Abstract
GABAA receptors are critical in controlling neuronal activity. Here, we examined the role for phospholipase C-related inactive protein type 1 (PRIP-1), which binds and inactivates protein phosphatase 1α (PP1α) in facilitating GABAA receptor phospho-dependent regulation using PRIP-1-/- mice. In wild-type animals, robust phosphorylation and functional modulation of GABAA receptors containing β3 subunits by cAMP-dependent protein kinase was evident, which was diminished in PRIP-1-/- mice. PRIP-1-/- mice exhibited enhanced PP1α activity compared with controls. Furthermore, PRIP-1 was able to interact directly with GABAA receptor β subunits, and moreover, these proteins were found to be PP1α substrates. Finally, phosphorylation of PRIP-1 on threonine 94 facilitated the dissociation of PP1α-PRIP-1 complexes, providing a local mechanism for the activation of PP1α. Together, these results suggest an essential role for PRIP-1 in controlling GABAA receptor activity via regulating subunit phosphorylation and thereby the efficacy of neuronal inhibition mediated by these receptors.
Keywords: GABA, receptor, phosphorylation, phosphatase, protein kinase, cAMP
Introduction
GABAA receptors are the predominant transducers of fast synaptic inhibitory neurotransmission in the brain. These receptors are heteropentamers that can be assembled from seven subunit classes with multiple members: α1-6, β1-3, γ1-3, δ, ϵ, π, and θ (Whiting et al., 1999), with the most common subtype in the brain composed of α, β, and γ2 subtypes (Whiting et al., 1999).
One major mechanism for regulating the functional properties of GABAA receptors is the modification of receptor structure via phosphorylation (Swope et al., 1999; Brandon et al., 2002a). Biochemical studies have revealed that β1-3 and γ2 subunits are phosphorylated by cAMP-dependent protein kinase A (PKA) and protein kinase C (PKC) (Moss et al., 1992a,b; Krishek et al., 1994; McDonald et al., 1998). PKA phosphorylates Serine 409 (S409) in the β1 and 408/9 in the β3 subunits to modulate GABAA receptor function (Porter et al., 1990; Moss et al., 1992b; Krishek et al., 1994; McDonald et al., 1998). The phosphorylation of GABAA receptors is regulated via the interaction of receptor β and γ subunits with a number of signaling proteins, including the βII isoform of PKC, the receptor for activated C kinase (RACK-1), and A-kinase anchoring protein (AKAP) 79/150 (Brandon et al., 1999, 2000, 2002b, 2003). These protein-protein interactions play critical roles in controlling GABAA receptor phosphorylation (Brandon et al., 1999, 2000, 2002b, 2003; Wang et al., 2002; Cai et al., 2002). To date, however, how specific protein phosphatases are targeted to GABAA receptors to facilitate dephosphorylation and hence dynamic functional modulation remains unknown.
Phospholipase C (PLC)-related inactive protein type 1 (PRIP-1) is a novel inositol 1,4,5-trisphosphate [Ins(1,4,5)P3] binding protein (Kanematsu et al., 1992; Yoshida et al., 1994), which is homologous to PLC-δ1 but catalytically inactive (Kanematsu et al., 1992, 1996, 2000; Yoshida et al., 1994; Takeuchi et al., 1996, 1997, 2000). PRIP-1 has a number of binding partners, including catalytic subunit of protein phosphatase 1α (PP1α) and GABAA receptor-associated protein (GABARAP) (Wang et al., 1999; Yoshimura et al., 2001; Kanematsu et al., 2002). A role for PRIP-1 in regulating GABAA receptor function is further suggested by the phenotype of PRIP-1 knock-out (PRIP-1-/-) mice, which have altered GABAA receptor pharmacology and behavior (Kanematsu et al., 2002). Recently, a second PRIP isoform, PRIP-2, has been identified, which, like PRIP-1, binds both GABARAP and PP1α, suggesting a central role for all PRIP isoforms in modulating GABAA receptor signaling (Uji et al., 2002).
Here, we examined the role of PRIP-1 in GABAA receptor phospho-dependent modulation using PRIP-1-/- mice. We focused on PKA-dependent modulation of GABAA receptor activity, because this has been extensively characterized using both biochemical and physiological methodologies (Moss et al., 1992a,b; McDonald et al., 1998; Brandon et al., 2002a, 2003). PKA-dependent phosphorylation and functional modulation of GABAA receptors containing the β3 subunit were greatly reduced in hippocampal slices from PRIP-1-/- mice. In PRIP-1-/- mice, elevated activity of PP1α was evident, which was responsible for reduced phosphorylation of GABAA receptor β3 subunit in these animals. PKA also phosphorylated PRIP-1 on threonine 94, an important residue for controlling the binding and activity of PP1α, providing a mechanism for the local activation of this phosphatase. Together, our results suggest that PRIP-1 plays a central role in controlling GABAA receptor phospho-dependent modulation and, therefore, the efficacy of synaptic inhibition mediated by these receptors.
Materials and Methods
Labeling and immunoprecipitation of brain slices for phosphorylation assay. Hippocampal slices (400 μm thick) from wild-type and PRIP-1-/- mice (Kanematsu et al., 2002) were prepared with a microslicer (LeicaVT1000S; Leica, Nussloch, Germany) and pooled in ice-cold oxygenated NaHCO3-buffered saline containing the following (in mm): 125 NaCl, 4 KCl, 26 NaHCO3, 1.5 MgSO4, 1.5 CaCl2, and 10 glucose, pH 7.4. The slices were transferred individually to polypropylene tubes containing 2 ml of fresh saline, gassed with a mixture of 95% O2-5% CO2, and maintained in a 30°C water bath. Labeling was performed by adding a 1.5 mCi of [32P]orthophosphate (specific activity, 8500-9120 Ci/mmol; PerkinElmer Life Sciences, Norwalk, CT) for 60 min. The radioactive saline was aspirated, and slices were washed twice with fresh saline and incubated in the presence of substances of interest. [32P]phosphate-labeled slices were homogenized as described previously (Snyder et al., 1998). Aliquots of the homogenate were used for the determinations of the protein concentration and total [32P]phosphate incorporation into trichloroacetic acid-insoluble protein. Protein G-Sepharose (10 μl of 75% slurry; Amersham Biosciences, Arlington Heights, IL) was added to each sample and gently rotated for 30 min at 4°C. This procedure was effective for minimizing nonspecific binding of extract proteins to the beads in the immunoprecipitation experiments. The beads were precipitated by centrifugation for 10 sec at 3000 rpm, and the supernatant was transferred to a new tube containing indicated antibodies and incubated for 2 hr, followed by the addition of 20 μl of protein G-Sepharose beads and incubation for an additional 1 hr at 4°C. The beads were precipitated by centrifugation and washed once with Buffer A composed of 20 mm Tris-HCl, pH 8.0, 150 mm NaCl, 5 mm EDTA, 1% Triton X-100, and 0.2% bovine serum albumin (BSA); three times with 1 ml of Buffer B containing Buffer A plus 0.1% SDS; three times with Buffer C containing 20 mm Tris-HCl, pH 8.0, 500 mm NaCl, 0.5% Triton X-100, and 0.2% BSA; and once with 1 ml of 50 mm Tris-HCl, pH 8.0. After the final wash, the beads were resuspended in 30 μl of a sample buffer for SDS-PAGE, followed by an electrophoresis, autoradiography, and analysis with a Fuji BAS 2500 image analyzer (Fujifilm, Tokyo, Japan). For the assay of phospho-S408/9 of β3 subunit, hippocampal slices (400 μm thick) from wild-type and PRIP-1-/- mice were extracted with Buffer A without BSA, followed by an SDS-PAGE and immunoblotting with antibodies specific to anti-phospho-S408/9 of β3 subunit (Brandon et al., 2002b, 2003; Jovanovic et al., 2004) or to anti-β2/3 subunit (clone 62-3G1; Upstate Biotechnology, Lake Placid, NY). Samples ranging from 20 to 50 μg were applied to each lane and detected by an ECL-plus kit (Amersham Biosciences).
In vitro kinase assay for PKA activity. Kinase activity in the brain extract was analyzed as described previously (Wang et al., 1998). Briefly, brain slices were incubated with agonists, washed with PBS, and homogenized with ice-cold extraction buffer containing 25 mm Tris-HCl, pH 7.4, 0.5 mm EDTA, 0.5 mm EGTA, 10 mm β-mercaptoethanol, 1 μg/ml leupeptin, 1 μg/ml aprotinin, and 0.5 mm phenylmethylsulfonyl fluoride. Equal amounts of the extract were added to a reaction mixture containing 40 mm Tris-HCl, pH 7.4, 20 mm MgCl2, 0.1 mg/ml BSA, 0.1 mm biotinylated PKA peptide substrate (Kemptide, LRRASLG; Promega, Madison, WI), 0.5 μCi [γ-32P]ATP (specific activity, 3000 Ci/mmol; PerkinElmer Life Sciences), and 0.1 mm ATP. The reaction was allowed for 5 min at 30°C and then terminated by the addition of 2.5 m guanidine hydrochloride. A 10 μl sample was spotted onto streptavidin-coated disks, washed repeatedly, dried, and counted for radioactivity. The PKA activities in the proximity of receptors were analyzed using the immunoprecipitates by anti-β3 antibodies. The immunoprecipitates prepared from either genotype of mice were incubated in the presence of 1 μm cAMP or 5′-AMP for 10 min at 30°C, and the supernatant was analyzed for the PKA activity assay as described above.
