Abstract
BACKGROUND:
Myocardial atrophy and left ventricular (LV) mass reductions are associated with fatigue and exercise intolerance. The relationships between the receipt of anthracycline-based chemotherapy (Anth-bC) and changes in LV mass and heart failure (HF) symptomatology are unknown, as is their relationship to LV ejection fraction (LVEF), a widely used measurement performed in surveillance strategies designed to avert symptomatic HF associated with cancer treatment.
METHODS AND RESULTS:
We performed blinded, serial assessments of body weight, LVEF and mass, LV-arterial coupling, aortic stiffness, and Minnesota Living with Heart Failure Questionnaire measures before and 6 months after initiating Anth-bC (n=61) and non–Anth-bC (n=15), and in 24 cancer-free controls using paired t and χ2 tests and multivariable linear models. Participants averaged 51±12 years, and 70% were women. Cancer diagnoses included breast cancer (53%), hematologic malignancy (42%), and soft tissue sarcoma (5%). We observed a 5% decline in both LVEF (P<0.0001) and LV mass (P=0.03) in the setting of increased aortic stiffness and disrupted ventricular-arterial coupling in those receiving Anth-bC but not other groups (P=0.11–0.92). A worsening of the Minnesota Living with Heart Failure Questionnaire score in Anth-bC recipients was associated with myocardial mass declines (r=−0.27; P<0.01) but not with LVEF declines (r=0.11; P=0.45). Moreover, this finding was independent of LVEF changes and body weight.
CONCLUSIONS:
Early after Anth-bC, LV mass reductions associate with worsening HF symptomatology independent of LVEF. These data suggest an alternative mechanism whereby anthracyclines may contribute to HF symptomatology and raise the possibility that surveillance strategies during Anth-bC should also assess LV mass.
Keywords: anthracyclines, atrophy, heart failure, leukemia, sarcoma
Decrements in left ventricular (LV) myocardial mass are associated with fatigue and heart failure (HF) symptomatology in the general population.1–3 This phenomenon has been reported in autopsy studies of patients with cancer4 and in imaging studies of childhood5 and adult6–8 cancer survivors late after anthracycline-based chemotherapy (Anth-bC). Although patients may develop HF symptomatology during or within weeks or decades after administration of Anth-bC, the relationship between change in LV mass and the onset of HF symptoms during and immediately after administration of Anth-bC is unknown. Moreover, most surveillance strategies implemented to detect early evidence of HF focus on serial assessment of LV ejection fraction (LVEF) to identify LV systolic dysfunction but have not evaluated LV mass.9,10
Accordingly, we performed this cohort analysis to assess time-dependent changes in LV mass and HF symptomatology from pre-chemotherapy to 6 months after initiating Anth-bC. In addition, we assessed serial changes in body mass index and patient weight—both of which can influence LV mass—as well as aortic stiffening and ventriculo-vascular coupling, all of which may contribute to exercise intolerance and HF symptomatology.11–13
METHODS
Study Population
This study complies with the Declaration of Helsinki. The Wake Forest Health Sciences Institutional Review Board approved this study, and all participants provided written, witnessed informed consent. Eligible patients scheduled to receive chemotherapy for breast cancer, soft tissue sarcoma, leukemia, or lymphoma were consecutively recruited (of whom 55 were reported in 2 prior studies of LV function14,15 and 45 were reported in a prior study of statin effects16) from the Comprehensive Cancer Center of Wake Forest Health Sciences. Cancer-free community volunteers of similar age to the patients with cancer were enrolled as comparators. Exclusions included those with cardiovascular magnetic resonance (CMR) contraindications. Solid tumors and lymphoma diagnoses were classified as either early or advanced staged (stages I and II versus III and IV). Chemotherapy regimens and the cumulative anthracycline dose were abstracted for each patient with cancer.
Study Design
CMR images were acquired at 1.5 Tesla using either a Magnetom Avanto (Siemens Medical Solutions, Malvern, PA) or Signa Twinspeed (General Electric Medical Systems, Waukesha, WI) scanner. Baseline CMR examinations were performed in controls and before chemotherapy administration in patients with cancer. Six months thereafter, all participants underwent imaging with identical protocols on the same scanner used at baseline. Cardiovascular parameters assessed with CMR included LVEF, LV mass, volumes, wall thickness, sphericity index, wall stress, LV and vascular elastance, aortic distensibility, and ventricular-arterial coupling assessments. Images were analyzed by individuals blinded to temporal sequence of images, participant identifiers, and clinical data. The 21-item Minnesota Living with Heart Failure Questionnaire (MLHFQ) was administered at each CMR examination to the cancer participants to assess the impact of symptoms associated with HF.17
CMR Image Acquisition and Analysis
LV Volumes and Mass
Using previously reported techniques,18 cine short-axis white-blood steady-state free precession images were acquired encompassing the LV in 8-mm thick planes separated by 2-mm gaps. Imaging parameters included a 40-cm field of view, 192×109 matrix, 10-ms repetition time, 1.12-ms echo time, 20° flip angle, 930 Hz/pixel bandwidth, and 40-ms temporal resolution. LV volumes (LV end-diastolic volume, LV end-systolic volume, and stroke volume), ejection fraction, and LV myocardial mass were calculated. Endocardial and epicardial contours were drawn at end-systole and end-diastole for each slice, excluding papillary muscles. LV volumes (LV end-diastolic volume, LV end-systolic volume, and stroke volume), LVEF, and LV myocardial mass were calculated.18 LV volumes and mass were indexed to body surface area, and myocardial mass was indexed to LV end-diastolic volume as a marker of LV remodeling.19
LV Shape
The sphericity index was measured to assess LV shape from an end-diastolic 4-chamber white blood image.20 Imaging parameters included a 40-cm field of view, 192×120 matrix, 2.3-ms repetition time, 1.05-ms echo time, 65° flip angle, 1130 Hz/ pixel bandwidth, and 7-mm slice thickness. Sphericity index was calculated as the short-axis diameter divided by the long-axis length. In addition, end-diastolic wall thickness was measured on midpapillary short-axis white blood images in the septal and lateral walls.21
LV Wall Stress and Elastance
LV end-systolic wall stress index was calculated from a pressure-independent, volume-based model using LV end-systolic volume and LV myocardial tissue volume.22 To determine the influence of LV mass and volume on functional measures, LV wall stress was indexed to 1 kPa of pressure without invasive pressure measurements to create a unitless index.22 The LV elastance index was calculated as the ratio of the end-systolic pressure to LV end-systolic volume index.23
Aortic Stiffness
Aortic stiffness was assessed as ascending aortic distensibility using phase-contrast CMR images of the proximal thoracic aorta in a subset of participants (41 Anth-bC and 10 non–Anth-bC). Phase-contrast imaging parameters included a 34- to 36-cm field of view, 256×192 matrix, 10-ms repetition time, 3- to 5-ms echo time, 15° to 20° flip angle, 491 Hz/pixel bandwidth, 5-mm slice thickness, and a through-plane velocity encoding of 150 cm/s.24 The ascending aorta was contoured on each frame of the magnitude image, and the velocity-time curve was extracted. Distensibility was calculated using a maximal lumen area averaged from the 3 peak velocity frames and the minimal lumen area averaged from 3 mid-diastole frames.24 In addition, the arterial elastance index was calculated as the ratio of end-systolic pressure to stroke volume index.23 The ventricular-arterial coupling was then calculated as the ratio of the LV to arterial elastance indices.23
HF Questionnaire
The MLHFQ17 was administered to patients with cancer at baseline and 6-month visits, and the total MLHFQ sum and domains including HF symptoms were scored according to previously published data.17 In addition, we divided the 21 questions into categories relevant to patients with cancer undergoing treatment (Table I in the Data Supplement), including financial stresses, fatigue, activities of daily living, sleep, factors that may be associated with chemotherapy effects, pleasure, HF symptoms, and emotional burden.
