Abstract
We used the [14C]-2-deoxyglucose method to study the location and extent of primate frontal lobe areas activated for saccades and fixation and the retrograde transneuronal transfer of rabies virus to determine whether these regions are oligosynaptically connected with extraocular motoneurons. Fixation-related increases of local cerebral glucose utilization (LCGU) values were found around the fundus of the inferior limb of the arcuate sulcus (AS) just ventral to its genu, in the dorsomedial frontal cortex (DMFC), cingulate cortex, and orbitofrontal cortex. Significant increases of LCGU values were found in and around both banks of the AS, DMFC, and caudal principal, cingulate, and orbitofrontal cortices of monkeys executing visually guided saccades. All of these areas are oligosynaptically connected to extraocular motoneurons, as shown by the presence of retrogradely transneuronally labeled cells after injection of rabies virus in the lateral rectus muscle. Our data demonstrate that the arcuate oculomotor cortex occupies a region considerably larger than the classic, electrical stimulation-defined, frontal eye field. Besides a large part of the anterior bank of the AS, it includes the caudal prearcuate convexity and part of the premotor cortex in the posterior bank of the AS. They also demonstrate that the oculomotor DMFC occupies a small area straddling the ridge of the brain medial to the superior ramus of the AS. Our results support the notion that a network of several interconnected frontal lobe regions is activated during rapid, visually guided eye movements and that their output is conveyed in parallel to subcortical structures projecting to extraocular motoneurons.
Keywords: saccades, frontal eye field, supplementary eye field, principal sulcus, cingulate eye fields, oculomotor
Introduction
The neural control of rapid eye movements (saccades) has been the object of considerable research (for review, see Moschovakis et al., 1996). At least two saccade-related areas are located in the frontal lobes, namely the frontal eye field (FEF) (Robinson and Fuchs, 1969) and the supplementary eye field (SEF) (Schlag and Schlag-Rey, 1987). The location and extent of both areas has been a matter of debate. For example, using electrical stimulation, sometimes of high intensity (e.g., 2 mA), saccades ranging in amplitude from 1 to 70° were evoked from a large periarcuate area, which includes the anterior bank of the arcuate sulcus (AS) and the prearcuate convexity (Robinson and Fuchs, 1969). A smaller region, occupying only a portion of the rostral bank of the AS and evoking smaller movements (1-30° in amplitude), was defined with stimuli of much lower intensity (<50 μA) (Bruce et al., 1985; Russo and Bruce, 1993). The dorsomedial frontal cortex (DMFC), which houses the SEF, encompasses areas 9, 8B, and 6 (from rostral to caudal); the latter was further subdivided into areas F2 and F3 caudally and areas F6 and F7 rostrally (Matelli et al., 1991). Based on electrically evoked eye movements, the SEF has been claimed to occupy a small region (10-15 mm2) in the rostral half of area F7 (Russo and Bruce, 1993), a bigger region (40 mm2) in the caudal half of area F7, and the rostralmost area F2 (Schlag and Schlag-Rey, 1987) or an even bigger region (70 mm2) extending through the whole anteroposterior length of areas F7, F6, and the rostral two thirds of areas F2 and F3 (Tehovnik and Lee, 1993).
The aims of the present study were to define better the location and extent of the primate FEF and SEF, to explore whether additional frontal lobe areas are involved in visually guided saccades, and to establish their connections to extraocular motoneurons. To this end, we used two different methods, the [14C]-2-deoxyglucose (14C-DG) technique to obtain high-resolution functional images of saccade-related frontal lobe areas and the highly specific retrograde transneuronal transfer of rabies virus (Ugolini, 1995; Tang et al., 1999; Graf et al., 2002; Morcuende et al., 2002) to reveal the distribution of frontal cortical neurons oligosynaptically connected to extraocular motoneurons. A brief description of some of our results has appeared in abstract form (Guldin et al., 2000).
Materials and Methods
Seven female rhesus monkeys (six Macaca mulatta and one Macaca fascicularis), weighing between 3 and 4 kg, were used in this study. All animals were purpose-bred by authorized suppliers within the European Union (Deutches Primatenzentum, Gottingen, Germany; Centre National de la Recherche Scientifique, Marseille, France) and were cared for in accordance with European Union regulations concerning biosafety and the use of live animals in research (directive 86/609) as well as with the National Institutes of Health's Principles of Laboratory Animal Care. In accordance with a protocol approved by the institutional animal use committee, functional brain images of cortical areas during saccades to visual targets were obtained from four of the Macaca mulatta monkeys at the Institute of Applied and Computational Mathematics (Foundation for Research and Technology-Hellas, Crete, Greece). The pattern of activation of the superior colliculus (SC) of these monkeys was described previously (Moschovakis et al., 2001). Experiments involving retrograde transneuronal labeling of oculomotor pathways with rabies virus were performed at the Centre National de la Recherche Scientifique at Gif-sur-Yvette in accordance with a protocol approved by the institutional animal use committee. Rabies virus was injected into the lateral rectus (LR) muscle of the left eye in three monkeys. Transneuronally labeled neurons found in the brainstem of these monkeys have been described previously (Graf et al., 1999, 2000; Ugolini et al., 2000, 2001; Grantyn et al., 2002).