Protein phosphatase activity assay. PP1α activity in the brain extract was assayed by the release of 32P-phosphate from phosphorylase a as described previously (Cohen et al., 1988). Slices were homogenized in a buffer containing 50 mm Tris-HCl, pH 7.0, 10 mm β-mercaptoethanol, 1 mm EDTA, 0.1 mm phenylmethylsulfonyl fluoride, 2 mm benzamidine, 1 μg/ml leupeptin, and 1 μg/ml aprotinin. Brain lysates were centrifuged, and PP1α was immunoprecipitated with goat polyclonal anti-PP1α (C-19; Santa Cruz Biotechnology, Santa Cruz, CA) for 1 hr, followed by the addition of 20 μl of protein G-Sepharose beads and incubation for 1 hr at 4°C. The beads were precipitated by centrifugation and washed three times with lysis buffer and three times with dilution buffer (50 mm imidazole-HCl, pH 7.2, 0.6 mm EGTA, pH 7.2, 0.3% β-mercaptoethanol, 3 mg/ml BSA) and then resuspended in 20 μl of dilution buffer. Assays were performed by mixing 20 μl of [32P]phosphorylase a (prepared by the incubation with phosphorylase b, phosphorylase kinase, and [γ-32P]ATP) and 20 μl of precipitated samples for 10 min at 30°C, followed by the addition of 200 μl of 25% trichloroacetic acid. The mixture was kept for 10 min at room temperature and centrifuged at 15,000 rpm for 5 min, and the aliquot was counted for radioactivity.
Dephosphorylation of GABAA receptor β3-subunit by PP1α, PP2A, and PP2B. Glutathione S-transferase (GST)-tagged intracellular loop of β3 subunit (residues 303-425) immobilized on glutathione Sepharose 4B beads (Amersham Biosciences) was phosphorylated with 10 μm ATP containing 2 μCi [γ-32P]ATP and 0.1 μg of the catalytic subunit of PKA, followed by washing with dephosphorylation buffer (50 mm Tris-HCl, pH 7.5, 1 mm MnCl2, 150 mm NaCl, 2 mm EGTA, and 1 mm DTT, 50 mm Tris-HCl, pH 8.5, 20 mm MgCl2, and 1 mm DTT, or 50 mm Tris-HCl, pH 7.0, 1 mm NiCl2, and 10 μg/ml calmodulin for PP1α, PP2A, or PP2B, respectively). The resulting beads were incubated in 50 μl of dephosphorylation buffer containing 0.5 or 1 U of PP1α, PP2A, or PP2B (Promega) at 30°C for 10 min. 32P, thus liberated, was counted by a scintillation counter, and the dephosphorylation was visualized by SDS-PAGE of GST-β3, followed by autoradiography.
Electrophysiology. Mice (2-3 weeks of age) were decapitated under pentobarbital anesthesia (50 mg/kg, i.p.). The brain was dissected and transversely sliced at a thickness of 350 μm using a microslicer. Slices containing the hippocampus were kept in an incubation medium (in mm: 124 NaCl, 5 KCl, 1.2 KH2PO4, 24 NaHCO3, 2.4 CaCl2, 1.3 MgSO4, and 10 glucose) saturated with 95% O2 and 5% CO2 at room temperature (21-24°C) for at least 1 hr before acute mechanical dissociation. The details of mechanical dissociation have been described previously (Rhee et al., 1999). Such mechanically dissociated neurons retained a short portion of their proximal dendrites. All electrical measurements were performed using the conventional whole-cell patch-recording mode at a holding potential of 0 mV using a patch-clamp amplifier (EPC-7; Heka, Lambrecht/Pfalz, Germany). Patch pipettes were made from borosilicate capillary glass (1.5 mm outer diameter, 0.9 mm inner diameter; G-1.5; Narishige, Tokyo, Japan) in two stages on a vertical pipette puller (PB-7; Narishige). The resistance of the recording pipettes filled with internal solution (in mm: 135 Cs-methanesulfonate, 5 TEA-Cl, 5 CsCl, 2 EGTA, 10 HEPES, and 4 Mg-ATP, pH 7.2, with Tris-base) was 3-4MΩ. During electrical measurements, some drugs were added to pipette solution. Electrode capacitance and liquid junction potential were compensated, but the series resistance was not compensated. Neurons were viewed under phase contrast on an inverted microscope (400×; Diapot; Nikon, Tokyo, Japan). The membrane currents were filtered at 1 kHz (E-3201A decade filter; NF Corporation, Yokohama, Japan), digitized at 4 kHz, and stored on a computer equipped with pClamp 8.0 (Axon Instruments, Union City, CA). When recording, 10 mV hyperpolarizing step pulses (30 msec in duration) were periodically delivered to monitor the access resistance. Neurons were consistently perfused with an external solution (in mm: 150 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 glucose, and 10 HEPES, pH 7.4, with Tris-base) by using the “Y-tube system” (Akaike and Harata, 1994). The external solution routinely contained 300 nm tetrodotoxin to block voltage-dependent Na+ channels and 10 μm 6-cyano-7-nitroquinoxaline-2,3-dione and 20 μm dl-2-amino-5-phosphonovaleric acid to block glutamatergic currents. All experiments were performed at room temperature (21-24°C).
In vitro phosphorylation of PRIP-1 by PKA. Recombinant full-length PRIP-1 or the short version [PRIP-1(82-232)] was first incubated in 50 μl of 40 mm Tris-HCl, pH 7.5, 5 mm magnesium acetate with 200 μm ATP, and 0.1 μg of the catalytic subunit of PKA for 30 min at 30°C. In some assays, 1 μCi of [γ-32P]ATP was included as a tracer.
Immobilized metal affinity chromatography and matrix-assisted laser desorption-ionization time-of-flight mass spectrometry. Full-length PRIP-1 phosphorylated by PKA in vitro as described above was subjected to SDS-PAGE, followed by an electrical transfer to a nitrocellulose membrane. The membrane was stained with Ponceau S, and the band of PRIP-1 was excised from the membrane and washed with deionized water. The pieces of membrane corresponding to the PRIP-1 band was then incubated with 0.5% polyvinylpyrrolidone in 0.1 m acetic acid for 30 min at 37°C and washed once with 50 mm ammonium bicarbonate, once with deionized water. Tryptic digestion was achieved by incubating the pieces of membrane in 30 μl of 50 mm ammonium bicarbonate with 0.375 ng of trypsin (sequencing grade; Promega) at 37°C overnight. Phosphopeptides present in tryptic digestion mixtures were purified by custom-made miniaturized Fe(III)-immobilized metal affinity chromatography (IMAC) columns as described previously (Stensballe et al., 2001). In brief, phosphopeptides were loaded slowly onto the Fe(III)-IMAC column, rinsed with 40 μl of 0.1 m acetic acid, 40 μl of 0.1 m acetic acid, and acetonitrile (3:1, v/v), and eluted twice with 5 μl of pH 10.5 by the addition of 25% ammonia. The eluate was acidified and loaded directly onto a nanoscale column packed with Poros Oligo R3 material (Applied Biosystems, Foster City, CA). The column was rinsed with 5% formic acid and then eluted with 1 μl of saturated matrix solution [2,5-dihydroxybenzoic (Sigma-Aldrich, Milwaukee, WI) in 50% acetonitrile/2.5% formic acid]. The matrix eluate was directly spotted onto the matrix-assisted laser desorption-ionization (MALDI) plate and recorded on a Reflex reflector time-of-flight (TOF) mass spectrometry (MS) (Bruker-Daltonics, Bremen, Germany).
In vitro pull-down assay. GST-PP1α fusion protein and recombinant short PRIP-1(82-232) were prepared as described previously (Yoshimura et al., 2001). Full-length GST-GABARAP was prepared as described previously (Kanematsu et al., 2002). Constructing strategies of GST-tagged loop regions of α1, α2, α3, α6, β1, β2, β3, γ2, δ, and ρ1 were described previously (Brandon et al., 1999; Hanley et al., 1999). The recombinant proteins were expressed in Escherichia coli and purified by affinity chromatography. Pull-down assays using these recombinant molecules were performed as described previously (Brandon et al., 1999; Hanley et al., 1999; Yoshimura et al., 2001; Kanematsu et al., 2002).
Immunopreciptation assay. The mouse brain slices, or COS-7 cells, transfected with PRIP-1 (wild-type or T94A mutant) were stimulated with agonists (forskolin or D1-specific agonist), followed by homogenization with a lysis buffer (50 mm Tris-HCl, pH 7.5, 0.5 mm EDTA, 2 mm EGTA, 1% Triton X-100, 2 mm benzamidine). Lysates were centrifuged, and the β3 subunit or PP1α was immunoprecipitated with goat polyclonal anti-β3 (C-20; Santa Cruz Biotechnology) or anti-PP1α (C-19; Santa Cruz Biotechnology) for 1 hr, followed by an addition of 20 μl of protein G-Sepharose beads and incubation for 1 hr at 4°C. The beads were precipitated by centrifugation and washed three times with a lysis buffer, followed by an SDS-PAGE and immunoblotting with antibodies specific to PRIP-1 or PP1α (Upstate Biotechnology) and detected by an ECL kit (Amersham Biosciences).
