Abstract
Essential blepharospasm (EB) causes difficulty in eyelid opening because of involuntary movements of the orbicularis oculi muscle. Patients with EB have functional visual loss due to sustained eyelid closure. We examined cerebral glucose metabolism in 39 patients with EB (12 men and 27 women; mean age, 52.1 years) by using positron emission tomography with 18F-fluorodeoxyglucose. Forty-eight eye open healthy subjects and 48 eye close healthy subjects served as controls. We analyzed and compared the data between the patients and controls by using both statistical parametric mapping (SPM) and regions of interest (ROIs). We defined ROIs on both sides of the posterior striate cortex, anterior striate cortex, extrastriate cortex, and thalamus. In SPM analysis, glucose hypometabolism were observed in both sides of the extrastriate cortex compared to eye open controls but not to eye close controls. We also observed a significant negative correlation between the Jankovic Rating Scale (JRS) sum score and relative glucose metabolism level in the striate cortex of these patients. ROI analysis, a significant correlation was observed between the JRS sum score and glucose metabolism level in the posterior (right: r = −0.53, P = .0005; left: r = −0.65, P = .00001) and anterior (right: r = −0.33, P = .04; left: r = −0.37, P = .02) striate cortices of patients with EB. We surmise that the interruption of visual input cause glucose hypometabolism in the visual cortex of patients with EB.
Keywords: Blepharospasm, Brain metabolism, Dystonia, Extrastriate cortex, Striate cortex
Highlights
-
•
Glucose hypometabolism (GM) in the visual cortex in patients with blepharospasm was observed.
-
•
GM level in the visual cortex of blepharospasm was same as that of eye close healthy subjects.
-
•
Negative correlation was observed between severity and GM level in the striate cortex of patients.
-
•
The results of this study reflect the visual symptoms of patients with blepharospasm.
1. Introduction
Essential blepharospasm (EB) is a form of focal dystonia of unknown cause characterized by involuntary spasms of the musculature of the upper face. EB causes difficulty in eyelid opening because of involuntary movements of the orbicularis oculi muscle. Most patients with EB have clinical symptoms of sustained eyelid closure, and patients with severe EB cannot independently open their eyes. In our previous study using positron emission tomography (PET) and 18F-fluorodeoxyglucose (FDG), we observed glucose hypermetabolism in the thalamus of patients with EB (Suzuki et al., 2007) and suggested that hyperactivity in the thalamus may be the cause of EB. Patients with EB have been known to have functional visual loss, and many patients have various difficulties in their life (Egan and LaFrance Jr, 2015; Pula, 2012). However, no study has examined the influence of the interruption of visual inputs on the visual cortex of patients with EB. In the current study, we hypothesized that the activity in the visual cortex of patients with EB decreases because of the interruption of visual inputs; therefore, we studied regional cerebral glucose metabolism in patients with EB by using FDG-PET.
2. Materials and methods
2.1. Participants
We studied 39 patients with EB (12 men and 27 women; mean age, 52.1 years). Each patient had bilateral blepharospasm, and the degree of blepharospasm was equal in the right and left eyelids. The duration of disease was 4.9 ± 5.0 years. The control subjects were 96 healthy volunteers (33 men and 63 women; mean age, 55.5 years). None of the participants had other neurologist-diagnosed neuro-psychiatric diseases or a family history of dystonic disorders. Neither the control subjects nor the patients with EB had taken any neuro-psychiatric drugs. The control subjects divided into two groups (eye open control group (48 subjects) and eye close control group (48 subjects) so that the sex ratio and the mean age were almost equal. We instructed the eye open control group and patients with EB to open their eyelids from FDG injection to PET scanning. On the other hand, we instructed the eye close control group to close their eyelids from FDG injection to PET scanning.
Informed consent was obtained from each subject before study participation, and the study protocol was approved by the Institutional Ethics Committee of Tokyo Metropolitan Institute of Gerontology. All the procedures conformed to the tenets of the Declaration of Helsinki.
Blepharospasm severity and frequency were assessed using scales ranging from 0 to 4 (severity: 0 = absent, 4 = most severe; frequency: 0 = none, 4 = persistent eye closure) in accordance with the Jankovic Rating Scale (JRS) (Jankovic and Orman, 1987) in each patient just before the PET examination. The average blepharospasm severity and frequency were 2.85 and 2.69, respectively. We hypothesized that the glucose metabolism level may be related to the strength (severity + frequency), i.e., the JRS sum score (Roggenkämper et al., 2006). We surmised that visual input during the period from FDG injection to PET scan is reflected in glucose metabolism in the visual cortex.
Among our patients, 19 with EB were conscious of photophobia that patients with blepharospasm frequently experienced dazzling or eye pain (Hallett, 2002).
