Abstract
Utilizing over 140,000 geocoded medical records for a diverse sample of children ages 2–12 living in Houston, Texas, we examine whether a comprehensive set of neighborhood social and environmental characteristics explain racial and ethnic disparities in childhood asthma. Adjusting for all individual risk factors, as well as neighborhood concentrated disadvantage, particulate matter, ozone concentration, and race/ethnic composition, reduced but did not fully attenuate the higher odds of asthma diagnosis among black (OR=2.59, 95% CI=2.39, 2.80), Hispanic (OR=1.22, 95% CI=1.14, 1.32) and Asian (OR=1.18, 95% CI=1.04, 1.33) children relative to whites.
Keywords: Childhood asthma, Neighborhoods, Race/ethnicity, Environment, Disparities
1. Introduction
Asthma, a leading chronic condition among children in the U.S., varies substantially across racial/ethnic groups. In 2012, 22% of non-Hispanic black children carried a diagnosis of asthma compared to 14% of Hispanic and 12% of non-Hispanic white children (Bloom et al., 2013). Research suggests that disparities in asthma prevalence between black and white children have increased in the last decade (Akinbami et al., 2014). The causes of asthma, which include a range of individual- and contextual-level risk factors, are complex (Gold and Wright, 2005). While it remains unclear why large disparities in childhood asthma persist, recent attention is focusing beyond individual level factors to the role of neighborhood context (Wilhelm et al., 2009; Williams et al., 2009; Rosenbaum, 2008).
Neighborhood factors contribute to pediatric asthma by exposing children to health risks and resources in the physical and social environment, which become biologically “embodied” and reflected in the social patterning of asthma prevalence (Kreiger, 2001: 673). Asthma prevalence is higher in low-income, urban communities (Williams et al., 2009; Bhan et al., 2015), and black and Hispanic children disproportionately live in disadvantaged and racially segregated areas, which are contextual characteristics associated with a host of negative health risks and outcomes (Williams and Collins, 2001; Williams et al., 2009; Lichter, 2013). Black and Hispanic children are therefore exposed to ecologically distinct physical and social environments that may contribute to the uneven distribution of asthma prevalence.
The purpose of this study is to examine whether racial and ethnic disparities persist in asthma prevalence after accounting for children’s differential exposure to health risks at the individual and neighborhood-level. Specifically, we draw from an ecological framework to examine whether three aspects of neighborhood context account, at least partially, for racial/ethnic disparities in childhood asthma: outdoor air pollution (including particulate matter and ozone levels), socioeconomic status (SES), and race/ethnic residential composition.
1.1. Physical and social neighborhood contributors to asthma disparities
Outdoor air pollution is an aspect of the physical environment associated with increased asthma prevalence in a community, especially among young children who have developing lungs and immature metabolic pathways (Guarnieri and Balmes, 2014). Ozone and fine particulate matter less than 2.5 micrograms (μm) in aerodynamic diameter (PM2.5) are pollutants commonly linked to worse asthma symptoms and to a lesser degree, asthma onset, though findings are not always consistent (Guarnieri and Balmes, 2014). For example, Akinbami et al. (2010) find that children (ages 3–17 years) who are chronically exposed to high levels of ozone and particulate matter at the county-level are more likely to suffer from asthma, and among children living in L.A., high ozone exposure is associated with worse asthma morbidity even after accounting for household and neighborhood-level risk factors (Wilhelm et al., 2009). Nishimura et al. (2013), however, find no association between early life exposure to PM2.5 and subsequent asthma onset among Latino and black children from five geographic regions in the U.S. and Puerto Rico. Beyond these inconsistencies, it is less clear whether disproportionate exposure to outdoor air pollution accounts, at least in part, for higher rates of asthma prevalence among black and Hispanic children. Higher levels of air pollution are generally found in lower-income, non-white communities (Gold and Wright, 2005; Ash and Fetter, 2004), and evidence suggests that children in low-income neighborhoods experience increased incidence of asthma diagnosis when ambient air pollution levels rise (Wendt et al., 2014). Several studies, however, find persistent racial disparities in asthma even after controlling for air pollution, suggesting that air pollutants are one of many asthma risk factors unevenly experienced by minorities (e.g. Rosenbaum, 2008; Astell-Burt et al., 2013).
Higher rates of asthma prevalence in low SES neighborhoods may also indicate differential health risks in the social environment. Neighborhood disadvantage, which exposes residents to a host of daily and chronic stressors, is characterized by a web of health risks including poverty, substandard housing and building infrastructure, unemployment, the erosion of social capital, and exposure to high levels of violence and crime (Williams et al., 2009; Gold and Wright, 2005). In turn, stress is associated with increased asthma severity (and possibly asthma onset) among children, particularly in early life (Chen et al., 2006; Gold and Wright, 2005). For instance, exposure to community violence has been linked to worse asthma morbidity among children and increased vulnerability to negative respiratory health effects of air pollution (Wright, 2011; Williams et al., 2009). Neighborhood disadvantage may also influence behavioral risk factors if residents adopt coping strategies (e.g. smoking) that threaten children’s respiratory health (Gold and Wright, 2005).
Racial residential composition is another aspect of the social environment that may contribute to racial disparities in childhood asthma. There are well-established race/ethnic disparities in housing and neighborhood conditions, which are not fully attributable to group differences in SES (Rosenbaum, 2008; Friedman and Rosenbaum, 2004). Specifically, black and Hispanic households are exposed to higher levels of substandard housing conditions (e.g. chipping paint, pest infestation, lower quality housing stock and inadequate heating), which are risk factors associated with asthma prevalence and increased asthma morbidity (Rosenbaum, 2008; Krieger and Higgins, 2002). Racial residential composition is also a powerful predictor of neighborhood quality and access to community resources (Logan, 2013; Massey and Denton, 1993). For instance, Logan (2013) finds that on average, affluent black and Hispanic households are exposed to higher levels of neighborhood poverty than poor white households. While racial residential segregation is typically linked to poor health outcomes (Williams and Collins, 2001), some evidence suggests that after accounting for area-level socioeconomic disadvantage, a protective effect of racial/ethnic density emerges, whereby race/ethnic minorities are healthier when they live in neighborhoods with a higher concentration of members from their own race/ethnic group (Bécares et al., 2012; Astell-Burt et al., 2013). The protective effects of racial/ethnic density, or co-ethnic composition, are theoretically attributed to the buffering effects of bolstered social cohesion, a strong sense of community, mutual social support and diminished exposure to racial discrimination and low-status stigma (Bécares et al., 2012; White and Borrell, 2011). In a review of 57 studies examining the (physical) health effects of ethnic density among adults, Bécares et al. (2012) conclude that higher levels of residential co-ethnic composition are often detrimental for U.S. blacks, though generally protective for U.S. Hispanics. Overall, the protective or harmful effect of ethnic density varied by physical health outcome and respondent race/ethnicity (Bécares et al., 2012). Taken together, racial residential composition, regardless of SES, influences children’s exposure to health risks and resources, and as such, is an important component for understanding the racial patterning of asthma prevalence among children who reside in neighborhoods of various race/ethnic compositions.
