Abstract
Background
Hematopoietic stem cell transplant (HSCT)-associated thrombotic microangiopathy (TA-TMA) is a well-known complication of HSCT and carries high risk of morbidity and mortality. A lack of consistent non-invasive diagnostic criteria can delay diagnosis and lead to irreversible organ damage.
Methods
Serum samples of 100 patients that underwent HSCT at Cincinnati Children’s Hospital were serially collected. Unbiased proteomic profiling by SELDI-TOF-MS was performed on serum from TA-TMA patients at baseline (pre-HSCT), 2 weeks before TMA diagnosis (pre-TMA), and at clinical TMA diagnosis. Two proteins with mass to charge ratios of 12–13 kDa were consistently elevated at the 2 week pre-TMA time point by SELDI-TOF, compared to control samples. Potential peptides were isolated and analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) on the Linear Trap Quadropole (LTQ). A MASCOTsearch identified haptoglobin fragments in the 12–17-kDa range. Western blot was performed to validate haptoglobin fragments as a potential biomarker.
Results
Western blot of TA-TMA patients showed haptoglobin fragments at 12, 14, and 17 kDa that varied between baseline, pre-TMA, and TMA time points for each patient. By densitometric analysis, the 17-kDa fragment in the pre-TMA samples differed significantly from TMA diagnosis (p < 0.0001). There was no significant difference in the concentrations of the 12-kDa and 14-kDa fragments.
Conclusion
The 17-kDa haptoglobin degradation product may represent a novel early serum biomarker for TA-TMA that could potentially allow for earlier diagnosis and intervention.
Keywords: Thrombotic microangiopathy, Hematopoietic stem cell transplant, Biomarker, Haptoglobin
Introduction
Hematopoietic stem cell transplant (HSCT)-associated thrombotic microangiopathy (TA-TMA) is a well-known complication of HSCT and carries a high risk of both morbidity and mortality. The endothelial dysfunction of TA-TMA can lead to multisystem disease resulting in renal, gastrointestinal, and central nervous system complications; pulmonary hypertension; and death [1]. Children with TA-TMA are 5 times more likely to die in the first year after transplant compared to those without TA-TMA [2]. In adult allogeneic HSCT recipients, those with TA-TMA were 4.3 times as likely to develop chronic kidney disease (CKD) and 9 times more likely to have hypertension than those without TA-TMA. The kidney is the most common organ affected by this small vessel injury and can present in the acute phase with hypertension, proteinuria, and decreased glomerular filtration rate (GFR) [2–5].
TA-TMA is a histologic diagnosis, with kidney biopsy findings demonstrating glomerular microthrombi and a characteristic C4d deposition in the renal arterioles [6]. Kidney biopsy, however, is not always feasible in these complex HSCT patients at risk for bleeding and complications. There have been multiple proposed diagnostic criteria for TA-TMA, including the Blood and Marrow Transplant Clinical Trials Network, and the International Working Group. Additional criteria have been proposed by Cho et al. as well as Jodele et al. [2, 7]. This lack of consistent non-invasive diagnostic criteria, however, can result in delayed diagnosis and irreversible organ damage. The need for a clinical biomarker panel that could predict the diagnosis of TA-TMA prior to current diagnostic criteria is critical for patient outcomes. There have been advances in treatment, including eculizumab, a monoclonal antibody directed towards complement component C5, that are promising in the treatment of TA-TMA [8–14]. Early detection and early intervention are important when implementing these therapies since they can prevent further injury via the complement cascade.
Serum proteomics is a rapidly developing field and a potential source for alternative biomarkers to aid in earlier diagnosis of TA-TMA. In this study, we conducted a serum proteomic analysis to identify proteins that were differentially expressed in patients who developed TA-TMA. The most promising protein discovered and validated was a 17-kDa haptoglobin degradation product. Haptoglobin is a plasma protein that binds free hemoglobin released from erythrocytes with high affinity and, thereby, inhibits its oxidative activity. The haptoglobin-hemoglobin complex is then removed by the reticuloendothelial system (mostly the spleen). In clinical settings, the haptoglobin assay is used to screen for and monitor intravascular hemolytic anemia [15]. To our knowledge, this is the first study to investigate haptoglobin degradation products as novel early biomarkers for TA-TMA.