Quantitative [14C]-2-deoxyglucose autoradiography. Animals were prepared for behavioral experiments by anchoring a metal bolt onto mandibular plates secured on the cranium with titanium screws (Synthes, Bettlach, Switzerland) for head immobilization. Search coils were sutured on the sclera to record the instantaneous position of the eyes (Remmel, 1984). All surgical procedures were performed under general anesthesia (sodium pentobarbital, 25 mg/kg, i.m.) and aseptic conditions. Eye position was digitized at a rate of 500 Hz using the Spike2 software (Cambridge Electronics Design, Cambridge, UK). Saccade targets were red dots (1.5° in diameter) presented on a video screen placed 23 cm from the animal. Monkeys were required to hold the position of their eyes within a circular window, 2.5° in diameter, surrounding the center of each target. A water delivery tube was placed close to their mouth, and successful completion of each trial was rewarded with water. Monkeys were trained for 3-6 months until they successfully completed >90% of the trials in the following behavioral tasks.
The first monkey was an untrained control, seated in front of the behavioral apparatus but receiving neither visual stimuli nor liquid rewards during the 14C-DG experiment. The second monkey had to fixate a central visual target located straight ahead and maintain fixation for the duration of the trial (4 sec). Inter-trial intervals were 0.2-0.3 sec. A third monkey was required to execute single-oblique up-left saccades, 20° in amplitude and 135° in direction. Each trial was initiated with the appearance of a central fixation target. The monkey had to fixate the target for 0.5-1 sec until it disappeared, and a peripheral target was illuminated, signaling that a saccade to it should be executed within 1 sec. The minimum latency to move the eyes after onset of each target was set to 0.1 sec to discourage anticipatory movements. The monkey was required to fixate the peripheral target for 0.5-1 sec until it disappeared. The animal was free to move its eyes spontaneously during the inter-trial interval (1-1.8 sec). The fourth monkey was required to perform sequential horizontal saccades to successively appearing visual targets: a sequence of single 5, 10, and 15° movements to the left, followed by two 30° movements to the right and a 30° movement to the left. The monkey had to fixate each target for 0.3-0.6 sec. Inter-trial intervals ranged between 0.5 and 0.8 sec.
The 14C-DG experiments were performed as described previously (Gregoriou and Savaki, 2001). The cerebral hemispheres were cut horizontally in a cryostat at -20°C to obtain ∼2500 serial sections, 20 μm thick, from each hemisphere. Preparation of the autoradiographs, their quantitative densitometric analysis, and calculation of the local cerebral glucose utilization (LCGU) values have been described previously (Savaki et al., 1993; Gregoriou and Savaki, 2001). The LCGU values (in μmol/100 gm/min) were calculated from the local 14C tissue concentration (densitometrically determined from the autoradiographs in reference to precalibrated standards) and the plasma 14C and glucose concentrations, according to the operational equation and the appropriate kinetic constants for the monkey (Sokoloff, 1977; Kennedy et al., 1978). The distribution of activity in the rostrocaudal extent of each section was determined by measuring LCGU values pixel by pixel (resolution, 60 μm/pixel) along a line parallel to the surface of the cortex, covering all cortical layers. Average LCGU values were calculated from sets of five adjacent 20 μm thick horizontal brain sections, throughout each cortical area examined. The number (n) of the sets of five sections used to obtain this value is indicated next to it in Table 1 (in parentheses, separately for each region and hemisphere). To normalize the LCGU values of affected cortical areas, we estimated the average LCGU value of nonaffected regions pooled across all monkeys. The statistical significance of differences in LCGU values in frontal cortical areas of different subjects was established with the Student's unpaired t test. Given that the LCGU values of corresponding areas in the two hemispheres of a normal resting monkey can differ by as much as 7% (Savaki et al., 1993), differences smaller than this were conservatively considered to arise by chance and were excluded from any additional statistical testing. The student's t test was used to evaluate the significance of LCGU values of the experimental monkeys that differed by >7% from those of the control. Also, the Kolmogorov-Smirnov test was used to evaluate the significance of differences in their distributions. The significance level we adopted in these tests was set to 0.001.
Table 1.