Results
Analysis of GABAA receptor β3 subunit phosphorylation in PRIP-1-/- mice
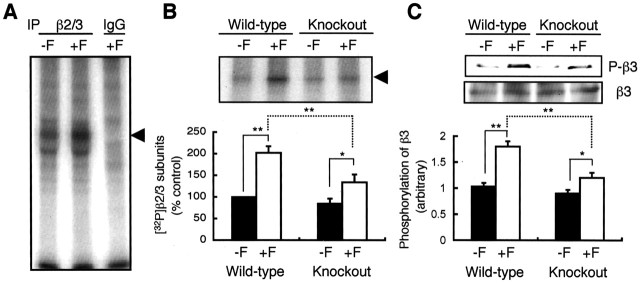
To study the possible role of PRIP-1 in GABAA receptor phospho-dependent regulation, we used PRIP-1-/- mice. Our studies focused on PKA-mediated modulation, because this is dependent on the direct phosphorylation of conserved serine residues within the intracellular domains of β subunit isoforms (Moss et al., 1992a; McDonald et al., 1998; Brandon et al., 2002a). For these studies, we used hippocampal slices from PRIP-1-/- mice or age-matched controls. Slices were labeled with 32P-orthophosphoric acid, and PKA was activated using forskolin. GABAA receptors were then immunoprecipitated with an antibody against the β2 and β3 subunits, because these are the major β subunit isoforms expressed in the hippocampus (Pirker et al., 2000). Under these conditions, the incorporation of the 32P into a major band with a molecular mass of 58 kDa was observed under basal conditions and was increased by forskolin treatment in slices from the control mice. This band was not evident when immunoprecipitations were performed using control IgG (Fig. 1A). Forskolin caused a twofold increase in incorporation of 32P radioactivity into β2/3 subunits of GABAA receptors from wild-type mice, but a much smaller increase was seen in slices from PRIP-1-/- mice (Fig. 1B). Previous studies have established that GABAA receptor β subunits are differentially phosphorylated by PKA. The β1 and β3 subunits are phosphorylated on serines 409 and 408/9, respectively, whereas the analogous residues in β2 are not PKA substrates (McDonald et al., 1998). To further analyze PKA phosphorylation of GABAA receptors, we used a phospho-specific antibody against S408/9 in the β3 subunit (Brandon et al., 2002b, 2003; Jovanovic et al., 2004). Forskolin produced an enhancement of S408/9 phosphorylation without modifying the overall levels of the β3 subunit in wild-type mice, but much smaller effects were seen in PRIP-1-/- mice (Fig. 1C).
Figure 1.
Phosphorylation of the β3 subunits of GABAA receptor by forskolin in wild-type and PRIP-1-/- mice. A, Hippocampal slices from wild-type mice were labeled with 1.5 mCi [32P]orthophosphate for 60 min and treated with 50 μm forskolin (+F) or vehicle only (-F). Homogenates (∼0.8 mg of hippocampal protein from two slices) were immunoprecipitated by anti-β2/3 antibodies (6.0 μg; clone 62-3G1; Upstate Biotechnology) or control IgG and protein G-Sepharose, followed by SDS-PAGE and autoradiography. B, Hippocampal slices from wild-type or PRIP-1-/- mice were labeled with [32P]orthophosphate and immunoprecipitated as described in A; a typical gel is shown (top) quantitated (bottom). Statistical analyses were performed using Student's t test (*p < 0.05, **p < 0.01; n = 6). C, Hippocampal slices were stimulated with forskolin (+F) for 5 min, and the extracts were analyzed by SDS-PAGE and immunoblotting. Typical immunoblots of hippocampal extracts by anti-phospho-S408/9 (P-β3) and the β2/3 subunit antibody subunit antibodies are shown in the top panels. The bottom panel shows a quantification of the blots using the phospho-specific antibody against the GABAA receptor β2/3 subunit. Results were expressed as the densities of anti-phospho-S408/9 relative to those of anti-β3. *p < 0.05 and **p < 0.01 using Student's t test (n = 4).
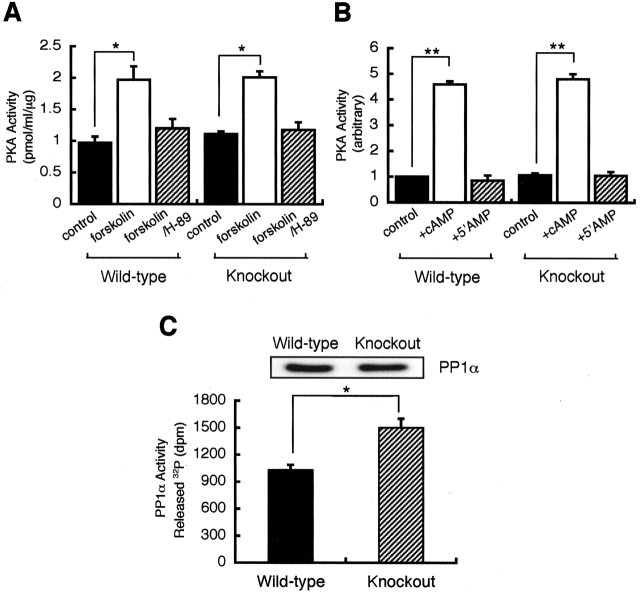
The differing effects of forskolin on GABAA receptor phosphorylation between wild-type and PRIP-1-/- mice may be attributable to either decreased activity of PKA or modified phosphatase activity. These possibilities were examined in hippocampal extracts from wild-type and PRIP-1-/- mice. Forskolin-treated hippocampal extracts from either wild-type or PRIP-1-/- animals exhibited an identical twofold enhancement in phosphorylation of Kemptide, an exogenous PKA substrate, which could be blocked by H-89, a PKA inhibitor (Fig. 2A). This observation suggests that there is no significant difference in PKA activity between PRIP-1-/- and control mice. GABAA receptors incorporating β3 subunits are associated with PKA activity in neurons, an interaction that is facilitated by AKAP79/150 (Brandon et al., 2003). Therefore, we assessed whether there were any differences in local PKA activity bound to GABAA receptors in PRIP-1-/- mice. GABAA receptors were immunoprecipitated from PRIP-1-/- mice and controls using an antibody against the β3 subunit. Precipitated material was then assessed for PKA activity using the Kemptide peptide in the presence and absence of cAMP or 5′-AMP. Phosphorylation of Kemptide was enhanced 4.5-fold in immunoprecipitates from both genotypes of mice, whereas 5′-AMP was without effect (Fig. 2B). The results clearly indicate that the PKA activities associated with GABAA receptors were similar between the control and mutant mice. These results suggest that PRIP-1 is unlikely to act as an AKAP-targeting PKA to GABAA receptors; more over, we were unable to demonstrate binding of either the catalytic or regulatory subunits of PKA to PRIP-1 (supplementary Fig. 1, available at www.jneurosci.org/cgi/content/full/24/32/7074/DC1).
Figure 2.
PKA and phosphatase activities in hippocampal extract from the wild-type and PRIP-1-/- mice. A, Hippocampal slices were stimulated with forskolin (50 μm) for 5 min or together with H-89 at 0.5 μm, which was added 15 min before the addition of forskolin. PKA activity in the extract was measured as described in Materials and Methods. Results are expressed as mean ± SE from three separate experiments. *p < 0.05 using Student's t test. B, Extracts of hippocampal slices were immunoprecipitated by anti-β3 antibodies, followed by the assay of PKA activity in the presence or absence of 1 μm cAMP or 5′-AMP. Results are expressed relative to those observed in the absence of cAMP of the wild-type mice in mean ± SE from three separate experiments. **p < 0.01 using Student's t test. C, Extracts of hippocampal slices were immunoprecipitated by anti-PP1α antibodies (6 μg; C-19; Santa Cruz Biotechnology), followed by the assay of the activities using the phosphorylated phosphorylase a (13,000 dpm of radioactivity) as a substrate for 10 min at 30°C. The results show the 32P-radioactivities released from phosphorylated phosphorylase a, representing mean ± SE of five independent experiments. The presence of similar amounts of PP1α in hippocampal extracts from both genotypes of mice was confirmed by immunoblotting as shown in the top panel. *p < 0.05 using Student's t test.
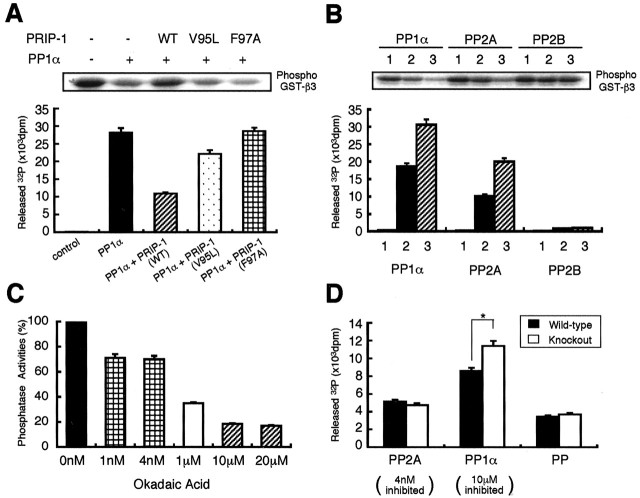
Next, we measured the level of PP1α and its activity in wild-type and PRIP-1-/- animals, because we have shown previously that PRIP-1 is a negative modulator of this phosphatase (Yoshimura et al., 2001). PP1α was immunoprecipitated from hippocampal extracts, and its activity was measured using [32P]phosphorylase a as a substrate. Similar amounts of PP1α were found in each mouse strain as measured by immunoblotting (Fig. 2C, top), but the activity was ∼43% higher in PRIP-1-/- mice compared with wild-type controls (Fig. 2C). This elevated level of PP1α activity in PRIP-1-/- mice may be significant for the reduced PKA-mediated phosphorylation of GABAA receptor subunits observed in hippocampal slices of these animals compared with controls. Therefore, we examined the dephosphorylation of the GABAA receptor β3 subunit using the intracellular domain of this receptor subunit expressed as a GST fusion protein (GST-β3). This protein was phosphorylated in vitro with the catalytic subunit of PKA and [γ-32P]ATP. Under these conditions, we established previously that only S408/9 within the intracellular domain of the β3 subunit is phosphorylated, because GST itself is not a substrate of PKA (Moss et al., 1992b; McDonald and Moss, 1997; Jovanovic et al., 2004). Phosphorylated GST-β3 was dephosphorylated by recombinant PP1α, which was inhibited by the addition of PRIP-1, whereas the mutant versions of this protein, V95L or F97A, exerted partial or no inhibition, respectively (Fig. 3A), consistent with the ability of PRIP-1 to bind and inactivate PP1α [Yoshimura et al. (2001), their Fig. 2Bd]. Next, we tested the ability of other purified phosphatases to dephosphorylate GST-β3 focusing on the roles of PP2A and PP2B. In common with PP1α, PP2A catalyzed the dephosphorylation of GST-β3, but dephosphorylation was not modified by the presence of PRIP-1 (T. Kanematsu and M. Hirata, unpublished observation), whereas PP2B was without effect (Fig. 3B). These observations are consistent with a recent report showing that phosphorylated GABAA receptor β3 subunits are not substrates of PP2B but can be dephosphorylated by PP2A activity in cortical neurons (Jovanovic et al., 2004). Therefore, the results indicate that both PP1α and PP2A can dephosphorylate the β3 subunit intracellular domain when phosphorylated on S408/9. Moreover, these results also reveal that PP1α dephosphorylation of GST-β3 can be negatively regulated by the interaction with PRIP-1.