Statistical Analysis
Cancer patient demographics were compared with controls using Student t tests for continuous variables and χ2 or Fisher exact tests for categorical variables. Cancer groups were classified based on the receipt of anthracycline (Anth-bC) or not. Serial data were compared using 2-sided paired Student t tests for each group, and between-group differences were assessed using general linear models adjusting for sex and group (and baseline value when assessing 6-month changes). The association of 6-month changes in CMR variables with HF questionnaire scores was assessed using general linear models adjusting for baseline HF score. Thereafter, adjusted models were fit to include body weight and other factors associated with LV remodeling. All continuous data are presented as mean±SD and categorical variables as number (percentage). All analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC) with P<0.05 considered statistically significant. The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results.
RESULTS
Study Participants and Cancer Treatment
Participant demographics are displayed in Table 1. The Anth-bC group included patients with breast cancer (53%), hematologic malignancies (42%), and sarcomas (5%) who received an average cumulative doxorubicin dose equivalent of 232±103 mg/m2. Other drugs administered included cyclophosphamide (62%), taxol (32%), and trastuzumab (16%). After completing Anth-bC treatment, 22 breast cancer participants received chest radiation therapy (with cardiac-sparing or limiting techniques with prone, respiration-controlled photon therapy) before the follow-up CMR examination (mean dose of 43.8±20.6 Gy). The non–Anth-bC group consisted mainly of women treated for breast cancer using a trastuzumab regimen with either docetaxel or taxol (n=13) and patients treated for a hematologic malignancy with either all transretinoic acid (n=1) or bendamustine/rituxan therapy (n=1).
Table 1.
Baseline Participant Demographics
| All Cancer Participants (n=76) |
Participants Receiving Anth-bC (n=61) |
Participants Receiving Non-Anth- bC (n=15) |
Noncancer Comparators (n=24) |
|
|---|---|---|---|---|
| Race | ||||
| White | 65 (86) | 51 (84) | 14 (93) | 21 (88) |
| Black | 11 (14) | 10 (16) | 1 (7) | 3 (13) |
| Sex | ||||
| Men | 20 (26) | 19 (31) | 1 (7)* | 10 (42) |
| Women | 56 (74) | 42 (69) | 14 (93)* | 14 (58) |
| Age, y | 51±13 | 51±14 | 54±12 | 52±8 |
| Height, cm | 169±11 | 170±11 | 166±7 | 172±10 |
| Weight, kg | 81±22 | 81±21 | 82±27 | 80±15 |
| Estimated GFR >60 mL/min per 1.73 meter2 | 72 (95) | 57 (93) | 15 (100) | … |
| Body mass index, m2/kg | 28±7 | 28±6 | 30±9 | 27±5 |
| Follow-up interval, mo | 5.8±1.5 | 6.0±1.5 | 5.2±0.90 | 6.1±4.4 |
| Cardiovascular risk factors | ||||
| Diabetes mellitus | 12 (16) | 11 (18) | 1 (7) | 1 (4) |
| Hypertension | 30 (39) | 23 (38) | 7 (47) | 6 (25) |
| Hyperlipidemia | 19 (25) | 16 (26) | 3 (20) | 5 (21) |
| Known CAD | 3 (4) | 3 (5) | 0 (0) | 0 (0) |
| Cardiovascular medication use | ||||
| Statins | 15 (20) | 13 (21) | 2 (13) | … |
| ACE inhibitors | 15 (20) | 12 (20) | 3 (20) | … |
| ARBs | 6 (8) | 5 (8) | 1 (7) | … |
| β-Blockers | 13 (17) | 8 (13) | 5 (33) | … |
| Primary cancer diagnosis | ||||
| Breast cancer | 40 (53) | 27 (44) | 13 (87) | … |
| Leukemia | 11 (14) | 11 (18) | … | … |
| Lymphoma | 21 (28) | 19 (31) | 2 (13) | … |
| Sarcoma | 4 (5) | 4 (7) | … | … |
| Received radiation therapy | 23 (30) | 22 (36) | 1 (7) | … |
Continuous variables reported as mean±SD. Categorical variables reported as number (percentage). P<0.05 considered statistically significant versus comparators. Cardiovascular medication use and GFR data not available for healthy comparators. ACE indicates angiotensin-converting enzyme; Anth-bC, anthracycline-based chemotherapy; ARB, angtiotensin receptor blockers; CAD, coronary artery disease; GFR, glomerular filtration rate.
Noncancer descriptors.