Metabolic effects in subregions of the lateral, medial, and dorsomedial prefrontal, cingulate, and orbitofrontal cortices
|
|
Sham control |
Fixation |
Single oblique saccades (20° amplitude) |
Sequential horizontal saccades (5, 10, 15, 30° amplitude) |
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
LCGU |
SD |
n
|
LCGU |
SD |
n
|
%C |
|
LCGU |
SD |
n
|
%C |
|
%F |
|
LCGU |
SD |
n
|
%C |
|
%F |
|
||||||||||||||||||
| Arcuate sulcus |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||
| Anterior bank | ||||||||||||||||||||||||||||||||||||||||
| Superior ramus | 49 | 2 | 48 | 50 | 2 | 49 | 2 | 55 | 4 | 45 | 12 | a,b | 10 | a,b | 55 | 3 | 40 | 12 | a,b | 10 | a,b | |||||||||||||||||||
| Inferior ramus (7 mm ventral) | 56 | 1 | 64 | 60 | 1 | 67 | 7 | 65 | 2 | 56 | 16 | a,b | 8 | a,b | 63 | 3 | 63 | 13 | a,b | 5 | ||||||||||||||||||||
| Inferior ramus (5 mm dorsal, genu) | 53 | 2 | 56 | 60 | 3 | 49 | 13 | a,b | 65 | 3 | 53 | 23 | a,b | 8 | a,b | 68 | 4 | 56 | 28 | a,b | 13 | a,b | ||||||||||||||||||
| Fundus (genu) | 54 | 2 | 13 | 63 | 2 | 13 | 17 | a,b | 73 | 1 | 13 | 35 | a,b | 16 | a,b | 77 | 1 | 11 | 43 | a,b | 22 | a,b | ||||||||||||||||||
| Posterior bank | ||||||||||||||||||||||||||||||||||||||||
| Superior ramus | 44 | 2 | 44 | 47 | 1 | 40 | 7 | 51 | 6 | 45 | 16 | a,b | 9 | a,b | 51 | 2 | 41 | 16 | a,b | 9 | a,b | |||||||||||||||||||
| Inferior ramus (7 mm ventral) | 51 | 1 | 69 | 53 | 5 | 63 | 4 | 61 | 4 | 65 | 20 | a,b | 15 | a,b | 63 | 4 | 63 | 24 | a,b | 19 | a,b | |||||||||||||||||||
| Inferior ramus (5 mm dorsal, genu) | 48 | 1 | 56 | 53 | 1 | 52 | 10 | a,b | 58 | 1 | 54 | 21 | a,b | 9 | a,b | 59 | 3 | 57 | 23 | a,b | 11 | a,b | ||||||||||||||||||
| Fundus (genu) | 51 | 2 | 13 | 63 | 1 | 13 | 24 | a,b | 68 | 2 | 13 | 33 | a,b | 8 | a,b | 74 | 1 | 14 | 45 | a,b | 17 | a,b | ||||||||||||||||||
| Prearcuate convexity | ||||||||||||||||||||||||||||||||||||||||
| Dorsal (area 8A) | 53 | 2 | 76 | 56 | 6 | 76 | 6 | 63 | 6 | 76 | 19 | a,b | 13 | a,b | 67 | 7 | 76 | 26 | a,b | 20 | a,b | |||||||||||||||||||
| Ventral (area 45) | 56 | 3 | 35 | 58 | 4 | 80 | 4 | 66 | 5 | 80 | 18 | a,b | 14 | a,b | 64 | 3 | 80 | 14 | a,b | 10 | a,b | |||||||||||||||||||
| FEF (areas 8A and 45) |
53 |
2 |
111 |
57 |
5 |
156 |
8 |
a,b
|
65 |
6 |
156 |
23 |
a,b
|
14 |
a,b
|
66 |
6 |
156 |
25 |
a,b
|
16 |
a,b
|
||||||||||||||||||
| Dorsomedial frontal |
49 |
2 |
44 |
54 |
2 |
42 |
10 |
a,b
|
56 |
2 |
22 |
14 |
a,b
|
4 |
|
60 |
2 |
34 |
22 |
a,b
|
11 |
a,b
|
||||||||||||||||||
| Principal sulcus |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||
| Lateral bank (anterior ½) | 56 | 4 | 68 | 54 | 3 | 66 | −4 | 57 | 4 | 67 | 2 | 6 | 58 | 4 | 67 | 4 | 7 | |||||||||||||||||||||||
| Medial bank (anterior ½) | 52 | 4 | 77 | 51 | 3 | 60 | −2 | 53 | 3 | 73 | 2 | 4 | 52 | 3 | 71 | 0 | 2 | |||||||||||||||||||||||
| Caudal tip (posterior ½) | 55 | 2 | 66 | 55 | 2 | 66 | 0 | 61 | 4 | 77 | 11 | a,b | 11 | a,b | 61 | 3 | 64 | 11 | a,b | 11 | a,b | |||||||||||||||||||