Methods
Patient population
This was an analysis of a previously collected, prospective study that was approved by the institutional review board. We enrolled 100 consecutive children and young adults from birth to 30 years of age who underwent HSCT at Cincinnati Children’s Hospital Medical Center from September 2010 to December 2011. All patients were prospectively monitored for TA-TMA. Complete blood counts were monitored daily, LDH was checked twice weekly, and serum haptoglobin and urine samples were collected weekly starting 3 weeks prior to conditioning (pre-HSCT), and until completion of the study. Serum, plasma, and urine samples were stored frozen at −80 °C. After completion of enrollment, samples were categorized as baseline (prior to starting HSCT conditioning regimen), no TMA (controls), pre-TMA (very first elevation of LDH and average 2 weeks prior to TA-TMA diagnosis), and TMA (at the time when study TA-TMA diagnostic criteria were met). All patients had assessment of renal function (GFR) prior to HSCT, as determined by serum creatinine estimated GFR and measured nuclear GFR.
TA-TMA definition
The diagnostic criteria proposed by Cho et al. [16] were used to diagnose TA-TMA in this cohort. These included (1) lactate dehydrogenase (LDH) above upper limit of normal, (2) de novo thrombocytopenia (< 50 × 109/L) or 50% decrease in platelet count, (3) de novo anemia with hemoglobin level below lower limit of normal or anemia that requires transfusion, (4) microangiopathic changes defined as schistocytes in peripheral blood or histologic evidence of microangiopathy on tissue specimen, and (5) absence of coagulopathy and negative Coombs test. All laboratory criteria had to occur simultaneously, and criteria 1 through 4 were considered positive only if documented changes were seen on at least two consecutive tests. The TMA diagnosis date was defined as the first date when all diagnostic criteria were met. ADAMTS13 was tested on all subjects with TMA to exclude the diagnosis of thrombotic thrombocytopenic purpura (TTP). Ninety patients underwent allogeneic transplants, and ten underwent autologous transplants. Thirty-nine percent (39/100) met study criteria for TMA. None of the ten patients who underwent autologous transplants developed TMA. Thirty-six of the 39 subjects with TMA were diagnosed within the first 100 days post-transplant [2].
Proteomic discovery
Unbiased proteomic profiling was performed on serum from ten control and ten TMA patients at baseline, 2 weeks before TMA diagnosis, and at TMA clinical diagnosis. Study samples were subjected in a blinded fashion to surface-enhanced laser desorption/ionization time of flight mass spectrometry (SELDI-TOF MS). Serum (both unfractionated and prefractionated using strong anion exchange fractionation protocol) was applied to five different protein chip surfaces (normal phase, anion exchange, cation exchange, metal affinity, and reverse phase). Acquired peaks were analyzed with ProteinChip Data Manager Software 3.0.7 (Bio-Rad Laboratories). Peak intensity was normalized to total ion current. Spectra at baseline were subtracted and clustered using default settings. Spectra were subjected to Expression Difference Mapping analysis which created peak clusters within a prescribed mass window. Peaks with a signal-to-noise ratio of 45 in a mass window of 0.3% found in at least 20% of spectra were identified as clusters. Peak intensity values were estimated using the average m/z value of the existing members of the cluster. Protein Chip Data Manager then utilized the Mann-Whitney test to calculate p values revealing the comparative levels of each peak across two defined groups and provided box and whisker and scatter plot visualization of peak intensity distributions. Receiver-operating characteristic (ROC) curves were generated, and the area under the curve (AUC) was calculated. SigmaStat3.5 was used for the analysis of clinical variables and demographics. p values were determined by chi-square, unpaired t test or Mann–Whitney rank sum analysis.
Identification of haptoglobin fragments
Two proteins with mass to charge ratios between 12 and 13 kDa were consistently elevated at the 2-week pre-TMA time point by SELDI-TOF, compared to control samples from the equivalent time point. Four additional unique patient pre-TMA serum samples were processed to isolate these proteins. Serum samples were processed using the Proteospin Abundant serum protein depletion kit (Norgen Biotek Corp, ON, Canada) per protocol. The depleted serum was then centrifuged using Millipore 50-kDa centrifugal filter units per kit instructions at 10,000 rpm for 20 min followed by 2 min at 3500 rpm to remove the concentrate. The samples were then subjected to SDS PAGE using Novex 18% tris-glycine1.0 mm gels, and Coomassie Blue staining. Proteins in the 12–17-kDa range that were overexpressed in the pre-TMA samples compared to baseline samples were excised from the gel. The gel sections were reduced, alkylated, and digested with trypsin. The resulting peptides were recovered and analyzed by Liquid chromatography-tandem mass spectrometry (LC-MS/MS) on the Linear Trap Quadropole (LTQ). A MASCOT search of homo-sapiens was used to identify the proteins. Two haptoglobin fragments were identified among the proteins isolated in the 12–17-kDa gel sections from pre-TMA sera.