Despite this potential importance of racial residential composition, we know very little about how neighborhood racial composition is associated with childhood asthma. Among the few studies that account for racial composition, researchers typically examine how the presence of a single race/ethnic group (e.g. percent black) influences asthma outcomes (equally) for all children (e.g. Holt et al., 2013; Rosenbaum, 2008; Wilhelm et al., 2009; Cagney et al., 2007). For instance, Holt et al. (2013) find no association between asthma diagnosis by age five and percent non-Hispanic black residents, while Rosenbaum (2008) finds no association between the percent of white residents and asthma prevalence among a racially and ethnically diverse sample of New York households. None of these studies, however, assess the effect of race/ethnic composition (i.e. race/ethnic density) for each race/ethnic group (e.g. percent Hispanic for Hispanic respondents) or whether the effect of co-ethnic composition differs by respondent race/ethnicity. In several U.S. metropolitan areas, including Houston, large-scale immigration from Latin American and Asian countries is creating a context of changing residential patterns. Neighborhoods of race/ethnic diversity are emerging but also coexisting alongside neighborhoods characterized by more traditional patterns of segregation (e.g. black-white) and white flight (Logan and Zhang, 2010; Logan, 2013). To reflect the diversifying landscape of the U.S. and to fill existing gaps, we utilize an indicator of racial composition – percent co-ethnic (also referred to hereafter as co-ethnic composition) – to assess how racial disparities in asthma prevalence are associated with various arrangements of racial/ethnic density for black, white, Hispanic and Asian respondents. To our knowledge, this is the first study to examine the effects of percent coethnic for childhood asthma and to test whether these effects differ based on a child’s race/ethnic status.
Racial disparities in childhood asthma therefore must be understood as resulting from multiple, concurrent health risks at the individual and neighborhood level (Williams et al., 2009). To capture asthma risk across multiple contextual levels, we link electronic medical records from over 200,000 children (ages 2–12) living in the Houston metropolitan region with census and historical ambient air quality data to create a detailed portrait of children’s physical and social environment. The Houston metropolitan area, as the most racially and ethnically diverse large metropolitan area in the U.S. (Emerson et al., 2012), is an ideal location for examining how racial differences in childhood asthma are related to neighborhood contexts that diverge from the traditional black-white color line (Logan, 2013). The current study has two primary goals. First, we establish how asthma prevalence differs among non-Hispanic black (black), Hispanic, Asian and non-Hispanic white (white) children in a large, ethnically diverse sample. Second, holding individual-level characteristics constant, we examine whether accounting for differential exposure to health risks in the neighborhood environment – including outdoor air pollution, concentrated disadvantage and racial residential composition – explains race/ethnic disparities in childhood asthma. Few studies have explored the role of neighborhood context in contributing to racial disparities in pediatric asthma, and to our knowledge, none of these studies include comprehensive measures of both the physical (e.g. outdoor air pollution) and social environments (e.g. neighborhood SES and racial/ethnic residential composition) (e.g. Rosenbaum, 2008; Holt et al., 2013; Pearlman et al., 2006).
2. Data and methods
Data for our project come from multiple sources. Health records from all inpatient pediatric visits to Texas Children’s Hospital (TCH) and all outpatient visits to Texas Children’s Pediatrics Associates (TCPA) in 2011–2013 were compiled. The TCPA data was generated from 50 clinics located in the Houston metropolitan area. To be included in our sample, children must have had an outpatient TCPA record, and they may have also had an inpatient record. Children who were only in our inpatient sample (most of whom live outside the Houston area) or had missing values for insurance (24%) or race/ethnic status (12%) were not retained. If a child was aged 2–12 at one or more visits, they were included in our records. If more than one child per household was in the data, we selected one child at random for a final sample size of 142,407 unique children ages 2–12 with visits to TCPA in 2011–2013. Contextual data at the Census tract geographic level come from two sources. First, U.S. American Community Survey data from 2009–2013 is utilized to characterize the racial/ethnic and socioeconomic composition of the children’s neighborhoods. Second, ozone and PM2.5data for the years 2010–2012 are gathered from the Texas Commission on Environmental Quality’s Texas Air Monitoring Information System. The individual-level health records were linked to children’s residential neighborhoods (Census tracts) and the contextual data, to create detailed information about the residential context in which the children live.
2.1. Measures
To assess whether a child had asthma, we utilized billing codes and descriptions in the patient records. If a child received an ICD9 diagnosis code in the first five diagnosis fields at any visit that contained the word “asthma,” the child was coded as having asthma. These billing code descriptions correspond to ICD9 codes which begin with 493. It is important to note that there may be asthmatic children in our data who are not coded as asthmatic, if they visited the doctor or were seen as an inpatient but did not receive a billing code for asthma. However, the rates of asthma we see in our data (described further in the results section) are comparable to other local and state estimates (Wickerham and Bhakta, 2013). Moreover, health records have potential advantages over parent-reported survey data, which may be prone to bias (Akinbami et al., 2014, 2005). We also control for the total number of medical visits a child made in 2011–2013, because the likelihood of receiving an asthma diagnosis may increase along with the number of visits.
Additional child-level data extracted from the medical record included age, gender, race/ethnicity, and insurance type. Child race/ethnicity was categorized as Non-Hispanic white (white), Non-Hispanic black (black), Hispanic, and Asian. Children’s insurance type was categorized as private or public. At the Census tract or neighborhood level, our measures include environmental air quality indicators as well as measures of socioeconomic and racial/ethnic composition. To determine air quality measures, daily maximum 8-h ozone concentrations and daily concentrations of PM2.5were used to calculate the average of quarterly means over three years (2010–2012) with data collected from the Houston Metropolitan area air monitoring network. Census tract concentrations were then estimated at the geographic center using a geostatistical method that incorporates data from nearby air monitors (Rodriquez and Alexeeff, 2014). Our measures of ozone and PM2.5 concentration indicate chronic, rather than acute, exposure to ambient air pollutants. We include ozone concentration as a continuous measure and PM2.5 as quartiles because the measures are collinear.
