SUMMARY
What we pay attention to is influenced by current task goals (goal-directed attention) [1,2], the physical salience of stimuli (stimulus-driven attention) [3–5], and selection history [6–12]. This third construct, which encompasses reward learning, aversive conditioning, and repetitive orienting behavior [12–18], is often characterized as a unitary mechanism of control that can be contrasted with the other two [12–14]. Here, we present evidence that two different learning processes underlie the influence of selection history on attention, with dissociable consequences for orienting behavior. Human observers performed an antisaccade task in which they were paid for shifting their gaze in the direction opposite one of two color-defined targets. Strikingly, such training resulted in a bias to do the opposite of what observers were motivated and paid to do, with associative learning facilitating orienting towards reward cues. On the other hand, repetitive orienting away from a target produced a bias to repeat this behavior even when it conflicted with current goals, reflecting instrumental conditioning of the orienting response. Our findings challenge the idea that selection history reflects a common mechanism of learning-dependent priority, and instead suggest multiple distinct routes by which learning history shapes orienting behavior. We also provide direct evidence for the idea that value-based attention is approach-oriented, which limits the effectiveness of attentional bias modification techniques that utilize incentive structures.
RESULTS
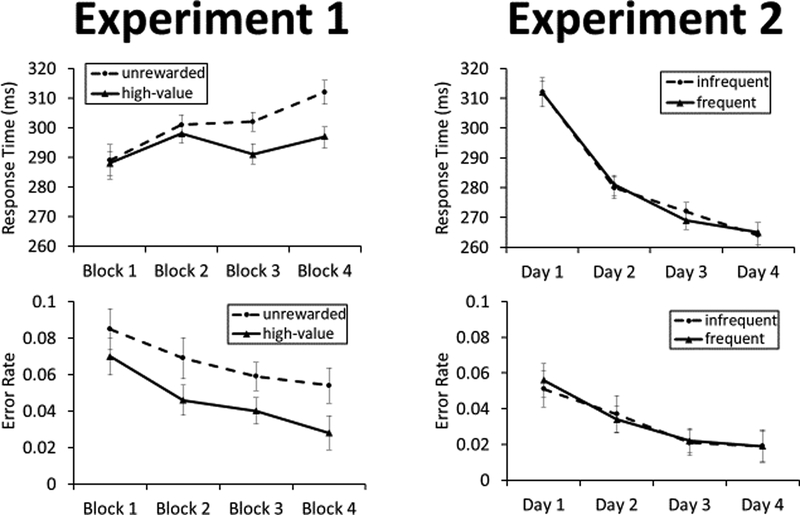
Healthy human participants (n = 30) performed an experiment comprising a training phase and a test phase. The training phase involved an antisaccade task in which participants moved their eyes in the direction opposite that of a colored square (Figure 1). Correct responses resulted in a monetary reward when the square was one of two colors (high-value target), creating a difference in associated value while matching other aspects of selection history [19,20] (see STAR Methods for details). Participants were faster [main effect of value: F = 10.29, p = 0.004, η2p = 0.284] and more accurate [main effect of value: F = 7.18, p = 0.013, η2p = 0.216] in generating an antisaccade to the high-value target as the task progressed, demonstrating motivated behavior consistent with the reward structure (Figure 2).
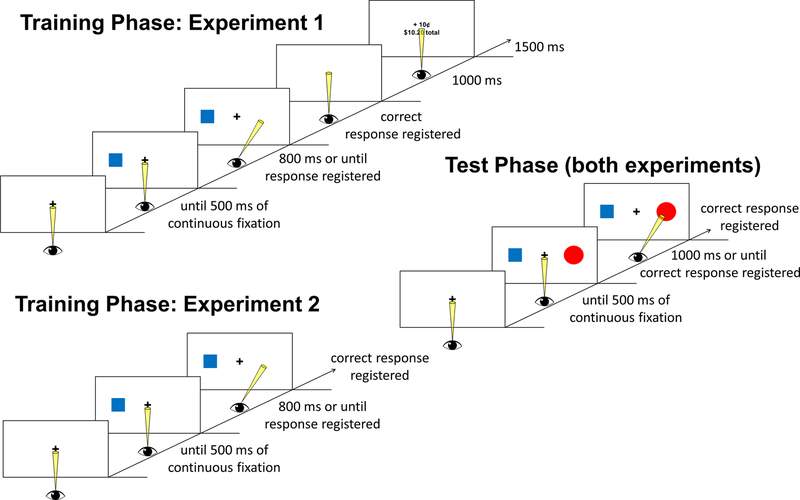
Figure 1.
