Abstract
Objectives
Pyogenic arthritis, pyoderma gangrenosum and acne (PAPA) syndrome is characterized by flares of sterile arthritis with neutrophil infiltrate and the overproduction of Interleukin (IL)-1β. The purpose of this study was to elucidate the potential role of neutrophil subsets and neutrophil extracellular traps (NETs) in the pathogenesis of PAPA.
Methods
Neutrophils and low-density granulocytes (LDGs) were quantified by flow cytometry. Circulating NETs were measured by ELISA and PAPA serum was tested for the ability to degrade NETs. The capacity of NETs from PAPA neutrophils to activate macrophages was assessed. Skin biopsies were analyzed for NETs and neutrophil gene signatures.
Results
Circulating LDGs are elevated in PAPA subjects. PAPA neutrophils and LDGs display enhanced NET formation compared to control neutrophils. PAPA sera exhibit impaired NET’s degradation and this is corrected with exogenous DNase1. Recombinant human IL-1β induces NET formation in PAPA neutrophils but not healthy control neutrophils. NET formation in healthy control neutrophils is induced by PAPA serum and this effect is inhibited by the IL-1 receptor antagonist, anakinra. NETs from PAPA neutrophils and LDGs stimulate IL-6 release in healthy control macrophages. NETs are detected in skin biopsies of PAPA patients in association with increased tissue IL-1β, IL-8 and IL-17. Furthermore, LDG gene signatures are detected in PAPA skin.
Conclusions
PAPA syndrome is characterized by an imbalance of NET formation and degradation that may enhance the half-life of these structures in vivo, promoting inflammation. Anakinra ameliorates NET formation in PAPA and this finding supports a role for IL-1 signaling in exacerbated neutrophil responses in this disease. The study also highlights other inflammatory pathways potentially pathogenic in PAPA, including IL-17 and IL-6, and these results may help to guide new therapeutic approaches in this severe and often treatment-refractory condition.
Keywords: neutrophil extracellular traps, PAPA syndrome, IL-1β, low-density granulocytes, anakinra
Introduction
Autoinflammatory disorders are characterized by abnormalities in the innate immune system resulting in excessive production of proinflammatory cytokines and exuberant inflammatory responses [1], [2]. One of these conditions is pyogenic arthritis, pyoderma gangrenosum and acne (PAPA) syndrome, a rare autosomal dominant inherited disorder caused by mutations in the proline/serine/threonine phosphatase-interaction protein 1 (PSTPIP1/CD2BP1) gene [3]. PSTPIP1 is a cytoskeleton-associated protein that acts as a bridge to proteins that interact with proline, glutamic acid, serine and threonine (PEST)-type protein tyrosine phosphatase (PTP; (PEST PTP)) and regulates actin reorganization [4], T cell activation [5], the phosphorylation status of Wiskott-Aldrich syndrome protein (WASP) [6] and cAbl tyrosine kinase [7], and the release of IL-1β [8]. The phosphorylation status of PSTPIP1 is likely important for its function [8]. Three missense mutations in PSTPIP1 account for most patients with PAPA syndrome [9], resulting in a hyperphosphorylated protein that interacts more avidly with the pyrin protein inflammasome and leading to dysregulated IL-1β and downstream TNF-α production in mononuclear cells [8], [10]. PAPA syndrome subjects present with a variable disease expressivity and severity of skin lesions. Within the clinical spectrum of disease, patients with milder presentations can often be effectively treated with the IL-1 receptor antagonist, anakinra [11], [12]. There are also patients who can achieve sustained clinical remission while taking the anti-TNF agents [10]. However, there are patients with highly refractory disease who require combination (anti-IL-1/anti-TNF) or even triple (anti-IL-1/anti-TNF/glucocorticoid) therapy to effectively suppress the intense inflammation effects resulting from this disease.
A putative role for neutrophils has been considered in PAPA syndrome, given the presence of neutrophilic dermatoses and sterile joint effusions with prominent neutrophilic infiltrates [8], [9], [13]. Indeed, PAPA patients have elevated circulating levels of various neutrophil serine proteases and antimicrobial proteins including myeloperoxidase (MPO), neutrophil elastase (NE), and lactoferrin (LF), suggesting global neutrophil activation and/or enhanced neutrophil cell death [9]. Modular analyses of peripheral blood mononuclear cells (PBMCs) from PAPA patients previously revealed an overexpression of genes expressed in neutrophils and other myeloid cells [1]. Among the upregulated neutrophil-associated genes were those encoding for neutrophil granule proteins including lipocalin 2 (LCN-2) and defensin A4 (DEFA4), which are highly expressed in low-density granulocytes (LDGs) [1], [14]. LDGs are a proinflammatory subset of neutrophils that were first described in systemic lupus erythematosus (SLE) as a population of granulocytes that, due to their “low buoyancy”, sediment within the PBMC fraction after density centrifugation [15]. In addition to SLE [14], [16], [17], [18], LDGs have been reported in other conditions such as ANCA-associated vasculitis [19] and psoriasis [20] but have yet to be described in autoinflammatory syndromes. LDGs represent a mixed population of neutrophils that express cell-surface markers of terminally differentiated neutrophils (CD15high/CD14l°/CD10+/CD16+) [16] despite in some cases having less segmented nuclear morphology that is characteristic of immature neutrophils [17]. Transcriptional analysis of lupus LDGs has been consistent with the presence of cells representing immature stages of neutrophil differentiation [14], [17].
Importantly, LDGs in SLE and other conditions have an enhanced capacity to spontaneously undergo neutrophil cell death through the formation of neutrophil extracellular traps (NETs). NETs are an extracellular meshwork of chromatin fibers decorated with immunostimulatory molecules and various enzymes and proteins from the cytosol and neutrophil granules [21], [18]. NETs can be induced with various infectious and sterile inflammatory stimuli including several cytokines [17], [21], [22], [23], [24]. During NET formation, various histones and other peptides become post-translationally modified by the enzyme peptidyl arginine deiminase 4 (PAD-4) through citrullination. Histone citrullination may contribute to chromatin decondensation, a hallmark of death by NET formation [25], [26]. Besides their antimicrobial role, NETs can cause vascular damage and stimulate immune cells in pleiotropic ways, including promoting type I interferons and proinflammatory cytokine synthesis by target cells if not readily cleared by host DNase enzymes [16], [17], [27]. Given the putative role of neutrophils in PAPA syndrome, we characterized neutrophil subsets in this condition and determined a potential role of NETs in the disease pathogenesis.