To assess social and economic disadvantage in neighborhoods we include an index of concentrated disadvantage, created based on the first dimension of a principal components factor analysis on percent of adults in the census tract living below the poverty line, the percent of households receiving public assistance, the percent of adult residents who are unemployed, and the percent of female-headed households with children (Sampson et al., 1997). To assess the race/ethnic composition of neighborhoods, we account for the percent of neighborhood residents who share the respondent’s race/ethnicity. This method of considering the presence of co-ethnic residents for each race/ethnic group allows us to test whether residential co-ethnic composition is differentially protective or harmful for asthma prevalence among a diverse sample of children, who live in neighborhoods characterized by various arrangements of race/ethnic composition.
2.2. Analysis
Because our data come from children who are nested within neighborhoods but who are also cross-classified within outpatient pediatric clinics, our analytic strategy relies on cross-classified multilevel logistic regression models (Rabe-Hesketh and Skrondal, 2012), predicting our binary outcome of whether the child ever received a diagnosis of asthma. These models allow us to take into account the fact that children from different neighborhoods access the same clinic; and that children from the same neighborhoods access different clinics. They also allow us to estimate the variance attributable to the clinic level as well as the neighborhood level. In addition, when one level of potential classification is ignored with clustered data, estimates may be biased (Dunn et al., 2015). In our data, children are nested within 1039 neighborhoods and 50 clinics. The average neighborhood has 137 children and the average clinic has 2848 children. Table 1 presents descriptive statistics about our individual-level and neighborhood-level data. Table 2 first presents results of a set of models that progressively add individual-level covariates (Table 2, Models 1–2). Next, we add neighborhood-level covariates for our environmental and socioeconomic measures, separately (Table 2, Models 3–4). We then estimate a model which includes all individual- and neighborhood-level covariates, including percent co-ethnic (Table 2, Model 5). Finally, we assess whether the effect of co-ethnic composition on asthma risk differs by children’s race/ethnic status (Table 2, Model 6).
Table 1.
Descriptive statistics for the full sample and by child’s race/ethnicity, mean or proportion and (standard deviation).
| Full sample (N=142,407) |
NH white (N=70,920) |
NH black (N=23,287) |
Hispanic (N=39,366) |
Asian (N=8834) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Child has asthma diagnosis | 0.06 | 0.04 | 0.13 | *** | 0.07 | *** | 0.04 | ||||||
| Total number of visits, 2011–2012 | 2.98 | (2.13) | 3.13 | (2.23) | 2.74 | (1.87) | *** | 2.95 | (2.16) | *** | 2.64 | (1.70) | *** |
| Child is male | 0.52 | 0.51 | 0.51 | 0.52 | 0.52 | ||||||||
| Child’s age (years) | 5.71 | (3.22) | 5.78 | (3.27) | 5.81 | (3.20) | 5.66 | (3.13) | *** | 5.17 | (3.10) | *** | |
| Insurance Status | |||||||||||||
| Private | 0.72 | 0.90 | 0.48 | *** | 0.48 | *** | 0.91 | ||||||
| Public | 0.28 | 0.10 | 0.52 | *** | 0.52 | *** | 0.09 | ||||||
| Neighborhood Characteristics | |||||||||||||
| Environmental | |||||||||||||
| Average ozone concentration, 2010–2012 | 25.91 | (1.05) | 26.22 | (1.09) | 25.58 | (0.85) | *** | 25.60 | (0.98) | *** | 25.70 | (0.72) | *** |
| Average PM2.5 concentration, 2010–2012 | 10.38 | (0.42) | 10.31 | (0.35) | 10.46 | (0.44) | *** | 10.50 | (0.48) | *** | 10.20 | (0.30) | *** |
| PM2.5 quartile 1 (low) | 0.19 | 0.21 | 0.15 | *** | 0.14 | *** | 0.33 | *** | |||||
| PM2.5 quartile 2 | 0.28 | 0.27 | 0.30 | *** | 0.27 | *** | 0.33 | *** | |||||
| PM2.5 quartile 3 | 0.32 | 0.40 | 0.22 | *** | 0.25 | *** | 0.29 | *** | |||||
| PM2.5 quartile 4 (high) | 0.21 | 0.12 | 0.33 | *** | 0.34 | *** | 0.05 | *** | |||||
| Socioeconomic | |||||||||||||
| Concentrated disadvantage (std) | 0.00 | (1.00) | −0.41 | (0.67) | 0.71 | (1.25) | *** | 0.43 | (0.98) | *** | −0.46 | (0.66) | *** |
| Race/Ethnic Composition | |||||||||||||
| Percent co-ethnic | 0.50 | (0.26) | 0.62 | (0.20) | 0.35 | (0.25) | *** | 0.45 | (0.25) | *** | 0.15 | (0.11) | *** |
p < 0.01
p < 0.05
p < 0.001
sig. differences between NH white children and indicated race/ethnic group.
Note: Co-ethnic represents the percent of neighborhood residents who share the respondent’s race/ethnicity.
Table 2.
Cross-classified logistic regression predicting asthma diagnosis, children ages 2–12.