2.2. Magnetic resonance imaging
Magnetic resonance imaging (MRI) scans were performed on each subject to screen for organic brain disorders by using a 1.5-T Signa Horizon scanner (General Electric, Milwaukee, WI). Trans-axial images with T1-weighted contrast (3DSPGR; TR = 9.2 ms, TE = 2.0 ms, matrix size = 256 × 256 × 124, and voxel size = 0.94 × 0.94 × 1.3 mm), and T2-weighted contrast (first spin echo; TR = 3000 ms, TE = 100 ms, matrix size = 256 × 256 × 20, and voxel size = 0.7 × 0.7 × 6.5 mm) were acquired. None of the subjects showed any abnormalities in brain morphology or contrast intensity.
2.3. PET data acquisition
PET scans were performed using the SET 2400W scanner (Shimadzu, Kyoto, Japan) at the Positron Medical Center, Tokyo Metropolitan Institute of Gerontology. A bolus of 2.5 MBq/kg (body weight) FDG was injected intravenously (Suzuki et al., 2007). All subjects remained supine position on the bed in a ready room separated by white walls and curtains. The room was bright at 200 lx. Patients with EB and eye open healthy subjects were instructed to fix white and solid ceiling from FDG injection to PET scanning. A 6-min emission scan in the three-dimensional acquisition mode was initiated 45 min after the FDG injection, and 50 trans-axial images with an inter-slice interval of 3.125 mm were acquired (matrix size = 128 × 128 × 63, and voxel size = 2.0 × 2.0 × 3.125 mm) in the scanning room at a brightness of 20 lx. We monitored the subjects by using a video camera during the scans and observed no extra movements in their face or other body parts (Suzuki et al., 2007). The tomographic images were reconstructed with the filtered back-projection method using a Butterworth filter (cut-off frequency, 1.25 cycles/cm; order, 2). Attenuation was corrected by performing a transmission scan by using a 68Ga/68Ge rotating source.
2.4. Data processing and statistical analysis
The PET images were processed using the statistical parametric mapping (SPM8) software (Friston et al., 1991) which was implemented in MATLAB (MathWorks, Sherborn, MA). Statistical parametric maps combine the general linear model and theoretical Gaussian fields to make statistical inferences about regional effects. All PET images were spatially normalized to a standard template produced by the Montreal Neurological Institute using an in house template of FDG-PET images and smoothed with a Gaussian filter for 16 mm at full width and half maximum (Suzuki et al., 2007) to increase the signal-to-noise ratio before statistical processing.
After the appropriate design matrix was specified, the subject and group effects were estimated according to the general linear model at each voxel. We examined the difference in cerebral glucose metabolism between each pair of groups (eye open controls minus patients with EB, eye close controls minus patients with EB, patients with EB minus eye open controls, patients with EB minus eye close controls, eye open controls minus eye close controls, and eye close controls minus eye open controls) by using two-sample t-tests and global normalization (Friston et al., 1991). Statistical inference on the SPM (Z) was corrected using the theory of Gaussian fields. To test the hypotheses regarding the region-specific group effects, the estimated group effects based on the general linear model were compared using linear contrasts between groups. This enabled us to test the null hypothesis that the contrast or linear mixture (subtraction) of the estimates is zero by using SPM {t}. We chose a height threshold of P < .05, family-wise error-corrected and excluded small areas that showed changes in cerebral glucose metabolism; the extent threshold was 150 voxels. To evaluate whether the identified changes in glucose metabolism correlated with disease severity, we performed regression analyses between glucose metabolism and the JRS sum score. We thresholded our results at P < .05, family-wise error-corrected for the cluster-level (Emoto et al., 2010) and small areas that showed glucose metabolism changes were excluded; the extent threshold was 150 voxels.
We also defined regions of interest (ROIs) interactively on normalized PET images by visual inspection with reference to the corresponding MR images. ROIs were placed over both sides of the posterior striate cortex, anterior striate cortex, extrastriate cortex, and thalamus, and we measured the cerebral glucose metabolism levels of each subject. The cerebral glucose metabolism level of each part was normalized to the mean level of the whole brain. We examined the difference in relative glucose metabolism level in each region between patients with EB and healthy subjects by using two-sample t-tests with Bonferroni's correction for multiple comparison (P < .006). Moreover, in patients with EB, regression analysis was performed between the relative glucose metabolism level in each region and the JRS sum score.
Nineteen of the 39 patients with EB complained of photophobia, such as dazzling or eye pain (Hallett, 2002) (Table 1). The average scores of severity and frequency in patients with EB and photophobia were 2.85 and 2.65, respectively, and the average scores of those without photophobia were 2.79 and 2.79, respectively. No difference was observed in the JRS sum scores between patients with photophobia and patients without photophobia. Difference in glucose metabolism level in each region between patients with photophobia and patients without photophobia was tested using Mann-Whitney U tests with Bonferroni's correction for multiple comparisons (P < .006).
Table 1.
