Abstract
Use of microfluidic devices to compartmentalize cultured neurons has become a standard method in neuroscience. This protocol shows how to use a pre-assembled multi-compartment chip made in a cyclic olefin copolymer (COC) to compartmentalize neurons differentiated from human stem cells. The footprint of these COC chips are the same as a standard microscope slide and are equally compatible with high resolution microscopy. Neurons are differentiated from human neural stem cells (NSCs) into glutamatergic neurons within the chip and maintained for 5 weeks, allowing sufficient time for these neurons to develop synapses and dendritic spines. Further, we demonstrate multiple common experimental procedures using these multi-compartment chips, including viral labeling, establishing microenvironments, axotomy, and immunocytochemistry.
Keywords: Neuroscience, Issue 147, Microfluidic chamber, cyclic olefin copolymer, neuron device, microfluidic chip, cultured neurons, multi-compartment chip, human stem cell derived neurons, neural stem cells, H9 stem cell line, chip, axon isolation
Introduction
Human stem cell differentiated neurons (hSC-neurons) are increasingly used for biological research. These neurons, which can be derived from human source material, are of great interest for translational research, including the study of traumatic brain injuries and neurodegenerative disorders such as Alzheimer’s disease. Thus, tools to improve and facilitate the study of hSC-neurons are in demand.
To study the unique polarized morphology of neurons, many researchers use multi-compartmentalized microfluidic devices1,2,3,4,5,6,7,8,9,10,11. These devices enable measurements and manipulations of long projection neurons with unique subcellular access. Multi-compartmentalized microfluidic devices consist of two parallel microfluidic compartments separated by microgrooves, which guide axonal growth. Neurons or neural stem cells (NSCs) are plated in the somatodendritic compartment, and then adhere to the bottom of the compartment surface after minutes. Differentiated neurons grow and extend their axons/projections through the microgroove region into an adjacent and isolated axonal compartment. In the past, these devices were exclusively made using poly (dimethylsiloxane) (PDMS) replica molding. PDMS devices have many drawbacks previously described12, including persistent hydrophobicity and the need to assemble to a glass coverslip immediately prior to use. Pre-assembled injection molded chips overcome many of these drawbacks and are sold commercially (see Table of Materials)12. The compartments of these chips are permanently made hydrophilic and the entire chip is injection molded in optically transparent cyclic olefin copolymer (COC).
This protocol demonstrates how to use this COC chip to differentiate human NSCs into excitatory neurons, and to separate and fluidically isolate their long neuronal projections. For this demonstration, neurons were differentiated from NIH-approved H9 stem cells. Similar procedures can be used to differentiate human induced pluripotent stem cells.
Protocol
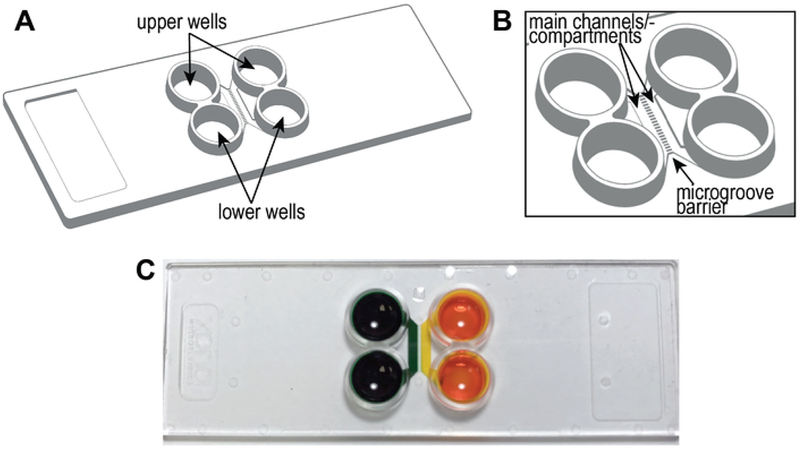
NOTE: Figure 1A,B shows a schematic of the pre-assembled COC chip, including the locations of the main channels or compartments, wells, and microgrooves. The compartmentalized chip can establish distinct fluidic microenvironments within a compartment as demonstrate by the isolation of food coloring dye (Figure 1C). The protocol for preparing the pre-assembled multi-compartment chip is given in Nagendran et al., Section 112.