Cardiac Magnetic Resonance Measurements
Overall, 6-month changes in LV remodeling parameters occurred in the group treated with anthracyclines; these changes were statistically significant and greater in magnitude than those observed among nonanthracycline-treated patients with cancer or cancer-free controls (Table 2). In Anth-bC recipients, notable results were LVEF and myocardial mass declines of similar magnitude (5% and 5 g, respectively; Figure 1). The LV wall stress index increased, whereas the ventricular-arterial coupling index decreased, primarily because of a change in the LV elastance index relative to the arterial elastance index (Table 2). In addition, Anth-bC recipients on a statin compared with those not on a statin demonstrated similar changes in LVEF (−3.7%±6.8% versus −4.2%±7.6%; P=0.82) and LV mass (−2.0±16.4 versus −5.4±16.4 g; P=0.53). Similarly, Anth-bC recipients with radiation therapy had no significant difference in LVEF change (−3.6%±5.4% versus −4.4%±8.3%; P=0.65) or LV mass change (−6.7±17.8 versus −3.5±15.5 g; P=0.47) compared with those without radiation therapy.
Table 2.
CMR Measurements of LV Remodeling at Baseline and 6 Months After Initiation of Chemotherapy
| All Cancer Patients (n=76) | Participants Receiving Anth-bC (n=61) |
Participants Receiving Non-Anth-bC (n=15) |
Cancer-Free Comparators (n=24) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (0 mo) |
Follow-Up (6 mo) |
P Value | Baseline (0 mo) |
Follow-Up (6 mo) |
P Value | Baseline (0 mo) |
Follow-Up (6 mo) |
P Value | Baseline (0 mo) |
Follow-Up (6 mo) |
P Value | |
| Cardiovascular measures at examination | ||||||||||||
| Heart rate, beats per min | 78±12 | 80±1 3 | 0.07 | 79±13 | 81±13* | 0.20 | 74±9 | 78±14 | 0.44 | 77±11 | 73±9 | 0.31 |
| Systolic blood pressure, mmHg | 119±20 | 112±1 5 | 0.01 | 118±21 | 110±14 | <0.01 | 119±18 | 118±15 | 0.79 | 122±14 | 117±16 | 0.18 |
| Diastolic blood pressure, mmHg | 70±13† | 65±11 * | 0.01 | 69±13† | 64±10* | <0.01 | 70±123† | 68±11 | 0.64 | 79±9 | 75±12 | 0.14 |
| Body surface area, m2 | 1.90±0.27 | 1.88±0.26 | 0.11 | 1.90±0.28 | 1.89±0.26 | 0.17 | 1.89±0.27 | 1.88±0.25 | 0.22 | 1.92±0.19 | 1.92±0.19 | 0.63 |
| Systolic function and biomechanical factors | ||||||||||||
| LVEF, % | 58±8t | 55±7* | <0.0001 | 59±8 | 54±7* | <0.0001 | 58±6 | 55±6 | 0.19 | 62 ±5 | 61 ±5 | 0.39 |
| LVEDV indexed, mL/m2 | 59±14 | 61 ±1 5 | 0.07 | 59±1 5 | 61 ±1 6 | 0.09 | 61 ±1 3 | 62±13 | 0.54 | 58±9 | 57±8 | 0.59 |
| LVESV indexed, mL/m2 | 25±8 | 28±8* | <0.0001 | 24±9 | 28±9* | <0.0001 | 25±6 | 27±6 | 0.14 | 23±6 | 22±5 | 0.75 |
| LV stroke volume indexed, mL/m2 | 34±9 | 34±9 | 0.44 | 34±9 | 33±9 | 0.42 | 35±8 | 35±9 | 0.93 | 36±5 | 35±5 | 0.27 |
| End-systolic wall stress index | 0.88±0.22 | 1.0±0.26* | <0.0001 | 0.86±0.23 | 1.0±0.27* | <0.0001 | 0.96±0.19† | 1.02±0.23 | 0.40 | 0.77±0.20 | 0.76±0.18 | 0.56 |
| Ventricular elastance index (ELVl) | 4.8±1.8 | 4.0±1.4* | <0.0001 | 4.9±1.9 | 4.0±1.5* | <0.001 | 4.5±1.3 | 4.0±0.9 | 0.11 | 5.2 ±1.6 | 4.9±1.3 | 0.28 |
| Geometric and atrophic factors | ||||||||||||
| LV myocardial mass, g | 103±27 | 102±28 | 0.08 | 105±28 | 100±26* | 0.03 | 97±21 | 99±23 | 0.66 | 115±23 | 116±23 | 0.25 |
| Septal wall thickness, mm | 7.8±1.5 | 7.7±1.3 | 0.11 | 7.7±1.5 | 7.6±1.4 | 0.12 | 8.1 ±1.6 | 8.0±1.2 | 0.72 | 7.2±1.2 | 7.3±1.2 | 0.10 |
| Lateral wall thickness, mm | 5.3±1.3 | 5.1 ±1.1 | <0.01 | 5.4±1.3 | 5.1±1.2 | <0.01 | 4.9±1.0 | 4.8±0.9 | 0.86 | 5.2±0.4 | 5.2±0.4 | 0.58 |
| Sphericity index | 0.51 ±0.07 | 0.52±0.07 | 0.31 | 0.52±0.07 | 0.52±0.07 | 0.78 | 0.50±0.05 | 0.50±0.05 | 0.81 | 0.51 ±0.05 | 0.52±0.05 | 0.36 |
| Arterial factors | ||||||||||||
| Ascending aorta distensibility, 10−3 mm Hg−1 | 1 68±1.28 | 1 86±1.60 | 0.41 | 1.68±1.31 | 1 98±1.70 | 0.28 | 1 69±1.23 | 1.32±0.85 | 0.75 | n/a | n/a | … |
| Arterial elastance index (Eal) | 3.3±1.0 | 3.2±0.8 | 0.44 | 3.3±1.0 | 3.2±0.9 | 0.43 | 3.2±0.9 | 3.2±0.8 | 0.92 | 3.1 ±0.6 | 3.1 ±0.6 | 0.65 |
| LV/arterial coupling factors | ||||||||||||
| Mass/LVEDV ratio, g/mL | 0.96±0.25 | 0.91 ±0.26* | 0.08 | 0.98±0.24 | 0.92±0.27* | 0.06 | 0.88±0.25 | 0.87±0.24 | 0.91 | 1.1 ±0.22 | 1.1 ±0.23 | 0.49 |
| Ventricular-arterial coupling (Eal/ ELVl) | 1.5±0.48 | 1.3±0.33* | <0.0001 | 1.5±0.52 | 1.3±0.34* | <0.0001 | 1.4±0.31 | 1. 3±0.31 | 0.20 | 1.7±0.34 | 1.6±0.31 | 0.38 |
Sphericity index and wall stress index are unitless parameters; wall stress is indexed to 1 kPa and LV volumes are indexed to body surface area. Continuous variables reported as mean±SD; P<0.05 considered statistically significant. Anth-bC indicates anthracycline-based chemotherapy; CMR, cardiovascular magnetic resonance; LV, left ventricular; LVEDV, LV end-diastolic volume; LVEF, LV ejection fraction; LVESV, LV end-systolic volume; and n/a, not acquired.