Validation of haptoglobin fragments
To validate the haptoglobin fragments as a potential biomarker, western blots were performed using Abcam polyclonal rabbit anti-haptoglobin antibody (1:2000) and Novex goat anti-rabbit HRP conjugate secondary antibody (1:50,000). Antibodies were diluted in SuperBlock (PBS) Blocking Buffer (Thermo Scientific, Waltham, MA). Samples from 13 patients from the initial study cohort were analyzed with available plasma samples at baseline, pre-TMA, and TMA time points. Each patient’s set of three samples was analyzed via Western blot to determine chronological changes in haptoglobin and its degradation products. In addition, three control patients with three time plasma samples at points similar to TMA patient’s pre-TMA and TMA diagnosis time point were used to compare to these 13 patients. Human haptoglobin was used as a positive control for the Western blots. Each sample underwent the same protocol of Proteospin followed by centrifugal unit filtration. Pierce™ BCA (bicinchoninic acid assay) protein assay kit (Thermo Fisher Scientific, Waltham, MA) was performed prior to SDS PAGE in order to load 10 μg of sample protein for each Western blot. The differences in volume were corrected with deionized water to allow for equal concentrations per gel. Samples underwent the same processing of denaturing with 4× running buffer with DTT. Gels were run for 4.5 h on an 18% gel at 4 °C. Western blotting membranes used included both novex PVDF membrane filter paper (0.2-μm pore) and Millipore Immobilon transfer membrane PVDF (0.34-μm pore). Amersham hyperfilm ECL was used for exposure. Membranes were scanned and analyzed using densitometry and Image J software. A linear mixed-effect model was used to compare densitometric analysis in TMA patients across time (baseline, pre-TMA, and TMA). The linear mixed-effect model properly accounts for the within subject correlation. Linear mixed effect model was fit using SAS (version 9.3).
Results
Proteomic discovery
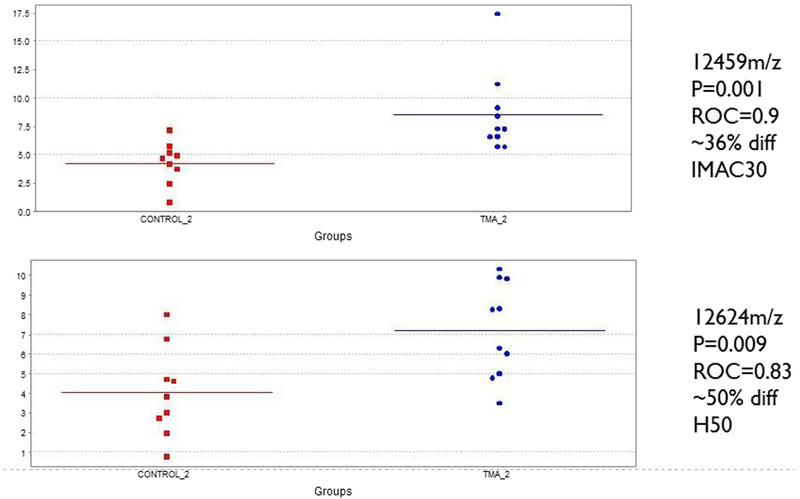
Unbiased proteomic profiling using SELDI-TOF was performed on serum from ten control and ten TMA patients at baseline, 2 weeks before TMA diagnosis, and at TMA clinical diagnosis. Two proteins with mass to charge ratios between 12 and 13 kDa were significantly elevated at the 2 week pre-TMA samples by SELDI-TOF, compared to control samples obtained at an equivalent time point. As shown in Fig. 1, a 12.5-kDa species displayed a peak intensity 36% greater in the pre-TMA samples compared to controls (ROC AUC 0.9, p = 0.001), and a 12.6-kDa species was elevated 50% in the pre-TMA samples (ROC AUC 0.83, p = 0.009).
Fig. 1.