Areas and coordinates for the maxima of regional glucose hypometabolism and negative correlation between glucose metabolism and disease severity in patients with essential blepharospasm.
| Area | x | y | z | Z score |
|---|---|---|---|---|
| A. Eye open controls minus essential blepharospasm | ||||
| Extrastriate cortex (L) | −24 | −88 | −8 | 6.39 |
| Extrastriate cortex (R) | 28 | −86 | −10 | 6.32 |
| B. Essential blepharospasm minus eye open controls | ||||
| Thalamus (R) | 8 | −28 | 6 | 6.03 |
| Thalamus (L) | −6 | −28 | 8 | 5.56 |
| C. Eye close controls minus essential blepharospasm | ||||
| None | ||||
| D. Essential blepharospasm minus eye close controls | ||||
| Thalamus (R) | 6 | −24 | 6 | 5.41 |
| Thalamus (L) | −6 | −24 | 6 | 5.04 |
| E. Eye open controls minus Eye close controls | ||||
| Extrastriate cortex (R) | 22 | −92 | 2 | 5.14 |
| Extrastriate cortex (L) | −20 | −94 | −2 | 4.96 |
| F. Eye close controls minus Eye open controls | ||||
| None | ||||
| G. Negative correlation between glucose metabolism and disease severity | ||||
| Striate cortex (L) | −14 | −86 | 0 | 4.07 |
| Striate cortex (R) | 12 | −88 | −10 | 3.79 |
3. Results
3.1. SPM analysis
In the SPM analysis, cerebral glucose hypometabolism was observed in both sides of the extrastriate cortex of patients with EB compared to eye open controls but not to eye close controls (Table 1 and Fig. 1). In contrast, cerebral glucose hypermetabolism was detected in both sides of the thalamus of patients with EB compared to both eye open and eye close controls. We observed a significant negative correlation between the JRS sum score and relative glucose metabolism level in the striate cortex of patients with EB (Table 1 and Fig. 2).
Fig. 1.
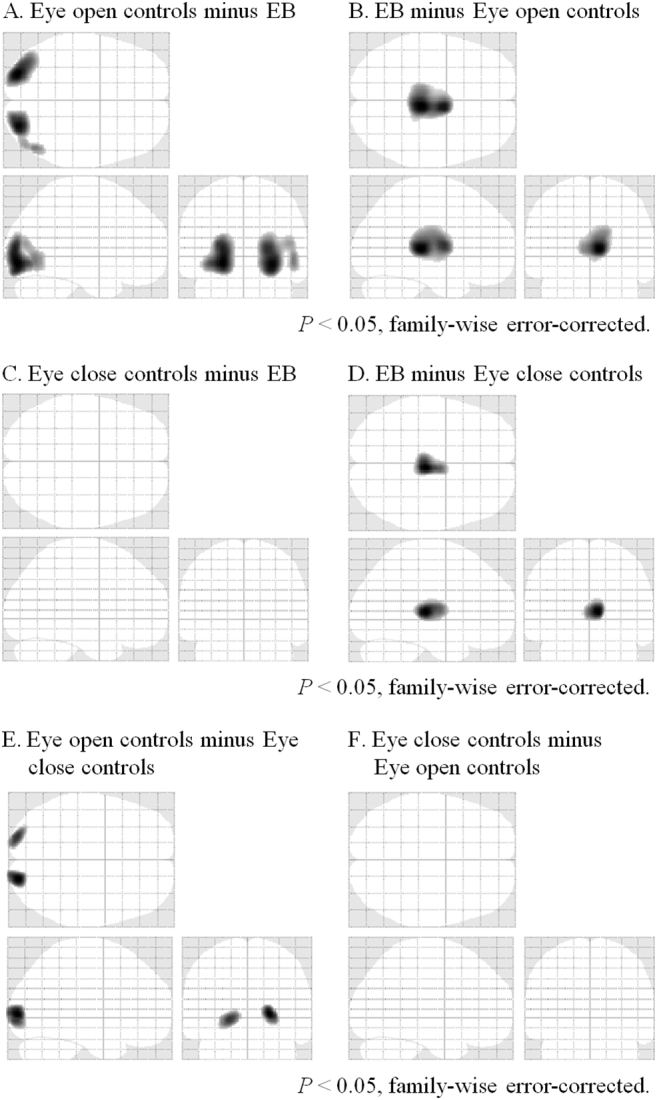
Areas of relative cerebral glucose metabolism in (A) eye open controls minus patients with essential blepharospasm (EB), (B) patients with EB minus eye open controls, (C) eye close controls minus patients with EB, (D) patients with EB minus eye close controls, (E) eye open controls minus eye close controls, and (F) eye close controls minus minus eye open controls (P < .05, family-wise error corrected) are shown.
Sagittal, transverse, and frontal views of a statistical parametric map rendered into a standard stereotactic space and projected onto a glass brain. Extent threshold: k = 150 voxels.
Fig. 2.
