Short abstract
Background
A mometasone furoate (MF) sinus implant (1350 mcg) was evaluated in 2 randomized controlled trials (RCTs) in 400 adults with nasal polyps (NP) who were candidates for revision endoscopic sinus surgery (RESS). We conducted a pooled analysis to evaluate the efficacy of MF implant in specific subgroups of NP patients.
Methods
Pooled data from 2 RCTs for 375 patients were analyzed across prespecified subjective and objective end points through day 90.
Results
At day 90, patients receiving implants and MF nasal spray (MFNS) experienced significant improvements in nasal obstruction/congestion (NO/C) score (P = .0095), bilateral polyp grade (BPG, P = .0008), and ethmoid sinus obstruction (P < .0001) compared to control using MFNS alone. Fewer treatment than control patients remained candidates for RESS (41.0% vs 69.3%, P < .0001). All subgroups experienced significant treatment effects, except NO/C in smokers (P = .0509) and patients without altered smell (P = .1873). Subgroups without asthma and with only 1 prior ESS experienced largest treatment effect on NO/C, and those with recent surgery <24 months and BPG >5 showed largest effect on endoscopic end points and RESS. Control patients with ESS <24 months were at 7 times highest risk for RESS (P < .0001). One (0.4%) patient experienced implant-related serious adverse event (epistaxis).
Conclusion
On pooled analysis, MF implants with MFNS showed more favorable results than MFNS alone across several subjective and objective end points at day 90 and may play an important role in management of NP patients, especially those who have allergic rhinitis, expanded polyposis, altered smell, or had most recent ESS < 24 months.
Keywords: bioabsorbable, chronic sinusitis, corticosteroid, ethmoid, endoscopic sinus surgery, polyposis, sinus surgery, stent
Introduction
Chronic rhinosinusitis (CRS) affects up to 12% of the U.S. adult population and results in 18 to 22 million U.S. physician office visits annually, representing considerable health-care expenditures.1–3 Patients who have CRS with nasal polyps (CRSwNP) exhibit a higher symptom burden, increased medication use, and a higher rate of revision endoscopic sinus surgery (RESS).3–5 Medical management with intranasal corticosteroid sprays (INCSs) is recommended as the initial treatment, supplemented with short courses of systemic corticosteroids as necessary to control symptom burden. The latter, while effective, result in numerous adverse effects limiting frequent use.6,7 The development of corticosteroids that are delivered directly to the nasal mucosa has addressed many of the systemic safety concerns.5,8,9 However, standard INCSs have limited access to ethmoid mucosa, the common site of origin for NP. 10 Moreover, poor compliance with daily dosing and improper head positioning further compromises drug delivery and efficacy. 10 ESS is reserved for CRSwNP patients who fail to improve or require excessive systemic corticosteroids. 6 Given the substantial morbidity of CRSwNP despite medical and surgical therapy, there is a need for novel treatment options to improve disease control. Early phase trials of systemic biologic agents (monoclonal antibodies) designed to shrink NP show promising results, 11 but the cost of these drugs will be high, and the durability of response remains unclear in the absence of continuous dosing. 12
Second-generation bioabsorbable corticosteroid-eluting sinus implants delivering 1350 mcg of mometasone furoate (MF) directly to the sinus mucosa have been developed for in-office treatment of recurrent NP in patients 18 years of age or older, who have had prior ethmoid sinus surgery. 13 MF implants are inserted under local anesthesia and have been evaluated in 4 clinical trials, including 2 double-blind randomized controlled trials (RCT) in 400 adult CRSwNP patients who were candidates for RESS.14–18 The first RCT showed positive trends and an acceptable safety profile but did not meet its efficacy end points.14,15 The reduction in nasal obstruction/congestion (NO/C) score and bilateral polyp grade (BPG) from baseline to day 90 reached statistical significance in a subset of patients with higher polyp burden (grade 2 or higher on each side).14,15 In the second larger RCT, patients receiving MF sinus implants and once-daily MF nasal spray (MFNS) demonstrated statistically significant improvements compared to controls receiving a sham procedure and once-daily MFNS across multiple prespecified primary and secondary efficacy end points. 16 Five secondary subjective and objective end points were adjusted for multiplicity, 4 of which demonstrated statistically significantly better values in the treatment group compared to control. The objective of this pooled analysis was to further evaluate the relation of demographic factors and baseline clinical characteristics to the efficacy outcomes of in-office placement of MF sinus implants. These results may help further define the role of MF sinus implants in the management of recurrent NP in subsets of patients. Understanding the differences among subgroups of the CRSwNP population may inform clinical decisions regarding which subset of the NP patient population may derive the greatest benefit.
Methods
Trial Design
The 2 RCTs had similar study designs (Table 1) and were conducted consecutively between January 2013 and August 2016.14–16 The study protocols were approved by the institutional review board of each participating site, and all patients provided written informed consent prior to study entry. All study patients were blinded by wearing eye masks and noise canceling headsets during the baseline procedure and each follow-up endoscopic examination at days 14, 30, 60, and 90. Paper symptom questionnaires were administered before endoscopic examination by research staff unaware of treatment assignment. All implants present at day 60 were removed to ensure blinded assessment of BPG (coprimary end point) at day 90 based on a centralized video-endoscopy review by the same independent panel of 3 sinus surgeons in both studies.
Table 1.
Description of 2 RCTs Conducted to Assess the Safety and Efficacy of In-office Placement of an MF Sinus Implant.
| Study | Trial Design Randomized/Completed Study | Treatment and Control | Follow-up Duration | Diagnosis and Eligibility Criteria | Coprimary Efficacy End Points | Secondary Efficacy End Points |
|---|---|---|---|---|---|---|
| RESOLVE14,15 | Phase 2/3Randomized 1:1, parallel group, double-blind (patients, assessors), concurrently controlled, multicenter (18 sites in United States) n = 100/98T: n = 53/52C: n = 47/46 | T: in-office bilateral placement of the MF sinus implant in the ethmoid sinuses plus daily MFNSC: in-office bilateral sham procedure plus daily MFNS | 6 monthsImplant removal by day 60 to ensure blinding |
|
|
Prespecified, not-adjusted for multiplicity:
|
| RESOLVE II 16 | Phase 3 pivotalRandomized 2:1, parallel group, double-blind (patients, assessors), concurrently controlled, multicenter (34 sites in United States) n = 300/298T: n = 201/200C: n = 99/98 | T: in-office bilateral placement of the MF sinus implant in the ethmoid sinuses plus daily MFNSC: in-office bilateral sham procedure plus daily MFNS | 90 daysImplant removal by day 60 to ensure blinding |
|
|
Prespecified and adjusted for multiplicity:
|
Abbreviations: BPG, bilateral polyp grade; C, control group; CRS, chronic rhinosinusitis; ESO, ethmoid sinus obstruction; ESS, endoscopic sinus surgery; INCS, intranasal corticosteroid spray; MF, mometasone furoate; MFNS, MF nasal spray; NO/C, nasal obstruction/congestion; NOSE, Nasal Obstruction Symptom Evaluation; NP, nasal polyps; RCT, randomized control trial; RESS, revision ESS; T, treatment group; VAS, visual analog scale.
For all scales, higher scores indicate worse outcomes.