Change in value statistically different from change in noncancer comparator group after adjustment for gender and baseline value.
Value statistically different from baseline value in noncancer comparator group after adjustment for sex.
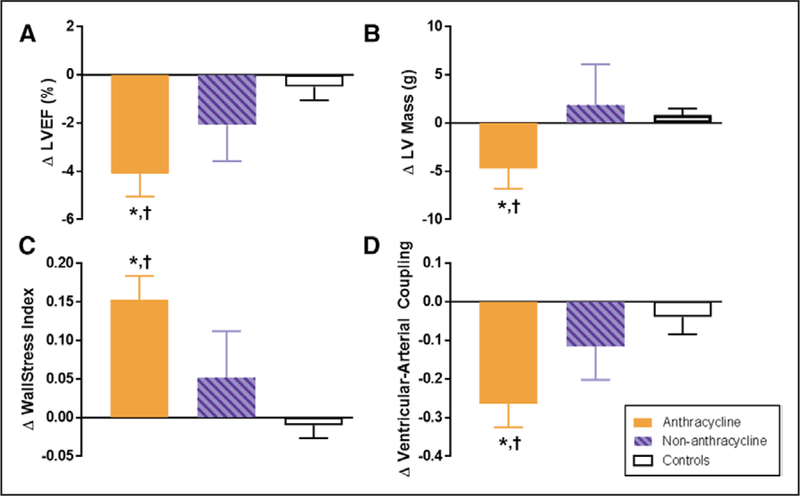
Figure 1. Six-month change in cardiovascular magnetic resonance (CMR)–derived left ventricular (LV) remodeling measurements after anthracycline-based chemotherapy.
Six-month change in CMR-derived measurements of left ventricular remodeling in adults treated with anthracycline-based chemotherapy (Anth-bC, orange), non–Anth-bC (purple) for breast cancer or hematologic malignancy and cancer-free comparators of similar age (white). Compared with cancer-free comparators, those receiving Anth-bC had a significant decrease in LV ejection fraction (LVEF; A; P<0.01) and LV myocardial mass (B; P=0.03) that occurred concurrently with increased end-systolic wall stress index (C; P<0.01) and reduced ventricular-arterial coupling (D; P<0.01). Changes among patients with cancer who received non–Anth-bC were not statistically different than those observed in noncancer comparators (P>0.15 for all). Data shown as mean±SEM.*P<0.05 for change from baseline. †P<0.05 vs change in controls.
We performed 2 analyses to gain insight into the confounding of cancer and Anth-bC cardiotoxicity.25 First, we compared the baseline LV mass of cancer participants diagnosed with early versus late stage cancer. There were no differences in LV mass between those with early versus late stage cancer at the time of entry into the study. Second, we recruited patients with cancer scheduled to receive non–Anth-Bc–associated potentially reversible myocardial dysfunction as a second comparator group in the era of multiagent therapy.25 In the 6-month study period, we observed no significant LV remodeling in our non–Anth-bC comparators (Figure 1).
HF Questionnaire
The MLHFQ total, domain, and category scores are included in Table 3 for cancer participants. Overall, all MLHFQ scores increased in patients with cancer during the first 6 months of treatment with similar rates of increased total MLHFQ score observed in Anth-bC and non–Anth-bC participants (57% and 60%, respectively). For Anth-bC patients, the total MLHFQ score and the domains of physical and other increased as well (Table 3). The HF symptom category score also increased from 0.9±1.6 to 2.0±2.5 in Anth-bC patients (P<0.001) but not in those treated with a nonanthracycline regimen (1.1±2.3–1.8±3.4; P=0.16; Table 3). Combining the categories of fatigue with HF symptoms yielded similar results. Anth-bC recipients on a statin demonstrated similar 6-month changes in total MLHFQ (10.5±31.5 versus 11.8±23.7; P=0.87) and HF symptom category (1.0±2.7 versus 1.2±2.3; P=0.78) compared with those not on a statin.
Table 3.
MLHFQ at Baseline and 6 Months After Initiation of Chemotherapy Treatment in Patients With Cancer
| All Cancer Participants (n=76) | Participants Receiving Anth-bC (n=61) | Participants Receiving Non-Anth-bC (n=15) |
||||
|---|---|---|---|---|---|---|
| Baseline (0 mo) | Follow-Up (6 mo) | Baseline (0 mo) | Follow-Up (6 mo) | Baseline (0 mo) | Follow-Up (6 mo) | |
| Total MLHFQ Score | 16.3±22.6 | 27.4+27.8* | 15.8+21.8 | 26.5+26.5† | 18.1+26.5 | 31.5+33.3‡ |
| Domains | ||||||
| Physical | 6.3+9.0 | 12.1 + 11.1* | 5.9+8.7 | 11.7+10.9* | 7.9+10.6 | 13.9+12.4‡ |
| Emotional | 4.3+6.4 | 57+7.2‡ | 4.4+6.4 | 5.6+6.8 | 4.1+6.8 | 6.6+9.0 |
| Other | 5.6+8.2 | 9.9+10.7§ | 5.5+8.0 | 9.6+10.2† | 6.1+9.6 | 11.1+12.8‡ |
| Categories | ||||||
| Financial | 2.2±3.1 | 3.2+3.8† | 2.1+2.9 | 3.1+3.7† | 2.6+4.2 | 3.5+3.9 |
| Fatigue | 1.8+2.8 | 3.7+3.4* | 1.6+2.6 | 3.6+3.5* | 2.4+3.4 | 4.3+3.2‡ |
| ADL | 2.8+3.7 | 4.9+4.4* | 2.7+3.6 | 4.6+4.3§ | 3.4+4.2 | 5.8+4.8† |
| Sleep | 1.6+2.7 | 2.6+3.2† | 1.6+2.6 | 2.5+3.0‡ | 2.0+3.0 | 2.9+3.7 |
| Chemotherapy side effects | 1.8+3.2 | 2.9+3.9‡ | 1.8+3.2 | 2.6+35 | 1.8+3.4 | 4.2+5.2† |
| Pleasure | 1.8+3.5 | 3.6+4.5* | 1.7+3.5 | 3.6+4.4§ | 2.1+3.3 | 3.5+5.2 |
| HF symptoms | 0.9+1.7 | 1.9+2.7§ | 0.9+1.6 | 2.0+2.5§ | 1.1+2.3 | 1.8+3.4 |
| Emotional burden | 3.5+5.3 | 4.6+6.1 | 3.6+5.3 | 4.5+5.9 | 3.0+5.3 | 5.2+7.3 |
Increased values indicate worsening of heart failure. ADL indicates activities of daily living; Anth-bC, anthracycline-based chemotherapy; HF, heart failure; and MLHFQ, Minnesota Living With Heart Failure Questionnaire. Significance shown for paired 2-sided t tests as follows:
P<0.0001.