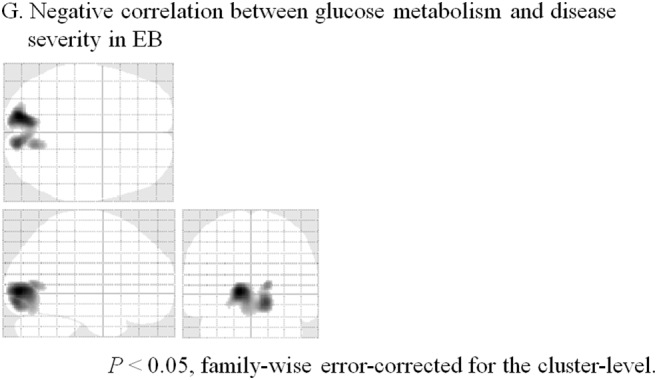
Areas of regression analysis of the correlation between glucose metabolism level and Jankovic Rating Scale sum score in patients with essential blepharospasm (P < .05, family-wise error-corrected for the cluster level).
Sagittal, transverse, and frontal views of a statistical parametric map rendered into a standard stereotactic space and projected onto a glass brain. Extent threshold: k = 150 voxels.
3.2. ROI analysis
In the ROI analysis, glucose hypometabolism was observed in both sides of the posterior striate cortex (right, P = .01; left, P = .001) and both sides of the extrastriate cortex (right, P = .000007; left, P = .000006) of patients with EB compared with eye open controls but not with eye close controls (Table 2). No significant change was observed in both sides of the anterior striate cortex. Glucose hypermetabolism was observed in both sides of the thalamus of patients with EB compared with both eye open controls (right, P = 6.6 × 10−12; left, P = 6.6 × 10−8) and eye close controls (right, P = 8.4 × 10−10; left, P = 7.1 × 10−7) (Table 2).
Table 2.
Average and standard deviation of glucose metabolism expressed as relative uptake.
| EB | Eye open controls | Eye close controls | |
|---|---|---|---|
| Posterior striate cortex (R) | 1.229 ± 0.050 | 1.266⁎ ± 0.052 | 1.220 ± 0.058 |
| Posterior striate cortex (L) | 1.227 ± 0.049 | 1.270⁎ ± 0.050 | 1.221 ± 0.055 |
| Anterior striate cortex (R) | 1.178 ± 0.051 | 1.169 ± 0.055 | 1.148 ± 0.045 |
| Anterior striate cortex (L) | 1.171 ± 0.050 | 1.177 ± 0.044 | 1.144 ± 0.049 |
| Extrastriate cortex (R) | 1.006 ± 0.035 | 1.049⁎ ± 0.040 | 1.011 ± 0.052 |
| Extrastriate cortex (L) | 0.996 ± 0.040 | 1.040⁎ ± 0.035 | 0.997 ± 0.048 |
| Thalamus (R) | 1.037 ± 0.025 | 0.974# ± 0.043 | 0.985# ± 0.040 |
| Thalamus (L) | 1.021 ± 0.029 | 0.971# ± 0.043 | 0.977# ± 0.041 |
Significant increase compared to EB (two-sample t-test with Bonferroni's correction for multiple comparison) (P < .05).
Significant decrease compared to EB (Two-sample t-test with Bonferroni's correction for multiple comparison) (P < .05).
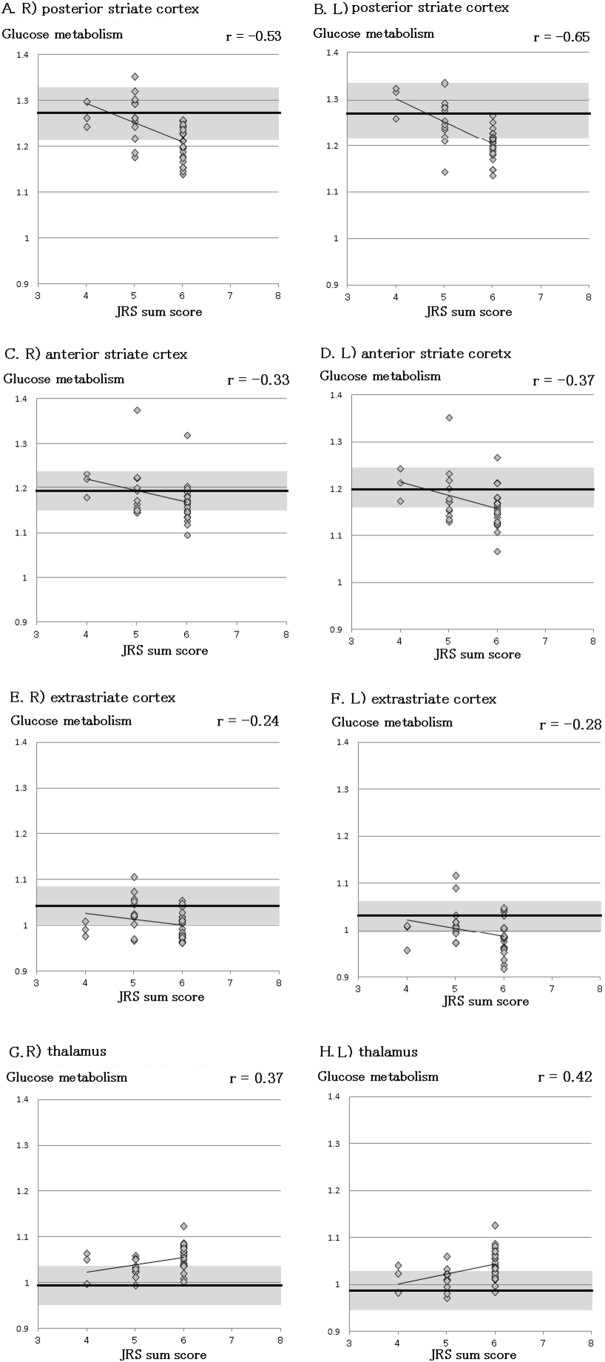
We observed a significant negative correlation between the JRS sum score and relative glucose metabolism level in the posterior (right: r = −0.53, P = .0005; left: r = −0.65, P = .00001) and anterior (right: r = −0.33, P = .04; left: r = −0.37, P = .02) striate cortices of patients with EB; however, no significant correlation was observed between the JRS sum score and relative glucose metabolism level in the extrastriate cortex (right: r = −0.23, P = .26; left: r = −0.28, P = .14) (Fig. 3). We also observed a significant positive correlation between the JRS sum score and relative glucose metabolism level in the thalamus (right: r = −0.37, P = .02; left: r = −0.42, P = .008) of patients with EB (Fig. 3). No significant correlation was observed between the JRS sum score and cerebral glucose metabolism level in the right or left thalamus. In contrast, relative glucose metabolism level in both sides of the thalamus was higher in patients with photophobia than in patients without photophobia (Table 3).
Fig. 3.