| Periprincipal convexity | ||||||||||||||||||||||||||||||||||||||||
| Dorsolateral prefrontal | 46 | 2 | 64 | 47 | 2 | 86 | 2 | 50 | 2 | 31 | 9 | a,b | 6 | 49 | 2 | 63 | 7 | 4 | ||||||||||||||||||||||
| Ventrolateral prefrontal |
51 |
2 |
32 |
55 |
2 |
32 |
8 |
a,b
|
60 |
2 |
39 |
18 |
a,b
|
9 |
a,b
|
59 |
1 |
23 |
16 |
a,b
|
7 |
|
||||||||||||||||||
| Orbitofrontal |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||
| Posterior to LOS (area 12) | 54 | 5 | 121 | 58 | 2 | 102 | 7 | 57 | 7 | 101 | 6 | −2 | 59 | 6 | 106 | 9 | a,b | 2 | ||||||||||||||||||||||
| 12 max effect | 57 | 2 | 20 | 67 | 3 | 21 | 18 | a,b | 69 | 4 | 21 | 21 | a,b | 3 | 70 | 2 | 17 | 23 | a,b | 4 | ||||||||||||||||||||
| Between LOS and MOS (11,13) | 50 | 4 | 82 | 55 | 3 | 70 | 10 | a,b | 54 | 6 | 72 | 8 | a,b | −2 | 57 | 6 | 63 | 14 | a,b | 4 | ||||||||||||||||||||
| 11,13 max effect | 56 | 4 | 16 | 63 | 2 | 21 | 13 | a,b | 65 | 2 | 21 | 16 | a,b | 3 | 71 | 3 | 21 | 27 | a,b | 13 | a,b | |||||||||||||||||||
| Between tip and MOS (10,14) |
46 |
1 |
45 |
46 |
2 |
77 |
0 |
|
45 |
2 |
82 |
−2 |
|
−2 |
|
48 |
5 |
74 |
4 |
|
4 |
|
||||||||||||||||||
| Medial prefrontal |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||
| Between tip and RS (areas 10,14) | 43 | 3 | 147 | 42 | 2 | 148 | −2 | 42 | 3 | 130 | −2 | 0 | 45 | 4 | 146 | 5 | 7 | |||||||||||||||||||||||
| Posterior to RS (areas 25,32) |
38 |
2 |
68 |
41 |
3 |
67 |
8 |
a,b
|
41 |
4 |
66 |
8 |
a,b
|
0 |
|
43 |
3 |
54 |
13 |
a,b
|
5 |
|
||||||||||||||||||
| Cingulate sulcus |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||
| Dorsal bank, caudal half | 43 | 3 | 36 | 43 | 3 | 49 | 0 | 44 | 3 | 38 | 2 | 2 | 47 | 2 | 38 | 9 | a,b | 9 | a,b | |||||||||||||||||||||
| Dorsal bank, rostral half | 45 | 3 | 50 | 50 | 3 | 39 | 11 | a,b | 48 | 2 | 46 | 7 | −4 | 53 | 4 | 46 | 18 | a,b | 6 | |||||||||||||||||||||
| Ventral bank, caudal half | 41 | 3 | 75 | 40 | 2 | 78 | −2 | 42 | 4 | 83 | 2 | 5 | 45 | 2 | 82 | 10 | a,b | 13 | a,b | |||||||||||||||||||||
| Ventral bank, rostral half |
43 |
4 |
51 |
44 |
4 |
44 |
2 |
|
49 |
5 |
43 |
14 |
a,b
|
11 |
a,b
|
49 |
3 |
44 |
14 |
a,b
|
11 |
a,b
|
||||||||||||||||||
Values represent the normalized mean of glucose utilization (LCGU) expressed in μmol/100 gm/min ± SD obtained from the right hemispheres of one sham control and three experimental monkeys performing fixation, single leftward oblique saccades, and sequential leftward horizontal saccades, respectively.
n, Number of sets of five adjacent horizontal sections used in obtaining mean LCGU values for each region in each hemisphere; %C and %F, experimental-to-control and experimental-to-fixation percentage differences, calculated as (experimental - control)/control × 100 and (experimental - fixation)/fixation × 100, respectively; 12 max effect, maximum effect within area 12; 11, 13 max effect, maximum effect within areas 11 and 13.
p < 0.001 (Student's unpaired t test).
p < 0.001 (Kolmogorov-Smirnov test).