| Model 1 |
Model 2 |
Model 3 |
Model 4 |
Model 5 |
Model 6 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O.R. | C.I. | O.R. | C.I. | O.R. | C.I. | O.R. | C.I. | O.R. | C.I. | O.R. | C.I. | |
| Total number of visits, 2011–2012 | 1.33 | (1.32, 1.35) | 1.33 | (1.32, 1.34) | 1.33 | (1.32,1.34) | 1.33 | (1.32,1.34) | 1.33 | (1.32,1.34) | 1.33 | (1.32,1.34) |
| Child’s race/ethnicity | ||||||||||||
| Non-Hispanic white | (Ref.) | (Ref.) | (Ref.) | (Ref.) | (Ref.) | |||||||
| Non-Hispanic black | 2.93 | (2.74, 3.14) | 2.84 | (2.65, 3.04) | 2.86 | (2.67,3.06) | 2.79 | (2.60,3.00) | 2.59 | (2.39,2.80) | 1.95 | (1.68,2.26) |
| Hispanic | 1.34 | (1.26, 1.43) | 1.29 | (1.21, 1.38) | 1.30 | (1.22,1.40) | 1.29 | (1.20,1.38) | 1.22 | (1.14,1.32) | 0.97 | (0.82,1.14) |
| Asian | 1.28 | (1.14, 1.44) | 1.29 | (1.15, 1.44) | 1.29 | (1.15,1.45) | 1.30 | (1.15,1.45) | 1.18 | (1.04,1.33) | 0.83 | (0.67,1.04) |
| Child is male | 1.53 | (1.46, 1.60) | 1.53 | (1.46, 1.60) | 1.53 | (1.46,1.60) | 1.53 | (1.46,1.60) | 1.53 | (1.46,1.60) | 1.53 | (1.46,1.60) |
| Child’s age (years) | 1.08 | (1.07, 1.08) | 1.08 | (1.07, 1.08) | 1.08 | (1.07,1.08) | 1.08 | (1.07,1.08) | 1.08 | (1.07,1.08) | 1.08 | (1.07,1.08) |
| Insurance status | ||||||||||||
| Private | (Ref.) | (Ref.) | (Ref.) | (Ref.) | ||||||||
| Public | 1.21 | (1.14, 1.29) | 1.21 | (1.14,1.29) | 1.19 | (1.12,1.27) | 1.19 | (1.12,1.27) | 1.19 | (1.12,1.27) | ||
| Neighborhood Characteristics | ||||||||||||
| Population density (ln) | 0.98 | (0.96,1.01) | 0.97 | (0.95,1.00) | 0.99 | (0.96,1.01) | 0.98 | (0.95,1.01) | ||||
| Environmental | ||||||||||||
| PM2.5 quartile 1 (Low) | (Ref.) | (Ref.) | (Ref.) | |||||||||
| PM2.5 quartile 2 | 0.99 | (0.91,1.08) | 0.98 | (0.90,1.07) | 1.00 | (0.92,1.09) | ||||||
| PM2.5 quartile 3 | 0.94 | (0.85,1.04) | 0.94 | (0.85,1.04) | 0.96 | (0.87,1.07) | ||||||
| PM2.5 quartile 4 (High) | 0.93 | (0.82,1.05) | 0.92 | (0.81,1.04) | 0.93 | (0.82,1.05) | ||||||
| Average ozone concentration, 2010–2012 | 1.01 | (0.97,1.06) | 1.02 | (0.97,1.06) | 1.03 | (0.99,1.08) | ||||||
| Socioeconomic | ||||||||||||
| Concentrated disadvantage (std) | 1.04 | (1.01,1.07) | 1.06 | (1.03,1.09) | 1.03 | (1.00,1.07) | ||||||
| Race/Ethnic Composition | ||||||||||||
| Percent co-ethnic | 0.80 | (0.72,0.89) | 0.54 | (0.43,0.67) | ||||||||
| Race/Ethnic Composition * Child Race/Ethnicity | ||||||||||||
| NHW * white co-ethnic | (Ref.) | |||||||||||
| NHB * black co-ethnic | 2.01 | (1.46,2.76) | ||||||||||
| H * Hispanic co-ethnic | 1.63 | (1.18,2.24) | ||||||||||
| A * Asian co-ethnic | 3.26 | (1.32,8.04) | ||||||||||
| Intercept (SE) | 0.01 | (0.01,0.01) | 0.01 | (0.01,0.01) | 0.01 | (0.00,0.02) | 0.01 | (0.01,0.01) | 0.01 | (0.00,0.02) | ||
| Random Effects Estimates | ||||||||||||
| Clinics | 0.65 | (0.53,0.81) | 0.63 | (0.51,0.77) | 0.63 | (0.51,0.77) | 0.62 | (0.50,0.77) | 0.62 | (0.50,0.76) | ||
| Neighborhoods | 0.10 | (0.06,0.17) | 0.09 | (0.05,0.17) | 0.09 | (0.05,0.17) | 0.09 | (0.05,0.17) | 0.08 | (0.04,0.17) | ||
| N=142,407 | ||||||||||||
Note: Children are cross-classified by neighborhoods and by clinic. For the fixed effect estimates, odds ratios (O.R.) are presented along with 95% confidence intervals. The intercept is a parameter estimate presented with its standard error in parentheses. Random effect estimates are presented as parameters and 95% confidence intervals.
Note: NHW: non-Hispanic white; NHB: non-Hispanic black; H: Hispanic; A: Asian.
2.3. Sensitivity analyses
To assess the robustness of our findings, we conducted a number of sensitivity analyses. First, we estimated models including patients who were missing values on insurance and race/ethnicity. Because our findings were substantively similar, we exclude children missing on those covariates in the models we present here. We also ran separate multilevel models with children clustered only within neighborhoods and then only within clinics. Although the fixed effect estimates were substantively similar across model specifications, the confidence intervals for the variance components were smallest using the cross-classified models and so we present those estimates. Further, although a relatively small proportion of our sample moved during the study period (15%) (as estimated from changes in address in their billing data) we estimated the fully-adjusted model (Table 2, Model 5) only for children who did not move. Results were virtually identical, so we include both movers and non-movers in the models presented here.
3. Results
Among our analytic sample of 142,407 pediatric patients ages 2–12, approximately 6% of children have a physician-diagnosis of asthma. Table 1 presents descriptive statistics for the full sample and by the child’s race/ethnic group. In terms of race/ethnicity, 50% of the children are white, 16% are black, 28% are Hispanic, 6% are Asian. On average, the children are 6 years old, and 72% are privately insured. Another 28% have public health insurance such as Medicaid or the Children’s Health Insurance Program (CHIP). Census tract air quality concentrations are measured in micrograms per cubic meter with an ozone range from 23.8 to 31.4, and low to high PM2.5 quartiles range from 6.8–9.9 (“low” PM2.5), 10.0–10.3, 10.4–10.7 and 10.8–11.5 (“high” PM2.5), respectively. In terms of PM2.5 exposure, 21% of children in our sample live in a “high” PM2.5 neighborhood, while 19% live in a “low” PM2.5 neighborhood. On average, children reside in neighborhoods where 50% of residents share their race/ethnic status.
Asthma diagnoses by race/ethnicity reveal stark differences, as just 4% of white and Asian children have an asthma diagnosis, while fully 13% of black and 7% of Hispanic children do. White and Asian children are much more likely to have private insurance (90% and 91%, respectively) relative to black and Hispanic children (48% and 48%, respectively). In terms of environmental measures, while whites are more likely to live in neighborhoods with higher levels of ozone, blacks and Hispanics live in neighborhoods with significantly higher levels of PM2.5. In fact, while 12% of white children live in the highest PM2.5 quartile neighborhoods, 33% of black children and 34% of Hispanic children live in these neighborhoods. Similarly, white and Asian children live in neighborhoods with far lower levels of concentrated disadvantage, relative to black and Hispanic children. Finally, children’s race/ethnicity is strongly associated with the racial/ethnic composition of their neighborhoods. Black, Hispanic and Asian children live in neighborhoods with significantly fewer co-ethnics compared to whites. We also tested (not shown) how children’s neighborhood co-ethnic composition was related to PM2.5 and found that higher PM2.5 was associated with higher levels of co-ethnic composition for black and Hispanic children relative to whites.