Figure 1: The pre-assembled COC, multicompartment microfluidic chip.
(A) A drawing of the chip identifying the upper and lower wells. The size of the chip is 75 mm × 25 mm, the size of standard microscope slides. (B) A zoomed in region showing the channels and microgrooves separating the channels. Additional details are provided in Nagendran et al.12. (C) This photograph illustrates the creation of isolated microenvironments within each compartment using food coloring dye. This entire figure has been reproduced from12.
1. Coating of multi-compartment chips for hSC-neurons
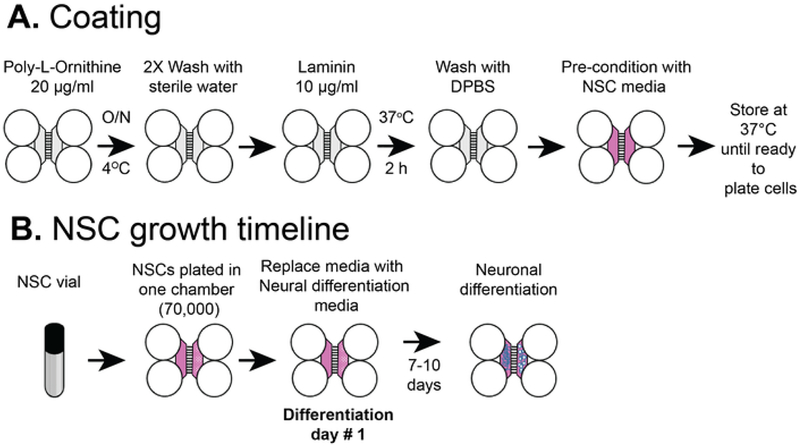
NOTE: Figure 2A shows an overview of the coating procedures.
Figure 2: Plastic compartmentalized chip coating and NSC cell plating timeline.
(A) Plastic multi compartment chips were coated with a pre-coating solution, and then poly-L-ornithine and laminin before pre-conditioning with NSC media. (B) NSCs were plated with 7 × 104 cells in the somatic compartment of the chip. The cells were grown in NSC media for 24 h and then the media was replaced with neural differentiation media. Differentiated neurons were observed by 7–10 days post differentiation.
Dissolve poly-L-ornithine in cell culture-grade distilled water to make 600 μL of solution per chip of a 20 μg/mL working solution.
-
Aspirate the remaining PBS left after preparing the chips from the wells. When aspirating, ensure that the pipet tip is away from the channel opening.
NOTE: The microfluidic channels must remain filled for the entirety of this procedure. Do not aspirate liquid from the channels/compartments, only the wells.
Load 150 μL of poly-L-ornithine working solution in the upper right well. Wait for 90 s or until liquid begins to fill the lower right well. Add 150 μL of media to the lower right well and wait for 5 min to let the liquid pass through the microgrooves.
-
Add 150 μL of media to the upper left well. Wait 90 s and then add 150 μL to the lower left well. Wrap the holder of the chip(s) with parafilm and place at 4 °C overnight.
NOTE: Alternatively, place the chip and holder at 37 °C for 1 h.
Aspirate the media from the wells, ensuring that the pipet tip is placed away from the channel opening. Add 150 μL of sterile water to the upper right well. Wait for 90 s and then add 150 μL to the lower right well. Wait for 5 min to let the liquid pass through the microgrooves.
Add 150 μL of sterile water to the upper left well, wait 90 s and then add another 150 μL to the lower left well.
Repeat steps 1.5–1.6.
Thaw laminin slowly at 2 °C to 8 °C and prepare a 10 μg/mL working solution.