The spatial distribution of intensity of the LCGU effects within the rostrocaudal and dorsoventral extent of the cortex in the anterior and posterior banks of the AS was reconstructed in two dimensions from the horizontal brain sections, as described previously (Dalezios et al., 1996; Savaki et al., 1997). The fundus of the AS was used for alignment of adjacent horizontal sections in each reconstructed map. In our horizontal sections, the floor of the AS contained parts of the agranular premotor cortical areas F2 and F7 and was included in the maps of the posterior bank. Because plotting resolution was set to 100 μm, each line of the two dimensional (2-D) reconstructed metabolic maps, parallel to the anteroposterior axis, represents the distribution of activity averaged over five adjacent serial sections, each 20 μm thick. To compare directly the sites of activation in all hemispheres studied, and because the arcuate sulci of different hemispheres are not identical, we considered as reference AS the one in the right hemisphere of the fixating control, and we normalized geometrically the AS of all other hemispheres accordingly. Thus, the anteroposterior and dorsoventral length of each bank of the AS were linearly transformed to fit the corresponding length of the reference AS. Cytoarchitectonic subdivisions in the frontal cortex within and near the AS were identified on Nissl-stained sections on the basis of known characteristics of areas F2-F7, 8, 45, 46, and 9 (Walker, 1940; Matelli et al., 1991).
Rabies virus experiments. All surgical procedures were performed aseptically under general anesthesia with acepromazine (0.5 mg/kg, i.m.) and ketamine (30 mg/kg, i.m.), preceded by premedication with valium and atropine. The LR muscle of the left eye was exposed for the injections by retracting the eyelids with blunt retractors, making an incision on the skin and conjunctiva and removing a small part of the orbital bone under a surgical microscope. The virus solution (110-120 μl) was pressure injected via a Hamilton (Reno, NV) syringe into the belly of the LR muscle, centered on its global layer. The tracer was the challenge virus standard fixed strain 11 of rabies virus (Ugolini, 1995). Concentrated virus (titer 7.8 × 1010 plaque-forming units per ml) was prepared by pelleting the supernatant of 72 hr infected baby hamster kidney 21 cells through a cushion of 25% glycerol. The virus stock was kept frozen at -80°C until use. Postoperative care involved local application of neomycin/hydrocortisone ophthalmic cream and daily administration of antibiotics (Clamoxyl retard; 15 mg/kg daily, i.m.) and analgesics (Aspegic; 60 mg/kg, i.m., twice each day).
After 2.5 d (experiment LR6) postinoculation (PI), 3 d PI (LR2), and 3.5 d PI (LR4), monkeys were administered a lethal dose of pentobarbital after induction of general anesthesia as described above. None of the animals showed behavioral changes or pathological symptoms at these times. They were perfused transcardially with PBS (pH 7.4), followed by 4% paraformaldehyde in 0.1 m phosphate buffer (PB; pH 7.4) and 10% sucrose in 0.1 m PB (pH 7.4). Brains were cut stereotaxically in two blocks (at 13 mm anterior to the interaural line). The two blocks and all extraocular muscles of the left eye were processed histologically as described previously (Grantyn et al., 2002). Briefly, the tissue was transferred to sucrose for cryoprotection and embedded in gelatin, which was then fixed. Subsequently, the brains were cut on a freezing microtome in 50 μm sections. For immunoperoxidase (PAP) staining, sections were incubated in 0.4% Triton X-100 (30 min), followed by 10% donkey serum (1 hr) and then overnight at 4°C in mouse monoclonal antibodies (dilution, 1:1000) directed against the rabies P protein (Laboratoire de Géné-tique des Virus, Gif-sur-Yvette, France). Subsequently, the sections were incubated in donkey anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA), diluted 1:200 (2 hr), and then in mouse PAP complex (Dako, High Wycombe, UK; Trappes, France) diluted 1:200 (2 hr). Peroxidase activity was detected through incubation in a metal enhanced diaminobenzidine substrate kit (Pierce, Rockford, IL). Specificity of the staining was checked by incubating positive controls (sections from brains having shown rabies immunolabeling in previous reactions) and negative controls (sections from noninfected rat brains and spinal cords) together with each series. After the reaction, sections were mounted on gelatin-coated slides, air dried, counterstained with cresyl violet (Sigma-Aldrich, St. Quentin Fallavier, France), and coverslipped with Entellan (Merck, Whitehouse Station, NJ).