Regression analysis in the visual cortex.
Regression analysis of the correlation between relative glucose metabolism and the Jankovic Rating Scale (JRS) sum score in the right posterior striate cortex (A), left posterior striate cortex (B), right anterior striate cortex (C), left anterior striate cortex (D), right extrastriate cortex (E), left extrastriate cortex (F), right thalamus (G) and left thalamus (H) are shown. Averages (1 standard deviation) of the healthy controls in each area are denoted by the thick line and the gray area.
Table 3.
Average and standard deviation of glucose metabolism expressed as relative uptake of patients with photophobia and without photophobia.
| Photophobia (+) | Photophobia (−) | P value | |
|---|---|---|---|
| Posterior striate cortex (R) | 1.236 ± 0.053 | 1.223 ± 0.047 | 2.25 |
| Posterior striate cortex (L) | 1.237 ± 0.047 | 1.218 ± 0.050 | 1.80 |
| Anterior striate cortex (R) | 1.170 ± 0.031 | 1.186 ± 0.064 | 2.74 |
| Anterior striate cortex (L) | 1.167 ± 0.030 | 1.174 ± 0.064 | 5.41 |
| Extrastriate cortex (R) | 1.002 ± 0.032 | 1.010 ± 0.038 | 3.69 |
| Extrastriate cortex (L) | 0.988 ± 0.031 | 1.003 ± 0.047 | 1.90 |
| Thalamus (R) | 1.061 ± 0.018 | 1.037 ± 0.051 | 0.03 |
| Thalamus (L) | 1.048 ± 0.023 | 1.018 ± 0.034 | 0.01 |
Mann-Whitney U test with Bonferroni's correction for multiple comparison.
4. Discussion
4.1. Cerebral glucose metabolism in the visual cortex of patients with EB
We observed glucose hypometabolism in both sides of the posterior striate cortex and extrastriate cortex of patients with EB compared to eye open controls. However, there was no difference in glucose metabolism distribution between patients with EB and eye close controls in the visual cortex. Moreover, a negative correlation was observed between the intensity of disease and the relative glucose metabolism level in the striate cortex but not in the extrastriate cortex. Studies have shown that the interruption of visual input decreases activity in the visual cortex. Veraart et al. (1990) observed glucose hypometabolism in the visual cortex of patients with late-onset blindness than in healthy subjects; however, they observed glucose hypermetabolism in the visual cortex of patients with early onset blindness by using PET. Several studies (Riedl et al., 2014; Mortensen et al., 2018) performed FDG-PET in the with eyes-closed and eyes-open resting-state conditions in healthy subjects, and they observed relative glucose metabolism in the both striate cortex and extrastriate cortex in the eyes-open minus eyes-closed contrast. The extrastriate cortex is divided into several areas according to functions, such as the V3, V4 (V8), and MT (+) (Zeki et al., 1991), and these areas are activated by visual stimuli that include specific factors, such as shape, color, and motion, respectively (Chawla et al., 1999; Suzuki et al., 2004). However, each part is hardly activated by other types of visual stimuli (Suzuki et al., 2004). These observations suggest that activity of the striate cortex is activated by increase in visual inputs, while activity of the extrastriate cortex is hardly dependent on the quantity of visual inputs.