Peak intensities of differentially expressed protein species identified by SELDI-TOF in samples from ten patients with thrombotic microangiopathy (TMA) at pre-TMA time point. Results for control serum samples are shown in red and the pre-TMA samples in blue. Top panel, IMAC30 chip. Bottom panel H50 chip. Area under the receiveroperating characteristic (ROC) curve for prediction of TMA in pre-TMA samples
Identification of haptoglobin fragments
Four pre-TMA serum samples were subjected to SDS-PAGE. Proteins in the 12–17-kDa range that were overexpressed in the pre-TMA samples compared to baseline controls were isolated and identified using LC-MS/MS. The peptide sequences are shown in Table 1, and represent ~ 17-kDa fragments of human haptoglobin.
Table 1.
Peptide sequence of species over-expressed in pre-TMA serum samples
| Peptide sequence | Predicted size (kDa) |
|---|---|
| MSALGAVIALLLWGQLFAVD SGNDVTDIADDGCPKPPEI AHGYVEHSVRYQCKNYY KLRTEGDGVYTLNDKKQ WINKAVGDKLPECEADD GCPKPPEIAHGYVEHSV RYQCKNYYKLRTEGDG VYTLNNEKQWINKAVG DKLPECEAVCGKPK |
17,081 |
| MSALGAVIALLLWGQLFAV DSGNDVTDIADDGCPKP PEIAHGYVEHSVRYQCK NYYKLRTEGDGVYTLN DKKQWINKAVGDKLPE CEADDGCPKPPEIAHGY VEHSVRYQCKNYYKLR TEGDGVYTLNNEKQWIN KAVGDKLPECEAVCGK PKNPANPVQR |
17,985 |
TMA thrombotic microangiopathy
Validation of haptoglobin fragments
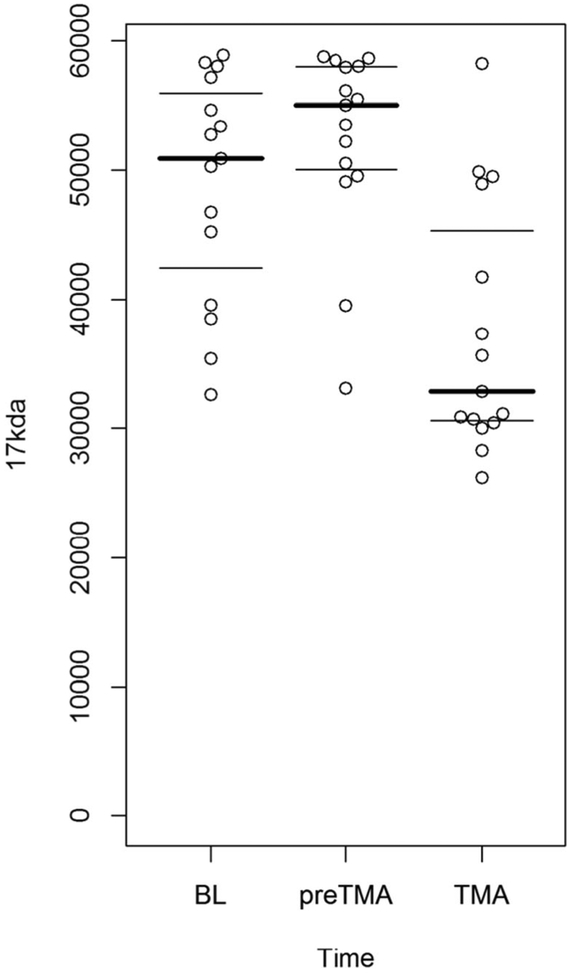
To validate the haptoglobin fragments as a potential biomarker, samples from 13 patients at baseline, pre-TMA, and TMA time points were tested. Each patient’s set of three samples was analyzed by Western blot to determine chronological changes in haptoglobin and its degradation products. Three controls who underwent BMT but did not develop TMA with serum samples at similar time points to TMA patients’ pre-TMA and TMA diagnosis time points were used for comparison. Their demographics are shown in Table 2. Western blot showed haptoglobin fragments at 12 kDa, 14 kDa, and 17 kDa that varied between baseline, pre-TMA, and TMA time points for each patient. By densitometric analysis, the 17-kDa fragment in the pre-TMA samples differed significantly from TMA diagnosis (p < 0.0001), as shown in Fig. 2. In addition, the 17 kDa fragment at TMA time point significantly decreased from baseline (p = 0.0012). There was no significant difference in the concentrations of the 12-kDa and 14-kDa fragments at baseline, pre-TMA, and TMA. In the control non-TMA samples, varying levels of all three fragments were present but remained stable during all time points.