Time course and trial events for each phase of the experiments. During training, participants performed antisaccades in response to color targets. In Experiment 1, one color predicted reward for a correct response while another target color was never rewarded. In Experiment 2, no explicit rewards were ever delivered, and one target color was more frequent than the other. The training phase comprised of a single session in Experiment 1 and four sessions in Experiment 2 (see STAR Methods for details). The test phase was the same for both experiments and consisted of a task in which participants made prosaccades to a circle target. The colors experienced during training were sometimes used for targets and distractors.
Figure 2.
Response time and error rate by target condition during the training phase of Experiment 1 and Experiment 2. Error bars reflect the within-subjects SEM.
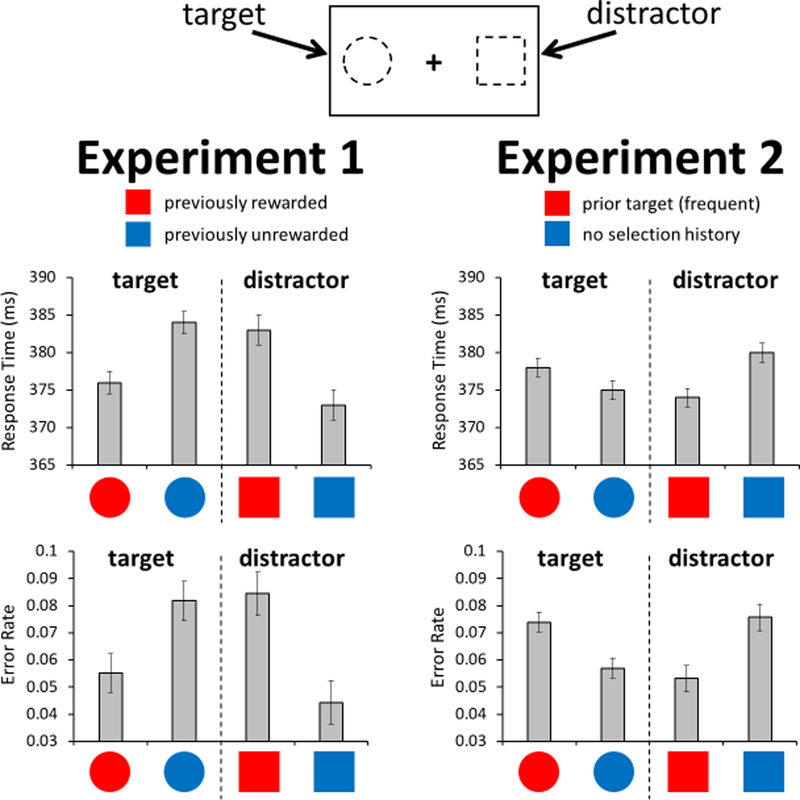
Immediately following the training phase, participants completed the test phase, which involved making a prosaccade to a shape-defined (circle) target. A colored square was presented opposite the target, which served as a task-irrelevant distractor (Figure 1). We compared response time and errors (eye movements directed towards the distractor) based on the reward history of the target and distractor colors, focusing our comparisons on the colors that were matched for prior target history but differed in associated value. When the target was rendered in the previously high-value color, participants were faster [t = 2.67, p = 0.013, d = 0.51] and marginally more accurate [t = 1.84, p = 0.077, d = 0.35] compared to when the target was rendered in the previously unrewarded color (Figures 3 and S1). Likewise, when the distractor was rendered in the previously high-value color, participants were slower [t = 2.60, p = 0.015, d = 0.50] and more error-prone [t = 2.51, p = 0.019, d = 0.48] compared to when the distractor was rendered in the previously unrewarded color. Even though participants were more highly rewarded and thus more motivated to look opposite a stimulus rendered in the high-value color during training, such training produced the exact opposite bias when probed in the test phase. That is, participants found it more difficult to move their eyes opposite the stimulus they were previously paid to look away from compared to an unrewarded color matched for selection history, and were in fact facilitated in looking towards this color when directed to do so.
