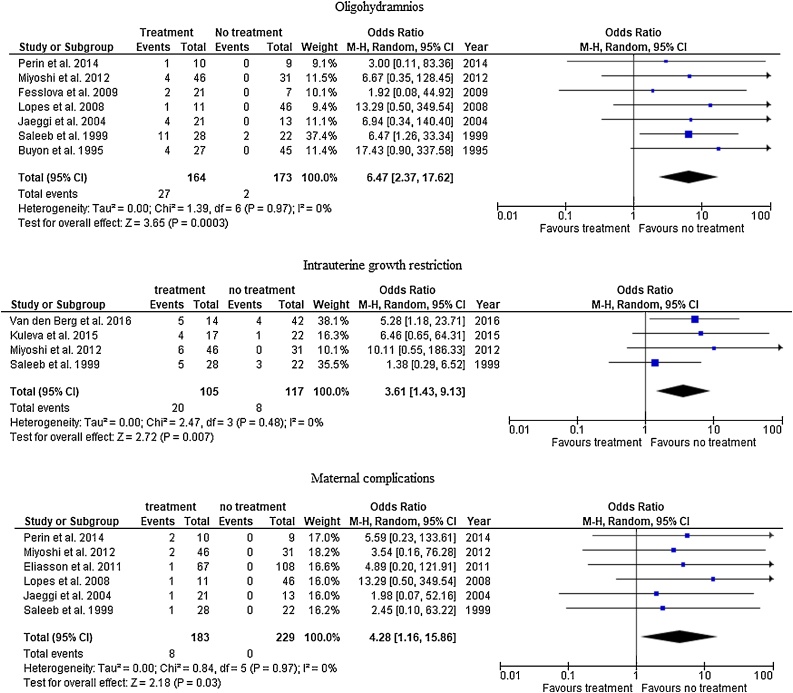
The incidence of oligohydramnios was documented in 7 studies. Among all treated fetuses, the incidence of oligohydramnios was significantly higher in the treated group (16.6% [27/164]) than untreated group (1.2% [2/173]) (OR 6.47, 95% CI 2.37–17.62). Similarly, 4 studies showed that the incidence of IUGR is 19% (20/105) in the treated group compared to 6.8% (8/117) in the untreated group, which is statistically significance (OR 3.61, 95% CI 1.43–9.13), I square value is 0%. Similarly, the incidence of maternal complications was higher among the treated than untreated group as reported in 6 studies (4.4% [8/183], 0% [0/229], OR 4.28, 95% CI 1.16–15.86). I square value is 0% (Fig. 4).
Fig. 4.
Complications of treatment.
Discussion
Several therapeutic options have been investigated to achieve early prenatal management of congenital heart diseases including CHB with the aim of reducing significant morbidity and mortality in utero [30]. In this systemic meta-analysis, we investigated the potential role of fluorinated steroids, alone or in combination with other medical options, to improve intrauterine or postnatal outcomes of CHB. According to our results, the use of fluorinated steroids, either alone or in combination with sympathomimetic drugs, did not reduce the rate of fetal death, neonatal death, or the rate of neonatal pacing compared to no intervention. Medical treatment showed superiority to no intervention in the incidence of CHB downgrading. This advantage is not evident with the use of fluorinated steroids alone. However, this effect does not seem to be clinically significant. On the other sides, as medical intervention was administered from the time of diagnosis to the time of delivery in most studies, our results also showed increased risk of oligohydramnios, IUGR and maternal complications among women receiving medical treatment for CHB compared to no intervention.