Table 2.
Patient demographics of patients who developed TMA and controls without TMA
| TMA (n=13) | Controls (n = 3) | |
|---|---|---|
| Age in years | 9.3 (0.6–18.7) | 17.0 (7.1–27.8) |
| Males | 8 (61%) | 2 (67%) |
| Caucasians | 8 (61%) | 2 (67%) |
| Donor unrelated | 11 (85%) | 2 (67%) |
| HLA full match | 9 (69%) | 3 (100%) |
| Myeloablative conditioning regimen | 7 (54%) | 2 (67%) |
| Time to TMA diagnosis from baseline (days ± SD) | 47 (±21) | N/A |
TMA thrombotic microangiopathy
Fig. 2.
Distribution of densitometric intensities of the 17-kDa haptoglobin fragment in 13 patients with thrombotic microangiopathy (TMA), in samples obtained at baseline (BL), pre-TMA, and at the time of clinical TMA diagnosis. The 17-kDa fragment in the pre-TMA samples differed significantly from TMA diagnosis (p < 0.0001). In addition, the 17-kDa fragment at TMA diagnosis significantly decreased from baseline (p =0.0012)
Discussion
The diagnosis of TA-TMA continues to be challenging without an invasive biopsy. The tissue diagnosis of TA-TMA is currently the gold standard, but these critically ill patients with high bleeding risks are often too unstable to undergo biopsy. There is great interest in alternative methods of diagnosing TA-TMA early to prevent long-term morbidity and mortality in these patients. Both the Blood and Marrow Transplant Clinical Trials Network for TMA [16]. Cho et al. performed a retrospective validation study which introduced the diagnosis of “probable-TMA” for early identification of this complication, which did not require to wait for renal or neurologic findings to make the diagnosis of TA-TMA [7]. This was also supported by Uderzo et al., who found that doubling of serum creatinine may have inadvertently excluded patients with TMA [2, 17]. However, renal manifestations are associated with higher rates of mortality and are, therefore, important in prognosis [7]. A decrease in the parent haptoglobin protein is included in the diagnostic criteria proposed by Cho et al., but a prospective study by Jodele et al. found that haptoglobin remained elevated 2 weeks longer in the patients with TMA who died from complications [2].
Recently, Jodele et al. proposed additional methods of diagnosis, including proteinuria > 30 mg/dL, hypertension requiring more than two antihypertensive medication, and elevated sC5b-9 [2]. Low haptoglobin was removed from the new diagnostic criteria given its elevated state, especially in patients with high-risk disease and lag behind LDH in posttransplant patients with TMA. Haptoglobin is an acute phase reactant that is produced in the liver, and its expression has been linked to inflammatory cytokines [18, 19]. At TMA diagnosis, parent haptoglobin levels were much higher in patients who died from TMA complications, thus likely representing a non-specific acute inflammatory state associated with poor overall outcomes. Previous studies have concluded that awaiting for low haptoglobin as diagnostic marker would delay TMA diagnosis, especially in high-risk patients. [20]. During early stages of active TMA, haptoglobin would be elevated or normal and a concurrent depletion of haptoglobin during hemolysis would be occurring prior to a measurable decrease in haptoglobin concentration. This balance of haptoglobin production as acute phase reactant in the setting of haptoglobin depletion and destruction in TMA increases the likelihood that a haptoglobin fragment would be elevated early on in the development of TMA.
In this study, we identified a 17-kDa haptoglobin degradation product as a potential serum biomarker of TA-TMA. This biomarker could be a valuable addition to both Cho’s and Jodele’s proposed diagnostic criteria to aid early diagnosis of TA-TMA before full clinical presentation of this disease resulting in organ injury. Our data suggests that haptoglobin degradation product is a more sensitive biomarker of TMA that measures full length haptoglobin in this patient population. The 17-kDa haptoglobin degradation product differed significantly from pre-TMA to clinical TMA diagnosis making it a potential early biomarker of this disease. Using this new 17-kDa degradation marker to screen for TA-TMA in conjunction with other laboratory TA-TMA markers could potentially allow for earlier diagnosis and earlier intervention, with perhaps less devastating outcomes by preventing further organ injury from an early detection point, especially that noninvasive biomarker panels are very desirable for diagnosis of TA-TMA due to complexity of obtaining diagnostic tissue in this severely ill patient population.