Figure 3.
Response time and error rate as a function of the selection history of the target and distractor color during the test phase of Experiment 1 and 2. In each experiment, three colors were used (previously high-value/frequent, previously unrewarded/infrequent, and never presented during training). Comparisons focus on the colors with and without reward history, matched for history as a former target (Experiment 1), and the colors with and without history as a former target (Experiment 2). Error bars reflect the within-subjects SEM. See also Figure S1.
Selection history encompasses not only learning from the outcomes of past orienting responses, but also the repetition of these orienting responses in the presence of a stimulus that signals the need to perform the action. In a second experiment, we manipulated the frequency of orienting away from different colors while removing any explicit rewards. A different group of participants (n = 30) completed four consecutive days of training in which they repeatedly performed an antisaccade in response to a frequent and infrequent color square. No reward feedback was provided (Figure 1). Participants improved in the speed and accuracy with which they executed the trained response across days [main effect of day: Fs > 21.21, ps < 0.001, η2p > 0.42], similarly for the frequent and infrequent colors [main effect of frequency and interaction: Fs < 0.83, ps > 0.48] (Figure 2). Then, on the fifth day of the experiment, participants completed the same test phase as in the prior experiment. We focused our comparisons on the frequent former target color and the color that never served as a target previously, reflecting the presence vs absence of selection history (compare to the presence vs absence of reward history in Experiment 1). Responses were numerically slower [t = 1.10, p = 0.279, d = 0.20] and significantly more error-prone [t = 2.37, p = 0.025, d = 0.43] when the target was rendered in the frequent color from training, compared to when the target was a color that never served as a target previously (Figures 3 and S1). Likewise, responses were faster [t = 2.12, p = 0.043, d = 0.39] and less error-prone [t = 2.32, p = 0.028, d = 0.42] when the distractor was rendered in the frequent color from training compared to when it was rendered in a color that never served as a target previously. That is, participants found it more difficult to orient towards the stimulus they had frequently oriented away from during training, and were facilitated in selecting a target when such selection also involved orienting away from this trained color. The pattern of results is directly opposite that of the first experiment, resulting in highly robust interactions between experiment and training history for all measures [Fs > 7.67, ps < 0.009, η2p > 0.12].
The infrequent target color produced an intermediate bias on all measures that did not differ significantly from either of the other two colors (ps > 0.16), which is consistent with the lack of a significant effect of frequency in the training data and suggests that both frequencies of repeated orienting across training days led to some learning. The interactions between experiments are maintained when comparing frequent and infrequent former targets in the current experiment [Fs > 5.56, ps < 0.023, η2p > 0.09], affirming the uniqueness of learned value in facilitating an approach-oriented bias. Consistent with a rapid influence of past orienting responses, there was some evidence for a bias to look away from the previously unrewarded color compared to the neutral color in Experiment 1, particularly for the response time measure (Figure S1), mirroring the pattern observed in Experiment 2 for the frequent target color and suggesting that the influence of unrewarded selection history could emerge over a similarly brief timescale (see Figure S1 for a full breakdown of the data across all conditions).