P<0.01–0.001.
P<0.05–0.01.
P<0.001–0.0001.
Associations of HF Questionnaire With CMR Measures of LV Remodeling
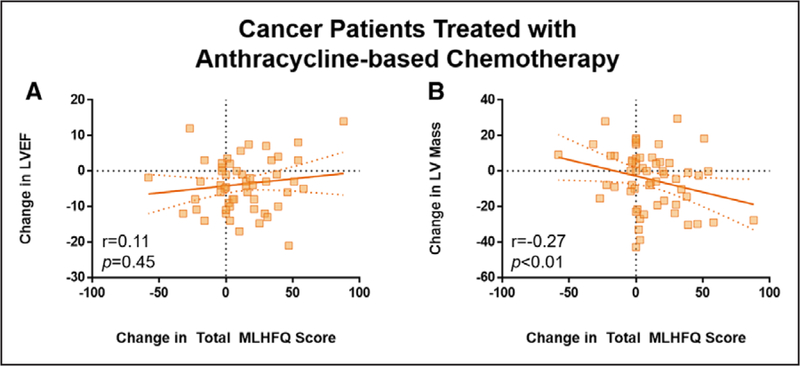
We were interested in whether LVEF or LV mass declines were closely associated with early HF symptomatology after receipt of chemotherapy. As shown in Figure 2, the change in total MLHFQ score in those receiving Anth-bC was associated with LV mass declines (r=−0.27; adjusted P<0.01) but not changes in LVEF (r=0.11; adjusted P=0.45). Associations of changes in LVEF and LV mass with MLHFQ score were not observed in patients treated with nonanthracycline regimens (P=0.07 and P=0.29, respectively).
Figure 2. Associations of cardiovascular magnetic resonance–derived changes in left ventricular (LV) remodeling with worsening Minnesota Living With Heart Failure Questionnaire in patients with cancer treated with anthracyclines.
Subclinical declines in LV ejection fraction (LVEF; A) were not associated with worsening of total Minnesota Living With Heart Failure Questionnaire (MLHFQ) score (P=0.45). Instead, atrophic remodeling (reduced myocardial mass; B) was associated with worsening total MLHFQ score (P<0.01) 6 mo after initiation of cancer treatment. Correlation of variables in (A) and (B) with P values for model adjusted for baseline MLHFQ score.
MLHFQ was associated with decrements in LV mass after accounting for LVEF (P<0.01) and body weight (P<0.01). Interestingly, MLHFQ scores trended toward an association with aortic distensibility (P=0.06) and were borderline associated with and LV wall stress index (P=0.05) after treatment with Anth-bC. MLHFQ scores were not associated with changes in body weight (P=0.57), LV elastance index (P=0.51), arterial elastance index (P=0.76), or ventricular-vascular coupling (P=0.62) after treatment with Anth-bC.
DISCUSSION
The results of this study indicate that LV mass declines (by 5%) as early as 6 months after initiating Anth-bC in comparison to nonanthracycline-based chemotherapies and healthy comparators. These declines in LV mass are associated with increases in HF symptomatology consistent with mild established HF.26 These findings occur independent of declines in LVEF—the standard imaging biomarker for cardiotoxicity—and, therefore, highlight the potential of an additional mechanism for the development of HF besides a decline in LVEF.
Myocardial Mass
Evidence suggests that anatomic LV remodeling precedes LV functional decline after Anth-bC.27 Furthermore, cardiotoxicity is a complex, multifactorial disease encompassing not only myocellular injury associated with LV systolic dysfunction but also mitochondrial injury, extracellular remodeling, myocellular apoptosis, and myocellular atrophy.28 Prior studies in pediatric and adult cancer survivors have shown that LV myocardial mass is reduced 2 to 20 years after receipt of Anth-bC.6–8,29–31 Our results indicate that LV mass may decline within 6 months after initiating Anth-bC (Table 2). This finding suggests an early association between the onset of LV mass reduction and receipt of Anth-bC in addition to other potential etiologies of LV mass loss (eg, aging, deconditioning, or the long-term effects of chemotherapy).
Prior studies have evaluated the relationship between LV mass and afterload. In those with cachexia from cancer, anorexia nervosa, or those who have experienced space travel or prolonged bed rest, where LV afterload is reduced, the LV mass falls.1,3,4,32,33 In the elderly or those with hypertension where LV afterload increases, the LV mass also increases. In this study, we observed a high LV afterload (similar to that in elderly HF patients13,34,35) but paradoxically found that LV mass decreased (Figure 1).
In addition to assessing LV mass, we also measured body weight throughout the study. Hellerstein and Santiago-Stevenson4 previously described in 1950 that as many as three quarters of patients with myocardial atrophy identified in a postmortem study experienced their atrophy in the setting of an underlying malignancy. In the current study, we observed decreases in LV mass without necessarily a concomitant decline in patient weight (Tables 1 and 2). This finding suggests a process other than cancer-associated cachexia can reduce LV mass within 6 months of initiating Anth-bC. Of note, symptomatology associated with HF was not associated with decreases in body weight.
Prior studies have demonstrated both declines and possible increases in mass after receipt of Anth-bC. Lipshultz et al5 have promoted the expression, Grinch syndrome, a form of cardiac remodeling that occurs in childhood cancer survivors, in which LV mass declines without necessarily a marked decline in LVEF.34 These individuals may be predisposed to development of restrictive cardiomyopathy later in life. In adult survivors of cancer who received Anth-bC a median of 88 months prior, Neilan et al6 reported that CMR identified reductions in LV mass (51±5 versus 71±12 g/m2; P<0.0001). Furthermore, in this cohort, LV mass index <57 g/m2 provided a sensitivity of 100% and specificity of 85% for prediction of cardiovascular events (P<0.001).6 Conversely, Narayan et al35 found that echocardiographic measures of LV mass may increase at 1 year after receipt of Anth-bC (Δ9.2 g; 95% confidence interval, 5.4–13.1 g) but then return to baseline at 2 to 3 years after treatment (change from baseline of Δ2.3 g and Δ7.5 g, respectively; P>0.05 for both).
