Abstract
Aim of the study
In this study we investigated Fas, FasL and Foxp3 expression in relation to liver graft rejection and its severity in autoimmune hepatitis (AIH) patients.
Material and methods
Twenty-three AIH patients including five post-transplant patients with acute rejection (AR) and 18 patients without AR (non-AR) were studied for Fas, FasL and Foxp3 gene expression in peripheral blood mononuclear cells on days 1, 3 and 7 after transplantation by real-time PCR. The relationships between gene expression and clinical features were determined.
Results
Real-time PCR showed various Fas gene expression levels with no significant difference between the days in AR patients (p = 0.52). In non-AR patients, Fas level increased from 0.98 ±0.24 fold on the first day to 1.89 ±0.42 fold on day 3 after transplantation (p < 0.01). In this group of patients, we also found a significant increase in FasL expression on day 7 (29.91 ±6.89 fold) compared to day 1 (13.50 ±7.44 fold, p < 0.05). Foxp3 gene expression in both groups showed decreased levels during the first week after transplantation. The decreased Foxp3 expression in AR patients was correlated with rejection activity index (r = 0.86, p < 0.0001).
Conclusions
Increased Fas and FasL gene expression levels in non-AR patients and decreased Foxp3 gene expression in both groups suggested the important role of these molecules in the alloreactive response after liver transplantation in AIH patients. Foxp3 expression might be useful for monitoring rejection severity.
Keywords: FOXP3, autoimmune hepatitis, Fas, FasL, liver allograft
Introduction
Liver transplantation is a life-saving and standard therapeutic approach for various diseases including cirrhosis, decompensated liver diseases, acute liver failure, hepatocellular cancer, viral hepatitis and auto-immune hepatitis (AIH) [1-3]. AIH is a chronic necro-inflammatory disease with ill-defined etiology [4-6]. Environmental triggers, failure of immune tolerance mechanisms and genetic predisposition are postulated to induce a T cell-mediated liver injury in AIH [7]. While local hepatic regulatory networks involving regulatory T cells are implicated in disease pathogenesis, animal models demonstrate that AIH may result from defective central immune tolerance. According to the presence of autoantibodies, two types of AIH are defined: type 1 is positive for antinuclear (ANA) and/or smooth muscle antibodies (SMA), and type 2 is positive for liver kidney microsomal antibody type 1 (LKM1) [7]. Currently, the Model for End-stage Liver Disease (MELD) score is used to determine eligibility of liver transplantation for patients with either acute or chronic AIH [8].
In the setting of transplantation, acute rejection is a well-known complication [9]. Numerous studies have proposed several mechanisms of graft rejection in liver transplantation such as the release of intragraft cytokines and induction of apoptosis by graft infiltrating lymphocytes [10-12]. T lymphocytes appear to be absolutely required in this rejection process [13]. T cells recognize allo-antigens on donor antigen-presenting cells (APCs) by direct presentation or interact with recipient APCs expressing donor tissue antigens. It has been shown that cytotoxic T lymphocytes (CTLs) alone are sufficient for the mediation of acute allograft rejection, but helper T lymphocytes (TH) also by secretion of interleukin 2 (IL-2) can induce clonal expansion of CTLs and upregulate their cytotoxic activity. The Fas/Fas ligand (FasL) pathway is a death-inducing pathway utilized by CTLs to destroy cells. Fas is widely expressed by many cell types with abundant expression in the thymus, liver, heart and kidney. FasL is expressed on activated CTLs and natural killer (NK) cells and has an important role in lymphocyte-mediated cytotoxicity. Increased expression of FasL may activate the cytotoxic pathway, leading to graft damage by activation of apoptosis [14]. On the other hand, regulatory T cells (Tregs) expressing CD4, CD25 and Foxp3 molecules can recognize alloantigens and induce transplantation tolerance. These cells have the ability to suppress immune responses both in vitro and in vivo [15, 16]. Tregs inhibit T cell proliferative responses through cell–cell contact or by secretion of suppressive cytokines such as IL-10 and/or TGF-β. Increasing evidence from animal transplantation research shows that Tregs can play a key role in promoting immunological unresponsiveness and tolerance to allograft transplants [17, 18]. Therefore, modulation of the number and function of these cells is suggested as new strategies for preventing graft rejection in patients with different diseases including AIH [19]. In order to use such approaches, providing sufficient information on how Tregs as well as various effector molecules of apoptosis such as Fas/FasL participate in graft rejection is required. For this purpose, in the present study we aimed to evaluate the expression levels of Fas, FasL and Foxp3 after liver transplantation in AIH patients with acute rejection (AR) compared to those without acute rejection (non-AR).