DISCUSSION
What we pay attention to is biased by past experience or selection history [6–18]. Initially, selection history effects on attention were thought to reflect varieties of top-down guidance [21]. More recently, the influence of selection history on attention has been described as reflecting a dedicated mechanism of control by which priorities are updated based on the outcomes of prior selection [12–18], constituting a third distinct factor in the control of attention [12–14]. Consistent with this idea, it has been proposed that correct task performance generates an internal reward signal that serves as a teaching signal to the perceptual system [22–25], mimicking the effects of learning from extrinsic rewards, which could explain why training with explicit rewards is not necessary to observe attentional capture by former target features [15].
Our findings provide clear evidence for two dissociable mechanisms underlying experience-driven attention, and argue against a common mechanisms framework. Using an antisaccade training task, in one experiment we varied whether a stimulus predicted reward while matching the need to orient away from the stimulus, while in a different experiment we varied the need to orient away from a stimulus while removing any reward feedback. When a stimulus predicts reward, the learning of this predictive relationship results in the stimulus gaining competitive priority in the visual system, biasing selection in its favor. This is in spite of the fact that participants were only ever rewarded for orienting away from the stimulus, providing powerful evidence for the role of associative learning in the orienting of attention [26–29]. On the other hand, repeatedly performing an orienting response in the presence of a particular stimulus facilitates the future engagement of this orienting response to the point of habit formation, reflecting a role for instrumental conditioning in the control of attention.
In a survey of the visual search literature, prior selection and reward were identified as two important factors governing the control of attention [30]. Our findings are consistent with this distinction, and further indicate that their influence is fundamentally dissociable. Our findings are also consistent with the idea that the effects of statistical learning on attention, which is related to history as a former target, are distinctly habit-based [31], which can be contrasted with the mechanisms of Pavlovian reward learning hypothesized to underlie value-driven attention [26–29]. However, a direct comparison of these hypothesized mechanisms has been lacking. By pitting these two sources of learning against each other, our study provides evidence for a distinctly reward-dependent mechanism of learning and a mechanism that is reward-independent, with different components of the same experience independently shaping attentional priority.
When individuals are rewarded for orienting to a stimulus [6,7,19,20], these two learning mechanisms will have the same consequence on future selection, with the resulting bias reflecting some combination of the two. This is the case, for example, in the context of addiction-related attentional biases resulting from drug use [31–34]. Distinguishing between different underlying components of experience-driven attention, and isolating their affects, may lend new insights into the relationship between attentional bias and clinical outcomes [34–37]. For example, the associative learning component we identify here reflects a form of visual attraction that appears to be approach-oriented in nature, even when approach conflicts with action-reward associations, which may reflect a fundamental aspect of incentive salience and its role in the addiction process [38,39].
Our findings also lend insight into why attentional bias modification techniques that provide incentives not to look at particular stimuli are limited in their effectiveness [36,37,40]. Although we paid people to explicitly look away from a particular stimulus, this training led to after-effects opposite what was incentivized. More effective attentional bias modification techniques should either not use incentives, as in our second experiment, or incentivize looking towards a different stimulus that might serve as a competitor for the target of reduced orienting [41].
The specific nature of the observed dissociation in learning mechanisms remains to be clarified. One possibility is that associative reward learning biases covert attention to reward-predictive cues, whereas unrewarded selection history specifically influences the oculomotor control system, producing habitual orienting responses. In the case of reward learning, this covert attentional bias would have competed with the practiced eye movement away from the stimulus in the present study, resulting in an opposing influence on oculomotor behavior. A related possibility is that learning from repetition involves the updating of specific stimulus-response mappings, whereas associative reward learning biases the representation of predictive stimulus features independently of task set. Our findings highlight two distinct learning influences, which should be further explored.