Load 150 μL of the laminin working solution to the upper right well. Wait for 90 s or until liquid begins to fill the lower right well. Pipet 150 μL to the lower right well. Wait for 5 min to let the laminin go through the microgrooves.
Add 150 μL of laminin to the upper left well. Wait 90 s and then add 150 μL to the lower left well. Incubate the chip within its holder for 2 h at 37 °C.
Rinse the chip with PBS (without Ca2+ and Mg2+). Load 150 μL of PBS to the upper right well. Wait for 90 s or until liquid begins to fill the lower right well. Add 150 μL of PBS to the lower right well and wait for 5 min to let the PBS go through the microgrooves.
Pipet 150 μL of PBS to the upper left well. After 90 s pipet 150 μL to the lower left well.
Aspirate PBS from the chip and then rinse the chip with NSC media (see Table of Materials). Load 150 μL of media to the upper right well. After 90 s or when liquid passes through the right channel into the lower right well, add 150 μL to the lower right well. Wait for 5 min to let the media flow through the microgrooves.
-
Add 150 μL of media to the upper left well and another 150 μL to the lower left well. Incubate the chip in a 37 °C incubator with 5% CO2 to pre-condition the chip until ready to seed NSCs.
NOTE: Chips can be pre-conditioned overnight if needed.
2. Seeding NSCs into the multicompartment chip
NOTE: This protocol used commercially purchased NSCs, unlike our previous publication which began with human embryonic stems cells5. In this protocol neural stem cells are plated directly into the plastic chips where they differentiate into neurons. The cells can be allowed to proliferate as NSCs (without differentiation) for up to 2 passages and stored in vials in liquid N2 for further use. This protocol uses NSC vials stored in liquid N2 after passage 1 or 2. Figure 2B shows an overview of the coating procedures.
-
Thaw NSCs according to manufacturer’s instructions.
NOTE: This procedure applies to H9-derived NSCs. Other cell lines will require cell density optimization.
Count the cell concentration using a hemocytometer and adjust using NSC media to get a concentration of 7 × 106 cells per mL.
Aspirate media from each well of the chip. Avoid aspirating media from the main channels.
-
Pipet 5 μL of the cell solution to the upper right well, followed by 5 μL to the lower right well (70,000 cells total). Ensure that the cells are pipetted towards the main channel openings. Use a microscope to check that cells have entered the channel. Wait 5 min for cells to adhere to the bottom of the chip.
NOTE: Either or both compartments can be used to load cells.
Pipet ~150 μL of NSC media to the upper right well and then 150 μL to the lower right well. Repeat on the left side. Incubate at 5% CO2, 37 °C within a suitable humidified container.
After 48 hours, aspirate NSC media from the wells and replace with neural differentiation media by adding 150 μL to each top well and each bottom well.
-
Culture neurons within a 5% CO2, 37 °C incubator within a suitable humidified container.
NOTE: Media should be replaced every 2–3 days. Because of the small volumes used in the chip, evaporation even within a humidified incubator can substantially impact cell viability. Use a suitable secondary container to provide additional humidity for long-term culture of cells with the chip (see Table of Materials).
3. Viral fluorescent labeling of hSC-neurons within the chip
NOTE: Multiple virus can be used to label neurons within the chip. Instructions below describe the use of G-deleted Rabies-mCherry or –eGFP virus. Potentially infectious materials must be handled according to applicable rules and regulations, and may require additional training and institutional approval.
Warm ~400 μL of fresh neural differentiation media per chip to 37 °C.
-
Remove 50 μL of media from one well of the axon compartment into a centrifuge tube and then add 100,000 viral units of modified rabies virus to the tube.
NOTE: Properly dispose spent pipet tips and centrifuge tubes containing virus.
-
Remove the remaining neural differentiation media from the axonal compartment and place into a centrifuge tube. Store at 37 °C.
NOTE: Make sure that the channels remain filled with liquid.