There are several implications of reduced LV mass in the setting of increased biomechanical stress and deteriorating LV function after cancer therapy. First, atrophic remodeling with increased wall stress can contribute to progressive myocardial fibrosis development, LV contractile dysfunction, reduced cardiac output, and the development of a cardiomyopathy.2,36–38 Second, inappropriate LV remodeling (reduced LV mass in setting of increased afterload) is associated with diminished exercise tolerance in other patient populations.37 Persistent decreases in LV mass and increases in LV wall stress could impact the implementation of therapeutic exercise training regimens that are rapidly evolving for the purpose of reducing cardiovascular morbidity and mortality in cancer survivors.39 This is supported by our finding showing impacts in the physical domain of the MLHFQ and its association with LV mass declines after therapy.
For the most part, current management strategies to identify cardiac abnormalities after receipt of Anth-bC involve the serial assessment of LVEF or global longitudinal strain.9 We and others have shown that HF symptomatology can worsen during receipt of potentially cardiotoxic chemotherapy and overall LVEF may also decline.14,30,32,40 In this study, HF symptomatology became more evident during the 6 months after initiating potentially cardiotoxic chemotherapy (Table 3). As expected, MLHFQ scores were low at initiation of chemotherapy and nearly doubled in 6 months, ending in the range consistent with mild, established HF.26 Unexpectedly, we found that worsening of the HF questionnaire score was associated with decrements in LV mass as opposed to the overall decline in ejection fraction. This association between LV mass and HF questionnaire score persisted after accounting for both changes in LVEF and changes in body weight.
Comparator Analyses
Data from our comparators demonstrate the longitudinal reproducibility of both CMR and our other methods. The accuracy and precision of CMR to measure cardiovascular structure and function is well known and thus permits smaller sample sizes.41
We performed 2 analyses to gain insight into the potential confounding of cancer and Anth-bC cardiotoxicity because a treatment-free cancer group could not be studied for the effect of cancer versus Anth-bC treatment.25 First, we compared the baseline LV mass of cancer participants diagnosed with early versus late stage cancer as we reasoned that if the presence of malignancy itself promoted a decrease in LV mass, then those individuals with more advanced cancer should exhibit smaller LV mass at baseline CMR relative to those presenting with less advanced disease. There were no differences in LV mass between those with early versus late stage cancer at the time of entry into the study. Although limited in nature, these data suggest that tumor burden does not exhibit a marked effect on LV mass before the start of therapy. Second, we recruited patients with cancer scheduled to receive non–Anth-bC–associated potentially reversible myocardial dysfunction as a second comparator group in the era of multiagent therapy.25 In the 6-month study period, we observed no significant LV remodeling in our non–Anth-bC comparators (Figure 1).
Limitations
We acknowledge the following limitations. First, although our comparators were similar to those receiving Anth-bC, our study design did not allow us to control for all potential confounders. Additional larger studies are needed be to determine whether other factors are associated with LV mass declines in those with cancer. Second, we used a noninvasive CMR-derived measurement of LV end-systolic wall stress normalized to 1 kPa. Although this represents an indexed volume-based wall stress measurement,22 it is proportional to the mass/ LV end-diastolic volume ratio commonly used in CMR studies (Table 2). Third, we are unable to compare early changes in LV mass, LVEF, and MLHFQ (<6 months) with late outcomes to determine if individuals with early disruptions to the cardiac plasticity and remodeling persist chronically and if these early changes occur in those at highest risk for HF and late cardiovascular events. Future studies will be aimed at addressing the need to capture late cardiovascular effects in patients with cancer undergoing cancer therapy. Fourth, we recruited cancer participants representative of our catchment area. Because of the predominance of white women with breast cancer, the generalizability of our interpretations to other cancers and race/ethnicities is limited. Finally, it is important to note that although our association of MLHFQ scores was associated with declines in LV mass were mildly associated with worsening MLHFQ scores (r=−0.27; P<0.01), future studies are required to determine other factors that may contribute to HF symptomatology in patients receiving potentially cardiotoxic chemotherapy, including objective maximal and submaximal measures of exercise intolerance.
Conclusions
In conclusion, in middle-aged adults, early declines in LV myocardial mass may occur 6 months after initiating Anth-bC. This observation occurs coincident with increased LV afterload and increases in HF questionnaire scores consistent with mild, established HF. These findings persist after accounting for LVEF, highlighting the potential independence of myocellular dysfunction from structural degradation and impaired adaptive remodeling after cardiotoxicity from anthracycline chemotherapy. Further studies are warranted to determine if this early decrease in LV mass is dose dependent, persists, or contributes to objective measures of exercise intolerance, fatigue, HF, or other cardiovascular events experienced by cancer survivors. Importantly, early declines in LV myocardial mass may represent an imaging biomarker for future interventions in this population even when LVEF is preserved.
Supplementary Material
WHAT IS NEW?
Myocardial mass declines of 5% occur as early as 6 months after receipt of anthracycline-based chemotherapy regimens in the setting of increased afterload.
In this acute period, patients with cancer who received anthracyclines experienced increased heart failure symptomatology consistent with mild, established heart failure.
Worsening heart failure scores were associated with reduced myocardial mass and not with left ventricular ejection fraction in patients with cancer who received anthracyclines.
WHAT ARE THE CLINICAL IMPLICATIONS?
Current consensus and position papers emphasize the role of systolic dysfunction (left ventricular ejection fraction and myocardial strain) in screening and monitoring patients with cancer at risk for cardiotoxicity.
Although previous studies have shown declines in myocardial mass and left ventricular ejection fraction after anthracycline treatment, our findings highlight the potential independence of myocellular dysfunction from structural degradation and impaired adaptive remodeling after cardiotoxicity from anthracycline chemotherapy.
Imaging of myocardial mass changes with cardiac magnetic resonance or echocardiography should be considered as an alternative or supplemental surveillance strategy, particular in those with preserved left ventricular ejection fraction.
Sources of Funding
This work was supported by National Institutes of Health grants R33CA12196, R01HL07648, R01CA167821, M01RR07122, and pilot funding from P30CA012197.