Results
Identification and characterization of LDGs in PAPA syndrome.
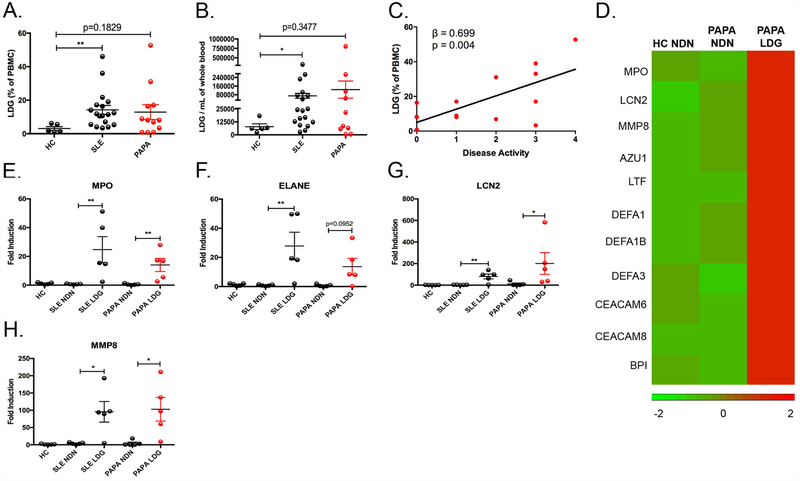
The demographic and clinical characteristics of patients studied are included in Supplementary Tables I and II. Among the subjects studied, genetic screening for PAPA mutations identified a family that included five PAPA subjects that all carried the A230T mutation (Supplementary Fig. 1). To determine if LDGs are present in the circulation of PAPA subjects, we quantified by FACS the presence of CD10−/+ /CD14l° /CD15 cells in the PBMC fraction as previously reported [14], [16], [28]. When compared to healthy controls, both SLE (n=20; range 3.4% - 46%) and PAPA subjects (n=12; range 0.7% – 53%) had higher percentage of LDGs within their total PBMC fraction compared to healthy control subjects (n=5; range 0.9% - 6.1%) (Fig. 1A). To determine the absolute number of LDGs, negative selection magnetic bead procedure was performed on PBMCs [14], [16]. We confirmed that both SLE and PAPA subjects had increased numbers of LDGs compared to healthy control subjects (Fig. 1B). We developed a scoring model to quantify disease activity in PAPA syndrome to determine its correlation with LDG numbers. PAPA disease activity significantly associated with the percentage (β = 0.699, P = 0.004) and absolute numbers (β = 0.623, P = 0.01) of LDGs (Fig. 1C and data not shown).
Fig. 1: Identification and characterization of LDGs in PAPA syndrome.
(A) Percentage of LDGs in the PBMC fraction of healthy controls (HC) (n=5), SLE patients (n=18), and PAPA patients (n=12). (B) Total LDG numbers per mL of whole blood in HC (n=5), SLE (n=18), and PAPA patients (n=10). (C) A linear regression analysis of the percentage of LDGs in PBMC fraction and disease activity (n=15). (D) RNASeq analysis of HC normal-dense neutrophils (NDN), PAPA NDN, and PAPA LDG (n=1/each group) identified upregulated expression of neutrophil granule genes in PAPA LDGs. (E–H) Total RNA was extracted from unstimulated HC NDN, SLE NDN and autologous LDGs, and PAPA NDN and autologous LDGs (n=5/group) and neutrophil granule gene expression was measured by qRT-PCR. Expression values were normalized to the expression of the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) housekeeping gene and fold induction over HC NDN was calculated. The results shown in A-H represent the mean ± SEM (*P ≤ 0.05; **P ≤ 0.01).
Previous reports suggest that lupus LDGs represent, at least in part, a subset of immature neutrophils, given the elevated expression of mRNAs encoding for granule proteins [14], [17]. We performed RNASeq analysis on PAPA LDGs and compared gene expression to autologous normal-density PAPA neutrophils (NDN) and healthy control neutrophils. Indeed, similar to lupus LDGs, PAPA LDGs have increased gene expression of mRNAs encoding for granule proteins (Fig. 1D, Supplementary Table 3). Findings were confirmed by qRT-PCR, where both PAPA LDGs and SLE LDGs express significantly higher mRNA levels of primary (MPO), secondary (LCN2), and tertiary (MMP8) granule proteins compared to healthy control neutrophils and autologous normal-density PAPA and lupus neutrophils (Fig. 1E–H). In summary, elevated levels of LDGs are found in PAPA syndrome at comparable levels to those in SLE, and they associate with PAPA syndrome disease activity. PAPA LDGs upregulate mRNAs encoding for granule proteins, suggesting an immature phenotype.
PAPA neutrophils undergo enhanced NET formation.
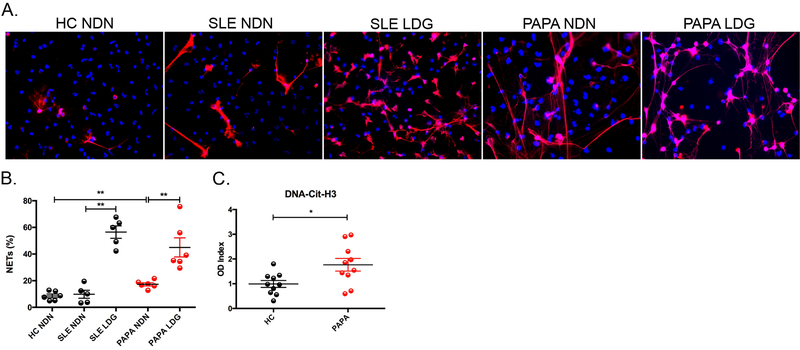
To further characterize neutrophil subsets in PAPA, we examined their ability to undergo spontaneous NET formation when compared to controls and SLE. Unstimulated LDGs from PAPA and SLE subjects displayed an enhanced ability to form NETs compared to autologous normal-density neutrophils and healthy control neutrophils (Fig. 2A and B). PAPA normal-density neutrophils also had significantly higher levels of NET formation compared to healthy control neutrophils (P = 0.0043) but to a lesser degree than LDGs (P = 0.0022) (Fig. 2A and B). To further confirm whether enhanced NET formation occurs in vivo in PAPA syndrome, we quantified NET remnants in circulation using an assay previously described by our group and others [18], [29]. PAPA subjects had significantly higher levels of citrullinated histone H3-DNA complexes compared to healthy control individuals, suggesting that NET formation is enhanced in vivo in PAPA neutrophils. (Fig. 2C).