Although the conceptual umbrella of selection history is useful for drawing distinctions between mechanisms of control that are not learning-dependent, the use of this term has also promoted an assumption that now appears untenable. The role of selection history in the control of attention reflects at least two dissociable mechanisms, and possibly more. For example, learning from punishment is also known to influence the control of attention [9,18,42], which may also differ mechanistically from the influence of reward. Aside from a shared dependence on learning, different components of selection history may be just as dissociable from each other as they are from the two other categories of attentional control (i.e., goal-directed and stimulus-driven). Future research needs to focus on differences within the domain of selection history, and reconsider the utility of grouping fundamentally different learning experiences into a common category of attentional control.
STAR METHODS
Contact for Reagent and Resource Sharing
Further information and requests for resources should be directed to and will be fulfilled by the Lead Contact, Haena Kim (hannah.kim@tamu.edu).
Experimental Model and Subject Details
Thirty human participants were recruited from the Texas A&M University community for Experiment 1 (14/15/1 male/female/no response, ages 18–26y [mean = 21.6y]), and thirty new participants were recruited for Experiment 2 (15/15 male/female, ages 18–33y [mean = 23.6y]). All reported normal or corrected-to-normal visual acuity and normal color vision. All procedures were approved by the Texas A&M University Institutional Review Board and conformed with the principles outlined in the Declaration of Helsinki. Written informed consent was obtained from each participant. Participants were compensated with their task earnings in Experiment 1 and at a rate of $10/hr in Experiment 2.
Methods Details
Apparatus.
A Dell OptiPlex equipped with Matlab software and Psychophysics Toolbox extensions [43] was used to present the stimuli on a Dell P2717H monitor. The participants viewed the monitor from a distance of approximately 70 cm in a dimly lit room. Eye position was monitored using an Eye Link 1000-plus desktop mount eye tracker (SR Research). Head position was maintained using an adjustable chin rest (SR Research).
Training Phase of Experiment 1.
Each trial consisted of a fixation display, a stimulus array, and a reward feedback display (see Figure 1). The fixation display remained on screen until eye position was registered within 1.1° of the center of the fixation cross for a continuous period of 500 ms, which triggered the beginning of a trial, and then for an additional 400–600 ms. The stimulus array, which consisted of a 4.7° × 3.4° color square appearing 12.2° center-to-center from fixation, was then presented for 800 ms or until an eye movement exceeding 8.2° in amplitude to the left or right was registered. The reward feedback display was presented for 1500 ms, and consisted of the money earned on the current trial along with the updated total earnings (if an eye movement exceeding 8.2° in amplitude in the direction of the color square was registered, the word “Incorrect” was presented in place of the money earned; if no eye movement exceeding 8.2° in amplitude in either direction was detected, the words “Too slow” were presented). A 1000 ms blank screen was inserted between the stimulus and feedback displays, and each trial concluded with a 1000 ms blank interval.
Participants were instructed to move their eyes opposite the color square on each trial as quickly as possible, and were informed that correct responses would sometimes be rewarded with a small amount of money. Each of two colors appeared equally-often across trials within a block, with each color appearing equally-often on each side of the display. Trials were presented in a random order. Correctly generating an antisaccade to one color target was associated with a reward of 15¢ (high-value color), while for the other target color a correct response always yielded 0¢ (unrewarded color). Red, green, and blue were used for the color of the squares, with each color serving as the high-value, unrewarded, and left-out color equally-often across participants. Participants were not explicitly informed of the reward contingencies, which had to be learned from experience in the task. Participants completed 4 runs of 60 trials.
Training Phase of Experiment 2.
The training phase was identical to that used in Experiment 1, with the exception that no monetary rewards were delivered and one color target appeared more frequently than the other. The frequent target color appeared on 90% of trials within a run, with the infrequent target color appearing on the remaining 10% of trials. Correct responses resulted in no feedback, with an errant saccade resulting in “Incorrect” feedback and a timeout resulting in “Too slow” feedback. Participants completed 7 runs of 80 trials on the first day of training, and 8 runs on each of days 2–4. Day 5 began with 2 runs of training before transitioning into the test phase. All 5 experiment sessions were completed on consecutive days.
Test Phase (both Experiments).