Figure 3.
Phosphatase activities in hippocampal extracts and dephosphorylation of β3 subunit. A, GST-tagged β3 subunit (200 pmol) immobilized on glutathione Sepharose 4B beads was phosphorylated with [γ-32P]ATP using the catalytic subunit of PKA. The beads were subjected to dephosphorylation by 1 U of PP1α, and released 32P was counted by a liquid scintillation counter. Recombinant PRIP-1 [wild type (WT)] or the mutant V95L or F97A (1 nmol) was included in the reaction mixture; these mutants reduce the binding to PP1α partially and totally, respectively (Yoshimura et al., 2001). Results are expressed as mean ± SE from four separate experiments. The top panel shows an autoradiogram of GST-β3 subunit after the treatment with PP1α alone or together with various versions of PRIP-1 molecule. B, GST-β3 subunit phosphorylated as described above was subjected to dephosphorylation by PP1α, PP2A, or PP2B, and released 32P was counted by a scintillation counter. Results are expressed as mean ± SE from four separate experiments. 1, 2, and 3 represent the presence of 0, 0.5, and 1 U of each phosphatase, respectively. The top panel shows an autoradiogram of GST-β3 subunit after the treatment with each phosphatase. C, Hippocampal extract (∼200 μg of protein) from the control mice was assayed for the phosphatase activities, which dephosphorylate phosphorylated GST-β3 subunit in the presence of various concentrations of okadaic acid. The absence of okadaic acid caused the release of 18,900 dpm (mean of 3 experiments), which was given a value of 100%. The relative radioactivities released in the presence of okadaic acid at concentrations indicated are shown. Results are expressed as mean ± SE from three separate experiments. D, The same experiments described in C were also performed using hippocampal extracts from the PRIP-1-/- mice, and the results were compared with those seen in the control mice. Results are expressed as mean ± SE from three separate experiments. *p < 0.05 using Student's t test.
Next analyzed was which of these phosphatases in hippocampal extracts was capable of dephosphorylating phosphorylated GST-β3. In these experiments, we used differing concentrations of okadaic acid to selectively inhibit either PP2A or PP1α. Okadaic acid at 1-4 nm, concentrations at which PP2A will be selectively inhibited (Favre et al., 1997), reduced the dephosphorylation of GST-β3 by hippocampal extracts to 72% of control. Increasing okadaic acid to 10-20 μm, concentrations sufficient to block both PP1α and PP2A (Favre et al., 1997), further reduced dephosphorylation of GST-β3 by hippocampal extracts to 20% of control (Fig. 3C). The remaining phosphatase activity was insensitive to okadaic acid or 100 nm calyculin A (data not shown), suggesting that an unknown enzyme may also participate in the dephosphorylation of GST-β3. Together, these results suggest that PP1α, PP2A, and the unknown phosphatase in hippocampal extracts all participate in dephosphorylation of GST-β3 but with PP1α playing the most predominant role. We also compared the dephosphorylation of GST-β3 by hippocampal extracts from wild-type and PRIP-1-/- mice using okadaic acid at differing concentrations to selectively inhibit PP1α and PP2A (Fig. 3D). These results revealed that phosphatase activity in the fraction inhibited by okadaic acid at concentrations from 4 nm to 10 μm, which probably represents PP1α, was 35% higher in the mutant mice compared with controls, but the other phosphatase activities remained unchanged.
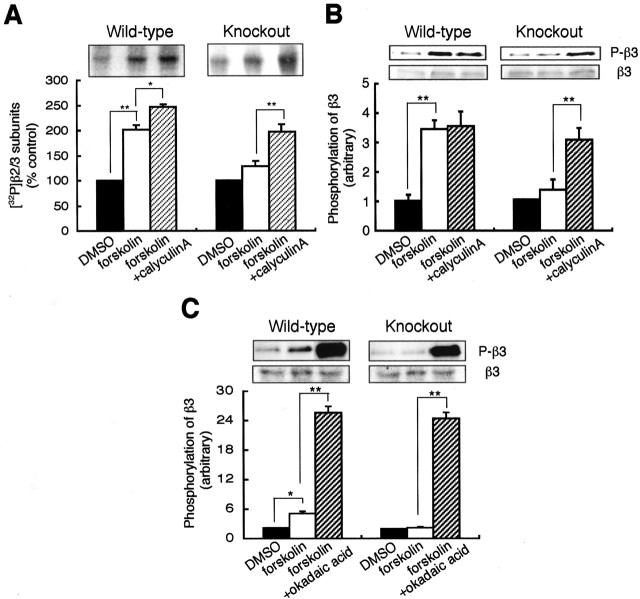
To examine whether this enhanced phosphatase activity in PRIP-1-/- mice may underlie the reduced GABAA receptor phosphorylation by PKA in these animals, hippocampal slices from PRIP-1-/- mice and controls were labeled with 32P-orthophosphate and treated with calyculin A or okadaic acid (Fig. 4). Phosphorylation of the GABAA receptor β2/3 subunits was then examined by immunoprecipitation. Compared with forskolin treatment alone, coapplication with calyculin A produced a robust increase in the phosphorylation of the β2/3 subunits in hippocampal slices from PRIP-1-/- mice. Similar effects of calyculin A and okadaic acid on the phosphorylation of S408/9 in theβ3 subunit were also evident, as measured via immunoblotting with a phospho-specific antibody against these residues (Fig. 4B,C). Together, our results suggest that enhanced activity of the phosphatase in PRIP-1-/- mice is responsible for the reduced PKA phosphorylation of the GABAA receptorβ subunits seen in hippocampal slices from these animals compared with wild-type controls.
Figure 4.
Effect of calyculin A and okadaic acid on the phosphorylation of the GABAA receptor β3 subunits. A, Hippocampal slices labeled with 1.5 mCi [32P]orthophosphate for 60 min were incubated for 5 min in the absence or presence of forskolin (50 μm) or forskolin plus 100 nm calyculin A. Receptors were immunoprecipitated by anti-β2/3 antibodies, followed by SDS-PAGE and autoradiography, as described in Figure 1. The experiments using the slices from the wild-type and mutant mice were independently performed; therefore, each control level of the phosphorylation was taken as 100% resulting from the labeling efficiency, and results are expressed as mean ± SE from four separate experiments. *p < 0.05 and **p < 0.01 by Student's t test. B, C, Hippocampal slices were stimulated with 50 μm forskolin (+F), or together with 100 nm calyculin A (B), or 1 μm okadaic acid (C) for 5 min, and the extracts were analyzed by SDS-PAGE and immunoblotting. Higher concentrations of okadaic acid (e.g., 10 μm) could not be used in the assay systems because of toxicity. Typical immunoblots of hippocampal extracts by anti-phospho-S408/9 of the β3 subunit antibodies (top panel) and anti-β2/3 subunit antibodies (bottom panel) are shown. The graph shows the summary of the results (means ± SE) from three independent preparations. Results were expressed as the densities of anti-phospho-S408/9 relative to those of the anti-β2/3 antibody. *p < 0.05 and **p < 0.01 using Student's t test.
GABAA receptor functional modulation in PRIP-1-/- mice
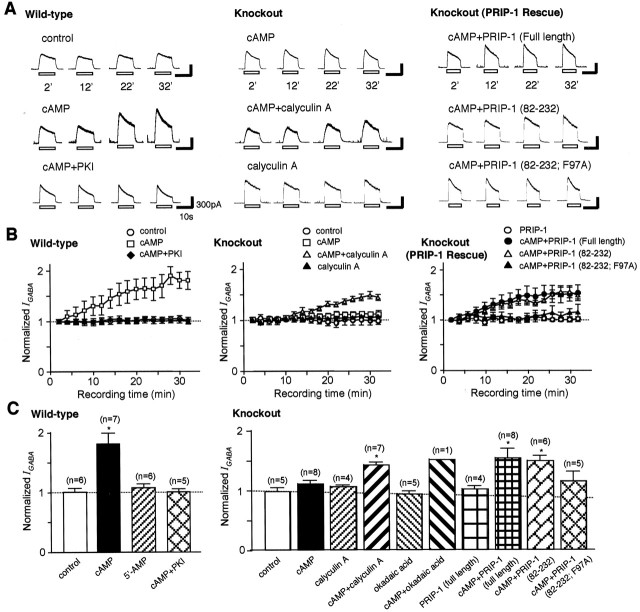
To assess the possible role of PRIP-1 in GABAA receptor phospho-dependent modulation, electrophysiological analyses were performed to measure GABA-evoked currents (IGABA) in hippocampal neurons from both genotypes of mice. Intracellular application of cAMP from the patch pipette, but not 5′-AMP, increased the peak of IGABA in control hippocampal CA3 cells by 70%, which was inhibited by simultaneous application with PKI (an inhibitory peptide of PKA), indicating the involvement of PKA-mediated phosphorylation (Fig. 5, left). This observation is in keeping with the analysis of recombinant receptor functional modulation by PKA activity, which has demonstrated that phosphorylation of GABAA receptors containing β3 subunits on S408/9 enhances receptor activity (McDonald et al., 1998). Neurons from PRIP-1-/- animals exhibited no increases in IGABA during activation of PKA (Fig. 5, right panels). These observations correlate well with the reduced phosphorylation of the β3 subunit seen in PRIP-1-/- animals, suggesting that enhanced phosphatase activity in these mice may be responsible for this loss of GABAA receptor functional modulation. To provide further evidence of this, recordings were made from PRIP-1-/- neurons in the presence of phosphatase inhibitors. A single application of calyculin A or okadaic acid caused little changes in IGABA, but coapplication of the phosphatase inhibitor with cAMP produced a similar enhancement of IGABA, mimicking the events observed in the control mice. To directly test the significance of PRIP-1 in these observations, purified PRIP-1 protein was introduced into the neurons from PRIP-1-/- animals via intracellular dialysis with the recording pipette. PRIP-1 alone had minimal effects on IGABA, but when cAMP was applied to neurons dialyzed with purified PRIP-1 protein, an enhancement of GABAA receptor activity was observed. Similar enhancement of IGABA was also seen when the short version of PRIP-1(82-232), which does not bind GABAA receptor β-subunits (see below), was applied together with cAMP; however, the mutant (F97A), which is not able to bind PP1α, did not enhance IGABA in the presence of cAMP.