Understanding the etiology and mechanism of CHB may clarify the theoretical basis of prenatal medical treatment. CHB secondary to fetal cardiac structural anomalies typically yields poor prognosis known for a congenital heart disease [31]. Poor prognosis is not fully understood. However, it is likely related to the underlying disease which contributes to complexity of care [31]. In these cases, in-utero administration of beta-receptor agonists may increase fetal heart rate to above 55 beats / minute. Beta-adrenergic agonists act on both atrial and ventricular rates with varying response due to defect in the A-V node or other anomalies of the conduction system of the heart [32]. They seem to act locally with no neural affection during the stimulation process of primary (i.e., atrial) and secondary (i.e., ventricular) pacemakers or even theoretical suggestion that there is a nodal pacemaker responsible for heart rate acceleration under effect of beta receptor agonists [32]. However, long-term outcomes including survival did not improve. Moreover, fetal Tachycardia with arrhythmia and maternal tachycardia were reported as complications [[30], [31], [32]]. On the other side, CHB secondary to maternal immunological disorders is likely related to immune response in form of inflammation and fibrosis, which subsequently damages conduction fibers and myocardium. Therefore, a proposed treatment approach would be to control immune response before permanent tissue damage occurs [33]. Nevertheless, plasmapheresis as well as intravenous injection of immunoglobulin (to decrease serum levels of anti- Ro and La antibodies) did not produce long term satisfactory results [[34], [35], [36]]. The familiar antimalarial agent Hydroxychloroquine (HCQ) plays a big modulating role in the action of toll-like receptor ligation and signaling which in turn affects the inflammatory process and fibrosis in cardiac tissue [37,38]. The effect of HCQ in decreasing the incidence of fetal heart block makes it a good alternative for traditional lines of treatment. However, HCQ use is still limited. This is mostly attributed to the lack of confirming prospective studies and its probable hearing and visual adverse effects [39]. Combination of plasmapheresis and immunosuppressive medications, including cyclophosphamide and azathioprine, was also investigated in pregnant women with Sjögren's syndrome with good results. However, evidence was limited to case reports [40] [41].
An alternative option is fluorinated steroid preparations, which have been investigated because of their anti-inflammatory proprieties, availability, easy administration and low cost [30,34,42]. It has been known that auto-antibodies have role in pathogenesis of congenital atrioventricular block through mediating several inflammatory processes of conductive system of fetal heart and reduction of L-type calcium channels [[43], [44], [45], [46]]. Pharmacologically placental 11ß-hydroxysteroid dehydrogenase complex inactivates maternal active prednisolone but minimally affects fluorinated steroids so dexamethasone and betamethasone are available to fetus in active form. [47]. On the other hand, non-fluorinated steroids are present in fetal circulation in inactive form because of immaturity of fetal hepatic function. [34]. Maternal administration of dexamethasone is effective in modulating immunological reactions and subsequent inflammation and fibrosis [27,48,49]. Initial studies have shown potential benefits when used alone or in combination with other medications [17,[50], [51], [52]], which ranges from regression of the disease to first degree or sinus rhythm [15,53,54] to resolution of pleural and/or pericardial effusion complicating CHB [15]. Steroid treatment was initiated around the twentieth week of gestation when universal sonographic examination is usually performed as the onset of disease process is thought to start as early as the sixteenth week of gestation [55,56]. Data on the role of fluorinated steroids to prevent the development of CHB among high risk population is still limited [34].
In this meta-analysis, fluorinated steroids were not superior to no-treatment except in the incidence of downgrading of CHB after initiation of treatment. However, some reviews have reported that transplacental steroid rarely reverse complete CHB, the fact that may explain the lack of improvement of outcomes despite steroid downgrading effect [57,58]. In addition, our results raise serious concern on the risk of oligohydramnios and IUGR, particularly as regimens described in these studies include high dose and/or prolonged use of corticosteroid. Brucato et al. [59] reported neurodevelopmental adverse effect of fluorinated steroids, which may be less prominent with betamethasone than dexamethasone. Several studies also reported the association between prenatal steroid administration and maternal adverse effects as gestational diabetes and hypertension, which is also consistent with our findings [16,[22], [23], [24],49,50,54,60]
The results of this review emerge from a total of more than 1000 cases, which presents an advantage of this study. The review investigated possible direct and indirect benefits of fluorinated steroids as well as their potential disadvantages. However, limitations include inconsistency in treatment regimens, retrospective nature of many studies and deficiency of some critical data including development of plural effusion and ascites with and without treatment.
In conclusion, fluorinated steroids do not provide significant benefit in fetuses with CHB. With the exception of CHB downgrading, it does not improve fetal or neonatal survival. On the other hand, prolonged regimens are associated with increased risk of fetal and maternal complications. Therefore, their use for this indication is not recommended.