Fig. 2. PAPA neutrophils and LDGs undergo enhanced NET formation.
(A) Representative images of unstimulated HC NDN, SLE NDN, SLE LDGs, PAPA NDN, and PAPA LDGs isolated from peripheral blood after 1 h (LDG) or 2 h (NDN) of incubation at 37°C. NETs were identified by colocalization of extracellular MPO (red) and DNA labeled with Hoechst33342 (blue). Original magnification x40. (B) Quantification of the percentage of cells undergoing NET formation are plotted as mean ± SEM (n=6 for HC and PAPA, n=5 for SLE, **P ≤ 0.01). (C) Circulating NET remnants in HC and PAPA subjects quantified (n=10 subjects/group) plotted as mean ± SEM (*P ≤ 0.05). The OD index was calculated by dividing the individual OD450 values by the average of HC OD450 values.
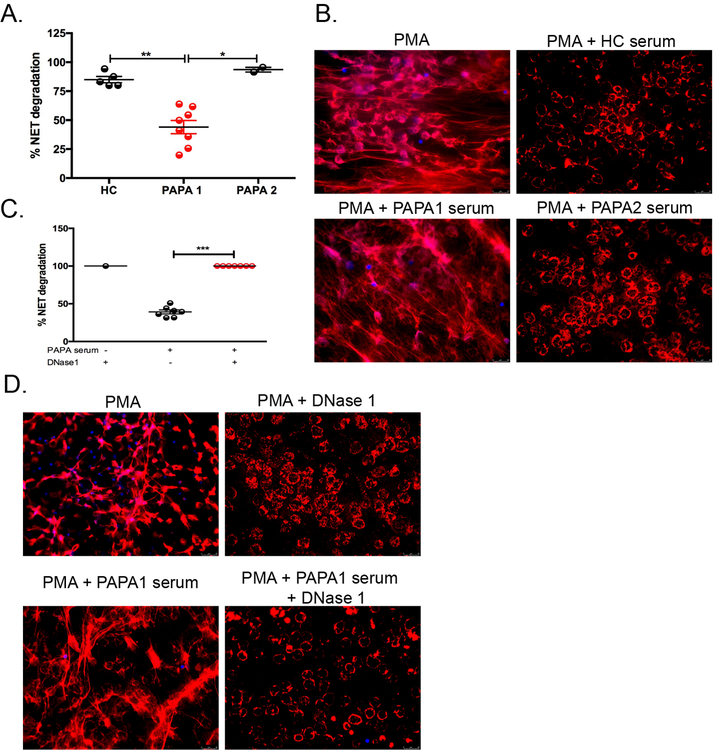
Previous studies have reported that sera from a subset of lupus patients degrades NETs inefficiently due to disruptions in DNase1 activity [30]. We tested the sera of 10 PAPA subjects for its ability to degrade NETs. We determined that 80% of the PAPA subjects’ sera (n=8, called “PAPA1”) was impaired in the ability to degrade NETs when compared to healthy control sera and a minor subset of PAPA sera (20%, n=2, called “PAPA2”) that efficiently degraded NETs (Fig. 3A and B). To determine if this deficiency was due to insufficient serum DNase I activity, we added recombinant DNase I to the “PAPA1” sera and this restored the ability to degrade NETs efficiently (Fig. 3C and D). Taken together, both normal-density neutrophils and LDGs in PAPA patients display enhanced NET formation in vitro and in vivo. Elevated levels of circulating NET products may be due to an imbalance in NET formation and degradation caused by insufficient DNase I activity in the sera of PAPA subjects.
Fig. 3. Impaired NET degradation in a subset of PAPA subjects.
(A and B) HC NDN were stimulated with PMA (2.5 μM) for 4 h to induce NETs and subsequently incubated with 1% serum from either HC (n=5) or PAPA subjects (n=10) for 16 h. “PAPA1” represents PAPA subjects who did not degrade NETs efficiently and “PAPA2” represents PAPA subjects that were efficient degraders of NETs. (C and D) HC NDN were stimulated with PMA (2.5 μM) for 4 h to induce NETs, followed by incubation with or without 1% serum from PAPA1 subjects (n=7) in the presence or absence of 1U/mL DNase1for 16 h. (A) Represents the mean ± SEM from three independent experiments (*P ≤ 0.05; **P ≤ 0.01; ***P < 0.001).
Serum from PAPA subjects induces NET formation in an IL-1-dependent manner.