In the test phase, the stimulus array now consisted of a square and a circle, presented equidistant from fixation on the left and right. The size and spacing of these shapes matched that used in the training phase. Participants were instructed to fixate the circle on each trial, regardless of the color of the shapes. Red, green, and blue were used for the shapes, with no color ever appearing twice in the same display. Each color served as the target and distractor equally-often in a run, with the color by position pairings fully crossed and counterbalanced. Trials were presented in a random order. A correct response was registered if eye position was measured at more than 8.2° from fixation in the direction of the target within the 1000 ms timeout limit. If the participant generated a saccade landing more than 8.2° from fixation in the direction of the distractor, the trial was scored as containing an errant eye movement. No rewards were provided, and feedback (the word, “Miss”) was provided for 1000 ms only if participants failed to generate a saccade towards the target within the timeout limit. Each trial ended with a 1000 ms blank interval. Participants completed 3 runs of 96 trials.
Measurement of Eye Position.
Head position was maintained throughout the experiment using an adjustable chin rest that included a bar upon which to rest the forehead (SR Research). Participants were provided a short break between each run of the task in which they were allowed to reposition their head to maintain comfort. Eye position was calibrated prior to each block of trials using 5-point calibration, and was manually drift corrected by the experimenter as necessary (the next trial could not begin until eye position was registered within 1.1° of the center of the fixation cross for 500 ms; see, e.g., [42]). During the presentation of the stimulus array, the X and Y position of the eyes was continuously monitored in real time, such that eye position was coded on line [26]. In the training phase, to ensure that only antisaccades were rewarded, an eye movement towards the target resulted in the termination of the trial and no reward. In the test phase, to maximize our sensitivity to measure selection bias, errant eye movements were recorded but did not result in the termination of the trial, such that participants could still fixate the target within the timeout limit and register a correct response even if gaze was initially directed towards the distractor.
Quantification and Statistical Analyses
The coding of response time and errant saccades was performed on line during each trial of the experiment as described above. Response times exceeding 3 SD of the mean of a given condition were trimmed. For Experiment 1, data from three participants were not analyzed due to an inability to remain alert for the duration of the task, resulting in poor tracking quality. For Experiment 2, 11 participants withdrew from the study before completing all five sessions and were replaced; all participants who completed the entire experiment produced usable data. The training phase data were subjected to 4 × 2 analyses of variance (ANOVAs) with epoch (block in Experiment 1 and day of training in Experiment 2) and target condition as factors, separately for response time and error rate. For the test phase, paired samples t-tests were performed separately for saccades with respect to target color and distractor color (to preserve independence of conditions), separately for response time and error rate. For each condition (bar) depicted in Figure 3, the conditional mean collapses across the color of the non-referenced stimulus (e.g., high-value target trials collapse across trials on which the distractor was the unrewarded color and the color not used during training). For assessment of interactions between experiments, experiment was added as a between-subjects factor for the aforementioned pairwise comparisons and subjected to 2 × 2 ANOVAs.
Data and Software Availability
Raw data for Experiments 1 and 2 are provided as Supplemental Material (Data S1).
Supplementary Material
Data S1. Raw data for the training and test phase of Experiments 1 and 2. Related to Figures 2 and 3.