Material and methods
Patients
Twenty-three female patients with AIH who underwent liver transplantation were followed for the occurrence of AR. Post-transplant AR was observed in five patients and in the rest (18 patients) AR did not occur. AR was diagnosed by means of clinical, laboratory, and histologic evidence. Blood samples were collected from both groups after 1, 3 and 7 days after liver transplantation. Clinical and paraclinical information of the patients, including total bilirubin, alanine transaminase (ALT), aspartate aminotransferase (AST), creatinine, rejection activity index (RAI), MELD score and age, were extracted from their files. The study was approved by the Ethics Committee of Shiraz University of Medical Sciences and informed consent was obtained from the patients.
Samples
Five milliliters of blood was taken from the patients and then peripheral blood mononuclear cells (PBMCs) were separated by Ficoll-Hypaque density gradient centrifugation. After washing, cells were counted and the concentration was adjusted to 2 × 106/ml.
RNA isolation and reverse transcription
Total RNA was extracted from PBMCs using the RNA easy mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The concentration and purity of the RNA samples were determined using spectrophotometer scan in the ultraviolet (UV) region and ethidium bromide visualization of intact 18S and 28S RNA bands after agarose gel electrophoresis. cDNA was prepared using 10 μl of RNA, dNTP and random primers and a Gene Amp RNA PCR kit (Takara Bio Inc, Otsu, Japan) according to the manufacturer’s protocol.
Quantitative real-time PCR
Fas, FasL and Foxp3 mRNA levels were quantified with reagents from the SYBR Premix Ex Taq TMII kit (Takara Bio Inc) using the Step One real-time PCR system (Applied Biosystems, Foster City, USA). The experiment was performed by two independent experiments in duplicate. Primers were designed not to amplify genomic DNA (exon-exon junction). The primer sequences are provided in Table 1. A melting curve analysis was performed in each run to ensure specificity of the primers. The housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control to normalize the variability in expression levels. The PCR conditions were as follows: one cycle at 95°C for 30 s, followed by 40 cycles at 95°C for 5 s, 57°C in regards to Fas, FasL, Foxp3 or 56°C for GAPDH. Data were collected using the Step One Real Time PCR system. The expression levels were analyzed using the 2− ΔΔCT method, normalized against GAPDH and presented as fold change.
Table 1.
Primer sequences used in real-time PCR
| Genes | Gene bank accession No. | Forward sequence | Reverse sequence | Size (bp) |
|---|---|---|---|---|
| GAPDH | NM_001256799.2 | GCGAGATCCCTCCAAAATCAA | GTTCACACCCATGACGAACAT | 81 |
| Fas | NM_000043.4 | GGGGTGGCTTTGTCTTCTTC | CCTTGGTTTTCCTTTCTGTGC | 102 |
| FasL | NM_000639.2 | CTCCGAGAGTCTACCAGCCA | TGGACTTGCCTGTTAAATGGG | 121 |
| Foxp3 | NM_001114377.1 | CAGCACATTCCCAGAGTTCCT | TCATTGAGTGTCCGCTGCTT | 132 |
Statistical analysis
All data were presented as mean ±SD. The comparisons among the groups were made using non-parametric tests of Mann-Whitney and Kruskal-Wallis. P-values of less than 0.05 were regarded as significant. Spearman’s rank correlation was applied to detect correlations between study parameters. The statistical analysis was performed by GraphPad Prism software version 6.0 (Inc, USA).
Results
Table 2 shows the characteristics of 23 liver transplanted AIH patients. All patients were female. AR patients were in the age range of 35 ±12 years with a MELD score range of 18-37. Non-AR patients were in the age range of 35.3 ±12.5 years and MELD score range of 18-30.
Table 2.