Patients
The study populations were consistent across both studies and comprised adults (18 years or older) with confirmed diagnosis of CRSwNP 19 who had prior ESS including bilateral total ethmoidectomy and were indicated for RESS because of refractory symptoms of NO/C and recurrent bilateral NP despite ongoing daily use of INCS and recent treatment with oral steroids. The inclusion and exclusion criteria as well as the criteria for RESS candidacy were similar in both studies (Table 1). However, the RESOLVE study entry criteria allowed for 1 sinus side to have NP grade 1, resulting in enrollment of 25 patients (8 treatment and 17 control) whose candidacy for RESS was, therefore, debatable. To reduce heterogeneity and ensure that all patients included in the pooled analysis had confirmed candidacy for RESS, the data for 375 patients with expanded NP bilaterally (grade ≥ 2 on each side) were used.
Interventions
Patients randomized to the treatment group underwent in-office bilateral placement in the ethmoid sinuses under local anesthesia of 2 MF implants (SINUVA® Sinus Implant, Intersect ENT, Inc., Menlo Park, CA, USA), designed to release 1350 mcg of MF over 90 days. The MF sinus implant, which is a combination drug/device product regulated as a drug, was investigational at the time the studies were conducted and subsequently approved in December 2017 under New Drug Application by the U.S. Food and Drug Administration (FDA) for the treatment of NP. Patients randomized to the control group underwent an in-office bilateral sham procedure, consisting of insertion of the delivery system containing the MF sinus implant into each ethmoid sinus followed by withdrawal without placement. All patients (treatment and control) were required to use MFNS (Nasonex Nasal Spray; Merck & Co., Inc., Whitehouse Station, NJ, USA) 200 mcg once daily (50 mcg twice in each nostril) through day 90 to ensure patient blinding and to offer control patients an FDA-approved treatment for NP. Patients were also encouraged to use saline rinses regularly.
Preexisting stable regimens for allergic rhinitis and asthma, including immunotherapy and inhaled corticosteroids, were maintained. Prohibited concomitant medications included systemic steroids, budesonide drops/irrigations, and nebulized steroids. Rescue treatments with antibiotics, oral steroids, or RESS were provided, if medically necessary. Patients who received prohibited steroids or surgery could continue the assigned treatment, and their most recent scores and videos prior to intervention were carried forward for postintervention time points in the analysis.
End Points
The prespecified efficacy end points for the pooled analysis were as follows: (1) NO/C score change from baseline to day 90 based on a reflective questionnaire, (2) BPG change from baseline to day 90 by the independent panel based on a blinded centralized video-endoscopy review and to each time point by clinical investigators, (3) ethmoid sinus obstruction (ESO) change from baseline to day 90 by the independent panel, and (4) proportion of patients still indicated for RESS at day 90 by clinical investigators.
Patients scored their NO/C symptoms at the time of follow-up visit using paper questionnaires on a 6-point scale in RESOLVE (0 = no symptoms, 1 = very mild, 2 = mild or slight problem, 3 = moderate, 4 = severe problem, and 5 = problem as bad as it can be) and on a 4-point scale in RESOLVE II (0 = no symptoms, 1 = mild symptoms, 2 = moderate symptoms, and 3 = severe symptoms). Because the scales differed between studies, the RESOLVE data for “mild” (scores 1 and 2) and “severe” (scores 4 and 5) symptoms were integrated with the RESOLVE II data for scores 1 and 3, respectively. BPG represents a sum of left and right polyp grades, each scored from 0 (no polyps) to 4 (NPs completely obstructing the nasal cavity). 20 The scale used in RESOLVE II included 3 intermediate grades (1.5, 2.5, and 3.5), which were added to the validated 5-point scale 20 used in RESOLVE to allow more sensitivity in quantifying the burden of polyposis in post-ESS patients with altered anatomy and varied amount of obstruction by polypoid edema (≥25%, ≥50%, and ≥75% of the ethmoid sinus/middle meatus, respectively). Because the BPG ranged from 0 to 8 in both studies, no remapping was required. ESO by polyps, edema, and/or scarring was determined on a 100-mm visual analog scale anchored at 0 (no obstruction) and 100 (complete obstruction). The proportion of patients still indicated for RESS was determined based on clinical investigator assessment. To be still indicated for RESS, a patient had to (1) continue to use MFNS once daily, (2) complain of NO/C and at least 1 more CRS symptom (postnasal discharge, facial pain/pressure, or altered sense of smell), and (3) have endoscopic evidence of NP (grade ≥ 2 at least unilaterally in RESOLVE and bilaterally in RESOLVE II).
Safety Evaluation
All adverse events (AEs) that occurred in both studies were collected and tabulated regardless of treatment group assignment and relationship to MF sinus implants.
Statistical Analysis
The data from 2 RCTs were analyzed according to the prespecified, FDA-approved statistical plans for integrated efficacy and safety evaluations prior to locking the database for RESOLVE II. Statistical analyses were performed by independent biostatisticians using an intent-to-treat (ITT) population, which included all patients who underwent randomization and in whom MF implant placement or sham procedure was attempted.
The efficacy outcomes were analyzed using the analysis of covariance (ANCOVA) model with the baseline values as a covariate and the study, site, and treatment group as fixed effects for continuous variables. Dichotomous variables were compared between treatment groups using the Cochran–Mantel–Haenszel test with study and site as stratification variables. The grades from the 3 independent reviewers for the same patient were averaged. The results for a given sinus were set as missing if 2 of the 3 independent reviewers could not provide grading. The results for a given patient were set as missing if the grade for 1 sinus was missing. Statistical summaries, confidence intervals (CIs), and P values were generated using SAS software, version 9.4 (SAS Institute, Inc., Cary, NC, USA). All statistical tests were 2-sided and interpreted at a 5% significance level. Descriptive statistics were provided for all outcome measures, displaying the mean and standard deviation for continuous data, and the count, percentage, and odds ratio for categorical data. All reported 95% CI are 2-sided. Because surgical and systemic steroid interventions prior to day 90 could confound the study results, a last-observation-carried-forward approach was used, where values obtained at the visit prior to such intervention were used for postintervention time points in the analysis. The results represent intervention-adjusted values. All available data at each time point were presented. No imputations for missing data were performed. All reported P values are 2-sided. We conducted subgroup analyses for major demographic factors (eg, age, gender, and race) and baseline clinical characteristics (eg, comorbidities, altered sense of smell, surgical history, smoking, and extent of NP). The subgroup-by-treatment interaction was included as a fixed effect in the ANCOVA model and as a stratification variable in the Cochran–Mantel–Haenszel test. Summary statistics included estimates of the treatment effect within each subgroups (mean difference or odds ratio between treatment groups, 95% CI), the overall treatment effect when accounting for subgroup effect, and the interaction of treatment by subgroup. To have reasonable ability to detect subgroup differences, analyses were omitted if a subgroup represented < 10% of the ITT population. All results of the pooled analysis were considered exploratory.
AEs were coded using Medical Dictionary for Regulatory Activities (MedDRA) version 17.0. All treatment emergent AEs (TEAEs) through day 90 are presented by MedDRA system organ class, preferred term, and maximum severity. A patient reporting more than 1 AE for a particular MedDRA preferred term was counted only once. A patient reporting several AEs with different preferred terms was counted under each term. Implant-related AEs comprised all TEAEs that were judged to be related to the study drug (MF), study device (implant), or implant procedure.