Financial disclosure
None to disclose.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.eurox.2019.100072.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Yan J., Varma S.K., Malhotra A., Menahem S. Congenital complete heart block: single tertiary centre experience. Heart Lung Circ. 2012;21(11):666–670. doi: 10.1016/j.hlc.2012.05.784. [DOI] [PubMed] [Google Scholar]
- 2.Brito-Zerón P., Izmirly P.M., Ramos-Casals M., Buyon J.P., Khamashta M.A. The clinical spectrum of autoimmune congenital heart block. Nat Rev Rheumatol. 2015;11(5):301. doi: 10.1038/nrrheum.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Puri S., Pooni P., Mohan B., Bindal V., Verma S., Verma S., Gupta R.K. Pregnancy with SLE and fetal congenital heart block: a case report. Cardiol Res. 2013;4(3):126. doi: 10.4021/cr278w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brucato A., Frassi M., Franceschini F., Cimaz R., Faden D., Pisoni M.P., Muscarà M., Vignati G., Stramba‐Badiale M., Catelli L. Risk of congenital complete heart block in newborns of mothers with anti‐Ro/SSA antibodies detected by counterimmunoelectrophoresis: a prospective study of 100 women. Arthritis Rheumatol. 2001;44(8):1832–1835. doi: 10.1002/1529-0131(200108)44:8<1832::AID-ART320>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 5.Grava C., Ruffatti A., Milanesi O., Favaro M., Tonello M., Calligaro A., Del Ross T., Todesco S. Isolated congenital heart block in undifferentiated connective tissue disease and in primary Sjögren’s syndrome: a clinical study of 81 pregnancies in 41 patients. Reumatismo. 2005;57(3):180–186. doi: 10.4081/reumatismo.2005.180. [DOI] [PubMed] [Google Scholar]
- 6.Ramsey‐Goldman R., Hom D., Deng J.S., Ziegler G.C., Kahl L.E., Steen V.D., Laporte R.E., Medsger T.A., Jr Anti‐SS‐a antibodies and fetal outcome in maternal systemic lupus erythematosus. Arthritis Rheumatol. 1986;29(10):1269–1273. doi: 10.1002/art.1780291013. [DOI] [PubMed] [Google Scholar]
- 7.Izmirly P.M., Saxena A., Kim M.Y., Wang D., Sahl S.K., Llanos C., Friedman D., Buyon J.P. Maternal and fetal factors associated with mortality and morbidity in a multi–racial/ethnic registry of anti-SSA/Ro–associated cardiac neonatal lupus. Circulation. 2011 doi: 10.1161/CIRCULATIONAHA.111.033894. p. CIRCULATIONAHA. 111.033894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breur J.M., Kapusta L., Cohen M.I., Crosson J.E., Boramanand N., Lubbers L.J., Friedman A.H., Brenner J.I., Vetter V.L. Pacemaker therapy in isolated congenital complete atrioventricular block. Pacing Clin Electrophysiol. 2002;25(12):1685–1691. doi: 10.1046/j.1460-9592.2002.01685.x. UDINK Ten Cate. [DOI] [PubMed] [Google Scholar]
- 9.Villain E. Indications for pacing in patients with congenital heart disease. Pacing Clin Electrophysiol. 2008;31:S17–S20. doi: 10.1111/j.1540-8159.2008.00948.x. [DOI] [PubMed] [Google Scholar]
- 10.Friedman D.M., Duncanson L., Glickstein J., Buyon J. A review of congenital heart block. Images Paediatr Cardiol. 2003;5(3):36. [PMC free article] [PubMed] [Google Scholar]
- 11.GA W, S.B, Welch V., Losos M., Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 3rd Symposium on Systematic Reviews: Beyond the Basics. 2000 [Google Scholar]
- 12.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 13.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 14.Collaboration, N.C.C.T.C . The Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen: 2014. Review manager (RevMan) [computer program] Version 53. [Google Scholar]