Strengths of this study include the prospective nature of the study, and the investigation of a novel biomarker to help identify TA-TMA before the current clinical diagnosis and organ injury. Although further studies are needed to validate our findings with a larger sample size, the 17-kDa haptoglobin degradation product has the potential to improve the outcomes of these at-risk patients. The use of unbiased proteomics and downstream conventional validation studies allowed for direct mapping of the 17-kDa product to the parent haptoglobin protein, and the ability to propose a diagnostic test specific for this fragment for predictive diagnosis. As previously discussed, haptoglobin levels vary depending on degree and severity of TMA disease activity and overall inflammatory state. On the contrary, haptoglobin degradation products would be detected irregardless of haptoglobin level during active TMA process and could serve as more accurate disease biomarker. We believe haptoglobin degradation products will likely be present in any type of TMA presenting with hemolysis and it is not exclusive to TA-TMA only [21] For that reason, haptoglobin degradation products likely will be detected in TMA occurring with lupus associated TMA, HELLP/Eclampsia, and atypical hemolytic-uremic syndrome, TTP and could serve as one of the early biomarkers in TMA diagnostic panels. We have the ability to develop an ELISA for rapid detection and protein quantification to the 17-kDa protein that can be adopted to clinical practice after its validation in the large prospective cohort.
Weaknesses of this study include the small sample size (n = 13 for patients that developed TMA) in the validation set. Furthermore, serum haptoglobin levels were not consistently checked on all patients included in this study at the same time points as the serum analyses for the haptoglobin degradation product, but available measurements of haptoglobin did not show a consistent trend. Additionally, the increase in 17-kDa fragment at the pre-TMA time point is represented as qualitative data extrapolated from densitometric analysis of Western blots. Since this fragment is also present in the control samples (but at consistent levels, and unchanged at various time points), we will need to quantify the increase in pre-TMA samples using tools available in standard clinical laboratories and development and validation of ELISA test for the haptoglobin degradation fragment. Development of a clinical grade assay for the 17-kDa haptoglobin fragment could significantly add to the early and timely diagnosis of TMA. We plan to develop this ELISA as we expand this testing to larger cohorts. Overall, the potential of haptoglobin degradation as a novel biomarker for early detection of TMA is an exciting new direction in the diagnosis of a severe complication of HSCT, with the potential for early intervention and further improved outcomes for affected patients.
Funding
This study was supported by NIH P50 DK096418.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
This was an analysis of a previously collected, prospective study that was approved by the institutional review board.
Informed consent Informed consent was obtained from all individual participants included in the study.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Laskin BL, Goebel J, Davies SM, Jodele S (2011) Small vessels, big trouble in the kidneys and beyond: hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Blood 118:1452–1462 [DOI] [PubMed] [Google Scholar]
- 2.Jodele S, Davies SM, Lane A, Khoury J, Dandoy C, Goebel J, Myers K, Grimley M, Bleesing J, El-Bietar J, Wallace G, Chima RS, Paff Z, Laskin BL (2014) Diagnostic and risk criteria for HSCT-associated thrombotic microangiopathy: a study in children and young adults. Blood 124:645–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glezerman IG, Jhaveri KD, Watson TH, Edwards AM, Papadopoulos EB, Young JW, Flombaum CD, Jakubowski AA (2010) Chronic kidney disease, thrombotic microangiopathy, and hypertension following T cell-depleted hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 6:976–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmeister PA, Hingorani SR, Storer BE, Baker KS, Sanders JE (2010) Hypertension in long-term survivors of pediatric hematopoietic cell transplantation. Biol Blood Marrow Transplant 16:515–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hingorani S (2016) Renal complications of hematopoietic-cell transplantation. N Engl J Med 374:2256–2267 [DOI] [PubMed] [Google Scholar]