Results
Demographics and baseline characteristics were similar between studies and well balanced between treatment groups on the pooled analysis (Table 2). The most common comorbidities were allergic rhinitis and asthma, which were reported in up to 73% and 79% of patients, respectively. Aspirin-exacerbated respiratory disease (AERD) was reported in up to 19% of patients and for the pooled analysis, this group was combined with 25% of patients with aspirin intolerance/allergy.
Table 2.
Demographic and Baseline Characteristics. a
| Variable |
RESOLVE14,15 |
RESOLVE II 16 |
Pooled analysis b |
|||
|---|---|---|---|---|---|---|
| Treatment (n = 53) | Control (n = 47) | Treatment (n = 201) | Control (n = 99) | Treatment (n = 246) | Control (n = 129) | |
| Age (years) | 47.8 ± 12.6 | 51.6 ± 13.1 | 50.5 ± 12.9 | 47.9 ± 12.4 | 50.0 ± 12.9 | 48.7 ± 12.4 |
| Male subjects, n (%) | 29 (54.7) | 31 (66.0) | 127 (63.2) | 56 (56.6) | 152 (61.8) | 77 (59.7) |
| Ethnicity, n (%) | ||||||
| Not Hispanic or Latino | 49 (92.5) | 46 (97.9) | 190 (94.5) | 92 (92.9) | 232 (94.3) | 121 (93.8) |
| Hispanic or Latino | 4 (7.5) | 1 (2.1) | 11 (5.5) | 7 (7.1) | 14 (5.7) | 8 (6.2) |
| Race, n (%) | ||||||
| White | 47 (88.7) | 44 (93.6) | 164 (81.6) | 80 (80.8) | 204 (82.9) | 107 (82.9) |
| Black or African American | 6 (11.3) | 2 (4.3) | 27 (13.4) | 13 (13.1) | 32 (13.0) | 15 (11.6) |
| Asian | 0 | 1 (2.1) | 4 (2.0) | 4 (4.0) | 4 (1.6) | 5 (3.9) |
| Other | 0 | 0 | 6 (3.0) | 2 (2.0) | 6 (2.4) | 2 (1.6) |
| CRS history, n (%) | ||||||
| Confirmed CRSwNP diagnosis | 53 (100) | 47 (100) | 201 (100) | 99 (100) | 246 (100) | 129 (100) |
| CRS symptoms despite ongoing use of INCS | ||||||
| Nasal obstruction, blockage or congestion | 48 (90.6) | 40 (85.1) | 185 (92.0) | 90 (90.9) | 226 (91.9) | 118 (91.5) |
| Postnasal discharge | 48 (90.6) | 44 (93.6) | 182 (90.5) | 83 (83.8) | 222 (90.2) | 112 (86.8) |
| Altered sense of smell | 42 (79.2) | 39 (83.0) | 174 (86.6) | 89 (89.9) | 210 (85.4) | 115 (89.1) |
| Facial pain, pressure, or fullness | 42 (79.2) | 32 (68.1) | 77 (38.3) | 44 (44.4) | 112 (45.5) | 64 (49.6) |
| ESS history, n (%) | ||||||
| Prior bilateral total ethmoidectomy | 53 (100) | 47 (100) | 201 (100) | 99 (100) | 246 (100) | 129 (100) |
| Number of prior ESS | ||||||
| 1 | 22 (41.5) | 16 (34.0) | 83 (41.3) | 41 (41.4) | 102 (41.5) | 51 (39.5) |
| 2 | 12 (22.6) | 15 (31.9) | 57 (28.4) | 36 (36.4) | 66 (26.8) | 43 (33.3) |
| 3 | 9 (17.0) | 8 (17.0) | 32 (15.9) | 7 (7.1) | 41 (16.7) | 13 (10.1) |
| 4 and more | 10 (18.9) | 8 (17.0) | 29 (14.4) | 15 (15.2) | 37 (15.0) | 22 (17.1) |
| Medical history, n (%) c | ||||||
| Asthma | 33 (62.3) | 31 (66.0) | 148 (73.6) a | 61 (61.6) | 179 (72.8) | 83 (64.3) |
| Allergic rhinitis | 41 (77.4) | 37 (78.7) | 155 (77.1) | 79 (79.8) | 189 (76.8) | 102 (79.1) |
| Aspirin intolerance/allergy | 15 (28.3) | 11 (23.4) | 46 (22.9) | 23 (23.2) | 60 (24.4) | 33 (25.6) |
| AERD | 11 (20.8) | 9 (19.1) | 30 (14.9) | 17 (17.2) | 40 (16.3) | 25 (19.4) |
Abbreviations: AERD, aspirin-exacerbated respiratory disease; CRS, chronic rhinosinusitis; CRSwNP, chronic rhinosinusitis with nasal polyps; ESS, endoscopic sinus surgery; INCS, intranasal corticosteroid spray; NP, nasal polyps; RESS, revision ESS; SD, standard deviation.
Values are means ± SD or as indicated. Treatment groups did not differ significantly, except in incidence of asthma in RESOLVE II (P = .0336).
Analysis of the pooled data from RESOLVE and RESOLVE II for patients with NP grade ≥ 2 on each side at screening and confirmed candidacy for RESS.
Medical history based on physician diagnosis as recorded in patient medical records.