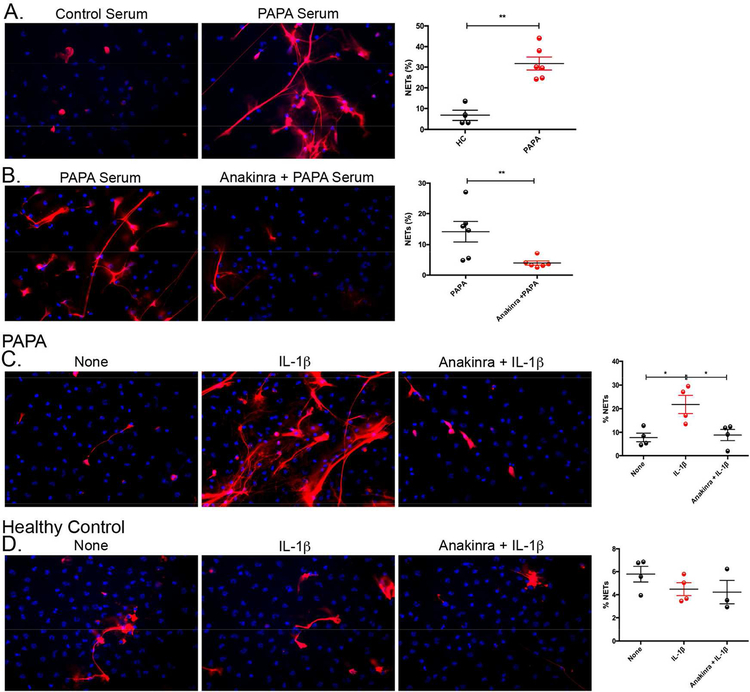
Healthy control neutrophils were incubated with healthy control or PAPA serum and NET formation was measured. PAPA, but not healthy control, sera induced significant NET formation in healthy control neutrophils (Fig. 4A). Moreover, this process was dependent on IL-1 signaling as healthy control neutrophils pretreated with the IL-1R antagonist anakinra, prior to addition of PAPA serum, had a significant decrease in NET formation compared to untreated neutrophils (Fig. 4B). These results suggest that increases in serum IL-1β levels in PAPA contribute to enhanced NET formation.
Fig. 4. PAPA serum induces NET formation in healthy control neutrophils in an IL-1-dependent manner.
(A) HC NDN were stimulated with 10% serum from either HC (n=4) or PAPA subjects (n=6) for 2.5 h and NET formation was quantified and reported as mean ± SEM (**P ≤ 0.01). (B) HC NDN were pretreated with/without Anakinra (149 μg/mL) for 15 min prior to stimulation with 10% PAPA serum (n=6) for 2.5 h and NET formation was quantified and plotted as mean ± SEM (**P ≤ 0.01). (C and D) PAPA or HC NDN were left unstimulated or stimulated with recombinant human IL-1β (100 ng/mL) for 2.5 h in the absence/presence of anakinra (149 μg/mL) pretreatment, respectively. C and D are the mean ± SEM from four different samples for each group (*P ≤ 0.05).
Since IL-1 blockade abrogates PAPA serum-induced NETosis, we examined if recombinant human IL-1β would induce NETs in PAPA neutrophils. Recombinant IL-1β induced significant NET formation in PAPA neutrophils and this induction was reduced to basal levels with anakinra pretreatment (Fig. 4C). In contrast, healthy control neutrophils and lupus neutrophils did not undergo enhanced NET formation in response to recombinant IL-1β stimulation (Fig. 4D and Fig. S2C). Previous studies have suggested that priming neutrophils with recombinant TNF-α can enhance their responsiveness to uric acid in gouty arthritis [31]. We primed healthy control and lupus neutrophils with recombinant TNF-α and stimulated with recombinant IL-1β to recapitulate the enhanced NETosis in PAPA neutrophils after IL-1β stimulation. We did not observe a significant increase in NETosis in healthy control and lupus neutrphils after TNF-α priming and IL-1β stimulation when compared to unstimulated controls (Fig. S2). To determine if the reason for increased responsiveness to IL-1 in PAPA neutrophils was due to elevated surface expression of IL-1R, we quantified this receptor by FACS in PAPA and healthy control neutrophils and found no significant difference (Fig. S3). Collectively, these results suggest that IL-1β is one of the circulating NET-inducing molecules found in PAPA patients and that PAPA neutrophils may be specifically primed to respond to IL-1β stimulation.
Proinflammatory cytokines are elevated in PAPA serum.
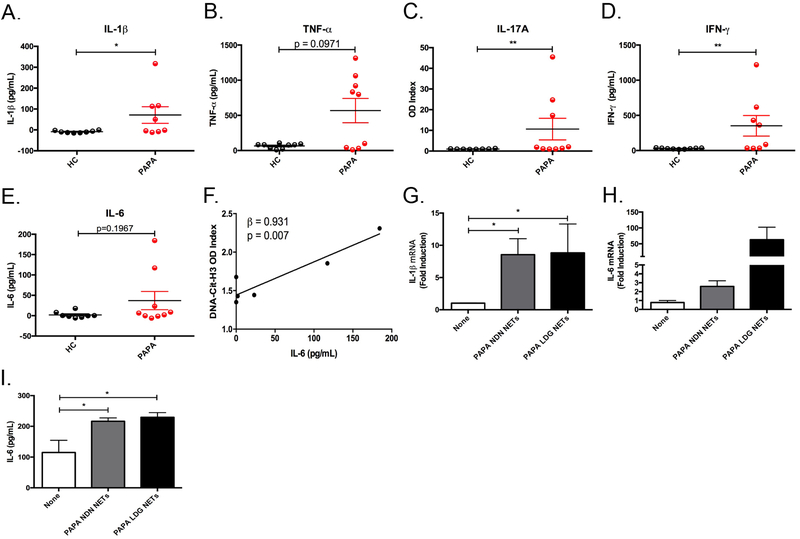
It has previously been shown that proinflammatory cytokines including IL-1β, TNF-α, IL-17A, and IFN-γ can induce/prime NET formation in neutrophils and that PAPA patients have elevated levels of IL-1β and TNF-α in circulation [8], [9], [10], [24], [32]. We next investigated if these cytokines were present in the serum of PAPA subjects to help explain the observed enhanced NET formation. PAPA subjects had significant elevations in circulating IL-1β, TNF-α, IL-6, IL-17A, and IFN-γ while most of these cytokines were largely undetected in healthy controls (Fig. 5). Even the PAPA subjects who were in clinical remission had detectable levels of circulating IL-1β and TNF-α (Fig. 5A–B). IL-17A, a cytokine not previously associated with PAPA but found in skin of patients with psoriasis [33], was elevated in three PAPA subjects with current or past history of pyoderma gangrenosum (PG) lesions (Fig. 5C and Supplementary Table 1). Linear regression analysis revealed that serum IL-6 levels correlated with circulating NET complexes (β = 0.931, P = 0.007), (Fig. 5F). Furthermore, NETs generated by PAPA neutrophils and LDGs significantly induced IL-1β and IL-6 mRNA gene expression and IL-6 protein release in healthy control monocyte-derived macrophages (Fig. 5G–I). In summary, PAPA is associated with elevated levels of proinflammatory cytokines that may contribute to dysregulated NET formation and these NETs in turn may enhance the production of proinflammatory mediators by macrophages.