To verify the injection site in the LR muscle and to rule out contamination of other eye muscles, all extraocular muscles of the left eye were dissected, cut in frozen sections, and immunoreacted for rabies virus as described above. To confirm that the virus was not taken up by motoneurons other than those innervating the LR muscle, we treated one series of sections through the trochlear, oculomotor, abducens, and facial nuclei with a dual-color immunofluorescence protocol for simultaneous detection of rabies and choline acetyltransferase (CAT), used here as a motoneuron-specific marker. Floating sections were incubated successively in the following: normal donkey serum in PBS; a mixture of mouse monoclonal antibodies (1:100) against the rabies P protein (Laboratoire de Génétique des Virus) and goat anti-CAT IgG (1:100; AB144P; Chemicon, Temecula, CA) overnight at 4°C; a mixture of FITC-conjugated donkey anti-mouse IgG (1:100; Jackson ImmunoResearch, West Grove, PA) and cyanin (Cy) 3-conjugated donkey anti-goat IgG (1:100; 2 hr; Jackson ImmunoResearch). Sections were then mounted on gelatin-coated slides, dried, coverslipped with Vectashield (Vector Laboratories, Burlingame, CA), and examined with a Leitz (Wetzlar, Germany) epifluorescence microscope. In all double-immunofluorescence protocols, specificity of the labeling was checked by omitting the primary or secondary antibody and using negative and positive controls.
One of every eight sections (400 μm spacing) was available for sampling labeled neurons. Using computer-assisted microscopy (Neurolucida; MicroBrightField, Colchester, VT), all rabies immunolabeled neurons were plotted in the coronal plane together with the cortex surface, the transition between layer VI and white matter, the corpus callosum, and the ventricles. To unfold the cortical gray matter of the frontal lobes, we used the method of Hubel and Wiesel (1972). Briefly, the cortical surface was piece-wise linearized with straight-line segments drawn tangentially and interrupted at points of high curvature (i.e., near the sulcal fundus and crown). Labeled neurons were projected onto the nearest line segment. Linear transformations of the plane (rotation and translation) were then used to bring line segments, along with associated neurons into a standard orientation, contiguous with neighboring segments. Except for the genu of the rostral bank of the AS, straightened representations were positioned parallel to each other and 400 μm apart (i.e., the distance between sections) and were aligned on surface landmarks characteristic of the unfolded region. In three to five sections adjacent to the genu, where the anterior bank of the AS (left) is almost parallel to the plane of section, cells were drawn as projections onto the plane of section. This enabled us to flatten the cortex without introducing a cut close to the genu, which would have artificially separated adjoining areas of dense labeling.
Results
Metabolic activity (i.e., LCGU values) was measured throughout the frontal lobes of the right hemisphere of two monkeys while they made repeated contraversive (left-ward) saccades to visual targets. The scatterplots of Figure 1 provide a pictorial summary of all saccades they executed during the critical first 10 min of the 14C-DG experiment. Briefly, most of the saccades executed by the first monkey were up-left, visually guided ones, 20° in amplitude and 135° in direction, with an average density of 7.1 saccades per degree2 (Fig. 1A). The second monkey performed leftward purely horizontal saccades 30, 15, 10, and 5° in amplitude (with an average density of 6.7, 7.5, 7.4, and 11.2 saccades per degree2, respectively) (Fig. 1B). To explore the metabolic effects induced by visually guided saccades, the LCGU values measured in these animals were compared with those of a sham control monkey (Table 1, %C columns). However, both animals also executed small (<2°) saccades to the target and fixation points (fixational saccades). Their density was 2.1 saccades/degree2 in the first animal (Fig. 1A) and 4.3 saccades/degree2 in the second (Fig. 1B). In contrast, neither animal executed a significant number of leftward spontaneous saccades in other directions or amplitudes. When the whole oculomotor range was divided into 4 degree2 bins, the density of such leftward spontaneous saccades was found to be 0.08 saccades/degree2 in the first (Fig. 1A) and 0.01 saccades/degree2 in the second (Fig. 1B). Also, both monkeys executing saccades were also required to fixate targets (for ∼4 min each during the critical first 10 min of the experiment). All of these facets of their oculomotor behavior must be reflected in the LCGU values measured in the right hemispheres of animals executing saccades when compared with those of a sham control monkey. Finally, both animals had to foveate the fixation point to begin a new trial, which was always to the right of the target of the previous trial and could be reached by a combination of spontaneous and visually guided rightward saccades controlled primarily by the left frontal lobes. If these rightward saccades were evenly distributed, saccade-related regions of the left frontal lobes might have been homogeneously activated and could thus have served as a control case for quantitative purposes. However, this was not the case for either animal. Accordingly, and to disambiguate the effects attributable to the large visually guided saccades from those resulting from fixation, values obtained from the right hemispheres of monkeys executing saccades were compared with those from a monkey rewarded for fixating a target (Table 1, %F columns); this animal spent ∼75% of the critical first 10 min of the experiment fixating the target. When the oculomotor space of this animal (Fig. 1C) was divided into 4 degree2 bins, the average density of saccades was found to be 0.02 saccades/degree2 (range, 0-1 saccades/degree2) except for a small region (<2°) near the fixation point (average density, 1.6 saccades/deg2). Thus, besides removing effects resulting from fixation, subtraction of metabolic effects found in the fixating monkey from those of the monkeys executing saccades removes effects resulting from fixational saccades. To reveal frontal cortical effects induced by fixation, glucose utilization values obtained from the fixating monkey were compared with those obtained from the sham control animal. In this case, our data do not allow us to distinguish effects attributable to fixation from those resulting from small fixational saccades.