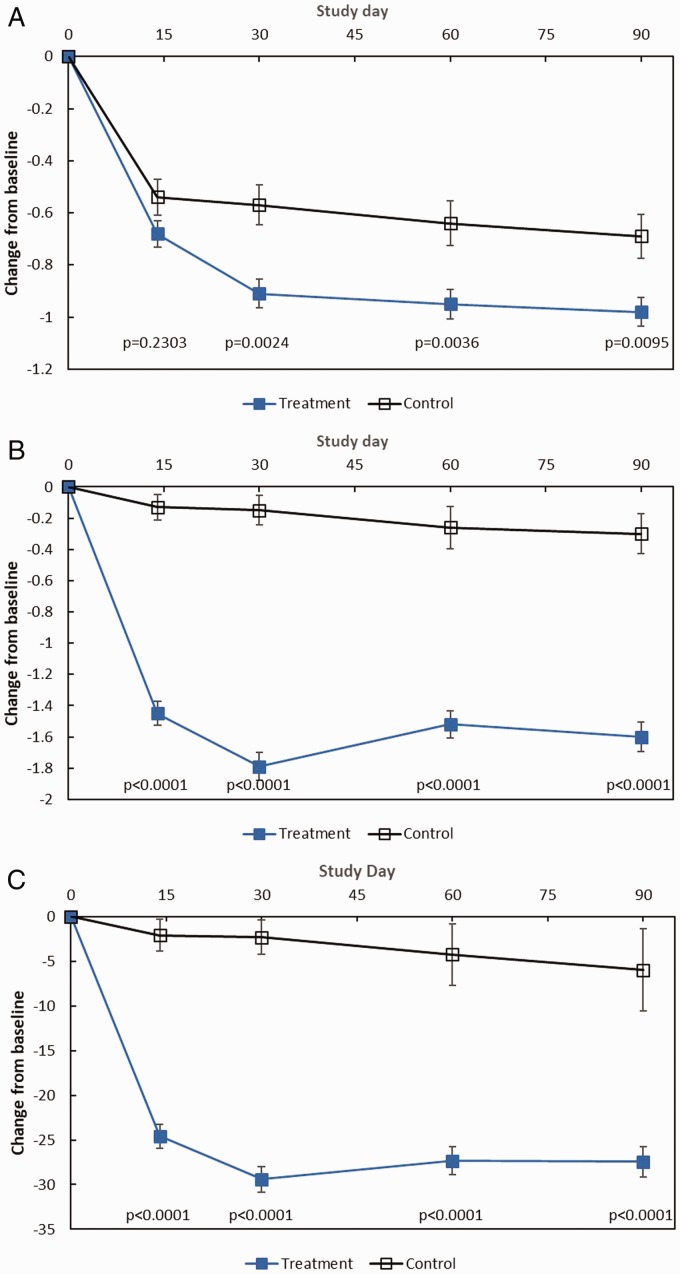
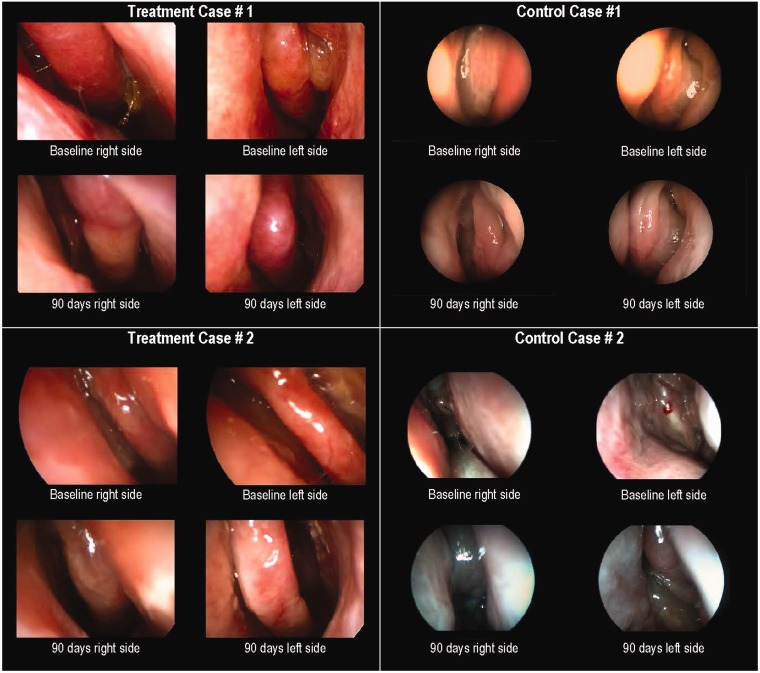
On pooled analysis, patients receiving MF implants and once-daily MFNS experienced significant improvements at day 90 across all subjective and objective outcomes compared to control patients on MFNS alone (Table 3). Significant improvements favoring the treatment group were sustained over time (Figure 1). Typical outcomes observed on endoscopy among treatment and control patients are presented in Figure 2.
Table 3.
Efficacy Outcomes.
| Outcome |
RESOLVE14,15,a |
RESOLVE II 16 |
Pooled Analysis b |
|||
|---|---|---|---|---|---|---|
| Treatment (n = 53) | Control (n = 47) | Treatment (n = 201) | Control (n = 99) | Treatment (n = 246) | Control (n = 129) | |
| NO/C | ||||||
| Baseline | ||||||
| Mean ± SD | 3.63 ± 1.176 | 3.48 ± 1.092 | 2.36 ± 0.488 | 2.35 ± 0.479 | 2.39 ± 0.580 | 2.35 ± 0.645 |
| Change from baseline to day 90 | ||||||
| n (%) | 43 (81.1) | 31 (66.0) | 177 (88.1) | 89 (89.9) | 244 (99.2) | 126 (97.7) |
| Mean ± SD | −1.40 ± 1.380 | −0.52 ± 1.379 | −0.93 ± 0.798 | −0.69 ± 0.791 | −0.98 ± 0.856 | −0.69 ± 0.942 |
| Treatment effect estimate (95% CI) c | −0.88 (−1.26 to −0.09) | −0.27 (−0.48 to −0.07) | −0.24 (−0.42 to −0.06) | |||
| P c | .0254 | .0248 d | .0095 | |||
| BPG | ||||||
| Baseline | ||||||
| Mean ± SD | 5.24 ± 0.649 | 5.42 ± 0.825 | 5.48 ± 1.132 | 5.43 ± 1.009 | 5.40 ± 1.107 | 5.34 ± 1.076 |
| Change from baseline to day 90 | ||||||
| n (%) | 40 (75.5) | 27 (57.4) | 195 (97.0) | 97 (98.0) | 238 (96.7) | 127 (98.4) |
| Mean ± SD | −0.80 ± 0.812 | −0.38 ± 1.049 | −0.56 ± 1.059 | −0.15 ± 0.907 | −0.60 ± 1.021 | −0.19 ± 0.950 |
| Treatment effect estimate (95% CI) c | −0.42 (−0.87 to −0.00) | −0.35 (−0.60 to −0.09) | −0.37 (−0.59 to −0.16) | |||
| P c | .0490 | .0073 | .0008 | |||
| ESO | ||||||
| Baseline | ||||||
| Mean ± SD | 70.59 ± 18.20 | 62.74 ± 25.673 | 69.16 ± 19.869 | 67.03 ± 18.552 | 69.94 ± 19.415 | 68.91 ± 19.097 |
| Change from baseline to day 90 | ||||||
| n (%) | 52 (98.1) | 47 (100) | 195 (97.0) | 97 (98.0) | 239 (97.2) | 127 (98.4) |
| Mean ± SD | −17.05 ± 19.361 | −5.57 ± 18.279 | −11.28 ± 18.108 | −1.87 ± 14.364 | −12.71 ± 18.619 | −2.96 ± 16.208 |
| Treatment effect estimate (95% CI) c | −9.83 (−17.2 to −2.43) | −7.96 (−12.1 to −3.83) | −8.97 (−12.7 to −5.22) | |||
| P c | .0099 | .0007 d | <.0001 | |||
| Proportion of patients indicated for RESS | ||||||
| Baseline n (%) | 53 (100.0) | 47 (100.0) | 201 (100) | 99 (100) | 246 (100.0) | 129 (100.0) |
| Day 90 n (%) | 25 (48.1) | 36 (78.3) | 78 (39.0) | 62 (63.3) | 100 (41.0) | 88 (69.3) |
| P e | .0022 | .0004 d | <.0001 | |||
| Odds ratio (95% CI) | 3.9 (1.6–9.4) | 2.7 (1.6–4.4) | 3.2 (2.1–5.1) | |||
Abbreviations: ANCOVA, analysis of covariance; BPG, bilateral polyp grade; CI, confidence interval; ESO, ethmoid sinus obstruction; ESS, endoscopic sinus surgery; MF, mometasone furoate; NO/C, nasal obstruction/congestion; NP, nasal polyps; RESS, revision ESS; SD, standard deviation; VAS, visual analog scale.