- 6.Laskin BL, Maisel J, Goebel J, Yin HJ, Luo G, Khoury JC, Davies SM, Jodele S (2013) Renal arteriolar C4d deposition: a novel characteristic of hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Transplantation 96:217–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho BS, Min CK, Eom KS, Kim YJ, Kim HJ, Lee S, Cho SG, Kim Y, Kim DW, Lee JW, Min WS, Kim CC (2008) Clinical impact of thrombotic microangiopathy on the outcome of patients with acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 41:813–820 [DOI] [PubMed] [Google Scholar]
- 8.Tsai HM (2013) Untying the knot of thrombotic thrombocytopenic purpura and atypical hemolytic uremic syndrome. Am J Med 126: 200–209 [DOI] [PubMed] [Google Scholar]
- 9.Jodele S, Fukuda T, Vinks A, Mizuno K, Laskin BL, Goebel J, Dixon BP, Teusink A, Pluthero FG, Lu L, Licht C, Davies SM (2014) Eculizumab therapy in children with severe hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Biol Blood Marrow Transplant 20:518–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jodele S, Laskin BL, Dandoy CE, Myers KC, El-Bietar J, Davies SM, Goebel J, Dixon BP (2014) A new paradigm: diagnosis and management of HSCT-associated thrombotic microangiopathy as multi-system endothelial injury. Blood Rev 29:191–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SS, Patel M, Yum K, Keyzner A (2015) Hematopoietic stem cell transplant-associated thrombotic microangiopathy: review of pharmacologic treatment options. Transfusion 55:452–458 [DOI] [PubMed] [Google Scholar]
- 12.De Fontbrune FS, Galambrun C, Sirvent A, Huynh A, Faguer S, Nguyen S, Bay JO, Neven B, Moussi J, Simon L, Xhaard A, Resche-Riggon M, O’Meara A, Fremeaux-Bacchi V, Veyradier A, Socié G, Coppo P, de Latour RP (2015) Transplantation 99:953–1959 [DOI] [PubMed] [Google Scholar]
- 13.Khosla J, Yeh AC, Spitzer TR, Dey BR (2017) Hematopoietic stem cell transplant-associated thrombotic microangiopathy: current paradigm and novel therapies. Bone Marrow Transplant 53:129–137 [DOI] [PubMed] [Google Scholar]
- 14.Jodele S, Fukuda T, Mizuno K, Vinks AA, Laskin BL, Goebel J, Dixon BP, Chima RS, Hirsch R, Teusink A, Lazear D, Lane A, Myers KC, Dandoy CE, Davies SM (2016) Variable eculizumab clearance requires pharmacodynamic monitoring to optimize therapy for thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 22:307–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shih AWY, Mcfarlane A, Verhovsek M (2014) Haptoglobin testing in hemolysis: measurement and interpretation. Am J Hematol 89:443–447 [DOI] [PubMed] [Google Scholar]
- 16.Cho BS, Yahng SA, Lee SE, Eom KS, Kim YJ, Kim HJ, Lee S, Min CK, Cho SG, Kim DW, Lee JW, Min WS, Park CW (2010) Validation of recently proposed consensus criteria for thrombotic microangiopathy after allogeneic hematopoietic stem-cell transplantation. Transplantation 90:918–926 [DOI] [PubMed] [Google Scholar]
- 17.Uderzo C, Bonanomi S, Busca A, Renoldi M, Ferrari P, Iacobelli M, Morreale G, Lanino E, Annaloro C, Volpe AD, Alessandrino P, Longoni D, Locatelli F, Sangalli H, Rovelli A (2006) Risk factors and severe outcome in thrombotic microangiopathy after allogeneic hematopoietic stem cell transplantation. Transplantation 82:638–644 [DOI] [PubMed] [Google Scholar]
- 18.Arredouani M, Matthijs P, Van Hoeyveld E, Kasran A, Baumann H, Ceuppens JL, Stevens E (2003) Haptoglobin directly affects T cells and suppresses T helper cell type 2 cytokine release. Immunology 108:144–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Kinzie E, Berger FG, Lim SK, Baumann H (2001) Haptoglobin, an inflammation-inducible plasma protein. Redox Rep 6:379–385 [DOI] [PubMed] [Google Scholar]
- 20.Robert L (2013) Serum haptoglobin in clinical biochemistry: change of a paradigm. Pathol Biol 61:277–279 [DOI] [PubMed] [Google Scholar]
- 21.Jodele S, Zhang K, Zou F, Laskin B, Dandoy CE, Myers KC, Lane A, Meller J, Medvedovic M, Chen J, Davies SM (2016) The genetic fingerprint of susceptibility for transplant-associated thrombotic microangiopathy. Blood 27:989–996 [DOI] [PMC free article] [PubMed] [Google Scholar]