Features of autoimmune hepatitis patients with and without acute rejection
| Patients | Number (%) | Age (years) | MELD | RAI | |||
|---|---|---|---|---|---|---|---|
| Range | Mean ±SD | Range | Mean ±SD | Range | Mean ±SD | ||
| Acute rejection | 5 (21.8) | 24-51 | 35 ±12 | 18-37 | 25.4 ±7.9 | 3-4 | 3.4 ±0.5 |
| Non-acute rejection | 18 (78.2) | 14-59 | 35.3 ±12.5 | 18-30 | 22.6 ±3.8 | – | – |
MELD – Model for End-stage Liver Disease scoring (1-40), RAI – rejection activity index
Gene expression levels by real-time PCR
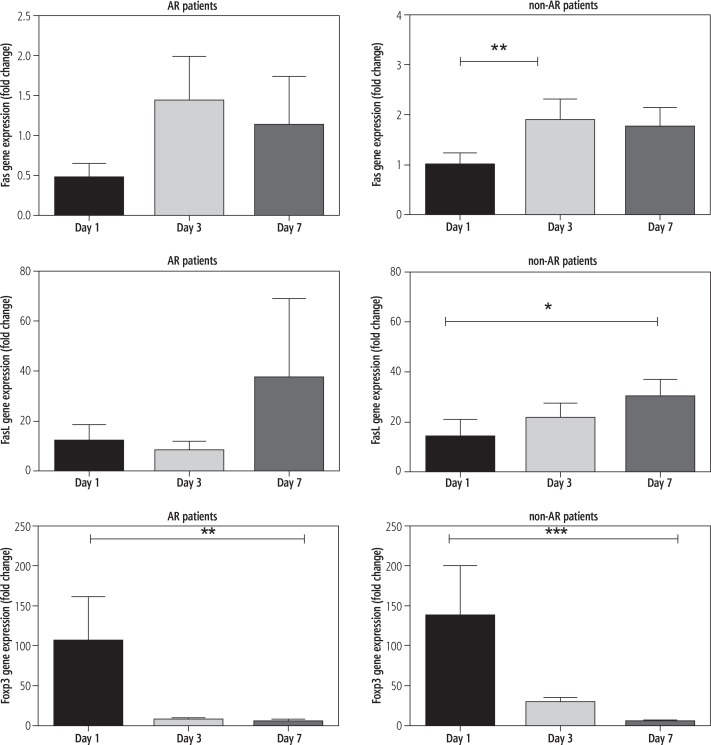
The gene expression levels of Fas, FasL and Foxp3 in PBMCs from the post-transplant patients on days 1, 3 and 7 were measured by real-time PCR. The results presented in Figure 1 show various levels of Fas gene expression with no significant changes up to 7 days in patients with AR (p = 0.52). In non-AR patients, we observed a significant increase in Fas gene expression levels from 0.98 ±0.24 fold on the first day of transplantation to 1.89 ±0.42 fold (p < 0.01) after 3 days and 1.74 ±0.40 fold after 7 days. In the same group of patients, FasL expression showed a significantly increased level from 13.5 ±7.44 fold on the first day to 29.91 ±6.89 fold after one week (p < 0.05).
Fig. 1.
Fas, FasL and Foxp3 gene expressions in peripheral blood mononuclear cells of post-liver transplant autoimmune hepatitis patients with acute rejection (AR) and without AR (non-AR) using real-time PCR technique
The study of Foxp3 gene expression in AR patients after transplantation showed decreased levels from 105.5 ±54.92 fold on the first day to 4.69 ±3.30 fold on day 7 (p < 0.01). Corresponding data for non-AR patients were 137.3 ±63.01 fold on the first day to 4.52 ±1.7 fold on day 7 (p < 0.001).
The initial Fas, FasL and Foxp3 gene expression on the first day showed no significant difference between AR and non-AR patients.
Correlation between gene expression levels and clinical features
The expression of genes was studied in relation to clinical and paraclinical features of the patients. There was no significant correlation between expression levels of Fas, FasL and Foxp3 genes with serum levels of AST, ALT and total bilirubin on different days after transplantation. However, we found a significant negative relationship between Foxp3 expression and RAI (r = –0.86, p < 0.0001), suggesting a possible association of decreased Tregs with severity of rejection.