Fig. 5. Proinflammatory cytokines are elevated in PAPA serum.
(A-E) Serum concentrations of IL-1β, TNF-α, IFN-γ, and IL-6 (A, B, D, and E) and OD Index for IL-17A (C) in HC and PAPA subjects (n=9/group). Results represent mean ± SEM (*P ≤ 0.05; **P ≤ 0.01). (F) A linear regression analysis of the OD Index from citrullinated histone (H3)-DNA complexes in the plasma from PAPA subjects and IL-6 protein in PAPA sera (n=6). (G-I) HC macrophages were stimulated with spontaneously formed NETs from PAPA NDN or LDGs for 2 h (G and H) or 24 h (I). (G and H) Total RNA was isolated and qRT-PCR was performed to measure IL-1β and IL-6 gene expression; results represent mean ± SEM from four independent experiments (*P ≤ 0.05). (I) Culture supernatants were analyzed by ELISA for IL-6 protein and results represent the mean ± SEM from four independent experiments (*P ≤ 0.05).
NETs are present in PAPA skin lesion.
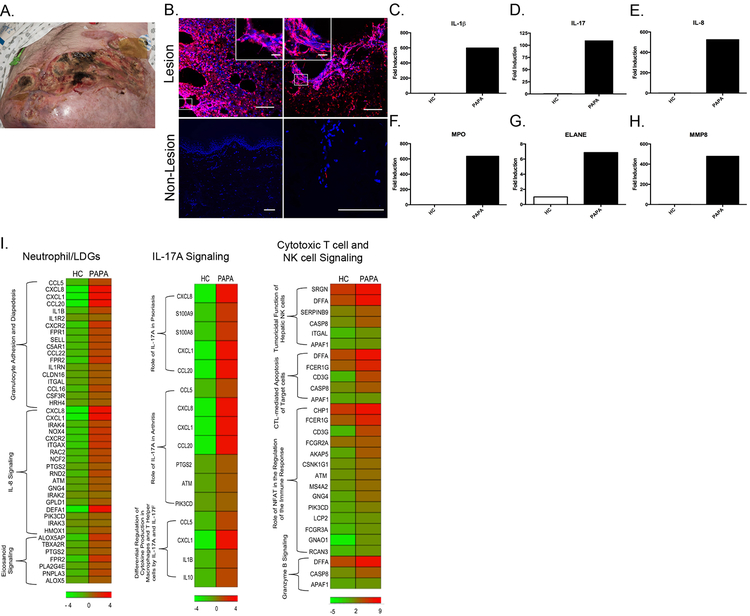
Infiltrating netting neutrophils were previously shown to be present in kidney, skin and placentas of lupus patients [14], [34] and have been described in tissues in other diseases [24], [29]. Immunohistochemistry was performed on the skin biopsy from a PAPA patient with active PG despite combination immunosuppressive therapy and in skin tissue from another PAPA patient with no active skin manifestations. A meshwork of extracellular traps (ETs) identified by positive extracellular citrullinated histone H4 (cit-H4) staining was present in the skin lesion but absent in the non-lesional tissue sample (Fig. 6A). We confirmed that the ETs in the skin lesion were derived from neutrophils because of colocalization of extracellular cit-H4 with neutrophil elastase (Fig. S4). RNA was purified from PAPA skin lesion as well as from healthy control skin. Transcripts of neutrophil granule genes previously found to be upregulated in lupus and vasculitis LDGs were quantified by real-time PCR [16], [19]. Neutrophil granule genes were highly expressed at the mRNA level in PAPA skin lesion and were significantly lower in skin tissue from a healthy control (Fig. 6F–H). This was confirmed at the protein level by quantifying skin MPO levels (Fig. S5). Expression of IL-1β mRNA and protein and IL-8 and IL-17A mRNA, but not TNF, IL-6 or IL-18, were significantly increased in the PAPA skin lesion (Fig. 6C–E, S5, and data not shown), when compared to control skin. RNAseq was performed on PAPA and healthy control skin samples to determine the transcriptional environment in active skin disease, (PG). IPA analysis revealed that upregulated pathways included IL-17A signaling, cytotoxic T cell, and NK cell signaling pathways (Fig. 6I, Supplementary Table 7). Overall, these results suggest that enhanced NET formation by various neutrophil subsets may contribute to tissue injury in PAPA and that mutations in PSTPIP1 may cause dysregulation of cytotoxic T cell and NK cell function that may further contribute to tissue destruction.
Fig. 6. NETs are present in PAPA skin lesion.
(A) Pyoderma gangrenosum lesion that developed in a PAPA patient with the A230T mutation. The lesion developed while receiving anakinra, infliximab and prednisone at doses up to 60 mg/daily. A skin biopsy taken from this lesion was obtained and used to perform experiments depicted in B-I. (B) Immunohistochemistry was performed on skin lesions from the PAPA patient described in A and in non-lesional skin sample from another PAPA subject. NETs are identified by colocalization of citrullinated histone H4 (cit-H4) (red) and DNA labeled with Hoechst33342 (blue). Main figure scale bar 100 μM, inset scale bar 20 μM. (C-H) Total RNA was extracted from HC skin biopsy and from lesional skin from the PAPA patient described in A. Proinflammatory cytokine and neutrophil granule gene expression were measured by qRT-PCR, normalized to the expression of GAPDH, and fold induction was calculated to HC skin. (I) RNASeq analysis of HC skin (n=1) and PAPA lesional skin (n=1) identified upregulated expression of neutrophil/LDG, IL-17A signaling, and cytotoxic T cell and NK cell signaling genes.