3.1. Individual and neighborhood associations with asthma diagnosis
Table 2 presents cross-classified logistic regression analyses predicting the odds of asthma diagnosis. Model 1 shows odds of asthma diagnosis by race/ethnic status and controls for sex, age, and number of clinic visits. Compared to whites, black children have nearly three times the odds of asthma diagnosis (OR=2.93, 95% CI=2.74, 3.14), while Hispanics and Asians have 34% and 28% higher odds, respectively. In terms of model fit, the significant clinic and neighborhood-level random intercepts support the use of multilevel cross-classified models and reveal the majority of the variation occurs at the clinic level rather than the neighborhood level. Model 2 controls for sex, age, number of clinic visits, and insurance status, which are all significantly related to the odds of asthma diagnosis. For instance, children with public insurance have 21% higher odds of asthma compared to those privately insured, while male and older children also have higher odds of asthma diagnosis. Accounting for these individual characteristics, however, does not significantly attenuate the higher odds of asthma among black, Hispanic and Asian children. Model 3 controls for individual characteristics and includes our indicators of the physical environment, ozone and fine particulate matter. Ozone is not significantly related to children’s asthma risk, and we also find no significant association between exposure to PM2.5 (at any level) and asthma risk.
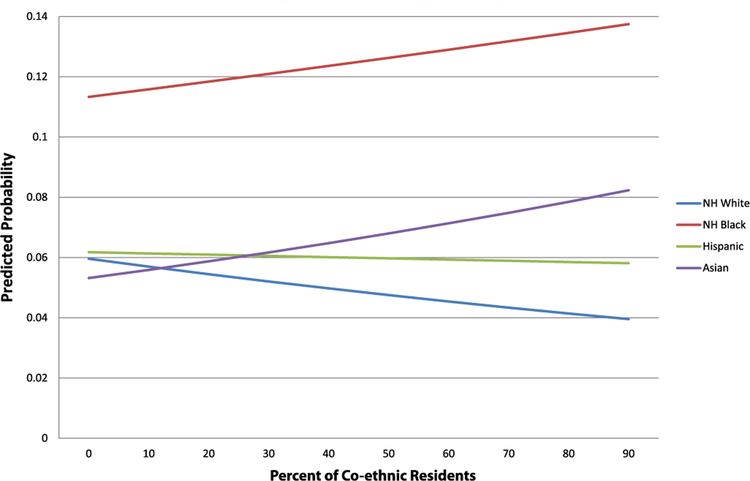
Model 4 controls for individual characteristics and focuses on neighborhood concentrated disadvantage, which is significantly related to asthma diagnosis. Each standard deviation increase in concentrated disadvantage is associated with 4% higher odds of asthma. Accounting for neighborhood disadvantage, however, does not significantly mitigate the overall race/ethnic disparities in asthma. Next, model 5 adjusts for all individual and neighborhood-level factors, including percent co-ethnic of children’s neighborhood. Overall, a child living in a neighborhood with a higher percentage of co-ethnic residents for his/her race/ethnic group has 20% lower odds of an asthma diagnosis. Also at the neighborhood-level, concentrated disadvantage remains marginally associated with higher odds of asthma diagnosis (OR=1.06, 95% CI=1.03, 1.09). Accounting for all the individual- and neighborhood-level characteristics reduces but does not fully attenuate the higher odds of asthma diagnosis among black, Hispanic and Asian children relative to white children. Compared to model 1, the black-white gap in asthma risk is reduced by roughly18%, but it still remains stark at 2.59 times as high for black children relative to white children. Finally, model 6 tests whether the influence of percent co-ethnic composition on children’s risk of asthma diagnosis varies by race/ethnicity. Relative to white children, who experience substantial reductions in asthma risk as the percent of white residents increases, black and Asian children’s asthma risk actually increases as percent co-ethnic increases. Hispanic children, relative to white children, experience a less steep decline in risk as percent co-ethnic increases. We depict this relationship in Fig. 1, which plots the predicted probability of asthma diagnosis by percent co-ethnic across each race/ethnic group. Fig. 1 shows that living in a neighborhood with a higher percentage of co-ethnic residents is protective for whites and to a lesser degree Hispanics, while the opposite is true for black and Asian children. It is important to note that while the association between percent co-ethnic and asthma risk differs significantly for black children relative to white children, percent co-ethnic does not have a major impact on the risk of asthma for black children, which remains high across levels of percent co-ethnic.
Fig. 1.
Predicted probability of asthma diagnosis, by % co-ethnic.
4. Discussion
The current study had two main goals. First, using medical records of in- and out-patient care linked with census and government air monitoring data, we documented physician-diagnosed asthma prevalence among a diverse sample of Hispanic, black, Asian and white children. Most studies of racial disparities in asthma are limited to black and white comparisons, and very few studies include Asians (Rosenbaum, 2008). We found that black and Hispanic children had significantly higher rates of asthma diagnosis compared to whites, and similar to prior research, that black children had the highest level of asthma prevalence (Akinbami et al., 2014, 2009; Rosenbaum, 2008).
Our second goal was to examine whether accounting for differential exposure to social and physical health risks at the neighborhood-level reduced, at least in part, the observed racial/ethnic disparities in asthma prevalence. Using cross-classified multilevel models, we find that two aspects of the social environment—concentrated disadvantage and co-ethnic composition—were related to risk of asthma diagnosis above and beyond individual and clinic-level characteristics. We speculate that higher asthma prevalence in certain neighborhoods may be attributable to the independent or interacting effect of health-damaging social (e.g. undermined collective efficacy and discrimination) and structural (e.g. gentrification and institutional discrimination) processes that we were unable to assess. In particular, neighborhood concentrated disadvantage increased children’s risk of asthma diagnosis. It is likely that several mechanisms, which we were unable to directly test, link neighborhood concentrated disadvantage with increased asthma risk, including, but not limited to, exposure to violence/crime, safety concerns, and the erosion of social cohesion, which materialize as health-damaging acute and chronic stressors (Williams et al., 2009; Gold and Wright, 2005; Chen et al., 2006; Wright, 2011). It is also possible that our measure of concentrated disadvantage captures household characteristics associated with asthma risk, such as dilapidated housing and indoor allergens/pests, known to differ by household SES and race/ethnic status (Rosenbaum, 2008; Camacho-Rivera et al., 2014).