Discussion
Acute rejection is known as an obstacle in allograft transplantation. The most important cells in this process are T lymphocytes. Overexpression of Fas and FasL can cause target organ damage due to the activation of CTLs [13] or mediate T cell apoptosis, which is implicated in immune suppression. An initial purpose of this study was to identify the changes in the gene expression levels of Fas and FasL in PBMCs of post-liver transplant AIH patients with AR. Our results showed no significant changes in the expression of these genes in AR patients during the first week of transplantation. In contrast, increased expression of Fas and FasL genes was observed in non-AR patients. Studies have shown that the effector CTL killing mechanism which leads to apoptosis in target cells involves a slow-acting lytic pathway of the Fas/FasL interaction and a fast-acting pathway of perforin/granzyme degranulation [20]. As we did not observe significant changes in the Fas and FasL expressions in AR patients, it is possible that graft rejection in this group of patients might have occurred due to the fast-acting perforin and granzyme pathway of CTL killing. Moreover, dysregulation of apoptosis has been reported to be a cause of autoimmunity. Thus, it is also possible that the lack of change in Fas/FasL expression in AR patients was related to the underlying disease. In a previous study by Zwolak et al., a significantly higher number of PBMCs bearing the Fas receptor in AIH compared to control subjects was observed [21]. This finding may suggest dysregulation of apoptosis in PBMCs of these patients.
It is known that infiltration of T cells with high levels of FasL in the graft can induce cell death and consequently graft rejection. An interesting finding among non-AR patients was the increased Fas and FasL expression in PBMCs during the first week after transplantation. This increase may on one hand reflect the increased activity of CTLs due to antigenic stimulation [22] and on the other hand might be considered as a positive role of these molecules in the liver engraftment. Through these cell surface molecules activated T cells can commit suicide. It has been shown that interaction of FasL-expressing cells with Fas-expressing T cells is important in maintenance of tolerance through induction of apoptosis in T cells. This finding was consistent with a previous study showing the possible contribution of enhanced apoptosis in PBMCs to prevent allograft rejection in cardiac transplanted patients [20].
Autoimmunity mostly is an outcome of a failure in natural Treg formation in the thymus to avoid early reactions against autoantigens. It has been shown that in AIH, expansion of Tregs through low-dose IL-2 treatment can inhibit T helper and cytotoxic T cell activation and stop hepatocyte damage. Moreover, in new experimental therapies in animal models, antigen-specific desensitization has been used to restore the immunological tolerance to AIH autoantigens and reduction of liver inflammation [23]. To investigate whether Tregs have a role in AR after liver transplantation, we analyzed the expression of Foxp3 gene in PBMCs of the patients. Foxp3+ Tregs in the graft by inhibition of effector T cells are involved in the alloimmune response and suppression of allograft rejection [19, 24]. We demonstrated a decreasing level of Foxp3 gene expression in both AR and non-AR patients during the first week after transplantation. This result was consistent with previous studies that have shown the decreased frequency of Tregs in the peripheral blood of transplanted patients [25, 26]. The reason for this reduced level of Foxp3 expression might be related to the infiltration of Tregs into the liver to suppress the alloreactive T cells [27]. Also, the possibility of inhibiting Tregs by immunosuppressive drugs should also be considered. Of note, in a previous study on AIH patients, no impairment of Foxp3+ Treg was reported [28].
On evaluation of the relationship between gene expression and clinical parameters, we found that in AR patients Foxp3 expression was negatively correlated with RAI, suggesting the important role of Tregs in AR severity. This result was consistent with an earlier study in which a significant negative correlation between decreased frequency of circulating CD4+CD25highFoxp3+ Tregs and RAI in heart transplant patients was found [26].
This study has some limitations. One limitation was the small size of samples, particularly in AR patients, which was due to a generally smaller number of liver transplant AIH patients with acute rejection. In order to increase the validity of data, more studies on a greater number of patients are needed.
To date, the majority of studies on immune mechanisms in AIH have been carried out on peripheral blood samples as a way of noninvasive screening [29, 30]. Although these correlation studies are helpful in understanding AIH, they do not entirely represent the inflammatory milieu of the liver. Analyzing immune cell interactions in inflammatory sites is important to understand the biology at tissue level. However, studies on local environmental regulatory mechanisms are difficult due to limited access to tissues [31, 32].
In conclusion, we found increased Fas and FasL gene expression in non-AR patients and decreased Foxp3 gene expression in both groups of patients, which suggests the importance of these molecules in the alloimmune response in liver transplanted AIH patients. We also found a negative correlation of Foxp3 gene expression with RAI, which may imply the usefulness of PBMC monitoring of Foxp3 expression to evaluate the severity of rejection.