The central visual field is represented at the posterior striate cortex, and the peripheral visual field corresponds to the anterior parts of the striate cortex (Horton, 2006). We observe objects by using the central view when reading characters or driving a car. A decrease in glucose metabolism in the visual cortex, especially the posterior striate cortex, may reflect functional visual loss in patients with EB.
The glucose metabolism level in the visual cortex in patients with EB was almost equal to that of eye close controls which visual input was interrupted. Moreover, factors other than interruption of visual input may influence glucose metabolism in the visual cortex in EB patients. Gawne et al. observed firing neurons in the visual cortex during blink in rhesus monkeys (Gawne and Martin, 2000; Gawne and Martin, 2002). There are several reports that activation in the visual cortex was observed during blink in darkness in human using fMRI and PET (Tsubota et al., 1999; Riedl et al., 2014). Generally in patients with EB, the frequency of blink will increase (Conte et al., 2013), while blinking is suppressed in severe cases because of excessive involuntary closure of the eyelids (Kranz et al., 2013). Frequency of blink may also influence glucose metabolism in the visual cortex. Recently, it has been reported that functional connectivity (FC) between cerebellum and visual cortex changes in dystonic diseases using resting-state functional MRI. Jochim et al. (2017) observed FC decrease between cerebellum and visual cortex in patients with EB/orofacial dystonia. And, Dresel et al. (2014) demonstrated increased negative FC of the cerebellum with visual cortex in patients with writer's cramp. Moreover, positive FC reduced between the cerebellum and visual network were found in musicians with embouchure dystonia (Haslinger et al., 2017). EB may markedly impair vision up to a functional blindness, which might result in altered feedback signals. These results may be an altered modulation of sensoric (i.e., visual) information and visuomotor integration, or may be interpreted as cerebellar deficit of visuomotor and visuotactile integration. On the other hand, Baker et al. (2003) examined brain activation in patients with EB during repeated blinking using fMRI, and they observed greater activation during spontaneous and voluntary blinking in EB patients compared with healthy subjects in the anterior visual cortex. This may be related to that glucose hypometabolism in the anterior striate cortex was not observed in the present study.
4.2. Cerebral glucose metabolism in the thalamus of patients with EB
We observed glucose hypermetabolism in both sides of the thalamus of patients with EB compared with healthy subjects. The results of functional imaging studies are often interpreted using the present anatomical model of information flow in the basal ganglia-thalamo-cortical motor circuit (Poston and Eidelberg, 2012; Tempel and Perlmutter, 1993). In our previous PET study, we also observed significant hypermetabolism in the thalamus (Suzuki et al., 2007). Other studies have also shown cerebral glucose hypermetabolism in the striatum and thalamus of patients with focal dystonia including blepharospasm (Esmaeli-Gustein et al., 1999; Galardi et al., 1996).
Regional cerebral glucose metabolism in the thalamus showed a significant positive correlation with the JRS sum score. Murai et al. (2011) examined a patient with blepharospasm who had five times higher cerebral glucose metabolism by using PET. The severity of blepharospasm changed during observation, and the severity was positively correlated with thalamic glucose metabolism. The activity of the thalamus may reflect the severity of blepharospasm.
4.3. Photophobia in patients with EB
The neuro-physiological cause of photophobia is not fully understood; however, studies have reported that light sensitivity thresholds in patients with blepharospasm are significantly lower than those in normal individuals (Adams et al., 2006). Emoto et al. (2010) also reported higher glucose hypermetabolism in the thalamus of patients with EB and photophobia than in patients with EB without photophobia by using PET. Recently, several “photophobia circuits” have been suggested in order to explain the pathology of photophobia (Digre and Brennan, 2012), and the thalamus is involved in these circuits (Noseda et al., 2010; Okamoto et al., 2009). It has been proposed that the thalamus is engaged in multisensory integration, and that the thalamus is a state- and gain-setting region in the brain. Studies have also reported that both the gain and precision of sensory signals are altered in the thalamus (Hirata et al., 2006). These observations, suggested that the thalamus may be activated by increased sensory inputs and processing of sensory signals in patients with photophobia.
5. Conclusion
Glucose hypometabolism was observed in both sides of the posterior striate cortex and extrastriate cortex in patients with EB. We observed a significant correlation between the JRS sum score and relative glucose metabolism level in the posterior and anterior striate cortices of patients with EB. It is surmised that interruption of the visual input is decreasing activity in the visual cortex in patients with EB.
Financial disclosures
Dr. Suzuki was supported by a Grants-in Aid for Scientific Research by Japan Society for the Promotion of Science (#21791725).
Dr. Kiyosawa was supported by the Benign Essential Blepharospasm Research Foundation.
Dr. Ishii was supported by a Grants-in-Aid for Scientific Research by Japan Society for the Promotion of Science (#20591038).
Dr. Wakakura reports no disclosures.