Figure 5.
Impaired PKA-dependent potentiation of GABAA receptor-mediated currents in PRIP-1-/- mice. A, Membrane currents (IGABA) activated by application of GABA (5 μm; open bars) were recorded from mechanically dissociated hippocampal CA3 neurons via patch-clamp recording after attaining the whole-cell configuration defined as t = 0 (left panel, wild type; right two panels, PRIP-1 knock-out). In each condition, cAMP (250 μm), 5′-AMP (250 μm), PKI (1 μg/ml), calyculin A (100 nm), okadaic acid (200 nm), or purified PRIP-1 (0.7 μg/ml) was added to the pipette solution. The shortversion of PRIP-1 (82-232) or its mutant (F97A) at 2.3 μg/ml was also used. Traces represent typical GABAA receptor-mediated currents recorded at 2, 12, 22, and 32 min after making whole-cell configuration in each condition. B, Time courses of the PKA-dependent potentiation of GABAA receptor-mediated currents (left panel, wild type; right two panels, PRIP-1 knock-out). To simplify the data obtained from different neurons, GABAA receptor-mediated currents were normalized to the first GABA response (at 2 min). C, Each column represents the relative value of GABA-induced currents measured at 32 min, which was normalized to that measured at 2 min after making whole-cell configuration. *p < 0.05 by Student's t test.
Together, our results illustrate that in PRIP-1-/- mice, phospho-dependent modulation of GABAA receptor function is compromised compared with wild-type animals resulting from enhanced phosphatase activity. This parallels the reduced PKA-mediated phosphorylation of the GABAA β subunits evident in these animals.
PRIP-1 interacts with GABAA receptors
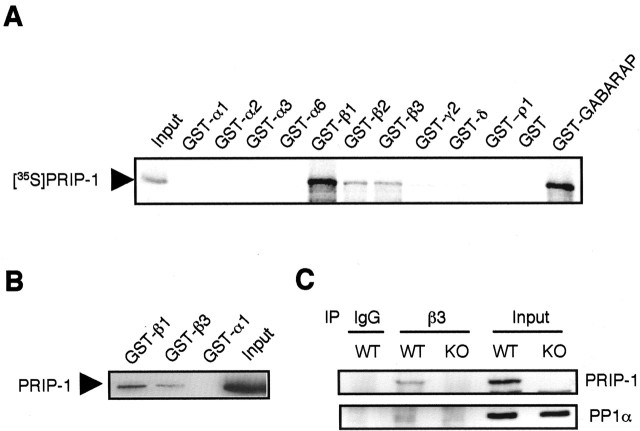
To assess the role of PRIP-1 in targeting of PP1α to GABAA receptors, we tested the possibility that PRIP-1 interacts with these receptors. For these experiments, PRIP-1 was labeled by in vitro translation using 35S-methionine and exposed to the intracellular domains of a range of GABAA receptor subunits expressed as GST fusion proteins. This approach revealed that PRIP-1 was able to bind to the intracellular domains of all GABAA receptor β subunit isoforms and weakly with γ2 subunit but not to those of the α1-6 and δ subunits or to the intracellular domain of the GABAC receptor ρ1 subunit (Fig. 6A). We also characterized the interaction between the intracellular domains of GABAA receptors and PRIP-1 in neuronal extracts using affinity purification followed by immunoblotting with antibody against PRIP-1. These results confirmed that PRIP-1 binds preferentially to GABAA receptor β subunits (Fig. 6B). Because β subunits are critical components for the assembly and cell surface targeting of GABAA receptors (Connolly et al., 1996a,b; Gorrie et al., 1997), these observations suggest that PRIP-1 is able to associate with most receptor subtypes in the brain.
Figure 6.
Interaction between PRIP-1 and GABAA receptors. A, Interaction of recombinant PRIP-1 with specific subunits of GABAA receptor. PRIP-1, [35S]-radiolabeled by invitro transcription and translation system, was analyzed for interaction with the intracellular domains of GABAA receptor and GABAC receptor subunits expressed as GST fusion protein, GST-GABARAP, and GST alone. Bound material was resolved by SDS-PAGE and analyzed by autoradiography. Similar results were seen in two other experiments. B, Interaction of PRIP-1 from mouse brains with the intracellular domains of GABAA receptor subunits. Brain extracts were applied to GST-immobilized beads containing the intracellular loops of α1,β1, or β3 subunit of GABAA receptors. Bound material was then probed with an anti-PRIP-1 antibody (1 μg; prepared in this laboratory) (Takeuchi et al., 2000). Two other experiments provided similar results. C, Interaction of PRIP-1 with GABAA receptors in brain extracts. Brain extracts (∼1 mg of protein) from either genotype of mice were immunoprecipitated by an antibody against the GABAA receptor β3 subunit. Precipitated material was separated by SDS-PAGE and then probed anti-PRIP-1 antibody or with anti-PP1α antibody. Similar results were seen in eight independent experiments. KO, Knock-out; WT, wild type.
We further investigated the interactions between PRIP-1 and GABAA receptors in neurons via immunoprecipitation. Brain extracts were immunoprecipitated with antibody against the β3 subunit followed by the immunoblotting with anti-PRIP-1 antibody. As shown in Figure 6C, PRIP-1 was immunoprecipitated with anti-β3 antibody but not with control IgG. No PRIP-1 could be detected immunoprecipitating with GABAA receptors from extracts prepared from PRIP-1-/- mice. Precipitated material was also blotted with antibody against PP1α. Although this protein could be detected immunoprecipitating with GABAA receptors from control, much lower levels were evident in PRIP-1-/- mice. This residual binding may reflect the targeting of PP1α to GABAA receptors via another anchoring protein, such as PRIP-2 (Uji et al., 2002), or direct association with GABAA receptor subunits. Together, these biochemical approaches strongly suggest a significant role for PRIP-1 in the targeting of PP1α to GABAA receptors.
PKA activity regulates the binding of PRIP-1 to PP1α
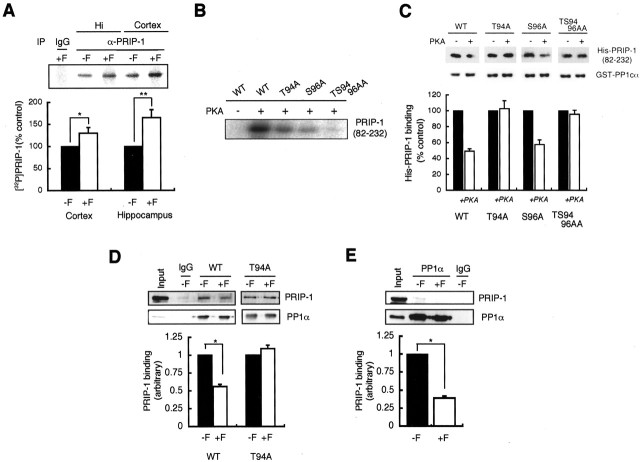
Previous studies have demonstrated that PRIP-1 is phosphorylated by PKA in vitro and that phosphorylation causes the dissociation of PP1α-PRIP-1 complexes (Yoshimura et al., 2001). To examine whether PRIP-1 is phosphorylated by PKA in vivo, cortical and hippocampal slices were labeled with 32P-orthophosphate and treated with forskolin for 5 min. Detergent-soluble extracts were then immunoprecipitated with anti-PRIP-1 antibody, followed by autoradiography. As shown in Figure 7A, basal phosphorylation of a band corresponding to PRIP-1 was observed in both slice types, but phosphorylation was enhanced during the activation of PKA with forskolin.
Figure 7.