-
Mix the 50 μL of virus solution with 150 μL of warmed media. Pipet 100 μL to one well of the axon compartment and 100 μL to the other axon compartment well. Incubate for 2 h within a 37 °C incubator.
NOTE: The difference in volumes between the axonal and somatic compartments helps ensure the virus stays in the axonal compartment during infection/incubation times.
-
Withdraw and dispose of virus-containing media.
CAUTION: Do not remove liquid within the enclosed microfluidic channels.
Gently pipet 75 μL of fresh media to one axon compartment well and let the media flow through the channel into other axon well.
Remove media from the axon compartment and dispose properly.
Repeat steps 3.6 and 3.7.
-
Fill the axon compartment with the saved media. Pipet an additional 50 μL fresh media, if needed, and then return the chip to the incubator. This additional fresh media is to count towards loss of media during liquid transfer and evaporation during the process.
NOTE: Visible fluorescent labeling using these modified rabies viruses occurs by 48 h and up to 8 days after which cell death of infected neurons generally occurs due to virus toxicity. Neuron imaging can be performed as with other standard culture platforms (e.g., glass bottom dishes), but special consideration is needed to maintain sufficient humidity to prevent evaporative losses.
4. Fluidic isolation, axotomy, and immunostaining within the chip
Follow fluidic isolation, axotomy, and immunostaining with the chip as described in Nagendran et al.12.
Representative Results
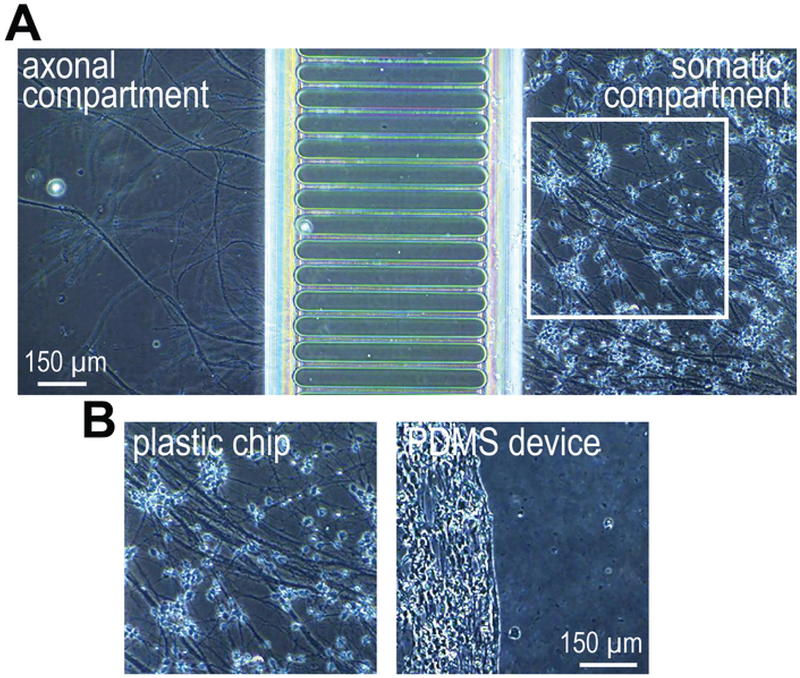
After one week (7–10 days) in the chip with differentiation media, NSCs differentiate into neurons and neuronal projections enter the axonal compartment (Figure 3). Within the chip, neurons attach and distribute evenly within the somatic compartment. In comparison, neurons in PDMS devices clump/aggregate as early as 5 days post addition of differentiation media, leading to compromised cell health as can be seen in the Figure 3B (day 13 magnified image). Neurons in the chips look healthy with bundled axons. Healthy neurons can be maintained within the chips for 4–5 weeks.
Figure 3: hSC-neuron growth comparison in chips and PDMS devices.
(A) A phase contrast image of hSC-neurons grown at 13 days after differentiation in the plastic multi compartment chip. (B) A zoomed in region of hSC-neurons cultured within the white box in (A) and an equivalent region within a PDMS device (right). hSC-neurons within the chips attach well. Aggregated neuron clusters form in PDMS-based devices. Representative of 2 independent experiments.