Acknowledgments
This work was supported by the Benign Essential Blepharospasm Research Foundation and Grants-in-Aid for Scientific Research No. 21791725 (YS) and No. 20591038 (KI) from the Japan Society for the Promotion of Science.
Footnotes
This work originated in the Positron Medical Center, Tokyo Metropolitan Institute of Gerontology.
References
- Adams W.H., Digre K.B., Patel B.C., Anderson R.L., Warner J.E., Katz B.J. The evaluation of light sensitivity in benign essential blepharospasm. Am J. Ophthalmol. 2006;142:82–87. doi: 10.1016/j.ajo.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Baker R.S., Andersen A.H., Morecraft R.J., Smith C.D. A functional magnetic resonance imaging study in patients with benign essential blepharospasm. Neuroophthalmol. 2003;23:11–15. doi: 10.1097/00041327-200303000-00003. [DOI] [PubMed] [Google Scholar]
- Chawla D., Buechel C., Edwards R., Howseman A., Josephs O., Ashburner J., Friston K.J. Speed-dependent responses in V5: a replication study. Neuroimage. 1999;9:508–515. doi: 10.1006/nimg.1999.0432. [DOI] [PubMed] [Google Scholar]
- Conte A., Defazio G., Ferrazzano G., Hallett M., Macerollo A., Fabbrini G., Berardelli A. Is increased blinking a form of blepharospasm? Neurology. 2013;80:2236–2241. doi: 10.1212/WNL.0b013e318296e99d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digre K.B., Brennan K.C. Shedding light on photophobia. J. Neuroophthalmol. 2012;32:68–81. doi: 10.1097/WNO.0b013e3182474548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresel C., Li Y., Wilzeck V., Castrop F., Zimmer C., Haslinger B. Multiple changes of functional connectivity between sensorimotor areas in focal hand dystonia. J. Neurol. Neurosurg. Psychiatry. 2014;85:1245–1252. doi: 10.1136/jnnp-2013-307127. [DOI] [PubMed] [Google Scholar]
- Egan R.A., LaFrance W.C., Jr. Functional vision disorder. Semin. Neurol. 2015;35:557–563. doi: 10.1055/s-0035-1563580. [DOI] [PubMed] [Google Scholar]
- Emoto H., Suzuki Y., Wakakura M., Horie C., Kiyosawa M., Mochizuki M., Kawasaki K., Oda K., Ishiwata K., Ishii K. Photophobia in essential blepharopsasm-a positron emission tomographic study. Mov. Disord. 2010;25:433–439. doi: 10.1002/mds.22916. [DOI] [PubMed] [Google Scholar]
- Esmaeli-Gustein B., Nahmias C., Thompson M., Kazdan M., Harvey J. Positron emission tomography in patients with benign essential blepharospasm. Ophthal. Plast. Reconstr. Surg. 1999;15:23–27. doi: 10.1097/00002341-199901000-00006. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Frith C.D., Liddle P.F., Frackowiak R.S. Comparing functional (PET) images: the assessment of significant change. J. Cereb. Blood Flow Metab. 1991;11:690–699. doi: 10.1038/jcbfm.1991.122. [DOI] [PubMed] [Google Scholar]
- Galardi G., Perani D., Grassi F., Bressi S., Amadio S., Antoni M., Comi G.C., Canal N., Fazio F. Basal ganglia and thalamo-cortical hypermetabolism in patients with spasmodic torticollis. Acta Neurol. Scand. 1996;94:172–176. doi: 10.1111/j.1600-0404.1996.tb07049.x. [DOI] [PubMed] [Google Scholar]
- Gawne T.J., Martin J.M. Activity of primate V1 cortical neurons during blinks. J. Neurophysiol. 2000;84:2691–2694. doi: 10.1152/jn.2000.84.5.2691. [DOI] [PubMed] [Google Scholar]
- Gawne T.J., Martin J.M. Responses of primate visual cortical neurons to stimuli presented by flash, saccade, blink, and external darkening. J. Neurophysiol. 2002;88:2178–2186. doi: 10.1152/jn.00151.200. [DOI] [PubMed] [Google Scholar]
- Hallett M. Blepharospasm: Recent advances. Neurology. 2002;59:1306–1312. doi: 10.1212/01.wnl.0000027361.73814.0e. [DOI] [PubMed] [Google Scholar]
- Haslinger B., Noé J., Altenmüller E., Riedl V., Zimmer C., Mantel T., Dresel C. Changes in resting-state connectivity in musicians with embouchure dystonia. Mov. Disord. 2017;32:450–458. doi: 10.1002/mds.26893. [DOI] [PubMed] [Google Scholar]
- Hirata A., Aguilar J., Castro-Alamancos M.A. Noradrenergic activation amplifies bottom-up and top-down signal-to-noise ratios in sensory thalamus. J. Neurosci. 2006;26:4426–4436. doi: 10.1523/JNEUROSCI.5298-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton J.C. Ocular integration in the human visual cortex. Can. J. Ophthalmol. 2006;41:584–593. doi: 10.1016/S0008-4182(06)80027-X. [DOI] [PubMed] [Google Scholar]