To allow comparison across the studies and with the pooled analysis results, the RESOLVE results for NO/C score (scale 0–5) and BPG by panel (scale 0–8) are presented for the subset of patients with NP grade ≥ 2 on each side, as reported by Han et al. 15 and Forwith et al. 14
Analysis of the pooled data from RESOLVE and RESOLVE II for patients with NP grade ≥ 2 on each side and confirmed candidacy for RESS at screening and 4 prespecified end points: NO/C score (scale 0–3) by patients using a reflective questionnaire, BPG (scale 0–8) based on a centralized, blinded video-endoscopy review by an independent panel of 3 sinus surgeons, ESO (VAS 0–100) by the panel, and proportion of patients indicated for RESS based on study criteria. (see “End points” section for details).
Based on the ANCOVA model with baseline value as a covariate and study, site, and treatment group as fixed effects. Values are adjusted for steroid and surgical interventions.
Prespecified secondary end point in RESOLVE II with P adjusted for multiplicity. NO/C score (scale 0–3) based on a daily diary, as reported by Kern et al. 16
Based on the Cochran–Mantel–Haenszel test with study and site as stratification variables.
Figure 1.
Pooled analysis efficacy outcomes through day 90. The changes from baseline to each time point in subjective and objective outcomes among treatment patients who received MF sinus implants bilaterally and control patients who underwent a sham procedure are shown. All patients (treatment and control) were required to use MFNS once daily. A, Change in NO/C (scale 0–3, with higher score indicating greater severity), as assessed by patients using a reflective questionnaire. B, Change in BPG (scale 0–8, with higher grade indicating greater severity), as assessed by clinical investigators. C, Change in ESO (VAS 0–100, with higher score indicating greater severity), as assessed by clinical investigators. All values are means with 2-sided standard error bars calculated based on ITT population. Data from patients who received surgical or medical intervention were imputed using most recent values prior to initiating or receiving intervention and represent intervention-adjusted values. P values based on the ANCOVA model with baseline value as a covariate and study, site, and treatment group as fixed effects. ANCOVA, analysis of covariance; BPG, bilateral polyp grade; ESO, ethmoid sinus obstruction; ITT, intent-to-treat; MF, mometasone furoate; MFNS, MF nasal spray; NO/C, nasal obstruction/congestion; VAS, visual analog scale.
Figure 2.
Endoscopic images of the ethmoid sinuses at baseline and 90 days after in-office bilateral placement of MF sinus implants (treatment) or sham procedure (control). All patients (treatment and control) were required to use MFNS once daily. MF, mometasone furoate; MFNS, MF nasal spray.
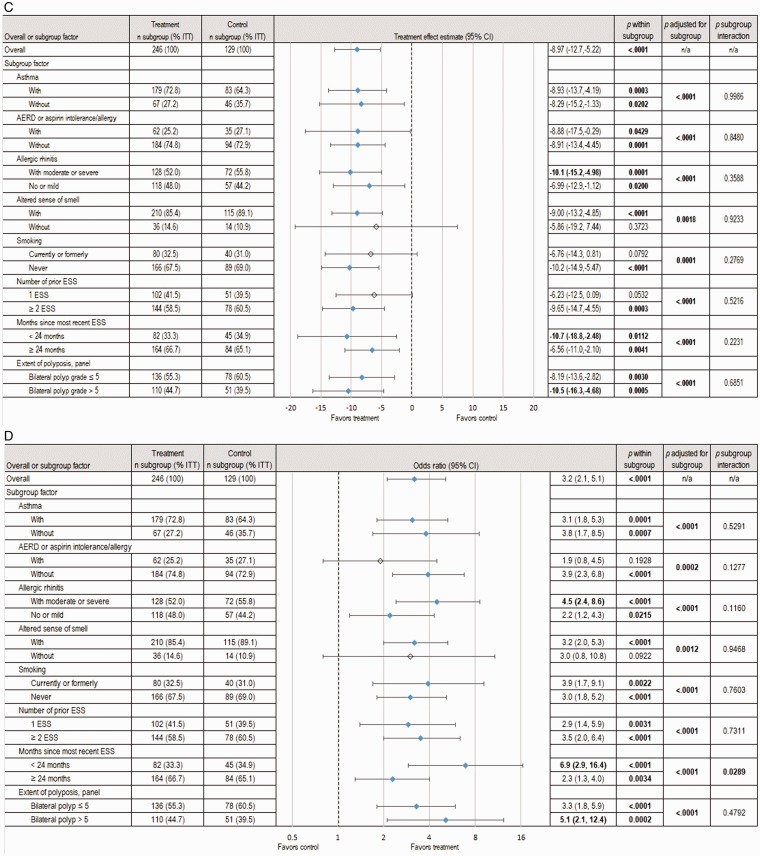
The subgroup analyses revealed significant between-group differences in subjective and objective outcomes at day 90 favoring the treatment group across most subgroups. Among the demographic subgroup factors (ie, age, gender, race), only gender subgroups were represented by more than 10% of the ITT population and were therefore analyzed. Both male and female subgroups demonstrated significant treatment effects in NO/C score (−0.23 [CI, −0.41 to −0.04], P = .0166), BPG (−0.37 [CI, −0.59 to −0.15], P = .0010), ESO (−9.17% [CI, −13.0 to −5.34], P < .0001) and proportion of RESS patients (male: 38.0% treatment vs 70.7% control, P < .0001; female: 45.7% treatment vs 67.3% control, P = .0101). However, the treatment by gender interaction effect was not statistically significant across all outcome measures (P > .5).
When adjusting for the key baseline characteristics, the overall MF implant effect on NO/C symptom improvement remained statistically significant across all subgroups, except for smoking status (P value adjusted for subgroup = .0509) and altered sense of smell (P = .1873) (Figure 3(A)). The largest improvements in NO/C score were experienced by patients without asthma (−0.40 [CI, −0.75 to −0.06], P = .0218) and those with only 1 prior ESS (−0.40 [CI, −0.72 to −0.08], P = .0142). The treatment effect on NO/C in the subgroup of patients with moderate-to-severe allergic rhinitis was significant and 2-fold larger compared to those without it (−0.28 [CI, −0.53 to −0.04], P = .02249 vs −0.14 [CI, −0.42, 0.14], P = .3245).
Figure 3.
Pooled analysis of subgroup factor effect on subjective and objective outcomes at day 90. The changes from baseline to day 90 in symptomatic and endoscopic outcomes across subgroups based on analysis of the pooled data from 2 RCTs, totaling 375 patients with NP grade ≥ 2 on each side at screening who were indicated for RESS, are shown. The treatment group underwent in-office bilateral placement of MF sinus implants, and the control group underwent a sham procedure. All patients (treatment and control) were required to use MFNS once daily. Summary statistics include estimates of the treatment effect (mean difference or odds ratio between treatment groups, 95% CI) within subgroups, the overall treatment effect when accounting for subgroups effect, and the interaction of treatment by subgroup. Subgroups representing <10% of ITT population were omitted. Treatment effects with P < .05 were considered statistically significant and are highlighted in bold font and with blue shaded diamonds. A, Change in NO/C score (scale 0–3, with higher scores indicating greater severity). B, Change in mean BPG (scale 0–8, with higher scores indicating greater severity), as determined based on a centralized blinded video-endoscopy review by an independent panel of 3 sinus surgeons. C, Change in ESO (VAS 0–100, with higher scores indicating greater severity), as determined by the independent panel. D, Proportion of patients still indicated for RESS. AERD, aspirin-exacerbated respiratory disease; CI, confidence interval; ESO, ethmoid sinus obstruction; ESS, endoscopic sinus surgery; ITT, intent-to-treat; MF, mometasone furoate; MFNS, MF nasal spray; n/a, not applicable; NO/C, nasal obstruction/congestion; NP, nasal polyps; RCT, randomized controlled trial; RESS, revision ESS; VAS = visual analog scale.