The results also indicate that the influence of co-ethnic composition on asthma prevalence varies by race/ethnicity. Living in a neighborhood where a higher percentage of residents share the child’s race/ethnic identity was associated with lower odds of asthma for white and Hispanic children but higher odds for black and Asian children. The protective effect of co-ethnic composition for white children is likely attributable to the well-established material and social benefits associated with predominately white neighborhoods that our indicator of neighborhood concentrated disadvantage was perhaps not able to fully capture (Logan, 2013). It is less clear why Hispanic children experience a protective effect of percent co-ethnic, though prior research focused on adult mortality and physical health also finds a protective effect of ethnic density among Hispanic adults (Bécares et al., 2012). Further, while Hispanics are the second most segregated minority group in the U.S., the nature and degree of residential segregation differs between Hispanics and blacks (Iceland and Wilkes, 2006; Massey and Denton, 1993). In the U.S black households experience the highest levels of residential segregation (e.g. hypersegregation) than any race/ethnic group (both historically and currently) as the result of independent and combined influences of individual, collective and institutional forms of racial discrimination (Massey and Denton, 1993). Further, areas with high black ethnic density are associated with a host of health-compromising characteristics (e.g. degree of poverty, unemployed, and institutional disinvestment) (Bécares et al., 2012). We speculate that our indicator of residential composition (i.e. percent co-ethnic)—which differs from formal measures of racial residential segregation—is capturing some but not all aspects of the uniquely disadvantaged ecological environments that many black children experience, which may override protective effects of black ethnic density for asthma prevalence. The mechanisms linking risk of asthma and co-ethnic composition are less clear for Asian children, who experienced increased risk of asthma when living among more co-ethnics.
It is possible that factors which were unable to assess, like immigration status and nativity, are important characteristics that influence the direct and moderating effect of race/ethnic density for Asian children’s risk of asthma. Significant heterogeneity exists among U.S. black, Hispanic and Asian populations, and future research should consider additional individual and community characteristics that may influence the effect of race/ethnic residential composition on child health outcomes (Bécares et al., 2012). Documenting the association between racial health disparities across various combinations of race/ethnic neighborhood composition, however, is an important first step for establishing how the diversifying demographic landscape of the U.S. is becoming sorted into distinct ecological contexts that promote (or hinder) well-being among residents. The Houston metropolitan region, characterized by race/ethnic diversity and a large immigrant population (Emerson et al., 2012), represents the future demographic profile of the U.S., and as such, is an ideal spatial context for exploring complex patterns of residential race/ethnic composition and racial disparities in childhood asthma.
We found little support for an association between air pollutants and increased odds of asthma diagnosis. Even in the unadjusted models, there was no association between PM2.5 and asthma prevalence or between ozone exposure and asthma. While several studies find an association between these pollutants and pediatric asthma, findings are not always consistent or in the expected direction (Guarnieri and Balmes, 2014). Discrepancies in findings, including our lack of association, may reflect major variation across studies in data and methods, including study design (e.g. cross-sectional versus longitudinal), spatial scale (e.g. census tract versus county or within-versus between-community comparisons), sample characteristics (e.g. age of children), asthma outcome (e.g. asthmatic episode or physician-diagnosis of asthma) and indicator and method for measuring air pollution (e.g. acute versus chronic exposure period) (Akinbami et al., 2010; Nishimura et al., 2013; Wilhelm et al., 2009; Astell-Burt et al., 2013). Our finding may also suggest that what is more relevant for asthma onset is exposure to air pollution during critical life stages (e.g. in utero) (Shankardass et al., 2015), which we were unable to measure, or short-term rather than long-term exposure (Guarnieri and Balmes, 2014). That is, while acute asthmatic episodes may relate strongly to spikes in air pollutants (e.g. Raun et al., 2014), long term average exposures of air pollutants may be less relevant for asthma onset than the sociodemographic environment. The findings might also reflect the unhealthful ambient levels of air pollution that affect the entire Houston Metropolitan area, including both affluent and poor neighborhoods, due to a variety of factors including emissions from cars, the presence of over 400 chemical manufacturing facilities along the largest petrochemical shipping corridor in the U.S., and meteorological patterns in warmer months that trap masses of unmoving air over the entire city (Sexton et al., 2007). Alternatively, multivariate regression looks at strength and significance of statistical relationships, and this approach can mask important geographic variability common with environmental hazards (Guo et al., 2005).
Overall, we find that racial and ethnic disparities in the odds of asthma diagnosis persist after accounting for key individual and neighborhood characteristics. The findings do not indicate, however, that racial disparities are attributable to genetic differences (Williams et al., 2009; Smith et al., 2005). Disadvantaged children live in distinct ecological environments of complex, interactive and cumulative health risks (Williams et al., 2009), and to the extent that these risks are biologically embodied and create population differences in health outcomes (Krieger, 2001), biological profiles of disease and illness stratified by race and class reflect disparities in environmental and social contexts, rather than inherent, genetic differences (Williams et al., 2009). Further, we found that variation at the clinic level is an important and understudied area of asthma disparities. Better understanding diagnosis practices and strategies across clinics in more and less advantaged areas may lend insights into the etiology of asthma disparities.
4.1. Strengths and limitations
This study does have some limitations. First, the cross-sectional nature of the data raises potential issues regarding exposure effects. We lack information on children’s complete residential history and cannot account for temporal ordering of neighborhood exposure and asthma diagnosis. Further, we were unable to account for nativity or country-of-origin, though prior research finds consistent heterogeneity within racial/ethnic subpopulations, including higher rates of asthma prevalence and morbidity among Puerto Ricans compared to other Hispanic subgroups (Gold and Wright, 2005; Rosenbaum, 2008). A major strength of our study is the use of electronic pediatric medical records from the largest set of pediatric clinics (that accepts private insurance as well as Medicaid and CHIP) in the nation, which provides a large and diverse sample of children who have visited clinics (typically for routine well-child visits) across the Houston metropolitan region. Using data from one hospital and one network of clinics, however, does not provide population-level estimates of asthma prevalence and may raise concerns over selection, particularly as it relates to socioeconomic and race/ethnic differences in hospitalization and clinic utilization. Research suggests that black and Latino children receive less consistent medical care and lower quality of care than white children (Flores et al., 2005), which could affect the likelihood of receiving an asthma diagnosis. While we were unable to account for unequal access and/or utilization of health services, our estimates of pediatric asthma prevalence, which estimate the likelihood of asthma diagnosis compared to sample children without an asthma diagnosis, are comparable to state and county-level estimates using data from the 2011 Texas Behavioral Risk Factor Surveillance System (BRFSS) (Wickerham and Bhakta, 2013). In addition, adjusting for neighborhood race/ethnic composition and clustering across clinics may account for some variation in access to care across neighborhoods and quality of care between TCPA clinics.