- Jankovic J., Orman J. Botulinum a toxin for cranial-cervical dystonia: a double-blind, placebo-controlled study. Neurology. 1987;37:616–623. doi: 10.1212/wnl.37.4.616. [DOI] [PubMed] [Google Scholar]
- Jochim A., Li Y., Gora-Stahlberg G., Mantel T., Berndt M., Castrop F., Dresel C., Haslinger B. Altered functional connectivity in blepharospasm/orofacial dystonia. Brain Behav. 2017;8 doi: 10.1002/brb3.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz G., Shamim E.A., Lin P.T., Kranz G.S., Hallett M. Long-term depression-like plasticity of the blink reflex for the treatment of blepharospasm. Mov. Disord. 2013;28:498–503. doi: 10.1002/mds.25329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen K.N., Gjedde A., Thompson G.J., Herman P., Parent M.J., Rothman D.L., Kupers R., Ptito M., Stender J., Laureys S., Riedl V., Alkire M.T., Hyder F. Impact of global mean normalization on regional glucose metabolism in the human brain. Neural. Plast. 2018;2018 doi: 10.1155/2018/6120925. (6120925) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai H., Suzuki Y., Kiyosawa M., Wakakura M., Mochizuki M., Ishiwata K., Ishii K. Positive correlation between severity of blepharospasm and thalamic glucose metabolism. Case Report Ophthalmol. 2011;2:50–54. doi: 10.1159/000324459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noseda R., Constandil L., Bourgeais L., Chalus M., Villanueva L. Changes of meningeal excitability mediated by corticotrigeminal networks: a link for the endogenous modulation of migraine pain. J. Neurosci. 2010;30:14420–14429. doi: 10.1523/JNEUROSCI.3025-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K., Thompson R., Tashiro A., Chang Z., Bereiter D.A. Bright light produces Fos-positive neurons in caudal trigeminal brainstem. Neuroscience. 2009;160:858–864. doi: 10.1016/j.neuroscience.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Poston K.L., Eidelberg D. Functional brain networks and abnormal connectivity in the movement disorders. Neuroimage. 2012;62:2261–2270. doi: 10.1016/j.neuroimage.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pula J. Functional vision loss. Curr. Opin. Ophthalmol. 2012;23:460–465. doi: 10.1097/ICU.0b013e328358c6dc. [DOI] [PubMed] [Google Scholar]
- Riedl V., Bienkowska K., Strobel C., Tahmasian M., Grimmer T., Förster S., Friston K.J., Sorg C., Drzezga A. Local activity determines functional connectivity in the resting human brain: a simultaneous FDG-PET/fMRI study. J. Neurosci. 2014;34:6260–6266. doi: 10.1523/JNEUROSCI.0492-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggenkämper P., Jost W.H., Bihari K., Comes G., Grafe S., for the NT 201 Blepharospasm Study Team Efficacy and safety of a new Botulinum Toxin Type A free of complexing proteins in the treatment of blepharospasm. J. Neural Transm. 2006;113:303–312. doi: 10.1007/s00702-005-0323-3. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Kiyosawa M., Mochizuki M., Wakakura M., Ishii K., Senda M. Oscillopsia associated with dysfunction of visual cortex. Jpn. J. Ophthalmol. 2004;48:128–132. doi: 10.1007/s10384-003-0040-0. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Mizoguchi S., Kiyosawa M., Mochizuki M., Ishiwata K., Wakakura M., Ishii K. Glucose hypermetabolism in the thalamus of patients with essential blepharospasm. J. Neurol. 2007;254:890–896. doi: 10.1007/s00415-006-0468-5. [DOI] [PubMed] [Google Scholar]
- Tempel L.W., Perlmutter J.S. Abnormal cortical responses in patients with writer's cramp. Neurology. 1993;43:2252–2257. doi: 10.1212/wnl.43.11.2252. [DOI] [PubMed] [Google Scholar]
- Tsubota K., Kwong K.K., Lee T.Y., Nakamura J., Cheng H.M. Functional MRI of brain activation by eye blinking. Exp. Eye Res. 1999;69:1–7. doi: 10.1006/exer.1999.0660. [DOI] [PubMed] [Google Scholar]
- Veraart C., De Volder A.G., Wanet-Defalque M.C., Bol A., Michel C., Goffinet A.M. Glucose utilization in human visual cortex is abnormally elevated in blindness of early onset but decreased in blindness of late onset. Brain Res. 1990;510:115–121. doi: 10.1016/0006-8993(90)90735-t. [DOI] [PubMed] [Google Scholar]
- Zeki S., Watson J.D.G., Lueck C.J., Friston K.J., Kennard C., Frackowiak R.S. A direct demonstration of functional specialization in human visual cortex. J. Neurosci. 1991;11:641–649. doi: 10.1523/JNEUROSCI.11-03-00641.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]