The largest treatment effects in BPG, ESO and proportion of patients still indicated for RESS were noted in the subgroups of patients with most recent ESS < 24 months, bilateral NP grade > 5, and moderate-to-severe allergic rhinitis (Figure 3(B) to (D)). The treatment by subgroup interaction effect was statistically significant only for indication for RESS in the subgroup of months since most recent ESS (< 24 months vs ≥ 24 months, P value subgroup interaction = .0289; Figure 3(D)). Among patients who had most recent surgery < 24 months, control patients were at 7 times higher risk of remaining indicated for RESS compared to treatment (odds ratio = 6.9, P < .0001; Figure 3(D)). Control patients were at least twice more likely than treatment patients to be indicated for RESS across all subgroups, and 3 times more likely in 13 of the 16 subgroups (Figure 3(D)).
Safety Outcomes
The overall incidence of AEs through day 90 in the pooled data was similar between the treatment groups (48.8% vs 47.9%, Table 4). AEs that occurred in > 1% of patients and more frequently in patients treated with MF implants and once-daily MFNS compared to control patients on MFNS alone were asthma worsening (4.7% vs 4.1%), headache (3.5% vs 3.4%), presyncope (2.4% vs 2.1%), and epistaxis (2.4% vs 1.4%). Implant-related AEs occurred in 15 (5.9%) patients, and the common AEs (> 1% of patients) were epistaxis (1.6%) and nasal discomfort (1.2%). Two (0.8%) patients reported 3 serious AEs (SAE)—asthma exacerbation, asthmatic bronchitis, and epistaxis—of which only 1 (0.4%) epistaxis was judged to be implant related.
Table 4.
AEs. a
| Category |
RESOLVE 15 |
RESOLVE II 16 |
Pooled Analysis |
|||
|---|---|---|---|---|---|---|
| Treatment (n = 53) | Control (n = 47) | Treatment (n = 201) | Control (n = 99) | Treatment (n = 254) | Control (n = 146) | |
| Patients with any AE | 33 (62.3) | 33 (70.2) | 91 (45.3) | 37 (37.4) | 124 (48.8) | 70 (47.9) |
| Patients with AE resulting in discontinuation | 0 | 0 | 0 | 0 | 0 | 0 |
| Patients with most common AEs (> 1% incidence rate in any group that occurred more commonly in the treatment group) | ||||||
| Nervous system disorders | 6 (11.3) | 5 (10.6) | 11 (5.5) | 3 (3.0) | 17 (6.7) | 8 (5.5) |
| Headache | 3 (5.7) | 2 (4.3) | 6 (3.0) | 3 (3.0) | 9 (3.5) | 5 (3.4) |
| Presyncope | 2 (3.8) | 3 (6.4) | 4 (2.0) | 0 | 6 (2.4) | 3 (2.1) |
| Respiratory, thoracic, and mediastinal disorders | 7 (13.2) | 5 (10.6) | 24 (11.9) | 9 (9.1) | 31 (12.2) | 14 (9.6) |
| Asthma (worsening) | 2 (3.8) | 2 (4.3) | 10 (5.0) | 4 (4.0) | 12 (4.7) | 6 (4.1) |
| Epistaxis | 3 (5.7) | 2 (4.3) | 3 (1.5) | 0 | 6 (2.4) | 2 (1.4) |
| Patients with any implant-related AE (>1% incidence rate) | 5 (9.4) | 0 | 10 (5.0) | 0 | 15 (5.9) | 0 |
| Epistaxis | 3 (5.7) | 0 | 1 (0.5) | 0 | 4 (1.6) | 0 |
| Nasal discomfort | 1 (8.3) | 0 | 2 (1.0) | 0 | 3 (1.2) | 0 |
| Patients with any SAE | 0 | 0 | 2 (1.0) | 1 (1.0) | 2 (0.8) | 1 (0.7) |
| Implant-related epistaxis | 0 | 0 | 1 (0.5) | 0 | 1 (0.4) | 0 |
Abbreviations: AE, adverse event; MedDRA, Medical Dictionary for Regulatory Activities; SAE, serious AE; PT, preferred term.
Values represent patient counts and percentages. Each AE was coded using MedDRA. A patient reporting more than 1 AE for a particular MedDRA PT is counted only once. A patient reporting several AEs with different PTs is counted under each term. Implant-related AEs comprise all AEs related to device, study drug, and/or implant procedure.
Discussion
The pooled analysis results support the safety and efficacy of sinus implants with 1350 mcg of MF for in-office treatment of CRS patients with NP who are candidates for RESS because of medically refractory symptoms and recurrent polyposis. Patients treated with MF sinus implants plus once-daily MFNS experienced improvements across several subjective and objective outcomes that were statistically superior to MFNS alone. The implants reduced NO/C score at day 90, a full month after their removal. Patients receiving MF sinus implants demonstrated greater reduction in BPG compared to controls at day 90, consistent with the observed greater decrease in ESO. The reduction in these objective endoscopic end points was identified in both studies based on a centralized blinded review of video endoscopies by the same independent panel of 3 sinus surgeons who were masked to both treatment assignment and patient clinical information. The clinical relevance of this magnitude of improvement with MF sinus implants was supported by a 59% reduction in the proportion of patients indicated for RESS at day 90 (from 100% at the onset to 41%), compared to 31% reduction (from 100% to 69%) among control patients. The control patients on once-daily MFNS were 3 times more likely to have surgery recommended at the end of the study compared to the treatment group who were treated with MF sinus implants plus once-daily MFNS. The observed reduction was considered clinically meaningful given that only patients who experienced significant improvements in both NO/C symptoms and BPG were deemed no longer indicated for RESS. The combination drug/device mode of action of MF sinus implants resulted in faster and greater treatment response compared to MFNS alone by mechanically opening the obstructed ethmoid sinus cavity, shrinking NP by targeted and sustained local delivery of corticosteroid to inflamed sinus mucosa, and providing superior access for topical therapy, which is one of the aims of ESS in patients with CRSwNP. Given the chronic nature of disease and high recurrence rate of NP, the ability of MF sinus implants to potentially avoid or delay another RESS could have positive implications for CRS patients and the health-care system. Ernst et al. have shown that the use of MF implants instead of RESS could result in substantial cost savings for payers in a single year even if the majority (>60%) of patients receive implants twice during that timeframe. 21 These cost savings are largely driven by substantially lower procedure-related costs associated with in-office MF implant placement. Therefore, repeat implantation with longer term follow-up as an alternative to RESS is of interest and is being studied (NCT03358329).
The results of this pooled analysis are compelling, given that the patient population consisted of CRSwNP patients exhibiting a high prevalence of comorbidities, such as allergic rhinitis, asthma, and AERD, and having failed prior RESS. The clinical significance supports the use of MF sinus implants plus once-daily MFNS as an effective treatment option for most of the evaluated subgroups compared to MFNS alone. The treatment effect of MF sinus implants plus MFNS appeared to be even greater compared to MFNS alone in patients with a larger extent of polyposis and exhibiting early NP recurrence post-ESS.