- 15.Buyon J., Waltuck J., Kleinman C., Copel J. In utero identification and therapy of congenital heart block. Lupus. 1995;4(2):116–121. doi: 10.1177/096120339500400207. [DOI] [PubMed] [Google Scholar]
- 16.Eliasson H. Isolated atrioventricular block in the fetus: a retrospective, multinational, multicenter study of 175 patients. Circulation. 2011 doi: 10.1161/CIRCULATIONAHA.111.041970. p. CIRCULATIONAHA. 111.041970. [DOI] [PubMed] [Google Scholar]
- 17.Fesslova V., Sonesson S.-E., Sharland G., Granath F., Simpson J.M., Carvalho J.S., Jicinska H., Tomek V., Dangel J., Zielinsky P. The impact of treatment of the fetus by maternal therapy on the fetal and postnatal outcomes for fetuses diagnosed with isolated complete atrioventricular block. Cardiol Young. 2009;19(3):282–290. doi: 10.1017/S1047951109004053. [DOI] [PubMed] [Google Scholar]
- 18.Jaeggi E., Laskin C., Hamilton R., Silverman E. The importance of the level of maternal anti-Ro/SSA antibodies as a prognostic marker of the development of cardiac neonatal lupus erythematosus: a prospective study of 186 antibody-exposed fetuses and infants. J Am Coll Cardiol. 2010;55(24):2778–2784. doi: 10.1016/j.jacc.2010.02.042. [DOI] [PubMed] [Google Scholar]
- 19.Jaeggi E.T., Fouron J.C., Silverman E.D., Ryan G., Smallhorn J., Hornberger L.K. Transplacental fetal treatment improves the outcome of prenatally diagnosed complete atrioventricular block without structural heart disease. Circulation. 2004;110(12):1542–1548. doi: 10.1161/01.CIR.0000142046.58632.3A. [DOI] [PubMed] [Google Scholar]
- 20.Kuleva M., Le Bidois J., Decaudin A., Villain E., Costedoat‐Chalumeau N., Lemercier D., Dumez Y., Ville Y., Bonnet D., Salomon L. Clinical course and outcome of antenatally detected atrioventricular block: experience of a single tertiary centre and review of the literature. Prenat Diagn. 2015;35(4):354–361. doi: 10.1002/pd.4547. [DOI] [PubMed] [Google Scholar]
- 21.Levesque K., Morel N., Maltret A., Baron G., Masseau A., Orquevaux P., Piette J.-C., Barriere F., Le Bidois J., Fermont L. Description of 214 cases of autoimmune congenital heart block: results of the French neonatal lupus syndrome. Autoimmun Rev. 2015;14(12):1154–1160. doi: 10.1016/j.autrev.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Lopes L.M., Tavares G.M.P., Damiano A.P., Lopes M.A.B., Aiello V.D., Schultz R., Zugaib M. Perinatal outcome of fetal atrioventricular block: one-hundred-sixteen cases from a single institution. Circulation. 2008;118(12):1268–1275. doi: 10.1161/CIRCULATIONAHA.107.735118. [DOI] [PubMed] [Google Scholar]
- 23.Miyoshi T., Maeno Y., Sago H., Inamura N., Yasukohchi S., Kawataki M., Horigome H., Yoda H., Taketazu M., Shozu M. Evaluation of transplacental treatment for fetal congenital bradyarrhythmia. Circ J. 2012;76(2):469–476. doi: 10.1253/circj.cj-11-1020. [DOI] [PubMed] [Google Scholar]
- 24.Perín F., del Rey M.R.V., Bronte L.D., Menduina Q.F., Nuñez F.R., Arguelles J.Z., de la Calzada D.G., Marin S.T., Malfaz F.C., Izquierdo A.G. Foetal bradycardia: a retrospective study in 9 Spanish centres. Anales de Pediatría (English Edition) 2014;81(5):275–282. doi: 10.1016/j.anpedi.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 25.Saleeb S., Copel J., Friedman D., Buyon J.P. Comparison of treatment with fluorinated glucocorticoids to the natural history of autoantibody‐associated congenital heart block: retrospective review of the research registry for neonatal lupus. Arthritis Rheum. 1999;42(11):2335–2345. doi: 10.1002/1529-0131(199911)42:11<2335::AID-ANR12>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 26.Shinohara K., Miyagawa S., Fujita T., Aono T., Kidoguchi K.-I. Neonatal lupus erythematosus: results of maternal corticosteroid therapy. Obstet Gynecol. 1999;93(6):952–957. doi: 10.1016/s0029-7844(99)00006-x. [DOI] [PubMed] [Google Scholar]