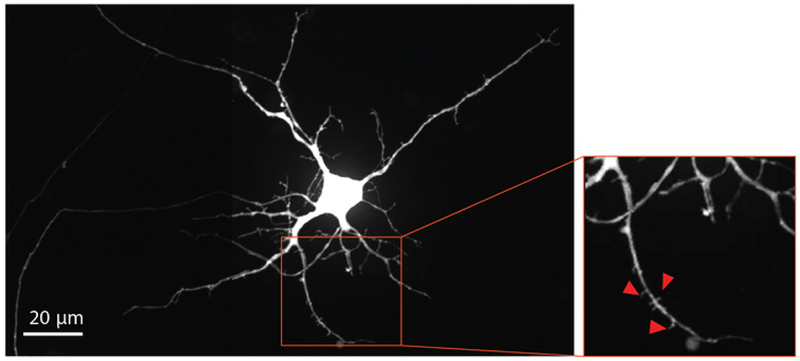
To visualize neuron maturation and dendritic spine development, modified rabies virus was delivered to the axonal compartment at Day 29 to retrograde label neurons, including dendritic spines, with mCherry fluorescent protein. Four days after rabies virus infection, neurons extending axons into the axonal compartment expressed mCherry. The neurons at differentiation day 33 showed formation of dendritic spines (Figure 4). Visualization of dendritic spines demonstrates that NSC derived neurons differentiated within the chips form mature synapses.
Figure 4: Human NSC derived neurons show dendritic spine morphology.
Retrograde labeled mCherry neuron at differentiation day 33 grown within the chip. The magnified region outlined in red, highlights the presence of dendritic spines, which provide evidence supporting the development of mature glutamatergic synapses. Red arrows point towards dendritic spines.
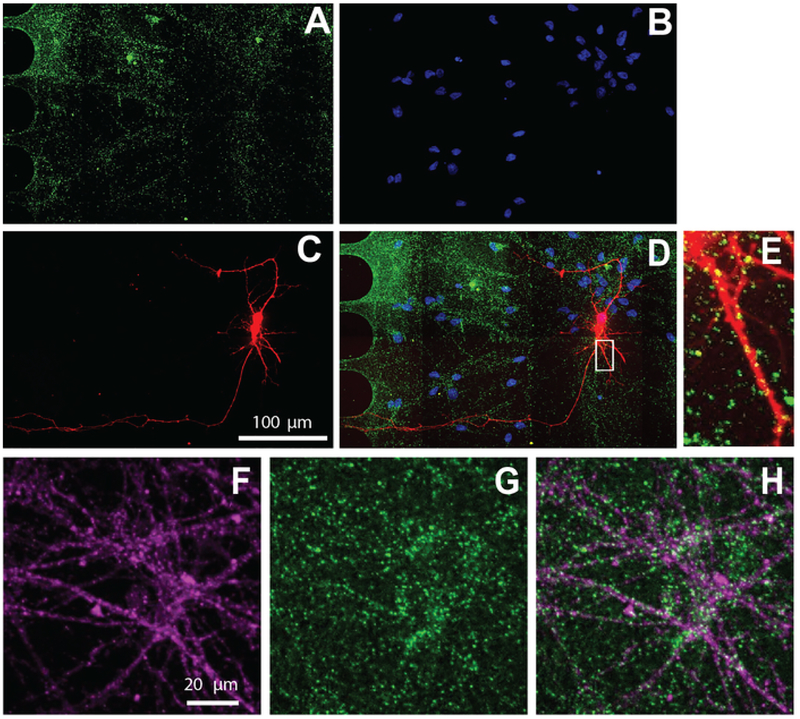
The multi-compartment chip is also compatible with immunocytochemistry to visualize the cellular localization of protein. After 26 days of maintaining neurons in differentiation media, neurons were labeled for the excitatory synaptic marker, vGlut1 (Figure 5). These results show that virally labeled neurons co-localize with vGlut1 (Figure 5E) and neuron specific marker, β-tubulin III (Figure 5F–H).