Discussion
Despite considerable advances into the molecular mechanism of inflammation in PAPA syndrome [8], the role that neutrophils subsets and NETs may play in the pathogenesis of this condition has yet to be explored. In recent years, some reports have revealed that neutrophils are dysregulated in PAPA syndrome [1], [9]. One particular study identified a granulocyte gene expression signature in PBMCs from PAPA patients, suggesting the presence of an abnormal subset of neutrophils called LDGs that were previously described in SLE [1], [14], [16], [17], [18]. We sought to determine if LDGs were indeed present in the blood from PAPA patients and if so, characterize their phenotype using LDGs from SLE as a benchmark. The results of these studies suggest that circulating LDGs are elevated in PAPA and at comparable levels relative to LDGs in SLE. We found that LDGs significantly associate with disease activity in PAPA, similar to what has been described in ANCA-associated vasculitis [19]. Previous studies have reported that LDGs express higher levels of transcripts for serine proteases and bactericidal proteins found in azurophilic granules, secondary granules, and tertiary granules of maturing neutrophils [14], [17]. Similar to SLE, this was confirmed in PAPA LDGs in our study [16], [35]. The expression of these genes is greatest during the myeloblast/promyelocytic stage of neutrophil differentiation suggesting that PAPA LDGs represent an immature neutrophil subset [17], [36]. It is unclear why neutrophil precursors would be prematurely released from the bone marrow in PAPA but previous studies have reported that proinflammatory cytokines/chemokines produced during an inflammatory episode or infection can trigger the proliferation and release of immature granulocytes [37]. Various chemokines including IL-8, macrophage inflammatory protein (MIP)-2, leukotriene B4, and complement component C5a have been shown to regulate neutrophil mobilization during inflammation [38], [39], [40]. Granulocyte-macrophage colony-stimulating factor (GMCSF) is one of the main cytokines that regulates the production and egress of neutrophils from the bone marrow [41]. Indeed, while circulating levels of GM-CSF have yet to be reported for PAPA syndrome, SLE PBMCs produce increased GM-CSF and IL-1 signaling can promote the production of this cytokine [42], [43]. Future studies should address the role of GM-CSF in neutrophil dysregulation in PAPA.
It was previously described that PAPA patients have elevated circulating levels of neutrophil granule enzymes suggesting a global neutrophil activation [9]. The marked release of neutrophil granule enzymes into the periphery could be a result of enhanced degranulation or dysregulated NET formation [14], [17], [21]. Indeed, spontaneous NET formation was enhanced in PAPA LDGs, similar to what has been observed in SLE. These results were confirmed by identifying circulating NET remnants in PAPA and that the majority of these subjects exhibited not only enhanced NET formation but also decreased ability of their serum to degrade NETs. Unlike SLE, where some serum samples could not rescue the ability to degrade NETs with the addition of recombinant DNase1 (suggesting the presence of DNase1-specific inhibitors) [30], all of the poor-degrading PAPA serum samples restored their ability to disassemble NETs with the addition of recombinant DNase1. These results suggest that DNase1 activity in mutant PAPA cells is not sufficient to degrade an overproduction of NETs and that DNase1-specific inhibitors do not play a major role in aberrant NET clearance in PAPA. Future studies should focus in understanding how DNase1 activity or levels may be perturbed in PAPA syndrome.
The enhanced NET formation in PAPA is likely driven by inflammatory stimuli characteristic of this disease, as serum from these patients stimulated NET formation in healthy control neutrophils in an IL-1 dependent manner. As control neutrophils formed NETs after stimulation with PAPA serum but not recombinant IL-1β, it is possible that PAPA neutrophils are primed due to chronic inflammation and other proinflammatory cytokines. It has previously been reported that priming of healthy neutrophils is necessary to induce responses to various stimuli, including TLR agonists and cytokines [44]. To support this hypothesis, the serum of PAPA subjects showed elevated levels of several proinflammatory cytokines that may prime neutrophils and make them more prone to NET formation. Furthermore, NETs derived from PAPA neutrophils and LDGs induced the release of IL-6 by macrophages, suggesting a vicious cycle where NETs are induced by proinflammatory cytokines and these NETs in turn enhance production of proinflammatory mediators. In a subset of PAPA subjects, markedly elevated levels of circulating IL-17 were detected. IL-17 has been associated with conditions characterized by skin inflammation including psoriasis and atopic dermatitis [45]. Of note, all the PAPA subjects with increased circulating IL-17A had history of cystic acne or PG and we found evidence of marked upregulation of IL-17 transcripts in PAPA skin. These observations should be systematically studied in future studies to better understand the source of cells contributing to higher IL-17 levels in PAPA, as well as the pathogenic role that this cytokine has in the disease and whether it could represent an important therapeutic target.
One of the most difficult aspects of treating PAPA syndrome is PG refractory to many immunosuppressive and immunomodulatory therapies [12], [46]. In proof of concept studies, we found evidence of significant NET formation in PG skin in PAPA but not in unaffected skin. This suggests that NETs may contribute to tissue destruction in PAPA syndrome. LDGs have been previously shown to cause vascular damage in lupus and netting neutrophils were reported in skin and kidneys from patients with dermatological and renal manifestations [14], [16]. Although the study by Villanueva et al was unable to determine whether these cells were neutrophils or LDGs, we made this differentiation in our PAPA skin lesion [14]. Neutrophils and LDGs share many similarities including surface markers and the ability to form NETs [16], [17] but they can be distinguished based on their gene expression of granule proteins [14], [17], [19]. Indeed, PAPA skin lesion expressed elevated mRNA levels of genes encoding for granule proteins previously reported for SLE LDGs. Given higher IL-1β, IΛ−17 and IL-8 expression in PAPA skin, it is possible that local production of these cytokines in the skin microenvironment promotes neutrophil recruitment, in situ skin NET formation and tissue damage. Indeed, RNASeq on PAPA skin tissue uncovered numerous pathways related to neutrophil processes including granulocyte adhesion, diapedesis and IL-17 and IL-8 signaling, further supporting the role of neutrophils in skin pathology in PAPA. Of note, we observed that eicosanoid signaling was upregulated in PAPA skin and Denny et al previously reported that SLE LDGs secrete enhanced levels of eicosanoids [16]. The RNASeq analysis also revealed that CTL, NK and granzyme B signaling were upregulated in PAPA skin. The role of these cytotoxic pathways in PAPA skin involvement remains to be better characterized in future studies.