- 27.Trucco S.M., Jaeggi E., Cuneo B., Moon-Grady A.J., Silverman E., Silverman N., Hornberger L.K. Use of intravenous gamma globulin and corticosteroids in the treatment of maternal autoantibody-mediated cardiomyopathy. J Am Coll Cardiol. 2011;57(6):715–723. doi: 10.1016/j.jacc.2010.09.044. [DOI] [PubMed] [Google Scholar]
- 28.Van den Berg N., Slieker M., van Beynum I., Bilardo C.M., de Bruijn D., Clur S.-A., Cornette J., Frohn-Mulder I., Haak M., van Loo-Maurus K. Fluorinated steroids do not improve outcome of isolated atrioventricular block. Int J Cardiol. 2016;225:167–171. doi: 10.1016/j.ijcard.2016.09.119. [DOI] [PubMed] [Google Scholar]
- 29.Izmirly P.M., Saxena A., Sahl S.K., Shah U., Friedman D.M., Kim M.Y., Buyon J.P. Assessment of fluorinated steroids to avert progression and mortality in anti-SSA/Ro-associated cardiac injury limited to the fetal conduction system. Ann Rheum Dis. 2016;75(6):1161–1165. doi: 10.1136/annrheumdis-2015-208311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donofrio M.T., Moon-Grady A.J., Hornberger L.K., Copel J.A., Sklansky M.S., Abuhamad A., Cuneo B.F., Huhta J.C., Jonas R.A., Krishnan A., Lacey S., Lee W., Michelfelder E.C., Rempel Sr., Silverman G.R., Spray N.H., Strasburger T.L., Tworetzky J.F., Rychik W. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation. 2014;129(21):2183–2242. doi: 10.1161/01.cir.0000437597.44550.5d. American Heart Association Adults With Congenital Heart Disease Joint Committee of the Council on Cardiovascular Disease in the, Y., Council on Clinical Cardiology, C.o.C.S., Anesthesia, Council on, C., Stroke, N. [DOI] [PubMed] [Google Scholar]
- 31.Strasburger J.F., Wakai R.T. Fetal cardiac arrhythmia detection and in utero therapy. Nat Rev Cardiol. 2010;7(5):277–290. doi: 10.1038/nrcardio.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cuneo B.F., Zhao H., Strasburger J.F., Ovadia M., Huhta J.C., Wakai R.T. Atrial and ventricular rate response and patterns of heart rate acceleration during maternal-fetal terbutaline treatment of fetal complete heart block. Am J Cardiol. 2007;100(4):661–665. doi: 10.1016/j.amjcard.2007.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hunter L.E., Simpson J.M. Atrioventricular block during fetal life. J Saudi Heart Assoc. 2015;27(3):164–178. doi: 10.1016/j.jsha.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saxena A., Izmirly P.M., Mendez B., Buyon J.P., Friedman D.M. Prevention and treatment in utero of autoimmune-associated congenital heart block. Cardiol Rev. 2014;22(6):263–267. doi: 10.1097/CRD.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pisoni C.N., Brucato A., Ruffatti A., Espinosa G., Cervera R., Belmonte-Serrano M., Sanchez-Roman J., Garcia-Hernandez F.G., Tincani A., Bertero M.T., Doria A., Hughes G.R., Khamashta M.A. Failure of intravenous immunoglobulin to prevent congenital heart block: findings of a multicenter, prospective, observational study. Arthritis Rheum. 2010;62(4):1147–1152. doi: 10.1002/art.27350. [DOI] [PubMed] [Google Scholar]