Acknowledgments
This work was extracted from a thesis written by Keivan Shams and received financial support from the Department of Immunology, Shiraz University of Medical Sciences (SUMS) (grant number 6860).
Disclosure
Authors report no conflict of interest.
References
- 1.Karjoo M, Banikazemi M, Saeidi M, et al. Review of natural history, benefits and risk factors pediatric liver transplantation. Int J Pediatr. 2016;4:1529–1544. [Google Scholar]
- 2.Liaskou E, Hirschfield GM, Gershwin ME. Mechanisms of tissue injury in autoimmune liver diseases. Semin Immunopathol. 2014;36:553–568. doi: 10.1007/s00281-014-0439-3. [DOI] [PubMed] [Google Scholar]
- 3.Meirelles Junior RF, Salvalaggio P, Rezende MB, et al. Liver transplantation: history, outcomes and perspectives. Einstein (Sao Paulo, Brazil) 2015;13:149–152. doi: 10.1590/S1679-45082015RW3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faisal N, Renner EL. Recurrence of autoimmune liver diseases after liver transplantation. World J Hepatol. 2015;7:2896. doi: 10.4254/wjh.v7.i29.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farid E, Isa HM, Al Nasef M, et al. Childhood autoimmune hepatitis in BahraIn: a tertiary center experience. Iran J Immunol. 2015;12:141. [PubMed] [Google Scholar]
- 6.Demkow U. Laboratory diagnosis of autoimmune hepatitis. Centr Eur J Immunol. 2010;35:37–40. [Google Scholar]
- 7.Rattanasiri S, McDaniel DO, McEvoy M, et al. The association between cytokine gene polymorphisms and graft rejection in liver transplantation: a systematic review and meta-analysis. Transpl Immunol. 2013;28:62–70. doi: 10.1016/j.trim.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Liberal R, Grant CR, Mieli-Vergani G, et al. Autoimmune hepatitis: a comprehensive review. J Autoimmun. 2013;41:126–139. doi: 10.1016/j.jaut.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Khalaf H, Mourad W, El-Sheikh Y, et al. Liver transplantation for autoimmune hepatitis: a single-center experience. Transplant Proc. 2007;39:1166–1170. doi: 10.1016/j.transproceed.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 10.Xie X-j, Ye Y-f, Zhou L, et al. Th17 promotes acute rejection following liver transplantation in rats. J Zhejiang Univ SciB. 2010;11:819–827. doi: 10.1631/jzus.B1000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warle MC, Farhan A, Metselaar HJ, et al. Cytokine gene polymorphisms and acute human liver graft rejection. Liver Transpl. 2002;8:603–611. doi: 10.1053/jlts.2002.33967. [DOI] [PubMed] [Google Scholar]
- 12.Krams SM, Egawa H, Quinn MB, et al. Apoptosis as a mechanism of cell death in liver allograft rejection. Transplantation. 1995;59:6. [PubMed] [Google Scholar]
- 13.Wang Y-L, Zhang Y-Y, Li G, et al. Correlation of CD95 and soluble CD95 expression with acute rejection status of liver transplantation. World J Gastroenterol. 2005;11:1700. doi: 10.3748/wjg.v11.i11.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Askenasy N, Yolcu ES, Yaniv I, et al. Induction of tolerance using Fas ligand: a double-edged immunomodulator. Blood. 2005;105:1396–1404. doi: 10.1182/blood-2004-06-2364. [DOI] [PubMed] [Google Scholar]
- 15.Zare H-R, Habibagahi M, Vahdati A, et al. Patients with active rheumatoid arthritis have lower frequency of nTregs in peripheral blood. Iran J Immunol. 2015;12:166–175. [PubMed] [Google Scholar]