ACKNOWLEDGEMENTS
Special thanks to Lindsey George and Victoria Fontenot for assistance with data collection. The reported research was supported in part by a start-up package to BAA from Texas A&M University and NARSAD Young Investigator Grant 26008 to BAA.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- 1.Wolfe JM, Cave KR, and Franzel SL (1989). Guided Search: An alternative to the feature integration model for visual search. J. Exp. Psychol. Hum. Percept. Perform 15, 419–433. [DOI] [PubMed] [Google Scholar]
- 2.Folk CL, Remington RW, and Johnston JC (1992). Involuntary covert orienting is contingent on attentional control settings. J. Exp. Psychol. Hum. Percept. Perform 18, 1030–1044. [PubMed] [Google Scholar]
- 3.Theeuwes J (1992). Perceptual selectivity for color and form. Percept. Psychophys 51, 599–606. [DOI] [PubMed] [Google Scholar]
- 4.Theeuwes J (2010). Top-down and bottom-up control of visual selection. Acta Psychol 135, 77–99. [DOI] [PubMed] [Google Scholar]
- 5.Itti L, and Koch C (2001). Computational modelling of visual attention. Nat. Rev. Neurosci 2, 194–203. [DOI] [PubMed] [Google Scholar]
- 6.Anderson BA, Laurent PA, and Yantis S (2011). Value-driven attentional capture. Proc. Natl. Acad. Sci. U. S. A 108, 10367–10371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Della Libera C, and Chelazzi L (2009). Learning to attend and to ignore is a matter of gains and losses. Psychol. Sci 20, 778–784. [DOI] [PubMed] [Google Scholar]
- 8.Hickey C, Chelazzi L, and Theeuwes J (2010). Reward changes salience in human vision via the anterior cingulate. J. Neurosci 30, 11096–11103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt LJ, Belopolsky AV, and Theeuwes J (2015). Attentional capture by signals of threat. Cogn. Emot 29, 687–694. [DOI] [PubMed] [Google Scholar]
- 10.Shiffrin RM, and Schneider W (1977). Controlled and automatic human information processing II: Perceptual learning, automatic attending, and general theory. Psychol. Rev 84, 127–190. [Google Scholar]
- 11.Chun MM, and Jiang YH (1998). Contextual cueing: Implicit learning and memory of visual context guides spatial attention. Cogn. Psychol 36, 28–71. [DOI] [PubMed] [Google Scholar]
- 12.Awh E, Belopolsky AV, and Theeuwes J (2012). Top-down versus bottom-up attentional control: A failed theoretical dichotomy. Trends Cogn. Sci 16, 437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Theeuwes J (2018). Visual selection: usually fast and automatic; seldom slow and volitional. J. Cogn 1(1): 29, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Failing M, and Theeuwes J (2018). Selection history: How reward modulates selectivity of visual attention. Psychon. Bull. Rev 25, 514–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sha LZ, and Jiang YV (2016). Components of reward-driven attentional capture. Atten. Percept. Psychophys 78, 403–414. [DOI] [PubMed] [Google Scholar]
- 16.Peck CJ, and Salzman CD (2014). Amygdala neural activity reflects spatial attention towards stimuli promising reward or threatening punishment. eLife 3, e04478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wentura D, Muller P, and Rothermund K (2014). Attentional capture by evaluative stimuli: gain- and loss-connoting colors boost the additional-singleton effect. Psychon. Bull. Rev 21, 701–707. [DOI] [PubMed] [Google Scholar]
- 18.Stankevich BA, and Geng JJ (2014). Reward associations and spatial probabilities produce additive effects on attentional selection. Atten. Percept. Psychophys 76, 2315–2325. [DOI] [PubMed] [Google Scholar]
- 19.Failing MF, and Theeuwes J (2014). Exogenous visual orienting by reward. J. Vis 14(5):6, 1–9. [DOI] [PubMed] [Google Scholar]
- 20.Anderson BA, and Halpern M (2017). On the value-dependence of value-driven attentional capture. Atten. Percept. Psychophys 79, 1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolfe JM, Butcher SJ, Lee C, and Hyle M (2003). Changing your mind: On the contributions of top-down and bottom-up guidance in visual search for feature singletons. J. Exp. Psychol. Hum. Percept. Perform 29, 483–502 [DOI] [PubMed] [Google Scholar]