Figure 1.
Scatterplots of the horizontal (ΔH) and vertical (ΔV) eye displacements of all visually guided saccades executed by the monkeys during the critical first 10 min of the 14C-DG experiment. A, B, Monkeys rewarded for executing oblique, up-left saccades 20° in amplitude and 135° in direction (A), and leftward horizontal saccades of 5, 10, 15, and 30° amplitude (B). C, Control monkey rewarded for fixating a centrally located visual target for the duration of the experiment.
Arcuate sulcus
Both fixation and saccadic eye movements induced significant enhancement of glucose consumption in the AS. More specifically, fixation induced pronounced enhancement of glucose consumption in the anterior and posterior banks of the AS (Fig. 2a, b, left; Table 1). To better define the extent of arcuate cortex associated with fixation, we generated 2-D reconstructions of the spatial distribution of metabolic activity in the AS (Fig. 3A, B) as described previously (Dalezios et al., 1996; Savaki et al., 1997). The average of the geometrically normalized 2-D reconstructions of metabolic activity in the AS of the two hemispheres of the fixation control monkey illustrates that the activated region is confined to the dorsalmost 5 mm of the inferior ramus of the AS and extends into both its anterior and posterior banks (Fig. 3C). In frontal sections, this region occupies the part of the genu of the AS lateral to the spur. Furthermore, the map generated after subtraction of the activity in the control monkey from that in the fixating monkey (Fig. 3E) demonstrates that fixation-related activation is more pronounced around the fundus of the sulcus.
Figure 2.

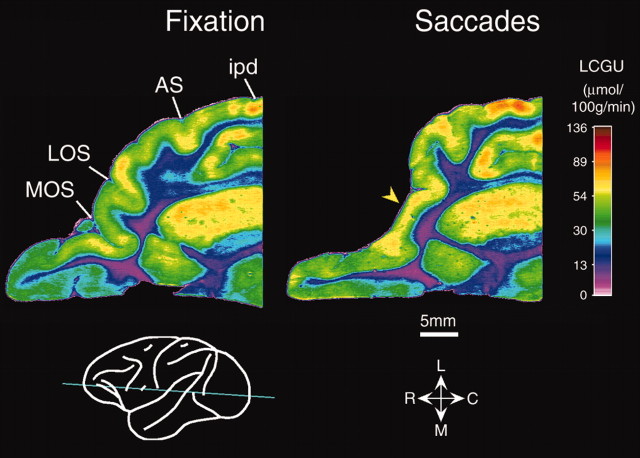
Quantitative, color coded, 14C-DG glucograms of the frontal cortex obtained from horizontal brain sections (a, b, c) through the arcuate sulcus (see corresponding levels in the diagrammatic representation of the monkey brain near the bottom on the left). Left column, Glucograms of sections from the fixating monkey. Right column, Glucograms of sections from the monkey executing visually guided, leftward horizontal saccades of 5, 10, 15, and 30° amplitude. Activations are illustrated within the anterior and the posterior banks of the AS in all sections, in the prearcuate convexity (yellow arrowheads), in area F4 (green arrowhead), in the caudal PS (black arrowhead), and in the orbitofrontal part of area 12 (red arrowhead). Color bar, LCGU values are in micromoles per 100 gm per minute; note that in this and the remaining glucograms, it obeys a saturating nonlinearity such that differences between gray and white matter are larger than they appear. C, Caudal; L, lateral; M, medial; R, rostral.
Figure 3.