PKA-mediated phosphorylation of PRIP-1 regulates interaction with PP1α. A, Cortical and hippocampal slices prelabeled with 1.5 mCi[32P]orthophosphate for 60 min were incubated for 5 min in the absence (-F) or presence (+F) of 50 μm forskolin. Each slice was lysed, and PRIP-1 was then immunoprecipitated with anti-PRIP-1 antibody. Precipitated material was separated by SDS-PAGE, followed by autoradiography. Results are expressed relative to the control slice for cortex and hippocampus in mean ± SE from four separate experiments. *p < 0.05 and **p < 0.01 using Student's t test. B, A short version of recombinant PRIP-1 encoding residues 82-232 was used to introduce the mutations (T94A, S96A, or TS94/96AA) to possible phosphorylation sites for PKA. Each recombinant PRIP-1 (82-232) (150 pmol) was first incubated in a volume of 50 μl with 1 μCi [γ-32P]ATP in the absence or presence of the catalytic subunit of PKA for 5 min at 30°C. The mixtures were subjected to SDS-PAGE and autoradiography. C, Pull-down assay. Wild-type (WT) or mutant PRIP-1(82-232) phosphorylated by PKA or ATP alone was incubated with GST-PP1α immobilized on glutathione Sepharose 4B beads. Material bound was then separated by SDS-PAGE and immunoblotting with antibodies to PRIP-1 [monoclonal antibodies to PRIP-1(95-232) prepared in this laboratory]. The top and bottom panels show a typical immunoblot and the summary of results from five independent experiments, respectively. Data in each case was normalized to binding seen in the absence of PKA. D, Interaction of PRIP-1 with PP1α in COS-7 cells. Cells expressing full-length wild-type PRIP-1 or mutant PRIP-1, in which threonine at 94 was changed to alanine (T94A), were stimulated with forskolin (10 μm) for 30 min. After cell lysis, PP1α was immunoprecipitated, and bound material was probed with anti-PRIP-1 antibody. The bottom panels show a summary of four independent experiments. Data are normalized to the level of PRIP-1 associated with PP1α in the absence of forskolin treatment. *p < 0.05 using Student's t test. E, Interaction of PRIP-1 with PP1α in neurons. Brain slices were stimulated with forskolin (50 μm) for 5 min, and the extracts were prepared. Immunoprecipitation was performed using anti-PP1α antibody, followed by immunoblotting with anti-PRIP-1 antibody. The top and bottom panels show a typical immunoblot and the summary of results from eight independent experiments, respectively. Data are normalized to untreated controls, which were given a value of 100%. *p < 0.05 using Student's t test.
To gain further information on the identity of residues within PRIP-1, which are phosphorylated, neuronal PRIP-1 phosphoprotein isolated by immunoprecipitation was digested with trypsin. Phosphopeptides were then purified by Fe(III) affinity chromatography [Fe(III)-IMAC] and analyzed by MALDI/TOF-MS (Stensballe et al., 2001). Results using MALDI-TOF analysis suggested one signal for the phosphopeptide (94-TVSFSSMPSEK-104; mass-to-charge ratio, 1279.5), and this peptide contained a single phosphate residue. This phosphopeptide contains the PP1α binding site (93-KTVSF-97) in PRIP-1, which we have identified previously in vitro (Yoshimura et al., 2001). To further analyze the phosphorylation of this peptide, we produced mutations in a fragment of PRIP-1(82-232) expressed as a His-tag fusion protein. These mutations converted residues T94, S96, and TS94/96 to alanine residues, respectively. As shown in Figure 7B, the phosphorylation of the mutants of T94A and S96A by PKA was reduced by a similar extent compared with the wild-type fusion protein. Together, these results demonstrate that both T94 and S96 are PKA substrates in PRIP-1(82-232). To measure the consequences of phosphorylation of PRIP-1 for PP1α binding, we performed in vitro pull-down assay using wild-type and mutant versions of PRIP-1(82-232) (Fig. 7C). The wild-type PRIP-1(82-232) and the S96A mutant exhibited decreased binding to PP1α after the phosphorylation by PKA. In contrast, the mutants of T94A and TS94/96AA showed no change in the binding of PP1α after exposure to PKA. These results indicate that the phosphorylation of T94 by PKA is likely responsible for the dissociation of PP1α from PRIP-1.
We next examined the regulated interaction between PRIP-1 and PP1α in COS-7 cells, which do not express PRIP-1 (Yoshimura et al., 2001). For these experiments, wild-type PRIP-1 or the T94A mutants were expressed in COS-7 cells, and PKA was stimulated by forskolin treatment. Cell extracts were then immunoprecipitated with anti-PP1α antibody, followed by immunoblotting with anti-PRIP-1 antibody. As shown in Figure 7D, activation of PKA reduced the amount of PRIP-1 coimmunoprecipitating with PP1α in cells expressing the wild-type protein but not in those expressing the T94A mutant or the overall levels of PP1α. We further examined whether PKA activity modulates the interaction between PP1α and PRIP-1 in hippocampal brain slices. As shown in Figure 7E, activation of PKA drastically reduced the level of PRIP-1 associated with PP1α, suggesting reduced interaction of these two proteins. Therefore, these results are consistent with the role for PKA in regulating the interaction between PP1α and PRIP-1 via direct phosphorylation of T94 within PRIP-1.
PRIP-1 is required for dopamine receptor modulation of GABAA receptor function
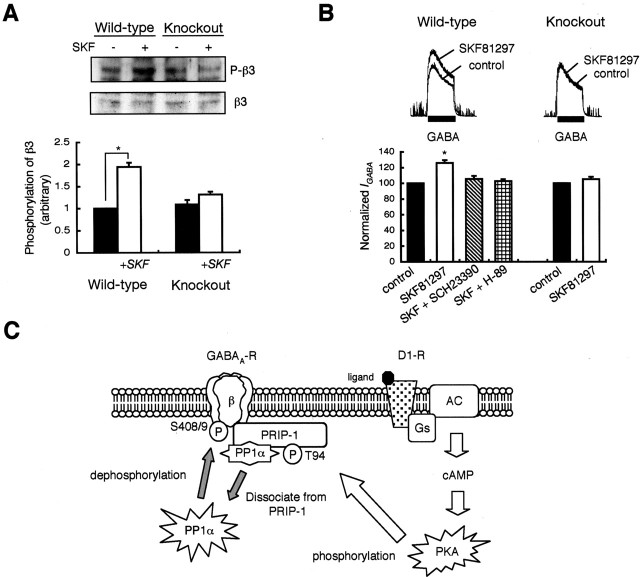
Finally, we examined whether PRIP-1 plays any role in regulating the phospho-dependent modulation of GABAA receptor function after the activation of endogenous signaling in neurons. For these studies, we focused on dopamine D1 receptors, which have been established previously to modify GABAA receptor function via a PKA-dependent mechanism (Hernandez et al., 2000). First, we examined the effects of D1 receptor activation on GABAA receptor β3 subunit phosphorylation in wild-type and PRIP-1-/- mice. Hippocampal slices from wild-type mice, but not from PRIP-1-/- mice, exhibited increased phosphorylation of β3 subunit on S408/9 in response to SKF81297, a D1-specific agonist (Fig. 8A). Using electrophysiological methods, the functional effects of D1 receptor activation on GABAA receptor activity were analyzed. Recordings made on hippocampal neurons from wild-type animals revealed that IGABA in these neurons was robustly increased by treatment with SKF81297, an effect that was abolished by the D1 antagonist SCH23390. Moreover, D1 receptor modulation of IGABA was also attenuated by H-89, a PKA inhibitor (Fig. 8B), confirming that D1 receptor modulation of IGABA is the event dependent on PKA activity. In contrast to our observations in wild-type mice, D1 receptor activation in hippocampal neurons from PRIP-1-/- mice had little effect on IGABA (Fig. 8B). In contrast, IGABA was positively modulated in PRIP-1-/- mice by D1 agonist after 15 min pretreatment with 100 nm okadaic acid (data not shown), in agreement with our biochemical analysis of receptor β3 subunit phosphorylation.
Figure 8.
PRIP-1 plays a critical role in GABAA receptor phospho-dependent functional modulation regulated by D1 receptors. A, Hippocampal slices were stimulated for 5 min with SKF81297 (1 μm) together with dopamine uptake inhibitor nomifensine (10 μm). After cell lysis, the extracts were analyzed by SDS-PAGE and immunoblotting with a phospho-specific antibody against S408/9 (P-β3) in the β3 subunit or total β3 (β3), as shown in the top panels. The graph shows the summary of the results (means ± SE) from four independent experiments. Results were expressed as the densities of anti-phospho-S408/9 relative to those of anti-β2/3 antibody. *p < 0.05 by Student's t test. B, Membrane currents (IGABA) activated by application of GABA (5 μm) were recorded from mechanically dissociated hippocampal CA3 neurons. Traces represent typical GABAA receptor-mediated currents recorded at 4 min after drug application. SCH23390 (3 μm) or H-89 (3 μm) was added together with SKF81297 (1 μm). *p < 0.05 using Student's t test. C, Possible role of PRIP-1 in phospho-regulation of GABAA receptors. Neurotransmitters such as dopamine, which increases intracellular cAMP, cause the activation of PKA. This will result in enhanced phosphorylation of the GABAA receptor β1 and β3 subunits, because PKA is specifically targeted to GABAA receptors via an association with AKAP150 (Brandon et al., 2003). This enhanced phosphorylation results in functional modulation of GABAA receptors, the nature of which is subtype dependent. Activated PKA at the same time phosphorylates PRIP-1 at residues, including T94, inducing the release of active PP1cα enhancing the dephosphorylation of receptor β-subunits and hence terminating receptor functional modulation (see Results).
Together, our results suggest that PRIP-1 is likely to play a significant role in the phospho-dependent modulation of GABAA receptor activity regulated by endogenous neuronal signaling pathways.
Discussion
PRIP-1 is a novel Ins(1,4,5)P3 binding protein, which has a number of binding partners, including GABARAP and PP1α (Takeuchi et al., 1996, 1997, 2000; Yoshimura et al., 2001; Kanematsu et al., 2002). Although the precise cellular function of PRIP-1 remains unknown, deletion of this protein in mice produces GABAA receptors with modified pharmacological properties and behavior, suggesting a role for PRIP-1 in facilitating receptor assembly or activity (Kanematsu et al., 2002). GABAA receptor function is subject to modulation by direct phosphorylation by a range of both serine-threonine and tyrosine protein kinases (Brandon et al., 2002a). Moreover, GABAA receptors are intimately associated with signaling complexes containing PKC isoforms Src tyrosine kinase, AKAP150, and RACK-1 (Brandon et al., 1999, 2002a,b, 2003), which are critical in controlling receptor phospho-dependent modulation. However, how protein phosphatases are targeted to GABAA receptors to ensure dynamic phospho-dependent modulation of receptor function remains to be addressed. The ability of PRIP-1 to bind the phosphatase PP1α may suggest that this protein plays a role as a scaffold facilitating the targeting of phosphatase activity to GABAA receptors.