Figure 5: Human NSC derived neurons cultured within chips exhibit excitatory synapses.
Immunostaining was performed at differentiation day 26. Neuron imaging was performed in the somatic compartment. A) vGlut1 (green) and (B) DAPI (blue) immunolabeling. (C) mCherry-labeled neurons (red) retrograde labeled using a modified rabies virus. (D) A merged fluorescent micrograph of (A-C). (E) Dendritic spines and vGlut1 positive puncta colocalized with mCherry-positive dendrites, shown in a zoomed in region from (D). Immunofluorescence micrographs of (F) neuron specific marker, β-tubulin III (magenta), and (G) vGlut1 (green). (H) Overlay of β-tubulin III, and vGlut1.
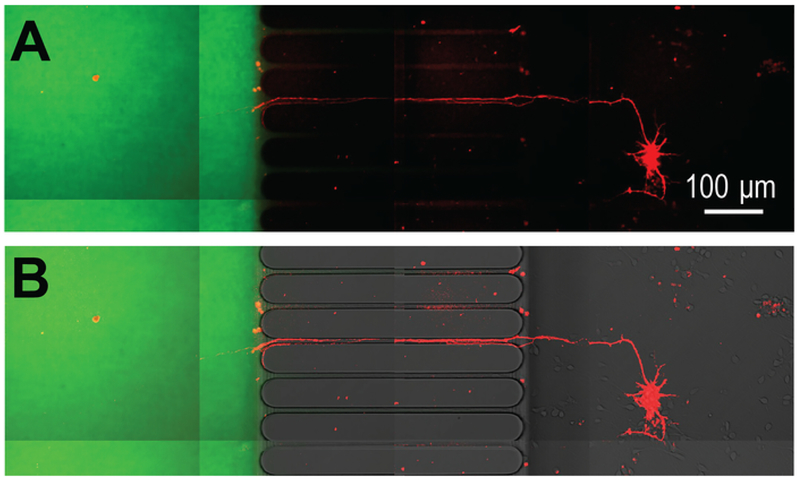
The ability to create distinct microenvironments isolated to axons was also demonstrated using Alexa Fluor 488 hydrazide, a low molecular weight fluorescent dye (Figure 6).
Figure 6: Virally transduced mCherry neurons extend projections into an axon localized microenvironment established within the preassembled COC chip.
(A) mCherry labeled neuron extend axons through the microgrooves of the chip and into an isolated axon compartment. Isolation of the axon compartment is visualized using Alexa Fluor 488 hydrazide. Imaging of neurons occurred at differentiation day 26 and 3 days after infection with the modified rabies virus. (B) The fluorescence image in (A) is merged with a DIC image. Note the location of the microgrooves.
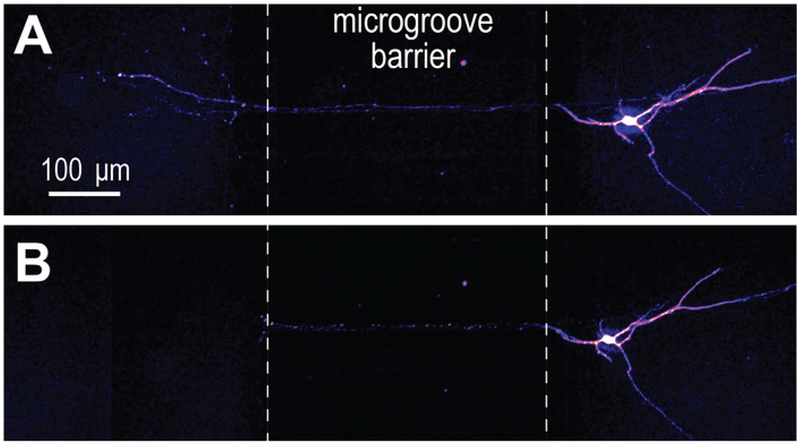
Axon injury studies are commonly performed within compartmentalized devices. A proof of principle experiment was performed to selectively injure axons of differentiated neurons with the pre-assembled COC chip (Figure 7). Results were equivalent to silicone compartmentalized devices6,13,14.