Footnotes
Disclosures
None.
REFERENCES
- 1.Hill JA, Olson EN. Mechanisms of disease: cardiac plasticity. N Engl J Med. 2008;358:1370–1380. [DOI] [PubMed] [Google Scholar]
- 2.Oriyanhan W, Tsuneyoshi H, Nishina T, Matsuoka S, Ikeda T, Komeda M. Determination of optimal duration of mechanical unloading for failing hearts to achieve bridge to recovery in a rat heterotopic heart transplantation model. J Heart Lung Transplant. 2007;26:16–23. doi: 10.1016/j.healun.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 3.Levine BD, Zuckerman JH, Pawelczyk JA. Cardiac atrophy after bed-rest deconditioning: a nonneural mechanism for orthostatic intolerance. Circulation. 1997;96:517–525. [DOI] [PubMed] [Google Scholar]
- 4.Hellerstein HK, Santiago-Stevenson D. Atrophy of the heart; a correlative study of 85 proved cases. Circulation. 1950;1:93–126, illust. [DOI] [PubMed] [Google Scholar]
- 5.Lipshultz SE, Scully RE, Stevenson KE, Franco VI, Neuberg DS, Colan SD, Silverman LB, Moslehi JJ, Cheng S, Sallan SE. Hearts too small for body size after doxorubicin for childhood ALL: Grinch syndrome. J Clin Oncol. 2014;32:10021–10021. [Google Scholar]
- 6.Neilan TG, Coelho-Filho OR, Pena-Herrera D, Shah RV, Jerosch-Herold M, Francis SA, Moslehi J, Kwong RY. Left ventricular mass in patients with a cardiomyopathy after treatment with anthracyclines. Am J Cardiol. 2012;110:1679–1686. doi: 10.1016/j.amjcard.2012.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Wolf D, Suys B, Maurus R, Benoit Y, Verhaaren H, Matthijs D, Otten J. Dobutamine stress echocardiography in the evaluation of late anthracycline cardiotoxicity in childhood cancer survivors. Pediatr Res. 1996;39:504–512. doi: 10.1203/00006450-199603000-00020. [DOI] [PubMed] [Google Scholar]
- 8.Iarussi D, Galderisi M, Ratti G, Tedesco MA, Indolfi P, Casale F, Di Tullio MT, de Divitiis O, Iacono A. Left ventricular systolic and diastolic function after anthracycline chemotherapy in childhood. Clin Cardiol. 2001;24: 663–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, Ga-name J, Sebag IA, Agler DA, Badano LP, Banchs J, Cardinale D, Carver J, Cerqueira M, DeCara JM, Edvardsen T, Flamm SD, Force T, Griffin BP, Jerusalem G, Liu JE, Magalhães A, Marwick T, Sanchez LY, Sicari R, Villarraga HR, Lancellotti P. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911–939. doi: 10.1016/j.echo.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Russell RR, Alexander J, Jain D, Poornima IG, Srivastava AV, Storozynsky E, Schwartz RG. The role and clinical effectiveness of multimodality imaging in the management of cardiac complications of cancer and cancer therapy. J Nucl Cardiol. 2016;23:856–884. doi: 10.1007/s12350-016-0538-8. [DOI] [PubMed] [Google Scholar]
- 11.Hundley WG, Kitzman DW, Morgan TM, Hamilton CA, Darty SN, Stewart KP, Herrington DM, Link KM, Little WC. Cardiac cycle-dependent changes in aortic area and distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol. 2001;38:796–802. [DOI] [PubMed] [Google Scholar]
- 12.Kitzman DW, Higginbotham MB, Cobb FR, Sheikh KH, Sullivan MJ. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank-Starling mechanism. J Am Coll Cardiol. 1991;17:1065–1072. [DOI] [PubMed] [Google Scholar]
- 13.Skaluba SJ, Litwin SE. Mechanisms of exercise intolerance: insights from tissue Doppler imaging. Circulation. 2004;109:972–977. doi: 10.1161/01.CIR.0000117405.74491.D2. [DOI] [PubMed] [Google Scholar]
- 14.Drafts BC, Twomley KM, D’Agostino R Jr, Lawrence J, Avis N, Ellis LR, Thohan V, Jordan J, Melin SA, Torti FM, Little WC, Hamilton CA, Hundley WG. Low to moderate dose anthracycline-based chemotherapy is associated with early noninvasive imaging evidence of subclinical cardiovascular disease. JACC Cardiovasc Imaging. 2013;6:877–885. doi: 10.1016/j.jcmg.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jordan JH, D’Agostino RB Jr, Hamilton CA, Vasu S, Hall ME, Kitzman DW, Thohan V, Lawrence JA, Ellis LR, Lash TL, Hundley WG. Longitudinal assessment of concurrent changes in left ventricular ejection fraction and left ventricular myocardial tissue characteristics after administration of cardiotoxic chemotherapies using T1-weighted and T2-weighted cardiovascular magnetic resonance. Circ Cardiovasc Imaging. 2014;7:872–879. doi: 10.1161/CIRCIMAGING.114.002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chotenimitkhun R, D’Agostino R Jr, Lawrence JA, Hamilton CA, Jordan JH, Vasu S, Lash TL, Yeboah J, Herrington DM, Hundley WG. Chronic statin administration may attenuate early anthracycline-associated declines in left ventricular ejection function. Can J Cardiol. 2015;31:302–307. doi: 10.1016/j.cjca.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rector TS, Cohn JN. Assessment of patient outcome with the Minnesota Living with Heart Failure questionnaire: reliability and validity during a ran domized, double-blind, placebo-controlled trial of pimobendan. Pimobendan Multicenter Research Group. Am Heart J. 1992;124:1017–1025. [DOI] [PubMed] [Google Scholar]
- 18.Hundley WG, Bluemke DA, Finn JP, Flamm SD, Fogel MA, Friedrich MG, Ho VB, Jerosch-herold M, Kramer CM, Manning WJ. ACCF/ACR/AHA/ NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on expert consensus documents. Circulation. 2010;121: 2462–2508. doi: 10.1161/CIR.0b013e3181d44a8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–2155. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hees PS, Fleg JL, Lakatta EG, Shapiro EP. Left ventricular remodeling with age in normal men versus women: novel insights using three-dimensional magnetic resonance imaging. Am J Cardiol. 2002;90:1231–1236. [DOI] [PubMed] [Google Scholar]
- 21.Chai JW, Chen WH, Chen HM, Chiang CM, Huang JL, Fu J, Chi-Chang Chen C, Lee SK. Correction of left ventricular wall thickening from short-axis cine MRI for basal-descent through-plane motion. J Magn Reson Imaging. 2011;33:464–473. doi: 10.1002/jmri.22462. [DOI] [PubMed] [Google Scholar]