To test this concept, we examined the phospho-dependent regulation of GABAA receptors in PRIP-1-/- mice. Specifically, we examined the regulation of GABAA receptors by PKA activity, as previous studies have shown that functional modulation of receptor by direct activation of this kinase or via dopamine D1 receptors is dependent on phosphorylation of receptor β subunits (Moss et al., 1992a,b; McDonald et al., 1998; Hernandez et al., 2000). In wild-type mice, activation of PKA in hippocampal slices increased phosphorylation of the GABAA receptor β3 subunit and enhanced GABAA receptor activity. This modulation of GABAA receptor phosphorylation and activity was significantly reduced in neurons from PRIP-1-/- animals. The reduced level of GABAA receptor PKA-dependent functional modulation in PRIP-1-/- animals suggests that PRIP-1 regulates either receptor phosphorylation or dephosphorylation. PRIP-1-/- mice exhibited equal levels of total cellular PKA activity. Moreover, the level of PKA associated with GABAA receptors, a process that is dependent on AKAP79/150 (Brandon et al., 2003), was similar in both control and mutant mice. These results suggest that PRIP-1 is unlikely to participate in regulation of PKA-mediated GABAA receptor phosphorylation. We established previously that PRIP-1 can interact with, and negatively regulate, the activity of PP1α (Yoshimura et al., 2001). Therefore, the loss of GABAA receptor functional modulation by PKA activity in PRIP-1-/- mice may be attributable to enhanced phosphatase activity. In agreement with this, whereas total levels of PP1α were equivalent in these mouse strains, PP1α activity in PRIP-1-/- mice was enhanced by ∼43%, as measured using phosphorylase a as a substrate. Using in vitro assays, we also established that phosphorylated GST-β3 subunit is dephosphorylated by PP1α, which is in agreement with previous observations by PP2A but not by PP2B (Jovanovic et al., 2004). We also compared the ability of hippocampal extracts prepared from wild-type and PRIP-1-/- mice to dephosphorylate phosphorylated GST-β3. Using okadaic acid to selectively inhibit PP1α and PP2A, we were able to establish that extracts prepared from PRIP-1-/- mice have enhanced PP1α activity, compared with controls. Together, our results suggest that enhanced activity of PP1α in PRIP-1-/- mice may be responsible for the compromised PKA-mediated GABAA receptor functional modulation in these animals. In agreement with this observation, okadaic acid or calyculin A partially restored PKA-mediated GABAA receptor β3 subunit phosphorylation and receptor functional modulation in PRIP-1-/- mice, as did the introduction of recombinant PRIP-1 via intracellular dialysis with the recording pipette. Because PRIP-1(82-232), which does not bind GABAA receptor β subunits, also restored the modulation of IGABA by cAMP in the mutant mice, inhibition of PP1α appears to be of primary significance for the effects of this protein on GABAA receptor function.
We further addressed the role of PRIP-1 in targeting PP1α to GABAA receptors using in vitro binding assays and immunoprecipitation. It was evident that PRIP-1 could selectively bind to the intracellular domains of receptor β subunits. PRIP-1 was also immunoprecipitated from the brains of wild-type mice with GABAA receptors containing β3 subunits but not from PRIP-1-/- mice. PP1α was also coimmunoprecipitated from wild-type brains with PRIP-1, but the level of PP1α coimmunoprecipitating was greatly reduced in PRIP-1-/- mice. Given PRIP-1 is capable of binding and inactivating PP1α, our results strongly suggest a significant role for this protein in targeting this phosphatase to GABAA receptors. Interestingly, our biochemical observations with PRIP-1-/- mice showed a less amount of anchored PP1α at GABAA receptors but an overall increase in total PP1α activity without any modification in the basal level of receptor phosphorylation compared with wild-type animals. Although enhanced PP1α activity may be expected to lead to modifications in GABAA receptor basal phosphorylation, this enhanced activity will not be localized to the environment of the receptor in mutant mice. Furthermore, complexes of PP1α and PRIP-1, localized to GABAA receptors, may exhibit low but significant levels of phosphates activity, because the binding site for PRIP-1 is distinct from the catalytic center of this enzyme (Egloff et al., 1997). Moreover, PP1α is not the only phosphatase associated with GABAA receptors, because PP2A can directly associate with and dephosphorylate these proteins (Jovanovic et al., 2004). In addition, PP2A has been shown to be responsible for regulating basal levels of β3 subunit phosphorylation in cultured neurons (Jovanovic et al., 2004). Therefore, both PP1α and PP2A anchored at GABAA receptors may play complex roles in regulating receptor phosphorylation under basal conditions.
Finally, we also analyzed how the interaction of PP1α with PRIP-1 is regulated, focusing on the role of phosphorylation, and our results revealed that PRIP-1 is phosphorylated after the activation of PKA in neurons. Phosphorylation was seen in one peptide of PRIP-1 between residues 94 and 104, which encompasses the PP1α binding site within this molecule. Phosphorylation of T94 by PKA in this region of PRIP-1 blocked the binding of PP1α, which we established previously to result in activation of this enzyme (Yoshimura et al., 2001).
Therefore, our observations suggest a model in which PRIP-1 plays a central role in mediating phospho-dependent modulation of GABAA receptors by PKA (Fig. 8C). In this model, G-coupled receptors activated by neurotransmitters, such as dopamine, after stimulation will increase intracellular cAMP levels activating anchored PKA at GABAA receptors (Brandon et al., 2003), enhancing GABAA receptor β subunit phosphorylation. PRIP-1 is also associated with GABAA receptors in neurons where, under basal conditions, it anchors an inactive or smaller pool of PP1α. Enhanced PKA activity will also phosphorylate PRIP-1 on T94 leading to dissociation and activation of PP1α, which will dephosphorylate GABAA receptor β subunits. Therefore, the level of GABAΑ receptor phosphorylation will be dependent on the relative rates of receptor β subunit and PRIP-1 phosphorylation by PKA. The later step will release active PP1α to catalyze dephosphorylation of GABAA receptor β subunits and is predicted to be slower than phosphorylation of the GABAA receptors, providing a mechanism for the dynamic control of GABAA receptor phosphorylation and function by PKA-dependent signaling pathway. The loss of PRIP-1 leads to elevated levels of active PP1α, which prevent PKA-stimulated phosphorylation of GABAA receptor β subunits.
It is becoming evident that phosphorylation plays an essential role in modulating the activity of ion channels, and central to this is the correct targeting of kinases and phosphatases to these proteins to facilitate dynamic phospho-dependent modulation (Fraser and Scott, 1999). Here, we have identified a role for PRIP-1 in targeting PP1α activity to GABAA receptors, and it is evident that PRIP-1 plays an important role in controlling the dynamics of receptor phosphorylation by PKA activity. Therefore, the ability of PRIP-1 to target PP1α to GABAA receptors and regulate the activity of this phosphatase suggests an important role for this protein in the control of synaptic inhibition.
Footnotes
This work was supported by the Japan-United Kingdom Research Cooperative Program of Japan Society for the Promotion of Sciences (JSPS) and by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (M.T., T.K., and M.H.), the Japan Epilepsy Research Foundation (M.H.), Uehara Memorial Foundation (T.K. and M.H.), Mochida Foundation for Medical and Pharmaceutical Research (T.K.), Takeda Science Foundation (T.K.), Novartis Foundation (Japan) for the Promotion of Science (M.H.), and Shimabara Foundation (M.H.). M.T. is a JSPS fellow and a recipient of the Iwadare Scholarship. Collaboration between Japan and Korea was initiated by the Annual Conference between Japan and Korea on Cellular Signaling for Young Scientists.
Correspondence should be addressed to Dr. Masato Hirata, Laboratory of Molecular and Cellular Biochemistry, Faculty of Dental Science, Kyushu University, Fukuoka 812-8582, Japan. E-mail: hirata1@dent.kyushu-u.ac.jp.