Aberrant neutrophil phenotypes have previously been described in other autoinflammatory conditions [47] and we now report that neutrophil subsets are dysregulated in PAPA syndrome in association with enhanced NET formation in blood and tissues that may further exacerbate inflammatory processes by other myeloid cells and contribute to the dysregulated immune microenvironment in this disease. Within the field of autoinflammatory diseases, where a number of conditions have had marked expansion in targeted treatment options, PAPA patients remain refractory to treatment in a number of cases. At times, this necessitates the use of dual biologic treatment such as anti-IL1/anti-TNF agents to try and decrease the inflammatory cascade. This study suggests that other pathways may potentially represent novel therapeutic targets. In addition to IL-17, future studies should address the role of specifically targeting NET formation as a therapeutic option in these group of conditions, as recently shown for autoimmune diseases [48]. A better understanding of neutrophil biology in syndromes with aberrant innate immune responses should provide better insight into the pathogenesis of the disease and further therapeutic development.
Supplementary Material
Acknowledgments:
Supported by the Intramural Research Program at NIAMS/NIH (ZIAAR041199 and the Office of Science and Technology NIAMS). This study utilized the high-performance computational capabilities of the Helix Systems at the NIH (http://helix.nih.gov).
Footnotes
Materials and Methods are included in Supplementary material
REFERENCES
- 1.Smith EJ, Allantaz F, Bennett L, Zhang D, Gao X, Wood G, et al. Clinical, Molecular, and Genetic Characteristics of PAPA Syndrome: A Review. Curr Genomics. 2010. November; 11(7):519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masters SL, Simon A, Aksentijevich I, Kastner DL. Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease (*). Annu Rev Immunol. 2009; 27:621–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wise CA, Gillum JD, Seidman CE, Lindor NM, Veile R, Bashiardes S, et al. Mutations in CD2BP1 disrupt binding to PTP PEST and are responsible for PAPA syndrome, an autoinflammatory disorder. Hum Mol Genet. 2002. April 15; 11(8):961–969. [DOI] [PubMed] [Google Scholar]
- 4.Spencer S, Dowbenko D, Cheng J, Li W, Brush J, Utzig S, et al. PSTPIP: a tyrosine phosphorylated cleavage furrow-associated protein that is a substrate for a PEST tyrosine phosphatase. J Cell Biol. 1997. August 25; 138(4):845–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badour K, Zhang J, Shi F, McGavin MK, Rampersad V, Hardy LA, et al. The Wiskott-Aldrich syndrome protein acts downstream of CD2 and the CD2AP and PSTPIP1 adaptors to promote formation of the immunological synapse. Immunity. 2003. January; 18(1):141–154. [DOI] [PubMed] [Google Scholar]
- 6.Cote JF, Chung PL, Theberge JF, Halle M, Spencer S, Lasky LA, et al. PSTPIP is a substrate of PTP-PEST and serves as a scaffold guiding PTP-PEST toward a specific dephosphorylation of WASP. J Biol Chem. 2002. January 25; 277(4):2973–2986. [DOI] [PubMed] [Google Scholar]
- 7.Cong F, Spencer S, Cote JF, Wu Y, Tremblay ML, Lasky LA, et al. Cytoskeletal protein PSTPIP1 directs the PEST-type protein tyrosine phosphatase to the c-Abl kinase to mediate Abl dephosphorylation. Mol Cell. 2000. December; 6(6):1413–1423. [DOI] [PubMed] [Google Scholar]
- 8.Shoham NG, Centola M, Mansfield E, Hull KM, Wood G, Wise CA, et al. Pyrin binds the PSTPIP1/CD2BP1 protein, defining familial Mediterranean fever and PAPA syndrome as disorders in the same pathway. Proc Natl Acad Sci U S A. 2003. November 11; 100(23):13501–13506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demidowich AP, Freeman AF, Kuhns DB, Aksentijevich I, Gallin JI, Turner ML, et al. Brief report: genotype, phenotype, and clinical course in five patients with PAPA syndrome (pyogenic sterile arthritis, pyoderma gangrenosum, and acne). Arthritis Rheum. 2012. June; 64(6):2022–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cortis E, De Benedetti F, Insalaco A, Cioschi S, Muratori F, D’Urbano LE, et al. Abnormal production of tumor necrosis factor (TNF) -- alpha and clinical efficacy of the TNF inhibitor etanercept in a patient with PAPA syndrome [corrected]. J Pediatr. 2004. December; 145(6):851–855. [DOI] [PubMed] [Google Scholar]
- 11.Dierselhuis MP, Frenkel J, Wulffraat NM, Boelens JJ. Anakinra for flares of pyogenic arthritis in PAPA syndrome. Rheumatology (Oxford). 2005. Mar; 44(3):406–408. [DOI] [PubMed] [Google Scholar]
- 12.Brenner M, Ruzicka T, Plewig G, Thomas P, Herzer P. Targeted treatment of pyoderma gangrenosum in PAPA (pyogenic arthritis, pyoderma gangrenosum and acne) syndrome with the recombinant human interleukin-1 receptor antagonist anakinra. Br J Dermatol. 2009. November; 161(5):1199–1201. [DOI] [PubMed] [Google Scholar]
- 13.Callen JP. Neutrophilic dermatoses. Dermatol Clin. 2002. July; 20(3):409–419. [DOI] [PubMed] [Google Scholar]
- 14.Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. 2011. July 1; 187(1):538–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hacbarth E, Kajdacsy-Balla A. Low density neutrophils in patients with systemic lupus erythematosus, rheumatoid arthritis, and acute rheumatic fever. Arthritis Rheum. 1986. November; 29(11):1334–1342. [DOI] [PubMed] [Google Scholar]
- 16.Denny MF, Yalavarthi S, Zhao W, Thacker SG, Anderson M, Sandy AR, et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I Interferons. Journal of immunology (Baltimore, Md : 1950). 2010 02/17; 184(6):3284–3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carmona-Rivera C, Kaplan MJ. Low-density granulocytes: a distinct class of neutrophils in systemic autoimmunity. Semin Immunopathol. 2013. July; 35(4):455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lood C, Blanco LP, Purmalek MM, Carmona-Rivera C, De Ravin SS, Smith CK, et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med. 2016. February; 22(2):146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grayson PC, Carmona-Rivera C, Xu L, Lim N, Gao Z, Asare AL, et al. Neutrophil-Related Gene Expression and Low-Density Granulocytes Associated With Disease Activity and Response to Treatment in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Arthritis Rheumatol. 2015. July; 67(7):1922–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin AM, Rubin CJ, Khandpur R, Wang JY, Riblett M, Yalavarthi S, et al. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J Immunol. 2011. July 1; 187(1):490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004. March 5; 303(5663):1532–1535. [DOI] [PubMed] [Google Scholar]