- 36.Friedman D.M., Llanos C., Izmirly P.M., Brock B., Byron J., Copel J., Cummiskey K., Dooley M.A., Foley J., Graves C., Hendershott C., Kates R., Komissarova E.V., Miller M., Pare E., Phoon C.K., Prosen T., Reisner D., Ruderman E., Samuels P., Yu J.K., Kim M.Y., Buyon J.P. Evaluation of fetuses in a study of intravenous immunoglobulin as preventive therapy for congenital heart block: results of a multicenter, prospective, open-label clinical trial. Arthritis Rheum. 2010;62(4):1138–1146. doi: 10.1002/art.27308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lafyatis R., York M., Marshak-Rothstein A. Antimalarial agents: closing the gate on Toll-like receptors? Arthritis Rheum. 2006;54(10):3068–3070. doi: 10.1002/art.22157. [DOI] [PubMed] [Google Scholar]
- 38.Alvarez D., Briassouli P., Clancy R.M., Zavadil J., Reed J.H., Abellar R.G., Halushka M., Fox-Talbot K., Barrat F.J., Buyon J.P. A novel role of endothelin-1 in linking Toll-like receptor 7-mediated inflammation to fibrosis in congenital heart block. J Biol Chem. 2011;286(35):30444–30454. doi: 10.1074/jbc.M111.263657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Izmirly P.M., Costedoat-Chalumeau N., Pisoni C.N., Khamashta M.A., Kim M.Y., Saxena A., Friedman D., Llanos C., Piette J.C., Buyon J.P. Maternal use of hydroxychloroquine is associated with a reduced risk of recurrent anti-SSA/Ro-antibody-associated cardiac manifestations of neonatal lupus. Circulation. 2012;126(1):76–82. doi: 10.1161/CIRCULATIONAHA.111.089268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aslan E., Tarim E., Kilicdag E., Simsek E. Sjögren’s syndrome diagnosed in pregnancy: a case report. J Reprod Med. 2005;50(1):67–70. [PubMed] [Google Scholar]
- 41.Yang C., Chen J., Lee S., Luo S. Successful preventive treatment of congenital heart block during pregnancy in a woman with systemic lupus erythematosus and anti-Sjogren’s syndrome A/Ro antibody. J Microbiol Immunol Infect. 2005;38(5):365. [PubMed] [Google Scholar]
- 42.Breur J., Visser G., Kruize A., Stoutenbeek P., Meijboom E. Treatment of fetal heart block with maternal steroid therapy: case report and review of the literature. Ultrasound Obstet Gynecol. 2004;24(4):467–472. doi: 10.1002/uog.1713. [DOI] [PubMed] [Google Scholar]
- 43.Alexander E., Buyon J.P., Provost T.T., Guarnieri T. Anti—Ro/SS‐a antibodies in the pathophysiology of congenital heart block in neonatal lupus syndrome, an experimental model. Arthritis & Rheum. 1992;35(2):176–189. doi: 10.1002/art.1780350209. [DOI] [PubMed] [Google Scholar]
- 44.Herreman G., Galezowski N. Maternal connective-tissue disease and congenital heart-block. N Engl J Med. 1985;312(20) doi: 10.1056/NEJM198505163122016. p. 1329–1329. [DOI] [PubMed] [Google Scholar]
- 45.Litsey S.E., Noonan J.A., O'Connor W.N., Cottrill C.M., Mitchell B. Maternal connective tissue disease and congenital heart block: demonstration of immunoglobulin in cardiac tissue. N Engl J Med. 1985;312(2):98–100. doi: 10.1056/NEJM198501103120206. [DOI] [PubMed] [Google Scholar]
- 46.Lee L.A., Coulter S., Erner S., Chu H. Cardiac immunoglobulin deposition in congenital heart block associated with maternal anti-Ro autoantibodies. Am J Med. 1987;83(4):793–796. doi: 10.1016/0002-9343(87)90918-1. [DOI] [PubMed] [Google Scholar]
- 47.Quinkler M., Oelkers W., Diederich S. Clinical implications of glucocorticoid metabolism by 11beta-hydroxysteroid dehydrogenases in target tissues. Eur J Endocrinol. 2001;144(2):87–97. doi: 10.1530/eje.0.1440087. [DOI] [PubMed] [Google Scholar]
- 48.Rosenthal D., Druzin M., Chin C., Dubin A. A new therapeutic approach to the fetus with congenital complete heart block: preemptive, targeted therapy with dexamethasone. Obstet Gynecol. 1998;92(4):689–691. doi: 10.1016/s0029-7844(98)00149-5. [DOI] [PubMed] [Google Scholar]