- 16.Shalini PU, Debnath T, Vidyasagar J, et al. A study on FoxP3 and Tregs in paired samples of peripheral blood and synovium in rheumatoid arthritis. Cent Eur J Immunol. 2015;40:431–436. doi: 10.5114/ceji.2015.55872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xia G, Shah M, Luo X. Prevention of allograft rejection by amplification of Foxp3+ CD4+ CD25+ regulatory T cells. Transl Res. 2009;153:60–70. doi: 10.1016/j.trsl.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.John K, Hardtke-Wolenski M, Jaeckel E, et al. Increased apoptosis of regulatory T cells in patients with active autoimmune hepatitis. Cell Death Dis. 2017;8:3219. doi: 10.1038/s41419-017-0010-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li W, Carper K, Zheng XX, et al. The role of Foxp3+ regulatory T cells in liver transplant tolerance. Transplant Proc. 2006;38:3205–3206. doi: 10.1016/j.transproceed.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 20.Hassin D, Garber OG, Meiraz A, et al. Cytotoxic T lymphocyte perforin and Fas ligand working in concert even when Fas ligand lytic action is still not detectable. Immunology. 2011;133:190–196. doi: 10.1111/j.1365-2567.2011.03426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zwolak A, Surdacka A, Daniluk J. Bcl-2 and Fas expression in peripheral blood leukocytes of patients with alcoholic and autoimmune liver disorders. Hum Exp Toxicol. 2016;35:799–807. doi: 10.1177/0960327115607078. [DOI] [PubMed] [Google Scholar]
- 22.Katsikis PD, Wunderlich ES, Smith CA, et al. Fas antigen stimulation induces marked apoptosis of T lymphocytes in human immunodeficiency virus-infected individuals. J Exp Med. 1995;181:2029–2036. doi: 10.1084/jem.181.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jimenez-Rivera C, Ling SC, Ahmed N, et al. Incidence and characteristics of autoimmune hepatitis. Pediatrics. 2015;136:e1237–1248. doi: 10.1542/peds.2015-0578. [DOI] [PubMed] [Google Scholar]
- 24.Dai Z, Li Q, Wang Y, et al. CD4+ CD25+ regulatory T cells suppress allograft rejection mediated by memory CD8+ T cells via a CD30-dependent mechanism. J Clin Invest. 2004;113:310–317. doi: 10.1172/JCI19727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He Q, Fan H, Li J, et al. Decreased circulating CD4+ CD25 high FOXP3+ T cells during acute rejection in liver transplant patients. Transplant Proc. 2011;43:1696–1700. doi: 10.1016/j.transproceed.2011.03.084. [DOI] [PubMed] [Google Scholar]
- 27.Demirkiran A, Kok A, Kwekkeboom J, et al. Low circulating regulatory T‐cell levels after acute rejection in liver transplantation. Liver Transpl. 2006;12:277–284. doi: 10.1002/lt.20612. [DOI] [PubMed] [Google Scholar]
- 28.Dijke IE, Korevaar SS, Caliskan K, et al. Inadequate immune regulatory function of CD4+CD25bright+FoxP3+ T cells in heart transplant patients who experience acute cellular rejection. Transplantation. 2009;87:1191–1200. doi: 10.1097/TP.0b013e31819ec2fb. [DOI] [PubMed] [Google Scholar]
- 29.Christen U, Hintermann E. Immunopathogenic mechanisms of autoimmune Hepatitis: how much do we know from animal models? Int J Mol Sci. 2016;17:E2007. doi: 10.3390/ijms17122007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grant CR, Liberal R, Holder BS, et al. Dysfunctional CD39 (POS) regulatory T cells and aberrant control of T helper type 17 cells in autoimmune hepatitis. Hepatology. 2014;59:1007–1015. doi: 10.1002/hep.26583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Longhi MS, Hussain MJ, Kwok WW, et al. Autoantigenspecific regulatory T cells, a potential tool for immune tolerance reconstitution in type 2 autoimmune hepatitis. Hepatology. 2011;53:536–547. doi: 10.1002/hep.24039. [DOI] [PubMed] [Google Scholar]
- 32.Oo YH, Weston CJ, Lalor PJ, et al. Distinct roles for CCR4 and Cxcr3 in the recruitment and positioning of regulatory T cells in the inflamed human liver. J Immunol. 2010;184:2886–2898. doi: 10.4049/jimmunol.0901216. [DOI] [PubMed] [Google Scholar]
- 33.Oo YH, Adams DH. Regulatory T cells and autoimmune hepatitis: what happens in the liver stays in the liver. J Hepatol. 2014;61:973–975. doi: 10.1016/j.jhep.2014.08.005. [DOI] [PubMed] [Google Scholar]