- 22.Roelfsema PR, and van Ooyen A (2005). Attention-gated reinforcement learning of internal representations for classification. Neural Comput. 17, 2176–2214. [DOI] [PubMed] [Google Scholar]
- 23.Roelfsema PR, van Ooyen A, and Watanabe T (2010). Perceptual learning rules based on reinforcers and attention. Trends Cogn. Sci 14, 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sasaki Y, Nanez JE, and Watanabe T (2010). Advances in visual perceptual learning and plasticity. Nat. Rev. Neurosci 11, 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seitz A, and Watanabe T (2005). A unified model for perceptual learning. Trends Cogn. Sci 9, 329–334. [DOI] [PubMed] [Google Scholar]
- 26.Le Pelley ME, Pearson D, Griffiths O, and Beesley T (2015). When goals conflict with values: Counterproductive attentional and oculomotor capture by reward-related stimuli. J. Exp. Psychol. Gen 144, 158–171. [DOI] [PubMed] [Google Scholar]
- 27.Bucker B, and Theeuwes J (2017). Pavlovian reward learning underlies value driven attentional capture. Atten. Percept. Psychophys 79, 415–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mine C, and Saiki J (2015). Task-irrelevant stimulus-reward association induces value-driven attentional capture. Atten. Percept. Psychophys 77, 1896–1907. [DOI] [PubMed] [Google Scholar]
- 29.Mine C, and Saiki J (2018). Pavlovian reward learning elicits attentional capture by reward-associated stimuli. Atten. Percept. Psychophys 80, 1083–1095. [DOI] [PubMed] [Google Scholar]
- 30.Wolfe JM, and Horowitz TS (2017). Five factors that guide attention in visual search. Nat. Hum. Behav 1, 0058. doi: 10.1038/s41562-017-0058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang YV (2017). Habitual vs goal-directed attention. Cortex 102, 107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Field M, Munafo MR, and Franken IHA (2009). A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychol. Bull 135, 589–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marissen MAE, Franken IHA, Waters AJ, Blanken P, van den Brink W, and Hendriks VM (2006). Attentional bias predicts heroin relapse following treatment. Addiction 101, 1306–1312. [DOI] [PubMed] [Google Scholar]
- 34.Field M, and Cox WM (2008). Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend. 97, 1–20. [DOI] [PubMed] [Google Scholar]
- 35.Anderson BA (2016). What is abnormal about addiction-related attentional biases? Drug Alcohol Depend. 167, 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Field M, Marhe R, and Franken IHA (2014). The clinical relevance of attentional bias in substance use disorders. CNS Spectr. 19, 225–230. [DOI] [PubMed] [Google Scholar]
- 37.Christiansen P, Schoenmakers TM, and Field M (2015). Less than meets the eye: reappraising the clinical relevance of attentional bias in addiction. Addict. Behav 44, 43–50. [DOI] [PubMed] [Google Scholar]
- 38.Robinson TE, and Berridge KC (1993). The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res. Rev 18, 247–291. [DOI] [PubMed] [Google Scholar]
- 39.Berridge KC, and Robinson TE (1998). What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Res. Rev 28, 309–369. [DOI] [PubMed] [Google Scholar]
- 40.Field M, Duka T, Tyler E, Schoenmakers T (2009). Attentional bias modification in tobacco smokers. Nicotine and Tob. Res 11, 812–822. [DOI] [PubMed] [Google Scholar]
- 41.Sigurjónsdóttir Ó, Björnsson AS, Ludvigsdottir SJ, and Kristjánsson Á (2015). Money talks in attention bias modification: Reward in a dot-probe task affects attentional biases. Vis. Cogn 23, 118–132. [Google Scholar]
- 42.Nissens T, Failing M, and Theeuwes J (2017). People look at the objects they fear: oculomotor capture by stimuli that signal threat. Cogn. Emot 31, 1707–1714. [DOI] [PubMed] [Google Scholar]
- 43.Brainard DH (1997). The psychophysics toolbox. Spat. Vis 10, 433–436. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Raw data for the training and test phase of Experiments 1 and 2. Related to Figures 2 and 3.
Data Availability Statement
Raw data for Experiments 1 and 2 are provided as Supplemental Material (Data S1).