Figure 7: Axotomy performed within the COC multicompartment chip.
(A) mCherry labeled neurons at differentiation day 33 were imaged before axotomy. ‘Fire’ color look-up table. (B) Immediately after axotomy, showing that axons are completely severed.
Discussion
The preassembled multi-compartment COC chip is an easy-to-use compartmentalized platform to differentiate and maintain human NSCs into neurons for long-term (>4 weeks). In this protocol, we demonstrate the differentiation of human NSCs into glutamatergic neurons, retrograde label neurons, perform immunocytochemistry, visualize dendritic spine morphology and perform axotomy. These chips are compatible with high resolution imaging and there is no autofluorescence with the COC12.
COC multi-compartment chips are functionally equivalent to silicone-based compartmentalized devices, and have benefits and drawbacks as described previously12. Table 1 compares multi-compartment COC chips and silicone devices for culturing hSC-neurons. The COC compartmentalized chips provide a better hydrophilic surface for attachment and maintenance of stem cells over a long culture period. PDMS-based devices need to be assembled and attached to glass coverslips. The hydrophobic nature of the PDMS devices causes aggregation of stem cells5; this leads to both challenges in imaging at the cellular level and greater susceptibility to physical damage due the movement of cell aggregates during media changes. The plastic chip overcomes these challenges. COC is gas impermeable, unlike PDMS, so air pockets trapped or formed within the channels must be removed by the user. The pre-coating solution reduces the possibility for air to become trapped in the channels. This solution consists of ethanol and other agents. A previously published protocol for culturing murine neurons within these plastic chips provides additional details about pipetting cells and media within the chips12. NSCs are more fragile that murine neurons, so must be handled more gently. It is also critical to mix the stem cells thoroughly before plating by gently pipetting them up and down.
Table 1:
Comparision of multi-compartment COC chips and silicone devices for culturing neurons
| Plastic multi-compartment chips | PDMS multi-compartment devices |
| isolate axons | isolate axons |
| establish microenvironments | establish microenvironments |
| axotomize neurons | axotomize neurons |
| optically transparent | optically transparent |
| compatible with high resolution imaging | compatible with high resolution imaging |
| compatible with fluorescence microscopy | compatible with fluorescence microscopy |
| fully assembled | assembly to substrate required |
| healthy axons >21 days | healthy axons >14 days |
| hydrophilic culturing surface | hydrophobic |
| gas impermeable | gas permeable |
| rounded microgrooves and channels | straight microgrooves |
| not compatible with laser ablation | Can be used for laser ablation when PDMS chambers are assembled on special laser ablation compatible slides. |
| device cannot be altered to remove top | top is removable for staining within microgrooves |
| not compatible with mineral oil-based immersion oils (silicone-based oils are fine) | compatible with mineral oil-based immersion oils |
| impermeable to small molecules and organic solvents | absorption of small molecules & organic solvents |
| No leakage issues with the chip | coating with poly-L-ornithine and laminin make the devices leaky |
| differentiated neurons remain evenly distributed (> 4 weeks) at the tested cell densities | differentiated neurons begin to aggregate after 3–4 days in culture at the tested cell densities |
Use of neurons derived from in vitro differentiated human stem cells is becoming increasingly popular in medicine and research. These neurons are important for research and clinical applications for many CNS disorders, including neurodegenerative diseases and traumatic brain injuries. These neurons closely resemble human fetal neurons15. In the future, appropriately aged neurons could be generated from stem cells to mimic age-related neuronal function and used in conjunction with these compartmentalized devices. These devices will facilitate research in diseases affecting axon health and function such as axon deficits in neurons of patients diagnosed with autism spectrum disorders and axonal regeneration after injury16,17.