- 49.Cuneo B.F., Lee M., Roberson D., Niksch A., Ovadia M., Parilla B.V., Benson D.W. A management strategy for fetal immune-mediated atrioventricular block. J Matern Neonatal Med. 2010;23(12):1400–1405. doi: 10.3109/14767051003728237. [DOI] [PubMed] [Google Scholar]
- 50.Jaeggi E.T., Fouron J.C., Silverman E.D., Ryan G., Smallhorn J., Hornberger L.K. Transplacental fetal treatment improves the outcome of prenatally diagnosed complete atrioventricular block without structural heart disease. Circulation. 2004;110(12):1542–1548. doi: 10.1161/01.CIR.0000142046.58632.3A. [DOI] [PubMed] [Google Scholar]
- 51.Copel J.A., Buyon J.P., Kleinman C.S. Successful in utero therapy of fetal heart block. Am J Obstet Gynecol. 1995;173(5):1384–1390. doi: 10.1016/0002-9378(95)90621-5. [DOI] [PubMed] [Google Scholar]
- 52.Friedman D.M., Kim M.Y., Copel J.A., Llanos C., Davis C., Buyon J.P. Prospective evaluation of fetuses with autoimmune-associated congenital heart block followed in the PR Interval and Dexamethasone Evaluation (PRIDE) Study. Am J Cardiol. 2009;103(8):1102–1106. doi: 10.1016/j.amjcard.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ciardulli A., D'Antonio F., Magro-Malosso E.R., Manzoli L., Anisman P., Saccone G., Berghella V. Maternal steroid therapy for fetuses with second-degree immune-mediated congenital atrioventricular block: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2018;97(7):787–794. doi: 10.1111/aogs.13338. [DOI] [PubMed] [Google Scholar]
- 54.Saleeb S., Copel J., Friedman D., Buyon J.P. Comparison of treatment with fluorinated glucocorticoids to the natural history of autoantibody‐associated congenital heart block: retrospective review of the research registry for neonatal lupus. Arthritis Rheumatol. 1999;42(11):2335–2345. doi: 10.1002/1529-0131(199911)42:11<2335::AID-ANR12>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 55.Buyon J.P., Winchester R. Congenital complete heart block. Arthritis Rheum. 1990;33(5):609–614. doi: 10.1002/art.1780330502. [DOI] [PubMed] [Google Scholar]
- 56.Brucato A., Tincani A., Fredi M., Breda S., Ramoni V., Morel N., Costedoat-Chalumeau N. Should we treat congenital heart block with fluorinated corticosteroids? Autoimmun Rev. 2017 doi: 10.1016/j.autrev.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 57.Hornberger L.K., Sahn D.J. Rhythm abnormalities of the fetus. Heart. 2007;93(10):1294–1300. doi: 10.1136/hrt.2005.069369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ciardulli A., D’Antonio F., Magro-Malosso E.R., Saccone G., Manzoli L., Radolec M., Berghella V. Maternal steroid therapy for fetuses with immune-mediated complete atrioventricular block: a systematic review and meta-analysis. J Matern Neonatal Med. 2017:1–261. doi: 10.1080/14767058.2017.1419182. (just-accepted) [DOI] [PubMed] [Google Scholar]
- 59.Brucato A., Astori M.G., Cimaz R., Villa P., Destri M.L., Chimini L., Vaccari R., Muscarà M., Motta M., Tincani A. Normal neuropsychological development in children with congenital complete heart block who may or may not be exposed to high-dose dexamethasone in utero. Ann Rheum Dis. 2006;65(11):1422–1426. doi: 10.1136/ard.2005.049866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hayashi T., Kaneko M., Kim K.-S., Eryu Y., Shindo T., Isoda T., Murashima A., Ito Y., Sago H. Outcome of prenatally diagnosed isolated congenital complete atrioventricular block treated with transplacental betamethasone or ritodrine therapy. Pediatr Cardiol. 2009;30(1):35–40. doi: 10.1007/s00246-008-9273-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.