- 22.Kenny EF, Herzig A, Kruger R, Muth A, Mondal S, Thompson PR, et al. Diverse stimuli engage different neutrophil extracellular trap pathways. Elife. 2017. June 2; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta AK, Hasler P, Holzgreve W, Gebhardt S, Hahn S. Induction of neutrophil extracellular DNA lattices by placental microparticles and IL-8 and their presence in preeclampsia. Hum Immunol. 2005. November; 66(11):1146–1154. [DOI] [PubMed] [Google Scholar]
- 24.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. 2013. March 27; 5(178):178ra140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith CK, Kaplan MJ. The role of neutrophils in the pathogenesis of systemic lupus erythematosus. Curr Opin Rheumatol. 2015. September; 27(5):448–453. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. 2009. January 26; 184(2):205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011. March 9; 3(73):73ra19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marini O, Costa S, Bevilacqua D, Calzetti F, Tamassia N, Spina C, et al. Mature CD10(+) and immature CD10(−) neutrophils present in G-CSF-treated donors display opposite effects on T cells. Blood. 2017. March 9; 129(10):1343–1356. [DOI] [PubMed] [Google Scholar]
- 29.Kessenbrock K, Krumbholz M, Schonermarck U, Back W, Gross WL, Werb Z, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009. June; 15(6):623–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hakkim A, Furnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A. 2010. May 25; 107(21):9813–9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yokose K, Sato S, Asano T, Yashiro M, Kobayashi H, Watanabe H, et al. TNF-alpha potentiates uric acid-induced interleukin-1beta (IL-1beta) secretion in human neutrophils. Mod Rheumatol. 2018. May; 28(3):513–517. [DOI] [PubMed] [Google Scholar]
- 32.Mitroulis I, Kambas K, Chrysanthopoulou A, Skendros P, Apostolidou E, Kourtzelis I, et al. Neutrophil extracellular trap formation is associated with IL-1beta and autophagy-related signaling in gout. PLoS One. 2011; 6(12):e29318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frieder J, Kivelevitch D, Menter A. Secukinumab: a review of the anti-IL-17A biologic for the treatment of psoriasis. Ther Adv Chronic Dis. 2018. January; 9(1):5–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marder W, Knight JS, Kaplan MJ, Somers EC, Zhang X, O’Dell AA, et al. Placental histology and neutrophil extracellular traps in lupus and pre-eclampsia pregnancies. Lupus Sci Med. 2016; 3(1):e000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003. March 17; 197(6):711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cowland JB, Borregaard N. The individual regulation of granule protein mRNA levels during neutrophil maturation explains the heterogeneity of neutrophil granules. J Leukoc Biol. 1999. December; 66(6):989–995. [DOI] [PubMed] [Google Scholar]
- 37.Cain DW, Snowden PB, Sempowski GD, Kelsoe G. Inflammation triggers emergency granulopoiesis through a density-dependent feedback mechanism. PLoS One. 2011; 6(5):e19957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Terashima T, English D, Hogg JC, van Eeden SF. Release of polymorphonuclear leukocytes from the bone marrow by interleukin-8. Blood. 1998. August 1; 92(3):1062–1069. [PubMed] [Google Scholar]
- 39.Jagels MA, Chambers JD, Arfors KE, Hugli TE. C5a- and tumor necrosis factor-alpha-induced leukocytosis occurs independently of beta 2 integrins and L-selectin: differential effects on neutrophil adhesion molecule expression in vivo. Blood. 1995. May 15; 85(10):2900–2909. [PubMed] [Google Scholar]
- 40.Burdon PC, Martin C, Rankin SM. The CXC chemokine MIP-2 stimulates neutrophil mobilization from the rat bone marrow in a CD49d-dependent manner. Blood. 2005. March 15; 105(6):2543–2548. [DOI] [PubMed] [Google Scholar]
- 41.Pollmacher T, Korth C, Schreiber W, Hermann D, Mullington J. Effects of repeated administration of granulocyte colony-stimulating factor (G-CSF) on neutrophil counts, plasma cytokine, and cytokine receptor levels. Cytokine. 1996. October; 8(10):799–803. [DOI] [PubMed] [Google Scholar]
- 42.Willeke P, Schluter B, Schotte H, Erren M, Mickholz E, Domschke W, et al. Increased frequency of GM-CSF secreting PBMC in patients with active systemic lupus erythematosus can be reduced by immunoadsorption. Lupus. 2004; 13(4):257–262. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki A, Takahashi T, Okuno Y, Tsuyuoka R, Fukumoto M, Nakamura K, et al. IL-1 production as a regulator of G-CSF and IL-6 production in CSF-producing cell lines. Br J Cancer. 1992. April; 65(4):515–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood. 2003. October 1; 102(7):2660–2669. [DOI] [PubMed] [Google Scholar]
- 45.Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev. 2008. June; 223:87–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stichweh DS, Punaro M, Pascual V. Dramatic improvement of pyoderma gangrenosum with infliximab in a patient with PAPA syndrome. Pediatr Dermatol. 2005. May-Jun; 22(3):262–265. [DOI] [PubMed] [Google Scholar]
- 47.Manukyan G, Petrek M, Kriegova E, Ghazaryan K, Fillerova R, Boyajyan A. Activated phenotype of circulating neutrophils in familial Mediterranean fever. Immunobiology. 2013. June; 218(6):892–898. [DOI] [PubMed] [Google Scholar]
- 48.Knight JS, Subramanian V, O’Dell AA, Yalavarthi S, Zhao W, Smith CK, et al. Peptidylarginine deiminase inhibition disrupts NET formation and protects against kidney, skin and vascular disease in lupus-prone MRL/lpr mice. Ann Rheum Dis. 2015. December; 74(12):2199–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.