The predictive value of comorbidities with regard to the effectiveness of MF sinus implants in improving symptoms of NO/C was higher in patients without asthma. Recidivism of NP requiring RESS or medical treatment with oral or topical steroids is known to be higher in asthmatic patients.4,22,23 However, objective endoscopic improvements in BPG and ESO were similar in all treated patients regardless of asthma status. Patients without asthma were slightly less likely to be recommended for RESS at study end, compared to patients with asthma. CRSwNP patients who also have asthma typically experience considerably greater total disease burden than those without. It is possible that the reported reduction in NO/C is commensurately influenced by their overall disease burden, though objective measures fail to show a marked difference in effect.
Patients with moderate-to-severe allergic rhinitis reported a statistically significant and greater reduction in symptoms of NO/C that was mirrored by objective outcomes, compared to patients with no or mild allergic rhinitis. More diseased patients with allergic rhinitis who received MF implants were less likely to be recommended for RESS than those with no-to-mild allergic rhinitis, compared to controls. Because INCSs are primary treatments for allergic rhinitis, patients with comorbid moderate-to-severe allergic rhinitis may experience a secondary benefit from MF delivered by implants. These results suggest that patients with CRSwNP and comorbid allergic rhinitis are particularly well suited for MF implants.
Patients with AERD reported subjectively less improvement in NO/C symptoms than patients without AERD. Objectively, patients with AERD were observed to have a slightly larger reduction in BPG and an essentially identical reduction in ESO versus patients without AERD, though the reduction in BPG did not reach statistical significance. These findings are consistent with other studies that show AERD patients to be a particularly recalcitrant subgroup of CRSwNP patients.
The magnitude of symptomatic improvement was moderate in current and former smokers and patients without altered olfaction. Smoking increases the risk of NP recurrence and shortens the time to RESS compared to nonsmokers even after 10 years following initial ESS. 24 Our findings of worse subjective and objective outcomes in current or former smokers are consistent with prior studies showing worse outcomes in this patient subgroup. Therefore, smoking cessation should be included in comprehensive treatment for all CRSwNP patients. Impaired sense of smell affects quality of life of CRS patients with NP and represents one of the main reasons that patients seek medical or surgical treatment.25–28 NP patients with impaired sense of smell reported significant improvement in both their smell and NO/C after undergoing MF sinus implant placement. 16 Similarly, patients with olfactory problems undergoing ESS reported more substantial symptom improvement than those without anosmia. 28
Patients who had undergone only 1 prior ESS noted a greater symptomatic improvement in NO/C compared to patients who had several prior ESS. However, objective measures showed larger improvement in patients who had 2 or more ESS. Although the interpretation of these findings remains uncertain, the subjective change experienced by CRS patients with NP after first ESS may be truly life changing, while those patients who have undergone several ESS previously may have tempered expectations and, therefore, rate their improvement as less dramatic. On objective outcomes measures, the results may reflect that some ethmoid cells missed at primary ESS are subsequently removed at RESS and that the ethmoid obstruction in these patients is more thoroughly soft tissue in nature.
The timing of most recent ESS did not affect subjective outcomes, but the objective outcomes were markedly better in patients who had undergone ESS less than 24 months prior. Additionally, patients receiving MF implants and once-daily MFNS in this subgroup were 7 times less likely to be indicated for RESS at the end of study than control patients on MFNS alone. These results suggest that MF implants may be more effective earlier in the treatment of recurrent NP after ESS, ideally within 24 months of the most recent ESS.
Finally, patients with extensive polyposis (BPG > 5) were seen to have superior objective improvement, with treatment effect size in BPG and ESO exceeding that in patients with BPG of 5 or less. Subjective improvements in NO/C showed the same trend, although results did not reach statistical significance.
The safety profile of MF sinus implants was supported by low incidence of implant-related SAEs (0.4%) and AEs (5.9%). The reported incidence rates of epistaxis (0.4% as SAE and 1.6% as AE) were well below 6% reported in the study with 200 mcg MFNS once daily, the only FDA-approved INCS for treatment of NP at the time the studies were conducted. 29 The favorable safety profile of MF sinus implants is further supported by high patient tolerance and satisfaction with the in-office placement procedure, 15 negligible systemic exposure, 18 and negligible ocular risk, 15 making it an appealing, minimally invasive topical therapy strategy for CRS patients with NP.
The interpretation of this pooled analysis should take into consideration several potential limitations. First, the scales used to assess outcomes differed slightly between studies. The NO/C symptom scales included in the pooled analysis were scored by patients using the same reflective questionnaire but differed in range. The mapping of the scores from the 0–5 scale in the first RCT to the 0–3 scale used in the second RCT was reasonable, given that both mild and both severe scores were grouped. The lack of remapping of the BPGs was reasonable, given that both studies reported the results on the same 0–8 scale. A second limitation was that the clinical investigators performing endoscopic grading and assessment of indication for RESS were not blinded to the treatment assignment, but this was mitigated by requiring real-time grading at each study visit (the investigators were not permitted to review prior grading) and providing the video endoscopies to the independent panel for centralized blinded grading of the coprimary end point. Finally, the use of ancillary therapies might have confounded the effect of the MF sinus implants compared with sham for the treatment of CRS patients with NP in the 2 RCTs. Although relevant data are inconclusive, there is evidence that analgesics, topical decongestants, INCS, antihistamines, and even saline irrigation may aid in the alleviation of sinonasal symptoms; however, both treatment groups followed standardized regimen of ancillary treatments in both trials, which minimized the possible confounding effect.
Conclusion
The morbidity and cost of treatment of CRS patients with NP are substantial, and there is a need for innovative treatment options. The present pooled analysis indicates that in-office placement of MF sinus implants plus once-daily MFNS demonstrated favorable results at day 90 compared to MFNS alone in improvement of NO/C symptoms and reduction in BPG and ESO in CRS patients with recurrent and medically refractory NP, representing an alternative for RESS. The subgroup analyses revealed that MF sinus implants may play an important role in management of NP patients, especially those who have allergic rhinitis, expanded polyposis, altered sense of smell, and most recent ESS within 24 months.
Acknowledgments
The authors thank Andy Mugglin, PhD, from Paradigm Biostatistics, Inc. for providing biostatistical advice.
Authors’ Note
The pooled analysis results were presented at the Annual Meeting of American Rhinologic Society on September 9, 2017 at Chicago, IL.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The study sponsor (Intersect ENT, Inc.) provided the investigational product, funding, and administrative and logistical support to the participating clinical sites. The sponsor was involved in the design and conduct of the RESOLVE and RESOLVE II studies, as they were U.S. FDA-regulated trials and assisted in monitoring and collection of data. The sponsor participated in the interpretation of the data and preparation of the manuscript. All authors reviewed and critiqued the draft manuscript and approved the final manuscript prior to submission for publication. J.P.S. received consultant fees from Intersect ENT, Inc.; R.C.K. has no conflict of interest to report; J.K.H. received consultant fees from Intersect ENT; K.D.F. has no conflict of interest to report; R.A.O. has no conflict of interest to report; S.K.W. has no conflict of interest to report; A.G. has no conflict of interest to report; K.E.M. received consulting fees from Intersect ENT; B.K. received consulting fees from Intersect ENT; S.H. received consulting fees from Intersect ENT; J.W.S. and A.K.G. are employees of and hold stock in Intersect ENT.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Authors participating in RESOLVE and RESOLVE II as national or principal investigators (J.P.S., R.C.K., K.D.F., R.A.O, S.K.W., and A.G.) received financial support for the research from the study sponsor. None of the authors received financial support for the authorship and publication of this article.