The inclusion of ambient air pollutants is an additional strength of our study, though one that presents several challenges. Although it is the leading methodology for measuring and regulating air pollution levels, estimates of air quality derived from stationary monitors and residential addresses may not accurately reflect individual exposure (Akinbami et al., 2010). We were also restricted by a limited and geographically concentrated number of stationary monitors throughout the Houston area, though we speculate that more comprehensive measures, perhaps via mobile monitors, might better capture particulate matter variation. Further, our study only includes two ambient air quality constituents in examining physical environment exposures, though evidence suggests that alternative air pollutants and air pollutant mixtures—including carbon monoxide (CO), nitrogen oxides (NO and NO2) and traffic-related air pollution (TRAP)—are important contributors to childhood asthma onset and asthmatic episodes (e.g. Wilhelm et al., 2009; Shankardass et al., 2015; Nishimura et al., 2013; Guarnieri and Balmes, 2014). Research shows, for example, that children living near major highways and roads have higher rates of asthma (Kim et al., 2004) and that diesel particulate matter is a cause of new-onset asthma (Pandya et al., 2002). Future research interested in the relationship between air pollutants and childhood asthma onset should examine multiple ambient air pollutants, as well as proximity to pollution sources. Finally, we lack information on potentially important confounders at the individual and household-level, including detailed family SES and indoor allergen exposure (e.g. household chemicals and smoke exposure) (Smith et al., 2005; Camacho-Rivera et al., 2014; Rosenbaum, 2008). Socioeconomic and race/ethnic factors have been found to influence indoor allergen exposure at the individual and neighborhood-level (Camacho-Rivera et al., 2014). Evidence suggests, however, that racial disparities remain even after controlling for individual and household risk factors (e.g. smoke exposure, indoor allergens, substandard housing and household income), indicating that other social and spatial scales, such as neighborhood context, warrant investigation (Rosenbaum, 2008; Smith et al., 2005).
5. Conclusion
Among children living in the Houston metropolitan region, several individual and neighborhood-level risk factors were associated with pediatric asthma diagnoses. At the neighborhood-level, concentrated disadvantage and residential co-ethnic composition were associated with an asthma diagnosis. Controlling for all the individual and neighborhood-level risk factors reduced but did not fully eliminate race/ethnic disparities in asthma prevalence. In particular, black children remained more than twice as likely to receive an asthma diagnosis than white children. Future research interested in racial disparities in pediatric asthma must contextualize the multiple, concurrent health risks that children experience by incorporating objective and subjective measures of neighborhood context, in addition to detailed information on family and household characteristics.
Supplementary Material
Acknowledgements
This project was supported by the Houston Endowment and by Rice University’s Faculty Initiatives Fund and Social Sciences Research Institute grants to the authors. These sponsors had no involvement in the data collection, analysis, writing, or decision to submit this article for publication.
Footnotes
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.healthplace.2017.01.006.
References
- Akinbami Lara J., Courtney D. Lynch, Parker Jennifer D., Woodruff Tracey J., 2010. The association between childhood asthma prevalence and monitored air pollutants in metropolitan areas, United States, 2001–2004. Environ. Res 110 (3), 294–301. [DOI] [PubMed] [Google Scholar]
- Akinbami Lara J., Jeanne E. Moorman, Garbe Paul L., Sondik Edward J., 2009. Status of childhood asthma in the United States, 1980–2007. Pediatrics 123 (Suppl. 3), S131–S145. [DOI] [PubMed] [Google Scholar]
- Akinbami Lara J., Jeanne E. Moorman, Simon Alan E., Schoendorf Kenneth C., 2014. Trends in racial disparities for asthma outcomes among children 0 to 17 years, 2001–2010. J. Allergy Clin. Immunol 134 (3), 547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinbami Lara J., Rhodes Julia, C., Marielena Lara, 2005. Racial and ethnic differences in asthma diagnosis among children who wheeze. Pediatrics 115 (5), 1254–1260. [DOI] [PubMed] [Google Scholar]
- Ash Michael, Fetter T. Robert, 2004. Who lives on the wrong side of the environmental tracks? Evidence from the EPA’s risk-screening environmental indicators model*. Soc. Sci. Q 85 (2), 441–462. [Google Scholar]
- Astell-Burt Thomas, Maynard Maria J., Lenguerrand Erik, Whitrow Melissa J., Molaodi Oarabile R., Seeromanie Harding, 2013. Effect of air pollution and racism on ethnic differences in respiratory health among adolescents living in an urban environment. Health Place 23, 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bécares Laia, Shaw Richard, Nazroo James, Stafford Mai, Albor Christo, Atkin Karl, Kiernan Kathleen, Wilkinson Richard, Pickett Kate, 2012. Ethnic density effects on physical morbidity, mortality, and health behaviors: a systematic review of the literature. Am. J. Public Health 102 (12), e33–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhan Nandita, Kawachi Ichiro, Glymour Maria M., Subramanian SV, 2015. Time trends in racial and ethnic disparities in asthma prevalence in the United States from the Behavioral Risk Factor Surveillance System (BRFSS) Study (1999–2011). Am. J. Public Health 105 (6), 1269–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom Barbara, Cohen Robin A., Freeman Gulnur, 2013. Summary health statistics for us Children: national health interview survey, 2012. Vital-. Health Stat. Ser. 10, Data Natl. Health Surv 254, 1–88. [PubMed] [Google Scholar]
- Cagney Kathleen A., Browning Christopher R., Wallace Danielle M., 2007. The Latino paradox in neighborhood context: the case of asthma and other respiratory conditions. Am. J. Public Health 97 (5), 919–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho-Rivera Marlene, Kawachi Ichiro, Bennett Gary G., Subramanian SV, 2014. Associations of neighborhood concentrated poverty, neighborhood racial/ethnic composition, and indoor allergen exposures: a cross-sectional analysis of Los Angeles households, 2006–2008. J. Urban Health 91 (4), 661–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Edith, Margaret D. Hanson, Paterson Laurel Q., Griffin Melissa J., Walker Hope A., Miller Gregory E., 2006. Socioeconomic status and inflammatory processes in childhood asthma: the role of psychological stress. J. Allergy Clin. Immunol 117 (5), 1014–1020. [DOI] [PubMed] [Google Scholar]