Copyright © 2004 Society for Neuroscience 0270-6474/04/247074-11$15.00/0
References
- Akaike N, Harata N (1994) Nystatin perforated patch recording and its application to analysis of intracellular mechanism. Jpn J Physiol 44: 433-473. [DOI] [PubMed] [Google Scholar]
- Brandon NJ, Uren JM, Kittler JT, Wang H, Olsen R, Parker PJ, Moss SJ (1999) Subunit-specific association of protein kinase C and the receptor for activated C kinase with GABA type A receptors. J Neurosci 19: 9228-9234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon NJ, Delmas P, Kittler JT, McDonald BJ, Sieghart W, Brown DA, Smart TG, Moss SJ (2000) Protein kinase C activity controls GABAA receptor phosphorylation and functional modulation in cortical neurons. J Biol Chem 275: 38856-38862. [DOI] [PubMed] [Google Scholar]
- Brandon NJ, Jovanovic JN, Moss SJ (2002a) Multiple roles of protein kinases in the modulation of γ-aminobutyric acidA receptor function and cell surface expression. Pharmacol Ther 94: 113-122. [DOI] [PubMed] [Google Scholar]
- Brandon NJ, Jovanovic JN, Smart TG, Moss SJ (2002b) Receptor for activated C kinase-1 facilitates protein kinase C-dependent phosphorylation and functional modulation of GABAA receptors with the activation of G-protein-coupled receptors. J Neurosci 22: 6353-6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon NJ, Jovanovic JN, Colledge M, Kittler JT, Brandon JM, Scott JD, Moss SJ (2003) A-kinase anchoring protein 79/150 facilitates the phosphorylation of GABAA receptors by cAMP-dependent protein kinase via selective interaction with receptor β subunits. Mol Cell Neurosci 22: 87-97. [DOI] [PubMed] [Google Scholar]
- Cai X, Flores-Hernandez J, Feng J, Yan Z (2002) Activity-dependent bidirectional regulation of GABA(A) receptor channels by the 5-HT(4) receptor-mediated signalling in rat prefrontal cortical pyramidal neurons. J Physiol (Lond) 540: 743-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Alemany S, Hemmings BA, Resink TJ, Stralfors P, Tung HY (1988) Protein phosphatase inhibitor-1 and inhibitor-2 from rabbit skeletal muscle. Methods Enzymol 159: 390-408. [DOI] [PubMed] [Google Scholar]
- Connolly CN, Krishek BJ, McDonald BJ, Smart TG, Moss SJ (1996a) Assembly and cell surface expression of heteromeric and homomeric gamma-aminobutyric acid type A receptors. J Biol Chem 271: 89-96. [DOI] [PubMed] [Google Scholar]
- Connolly CN, Wooltorton JR, Smart TG, Moss SJ (1996b) Subcellular localization of gamma-aminobutyric acid type A receptors is determined by receptor beta subunits. Proc Natl Acad Sci USA 93: 9899-9904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egloff MP, Johnson DF, Moorhead G, Cohen PTW, Cohen P, Barford D (1997) Structural basis for the recognition of regulatory subunits by the catalytic subunit of protein phosphatase 1. EMBO J 16: 1876-1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre B, Turowski P, Hemmings BA (1997) Differential inhibition and posttranslational modification of protein phosphatase 1 and 2A in MCF7 cells treated with calyculin-A, okadaic acid, and tautomycin. J Biol Chem 272: 13856-13863. [DOI] [PubMed] [Google Scholar]
- Fraser IDC, Scott JD (1999) Modulation of ion channels: a “current” view of AKAPS. Neuron 23: 423-426. [DOI] [PubMed] [Google Scholar]
- Gorrie GH, Vallis Y, Stephenson A, Whitfield J, Browning B, Smart TG, Moss SJ (1997) Assembly of GABAA receptors composed of α1 and β2 subunits in both cultured neurons and fibroblasts. J Neurosci 17: 6587-6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley JG, Koulen P, Bedford FK, Gordon-Weeks PR, Moss SJ (1999) Microtubule associated protein MAP-1B links GABAC receptors to the cytoskeleton at retinal synapses. Nature 397: 66-69. [DOI] [PubMed] [Google Scholar]
- Hernandez JF, Hernandez S, Snyder S, Yan Z, Fienberg A, Moss SJ, Green-gard P, Surmier J (2000) D1 dopamine receptor activation reduces GABAA receptor currents in neostriatal medium spiny neurones through a protein kinase A/DARP-32/PP1 signaling cascade. J Neurophysiol 83: 2996-3004. [DOI] [PubMed] [Google Scholar]
- Jovanovic JN, Thomas P, Kittler JT, Smart TG, Moss SJ (2004) Brain-derived neurotrophic factor modulates fast synaptic inhibition by regulating GABAA receptor phosphorylation, activity, and cell-surface stability. J Neurosci 24: 522-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanematsu T, Takeya H, Watanabe Y, Ozaki S, Yoshida M, Koga T, Iwanaga S, Hirata M (1992) Putative inositol 1,4,5-trisphosphate binding proteins in rat brain cytosol. J Biol Chem 267: 6518-6525. [PubMed] [Google Scholar]
- Kanematsu T, Misumi Y, Watanabe Y, Ozaki S, Yoshida M, Koga T, Iwanaga S, Hirata M (1996) A new inositol 1,4,5-trisphosphate binding protein similar to phospholipase C-δ1. Biochem J 313: 319-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanematsu T, Yoshimura K, Hidaka K, Takeuchi H, Katan M, Hirata M (2000) Domain organization of p130, PLC-related catalytically inactive protein, and structural basis for the lack of enzyme activity. Eur J Biochem 267: 2731-2737. [DOI] [PubMed] [Google Scholar]
- Kanematsu T, Jang I-S, Yamaguchi T, Nagahama H, Yoshimura K, Hidaka K, Matsuda M, Takeuchi H, Misumi Y, Nakayama K, Yamamoto T, Akaike N, Hirata M, Nakayama K (2002) Role of the PLC-related, catalytically inactive protein p130 in GABAA receptor function. EMBO J 21: 1004-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishek BJ, Xie X, Blackstone C, Huganir RL, Moss SJ, Smart TG (1994) Regulation of GABAA receptor function by protein kinase C phosphorylation. Neuron 12: 1081-1095. [DOI] [PubMed] [Google Scholar]
- McDonald BJ, Moss SJ (1997) Conserved phosphorylation of the intracellular domains of GABAA receptor β2 and β3 subunits by cAMP-dependent protein kinase, cGMP-dependent kinase, protein kinase C, and Ca2+/calmodulin type II-dependent kinase. Neuropharmacology 36: 1377-1385. [DOI] [PubMed] [Google Scholar]
- McDonald BJ, Amato A, Connolly CN, Benke D, Moss SJ, Smart TG (1998) Adjacent phosphorylation site on GABAA receptor β subunits determine regulation by cAMP-dependent protein kinase. Nat Neurosci 1: 23-28. [DOI] [PubMed] [Google Scholar]
- Moss SJ, Doherty CA, Huganir RL (1992a) Identification of the cAMP-dependent protein kinase and protein kinase C phosphorylation sites within the major intracellular domains of the β1, γ2S, and γ2L subunits of the γ-aminobutyric acid type A receptor. J Biol Chem 267: 11470-11476. [PubMed] [Google Scholar]
- Moss SJ, Smart TG, Blackstone CD, Huganir RL (1992b) Functional modulation of GABAA receptors by cAMP-dependent protein phosphorylation. Science 257: 661-665. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G (2000) GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience 101: 815-850. [DOI] [PubMed] [Google Scholar]
- Porter NM, Twyman RE, Uhler MD, McDonald RL (1990) Cyclic AMP-dependent protein kinase decreases GABAA receptor current in mouse spinal neurons. Neuron 5: 789-796. [DOI] [PubMed] [Google Scholar]
- Rhee JS, Ishibashi H, Akaike N (1999) Calcium channels in the GABAergic presynaptic nerve terminals projecting to Meynert neurons of the rat. J Neurochem 72: 800-807. [DOI] [PubMed] [Google Scholar]
- Snyder GL, Fienberg AA, Huganir RL, Greengard P (1998) A dopamine/D1 receptor/protein kinase A/dopamine- and cAMP-regulated phosphoprotein (Mr 32 kDa)/protein phosphatase-1 pathway regulated dephosphorylation of the NMDA receptor. J Neurosci 18: 10297-10303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensballe A, Andersen S, Jensen ON (2001) Characterization of phosphoproteins from electrophoretic gels by nanoscale Fe(III) affinity chromatography with off-line mass spectrometry analysis. Proteomics 1: 207-222. [DOI] [PubMed] [Google Scholar]
- Swope SL, Moss SJ, Raymond LA, Huganir RL (1999) Regulation of ligand-gated ion channels by protein phosphorylation. Adv Second Messenger Phosphoprotein Res 33: 49-78. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Kanematsu T, Misumi Y, Yaakob HB, Yagisawa H, Ikehara Y, Hirata M (1996) Localization of a high affinity inositol 1,4,5-trisphosphate/inositol 1,4,5,6-tetrakiphosphate binding domain to the pleckstrin homology module of a new 130-kDa protein: characterization of the de terminants of structural specificity. Biochem J 318: 561-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Kanematsu T, Misumi Y, Sakane F, Konishi H, Kikkawa U, Watanabe Y, Katan M, Hirata M (1997) Distinct specificity in the binding of inositol phosphates by pleckstrin homology domains of pleckstrin, RAC-protein kinase, diacylglycerol kinase and a new 130-kDa protein. Biochim Biophys Acta 1359: 275-285. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Oike M, Paterson HF, Allen V, Kanematsu T, Ito Y, Erneux C, Katan M, Hirata M (2000) Inhibition of calcium signalling by p130,PLC-related catalytically inactive protein: critical role of the p130PH domain. Biochem J 349: 357-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uji A, Matsuda M, Kukita T, Maeda K, Kanematsu T, Hirata M (2002) Molecules interacting with PRIP-2, a novel Ins(1,4,5)P3 binding protein type 2: comparison with PRIP-1. Life Sci 72: 443-453. [DOI] [PubMed] [Google Scholar]
- Wang H, Bedford FK, Brandon NJ, Moss SJ, Olsen RW (1999) GABAA receptor associated protein links GABAA receptors and the cytoskeleton. Nature 397: 69-72. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhu Y, Sharma K (1998) Transforming growth factor-β1 stimulates protein kinase A in mesangial cells. J Biol Chem 273: 8522-8527. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhong P, Yan Z (2002) Dopamine D4 receptors modulate GABAergic signaling in pyramidal neurons of prefrontal cortex. J Neurosci 22: 9185-9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting PJ, Bonnert TP, McKernan RM, Farrer S, Le Bourdelles B, Heavens RP, Smith DW, Hewson L, Rigby MR, Sirinathsinghji DJ, Thompson SA, Wafford KA (1999) Molecular and functional diversity of the expanding GABA-A receptor gene family. Ann NY Acad Sci 868: 645-653. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Kanematsu T, Watanabe Y, Koga T, Ozaki S, Iwanaga S, Hirata M (1994) d-myo-Inositol 1,4,5-trisphosphate binding proteins in rat brain membranes. J Biochem (Tokyo) 115: 973-980. [DOI] [PubMed] [Google Scholar]
- Yoshimura K, Takeuchi H, Sato O, Hidaka K, Doira N, Terunuma M, Harada K, Ogawa Y, Ito Y, Kanematsu T, Hirata M (2001) Interaction of p130 with, and consequent inhibition of, the catalytic subunit of protein phosphatase 1α. J Biol Chem 276: 17908-17913. [DOI] [PubMed] [Google Scholar]