References
- 1.Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for U.S. adults: national health interview survey, 2012. Vital Health Stat 10. 2014; 260:1–161. [PubMed] [Google Scholar]
- 2.Bhattacharyya N, Orlandi RR, Grebner J, Martinson M. Cost burden of chronic rhinosinusitis: a claims-based study. Otolaryngol HNS. 2011; 144:440–445. [DOI] [PubMed] [Google Scholar]
- 3.Caulley L, Thavorn K, Rudmik L, Cameron C, Kilty SJ. Direct costs of adult chronic rhinosinusitis by using 4 methods of estimation: results of the US Medical Expenditure Panel Survey. J Allergy Clin Immunol. 2015; 136:1517–1522. [DOI] [PubMed] [Google Scholar]
- 4.DeConde AS, Mace JC, Levy JM, Rudmik L, Alt JA, Smith TL. Prevalence of polyp recurrence after endoscopic sinus surgery for chronic rhinosinusitis with nasal polyposis. Laryngoscope. 2016; 127:550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fandino M, Maconald K, Lee J, Witterick I. The use of postoperative topical corticosteroids in chronic rhinosinusitis with nasal polyps: a systematic review and meta-analysis. Am J Rhinol Allergy. 2013; 27:e146–e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orlandi RR, Kingdom TT, Hwang PH, et al. International consensus statement on allergy and rhinology: rhinosinusitis. Int Forum Allergy Rhinol. 2016; 6(Suppl 1):S22–S209. [DOI] [PubMed] [Google Scholar]
- 7.Poetker DM. Oral corticosteroids in the management of adult chronic rhinosinusitis with and without nasal polyps: an evidence-based review with recommendations. Int Forum Allergy Rhinol. 2013; 3:104–120. [DOI] [PubMed] [Google Scholar]
- 8.Sastre J, Mosges R. Local and systemic safety of intranasal corticosteroids. J Investig Allergol Clin Immunol. 2012; 22:1–12. [PubMed] [Google Scholar]
- 9.Wei C, Adappa N, Cohen N. Use of topical nasal therapies in the management of chronic rhinosinusitis. Laryngoscope. 2013; 123:2347–2359. [DOI] [PubMed] [Google Scholar]
- 10.Sanan A, Rabinowitz M, Rosen M, Nyquist G. Topical therapies for refractory chronic rhinosinusitis. Otolaryngol Clin North Am. 2017; 50:129–141. [DOI] [PubMed] [Google Scholar]
- 11.Bachert C, Mannent L, Naclerio RM, et al. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. JAMA. 2016; 315:469–479. [DOI] [PubMed] [Google Scholar]
- 12.Lam K, Kern RC, Luong A. Is there a future for biologics in the management of chronic rhinosinusitis? Int Forum Allergy Rhinol. 2016; 6:935–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.SINUVA (mometasone furoate) Sinus Implant Prescribing Information. In: US FDA (ed.). December 2017, ID: 4192542..
- 14.Forwith KD, Han JK, Stolovitzky JP, et al. RESOLVE: bioabsorbable steroid-eluting sinus implants for in-office treatment of recurrent sinonasal polyposis after sinus surgery: 6-month outcomes from a randomized, controlled, blinded study. Int Forum Allergy Rhinol. 2016; 6:573–581. [DOI] [PubMed] [Google Scholar]
- 15.Han JK, Forwith KD, Smith TL, et al. RESOLVE: a randomized, controlled, blinded study of bioabsorbable steroid-eluting sinus implants for in-office treatment of recurrent sinonasal polyposis. Int Forum Allergy Rhinol. 2014; 4:861–870. [DOI] [PubMed] [Google Scholar]
- 16.Kern RC, Stolovitzky JP, Silvers SL, et al. A phase 3 trial of mometasone furoate sinus implants for chronic sinusitis with recurrent nasal polyps. Int Forum Allergy Rhinol. 2018; 8:471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lavigne F, Miller SK, Gould AR, Lanier BJ, Romett JL. Steroid-eluting sinus implant for in-office treatment of recurrent nasal polyposis: a prospective, multicenter study. Int Forum Allergy Rhinol. 2014; 4:381–389. [DOI] [PubMed] [Google Scholar]
- 18.Ow R, Groppo E, Clutter D, Gawlicka AK. Steroid-eluting sinus implant for in-office treatment of recurrent polyposis: a pharmacokinetic study. Int Forum Allergy Rhinol. 2014; 4:816–822. [DOI] [PubMed] [Google Scholar]
- 19.Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol HNS. 2007; 137:S1–S31. [DOI] [PubMed] [Google Scholar]
- 20.Meltzer EO, Hamilos DL, Hadley JA, et al. Rhinosinusitis: developing guidance for clinical trials. J Allergy Clin Immunol. 2006; 118:S17–S61. [DOI] [PubMed] [Google Scholar]
- 21.Ernst FR, Imhoff RJ, DeConde A, Manes RP. Budget impact of a steroid-eluting sinus implant versus sinus surgery for adult chronic sinusitis patients with nasal polyps. J Manag Care Spec Pharm. Published online March 6, 2019:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bachert C, Holtappels G. Pathophysiology of chronic rhinosinusitis, pharmaceutical therapy options. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2015; 14:Doc09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Batra PS, Tong L, Citardi MJ. Analysis of comorbidities and objective parameters in refractory chronic rhinosinusitis. Laryngoscope. 2013; 123(Suppl 7):S1–S11. [DOI] [PubMed] [Google Scholar]
- 24.Wu AW, Ting JY, Platt MP, Tierney HT, Metson R. Factors affecting time to revision sinus surgery for nasal polyps: a 25-year experience. Laryngoscope. 2014; 124:29–33. [DOI] [PubMed] [Google Scholar]
- 25.Alobid I, Mullol J. Role of medical therapy in the management of nasal polyps. Curr Allergy Asthma Rep. 2012; 12:144–153. [DOI] [PubMed] [Google Scholar]
- 26.Blomqvist E, Lundblad L, Anggards A, Haraldsson P-O, Stjarne P. A randomized controlled study evaluating medical treatment versus surgical treatment in addition to medical treatment of nasal polyposis. J Allergy Clin Immunol. 2001; 107:224–228. [DOI] [PubMed] [Google Scholar]
- 27.Hox V, Bobic S, Callebaux I, Jorissen M, Hellings PW. Nasal obstruction and smell impairment in nasal polyp disease: correlation between objective and subjective parameters. Rhinology. 2010; 48:426–432. [DOI] [PubMed] [Google Scholar]
- 28.Rudmik L, Smith TL. Olfactory improvement after endoscopic sinus surgery. Curr Opin Otolaryngol HNS. 2012; 20:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stjarne P, Mosges R, Jorissen M, et al. A randomized controlled trial of mometasone furoate nasal spray for the treatment of nasal polyposis. Arch Otolaryngol Head Neck Surg. 2006; 132:179–185. [DOI] [PubMed] [Google Scholar]