Acknowledgments
The authors acknowledge support from Xona Microfluidics, LLC, the National Institute of Mental Health (R42 MH097377), and the National Institute of Neurological Disorders and Stroke (R41 NS108895, P30 NS045892). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures
S.P. and T.N. declare no competing financial interests. V.P. is an employee of Xona Microfluidics, LLC. J.H. is a member of Xona Microfluidics, LLC. A.M.T. is an inventor of the microfluidic chamber/chip (US 7419822 B2, EPO 2719756 B1) and is a member of Xona Microfluidics, LLC.
Video Link
The video component of this article can be found at https://www.jove.com/video/59250/
References
- 1.Taylor AM, Wu J, Tai HC, Schuman EM Axonal translation of beta-catenin regulates synaptic vesicle dynamics. The Journal of Neuroscience. 33 (13), 5584–5589 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Virlogeux A et al. Reconstituting Corticostriatal Network on-a-Chip Reveals the Contribution of the Presynaptic Compartment to Huntington’s Disease. Cell Reports. 22 (1), 110–122 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Neto E et al. Compartmentalized Microfluidic Platforms: The Unrivaled Breakthrough of In Vitro Tools for Neurobiological Research. The Journal of Neuroscience. 36 (46), 11573–11584 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinto MJ et al. The proteasome controls presynaptic differentiation through modulation of an on-site pool of polyubiquitinated conjugates. The Journal of Cell Biology. 212 (7), 789–801 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bigler RL, Kamande JW, Dumitru R, Niedringhaus M, Taylor AM Messenger RNAs localized to distal projections of human stem cell derived neurons. Scientific Reports. 7 (1), 611 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagendran T et al. Distal axotomy enhances retrograde presynaptic excitability onto injured pyramidal neurons via trans-synaptic signaling. Nature Communications. 8 (1), 625 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Laar VS et al. Evidence for compartmentalized axonal mitochondrial biogenesis: Mitochondrial DNA replication increases in distal axons as an early response to Parkinson’s disease-relevant stress. The Journal of Neuroscience. (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.del Río JA, Ferrer I, Gavín R Role of cellular prion protein in interneuronal amyloid transmission. Progress in Neurobiology. 165–167 87–102 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Jia L et al. MiR-34a Regulates Axonal Growth of Dorsal Root Ganglia Neurons by Targeting FOXP2 and VAT1 in Postnatal and Adult Mouse. Molecular Neurobiology. (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor AM, Dieterich DC, Ito HT, Kim SA, Schuman EM Microfluidic local perfusion chambers for the visualization and manipulation of synapses. Neuron. 66 (1), 57–68 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menon S et al. The E3 Ubiquitin Ligase TRIM9 Is a Filopodia Off Switch Required for Netrin-Dependent Axon Guidance. Developmental cell. 35 (6), 698–712 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagendran T, Poole V, Harris J, Taylor AM Use of Pre-Assembled Plastic Microfluidic Chips for Compartmentalizing Primary Murine Neurons. Journal of visualized experiments : JoVE. (141) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor AM et al. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nature Methods. 2 (8), 599–605 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor AM et al. Axonal mRNA in uninjured and regenerating cortical mammalian axons. The Journal of Neuroscience. 29 (15), 4697–4707 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oh JH, Jung CR, Lee MO, Kim J, Son MY Comparative analysis of human embryonic stem cell derived neural stem cells as an in vitro human model. International Journal of Molecular Medicine. 41 (2), 783–790 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lazar M, Miles LM, Babb JS, Donaldson JB Axonal deficits in young adults with High Functioning Autism and their impact on processing speed. Neuroimage Clinical. 4, 417–425 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DePaul MA, Lin CY, Silver J, Lee YS Combinatory repair strategy to promote axon regeneration and functional recovery after chronic spinal cord injury. Scientific Reports. 7 (1), 9018 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]