- Dunn Erin C., Tracy K. Richmond, Milliren Carly E., Subramanian SV, 2015. Using cross-classified multilevel models to disentangle school and neighborhood effects: an example focusing on smoking behaviors among adolescents in the United States. Health Place 31, 224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson MO, Bratter J, Howell J, Jeanty PW, Cline M, 2012. Houston region grows more racially/ethnically diverse, with small declines in segregation. A joint report analyzing census data from 1990, 2000, and 2010 Houston: rice University; 2012 Kinder Inst. Urban Res. Hobby Cent. Study Tex. [Google Scholar]
- Flores Glenn, Olson Lynn, Tomany-Korman Sandra C., 2005. Racial and ethnic disparities in early childhood health and health care. Pediatrics 115 (2), e183–e193. [DOI] [PubMed] [Google Scholar]
- Friedman Samantha, Rosenbaum Emily, 2004. Nativity status and racial/ethnic differences in access to quality housing: does homeownership bring greater parity? Hous. Policy Debate 15 (4), 865–901. [Google Scholar]
- Gold Diane R., Wright Rosalind, 2005. “Population disparities in asthma. Annu. Rev. Public Health 26, 89–113. [DOI] [PubMed] [Google Scholar]
- Guarnieri Michael, Balmes John R., 2014. Outdoor air pollution and asthma. Lancet 383 (9928), 1581–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Diansheng, Gahegan Mark, MacEachren Alan M., Zhou Biliang, 2005. Multivariate analysis and geovisualization with an integrated geographic knowledge discovery approach. Cartogr. Geogr. Inf. Sci 32 (2), 113–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt Elizabeth W., Katherine P. Theall, Felicia A. Rabito, 2013. Individual, housing, and neighborhood correlates of asthma among young urban children. J. Urban Health 90 (1), 116–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iceland J, Wilkes R, 2006. Does socioeconomic status matter? Race, class, and residential segregation. Soc. Probl 53 (2), 248–273. [Google Scholar]
- Kim Janice J., Smorodinsky Svetlana, Lipsett Michael, Singer Brett C., Hodgson Alfred T., Ostro Bart, 2004. Traffic-related air pollution near busy roads: the East Bay Children’s Respiratory Health Study. Am. J. Respir. Crit. Care Med 170 (5), 520–526. [DOI] [PubMed] [Google Scholar]
- Krieger Nancy, 2001. Theories for social epidemiology in the 21st century: an ecosocial perspective. Int. J. Epidemiol 30 (4), 668–677. [DOI] [PubMed] [Google Scholar]
- Kreiger James, Higgins Donna, 2002. Housing and health: time again for public action. Am. J. Public Health 92 (7), 758–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichter Daniel T., 2013. Integration or fragmentation? Racial diversity and the American future. Demography 50 (2), 359–391. [DOI] [PubMed] [Google Scholar]
- Logan John R., 2013. The persistence of segregation in the 21st century metropolis. City Community 12 (2), 160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan John R., Zhang Charles, 2010. Global neighborhoods: new pathways to diversity and separation. AJS; Am. J. Sociol 115 (4), 1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey Douglas S., Denton NA, 1993. American apartheid: segregation and the making of the underclass Harvard University Press. [Google Scholar]
- Nishimura Katherine K., Galanter Joshua M., Roth Lindsey A., Oh Sam S., Thakur Neeta, Nguyen Elizabeth A., Thyne Shannon, et al. , 2013. Early-life air pollution and asthma risk in minority children. The GALA II and SAGE II studies. Am. J. Respir. Crit. Care Med 188 (3), 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya Robert J., Solomon Gina, Kinner Amy, Balmes John R., 2002. Diesel exhaust and asthma: hypotheses and molecular mechanisms of action. Environ. Health Perspect 110 (Suppl. 1), 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman Deborah N., Zierler Sally, Meersman Stephen, Kim Hyun K., Viner-Brown Samara I., Caron Colleen, 2006. Race disparities in childhood asthma: does where you live matter? J. Natl. Med. Assoc 98 (2), 239. [PMC free article] [PubMed] [Google Scholar]
- Rabe-Hesketh Sophia, Skrondal Anders, 2012. Multilevel and Longitudinal Modeling Using Stata 3rd edition. Stata Press, College Station, TX. [Google Scholar]
- Raun Loren H., Katherine B. Ensor, Persse David, 2014. Using community level strategies to reduce asthma attacks triggered by outdoor air pollution: a case crossover analysis. Environ. Health 13 (1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriquez M, Alexeeff GV, 2014. California communities environmental health screening tool, version 2.0
- Rosenbaum Emily, 2008. Racial/ethnic differences in asthma prevalence: the role of housing and neighborhood environments. J. Health Soc. Behav 49 (2), 131–145. [DOI] [PubMed] [Google Scholar]
- Sampson Robert J., Stephen W. Raudenbush, Earls Felton, 1997. Neighborhoods and violent crime: a multilevel study of collective efficacy. Science 277 (5328), 918–924. [DOI] [PubMed] [Google Scholar]
- Sexton K, Linder SH, Marko D, Bethel H, Lupo PJ, 2007. Comparative assessment of air pollution–related health risks in Houston. Environ. Health Perspect 115 (10), 1388–1393. 10.1289/ehp.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankardass K, Jerrett M, Dell SD, Foty R, Stieb D, 2015. Spatial analysis of exposure to traffic-related air pollution at birth and childhood atopic asthma in Toronto, Ontario. Health Place 34, 287–295. [DOI] [PubMed] [Google Scholar]
- Smith Lauren A., Hatcher-Ross Juliet L., Wertheimer Richard, Kahn Robert S., 2005. Rethinking race/ethnicity, income, and childhood asthma: racial/ethnic disparities concentrated among the very poor. Public Health Rep 120 (2), 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt Judy K., Symanski Elaine, Stock Thomas H., Chan Wenyaw, Du Xianglin L., 2014. Association of short-term increases in ambient air pollution and timing of initial asthma diagnosis among medicaid-enrolled children in a metropolitan area. Environ. Res 131, 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White Kellee, Borrell N. Luisa, 2011. Racial/ethnic residential segregation: framing the context of health risk and health disparities. Health Place 17 (2), 438–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickerham and Bhakta, 2013. Asthma Hospitalization Rates and Prevalence Among Children in the Greater Houston Area, Texas, 2011 file:///C:/Users/MackenzieBrewer/Downloads/AsthmaHospDischarges_RatesPrevalenceChildrenGreaterHouston.pdf.
- Wilhelm Michelle, Qian Lei, Ritz Beate, 2009. Outdoor air pollution, family and neighborhood environment, and asthma in LA FANS children. Health Place 15 (1), 25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams David R., Collins Chiquita, 2001. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep 116 (5), 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams David R., Sternthal Michelle, Wright Rosalind J., 2009. Social determinants: taking the social context of asthma seriously. Pediatrics 123 (Suppl. 3), S174–S184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright Rosalind J., 2011. Epidemiology of stress and asthma: from constricting communities and fragile families to epigenetics. Immunol. Allergy Clin. N. Am 31 (1), 19–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.