- 22.Alter P, Rupp H, Stoll F, Adams P, Figiel JH, Klose KJ, Rominger MB, Maisch B. Increased end diastolic wall stress precedes left ventricular hypertrophy in dilative heart failure–use of the volume-based wall stress index. Int J Cardiol. 2012;157:233–238. doi: 10.1016/j.ijcard.2011.07.092. [DOI] [PubMed] [Google Scholar]
- 23.Bombardini T, Costantino MF, Sicari R, Ciampi Q, Pratali L, Picano E. End-systolic elastance and ventricular-arterial coupling reserve predict cardiac events in patients with negative stress echocardiography. Biomed Res Int. 2013;2013:235194. doi: 10.1155/2013/235194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaosuwannakit N, D’Agostino R Jr, Hamilton CA, Lane KS, Ntim WO, Lawrence J, Melin SA, Ellis LR, Torti FM, Little WC, Hundley WG. Aortic stiffness increases upon receipt of anthracycline chemotherapy. J Clin Oncol. 2010;28:166–172. doi: 10.1200/JCO.2009.23.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suter TM, Ewer MS. Cancer drugs and the heart: importance and management. Eur Heart J. 2013;34:1102–1111. doi: 10.1093/eurheartj/ehs181. [DOI] [PubMed] [Google Scholar]
- 26.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM, Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–2150. [DOI] [PubMed] [Google Scholar]
- 27.Reichek N, Glick J, Davis E, Keller C, Alavi A. Anatomic remodeling of the left-ventricle precedes functional impairment in adriamycin cardiac toxicity. Clinical Research. 1979;27:A198–A198. [Google Scholar]
- 28.Moslehi J, Amgalan D, Kitsis RN. Grounding cardio-oncology in basic and clinical science. Circulation. 2017;136:3–5. doi: 10.1161/CIRCULATIONAHA.117.025393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ganame J, Claus P, Eyskens B, Uyttebroeck A, Renard M, D’hooge J, Gewillig M, Bijnens B, Sutherland GR, Mertens L. Acute cardiac functional and morphological changes after Anthracycline infusions in children. Am J Cardiol. 2007;99:974–977. doi: 10.1016/j.amjcard.2006.10.063. [DOI] [PubMed] [Google Scholar]
- 30.Tham EB, Haykowsky MJ, Chow K, Spavor M, Kaneko S, Khoo NS, Pagano JJ, Mackie AS, Thompson RB. Diffuse myocardial fibrosis by T1-mapping in children with subclinical anthracycline cardiotoxicity: relationship to exercise capacity, cumulative dose and remodeling. J Cardiovasc Magn Reson. 2013;15:48. doi: 10.1186/1532-429X-15-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armstrong GT, Plana JC, Zhang N, Srivastava D, Green DM, Ness KK, Daniel Donovan F, Metzger ML, Arevalo A, Durand JB, Joshi V, Hudson MM, Robison LL, Flamm SD. Screening adult survivors of childhood cancer for cardiomyopathy: comparison of echocardiography and cardiac magnetic resonance imaging. J Clin Oncol. 2012;30:2876–2884. doi: 10.1200/JCO.2011.40.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kazemi-Bajestani SM, Becher H, Fassbender K, Chu Q, Baracos VE. Concurrent evolution of cancer cachexia and heart failure: bilateral effects exist. J Cachexia Sarcopenia Muscle. 2014;5:95–104. doi: 10.1007/s13539-014-0137-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.St John Sutton MG, Plappert T, Crosby L, Douglas P, Mullen J, Reichek N. Effects of reduced left ventricular mass on chamber architecture, load, and function: a study of anorexia nervosa. Circulation. 1985;72:991–1000. [DOI] [PubMed] [Google Scholar]
- 34.Bansal N, Franco VI, Lipshultz SE. Anthracycline cardiotoxicity in survivors of childhood cancer: clinical course, protection, and treatment. Prog Pediatr Cardiol. 2014;36:11–18. doi: 10.1016/j.ppedcard.2014.09.012. [DOI] [Google Scholar]
- 35.Narayan HK, Finkelman B, French B, Plappert T, Hyman D, Smith AM, Margulies KB, Ky B. Detailed echocardiographic phenotyping in breast cancer patients: associations with ejection fraction decline, recovery, and heart failure symptoms over 3 years of follow-up. Circulation. 2017;135:1397–1412. doi: 10.1161/CIRCULATIONAHA.116.023463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lipshultz SE, Lipsitz SR, Sallan SE, Dalton VM, Mone SM, Gelber RD, Colan SD. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23:2629–2636. doi: 10.1200/JCO.2005.12.121. [DOI] [PubMed] [Google Scholar]
- 37.Borow KM, Colan SD, Neumann A. Altered left ventricular mechanics in patients with valvular aortic stenosis and coarction of the aorta: effects on systolic performance and late outcome. Circulation. 1985;72:515–522. [DOI] [PubMed] [Google Scholar]
- 38.Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, Yeh ET. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18:1639–1642. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 39.Hornsby WE, Douglas PS, West MJ, Kenjale AA, Lane AR, Schwitzer ER, Ray KA, Herndon JE 2nd, Coan A, Gutierrez A, Hornsby KP, Hamilton E, Wilke LG, Kimmick GG, Peppercorn JM, Jones LW. Safety and efficacy of aerobic training in operable breast cancer patients receiving neoadjuvant chemotherapy: a phase II randomized trial. Acta Oncol. 2014;53:65–74. doi: 10.3109/0284186X.2013.781673. [DOI] [PubMed] [Google Scholar]
- 40.Bowles EJ, Wellman R, Feigelson HS, Onitilo AA, Freedman AN, Delate T, Allen LA, Nekhlyudov L, Goddard KA, Davis RL, Habel LA, Yood MU, McCarty C, Magid DJ, Wagner EH; Pharmacovigilance Study Team. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst. 2012;104:1293–1305. doi: 10.1093/jnci/djs317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bellenger NG, Davies LC, Francis JM, Coats AJ, Pennell DJ. Reduction in sample size for studies of remodeling in heart failure by the use of cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2000;2: 271–278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.