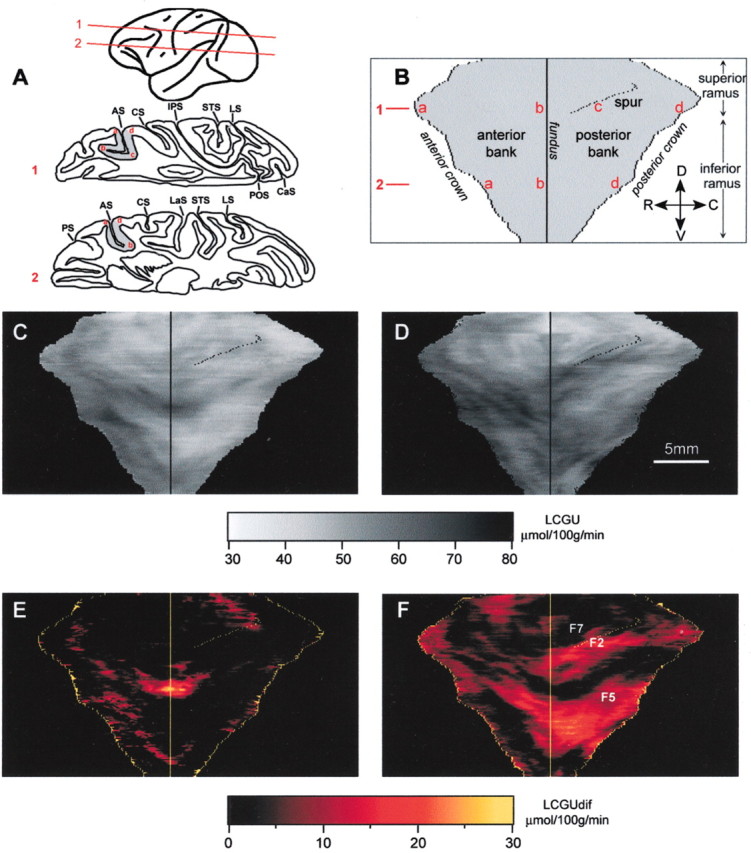
2-D maps of metabolic activity in the unfolded arcuate sulcus. A, Schematic representation of two horizontal sections at two different dorsoventral levels (1 and 2, indicated in the diagrammatic representation at the top) through the right hemisphere. The gray area in the two sections indicates the portion of the anterior and posterior banks of the AS, which was reconstructed. a-d (in red) indicate the crown of the anterior bank of the AS (a), the fundus of the AS (b; used as the point of alignment of adjacent sections), the posterior tip of the medial bank of the AS (c), and the crown of the posterior bank of the AS (d). B, Schematic representation of the geometrically normalized 2-D maps. Solid vertical line indicates the fundus of the AS. Parts of the reconstruction to the left and right of the fundus correspond to the anterior and posterior banks of the arcuate sulcus, respectively. a-d(in red)indicate the same landmarks as in A.C, Quantitative 2-D map of metabolic activity(LCGU values in micromoles per 100 gm per minute) obtained after averaging and geometrically normalizing the unfolded AS of both hemispheres of the fixating monkey. D, Quantitative 2-D map of metabolic activity obtained after averaging and geometrically normalizing the unfolded AS of the two hemispheres contralateral to the visually guided saccades executed by two monkeys. E, Image generated after subtracting the 2-D map of metabolic activity of the AS of the control monkey from that of the fixating animal shown in C. F, Image generated after subtracting the 2-D map of metabolic activity in the AS of the fixating monkey (averaged over both of its hemispheres) from the 2-D map of metabolic activity in the AS of two monkeys executing saccades (again averaged over two hemispheres contralateral to the visually guided saccades). F2, F5, and F7 are cytoarchitectonically identified areas in the posterior bank of the arcuate sulcus. Length scale in D applies to B-F. Gray scale bar indicates LCGU values in micromoles per 100 gm per minute and applies to C and D. Color bar indicates the difference between the LCGU values found in one pixel in two different maps (LCGUdif; also in micromoles per 100 gm per minute) and applies to E and F. CaS, Calcarine sulcus; CS, central sulcus; D, dorsal; IPS, intraparietal sulcus; LaS, lateral sulcus; LS, lunate sulcus; POS, parietoccipital sulcus; STS, superior temporal sulcus; V, ventral. Other abbreviations as in Figure 2.
The average of the geometrically normalized 2-D reconstructions of AS activity in the two monkeys executing saccades (Fig. 3D), and the map generated after the metabolic activity of the fixating monkey was subtracted from it (Fig. 3F), demonstrate that saccade-related activation occupies portions of the anterior bank of both the superior and inferior limbs and extends to the posterior bank of the AS. In particular, saccade-related activation was found in the anterior bank of the superior ramus of the AS, a region not activated during fixation (Fig. 3, Table 1). Moreover, saccades induced significant enhancement of glucose consumption in the dorsalmost 5 mm (Table 1, genu) of the anterior bank of the inferior ramus, which was more marked than the enhancement induced by fixation (Figs. 2, 3). The maximal saccade-related effect was found in the fundus of the sulcus (Table 1, genu). A second more ventral region, the ventralmost 7 mm of the anterior bank of the inferior ramus, was also activated (Table 1). The saccade-related region of pronounced metabolic activity in the anterior bank of the AS extended beyond the crown of the sulcus into the exposed convexity bounded by the superior ramus of AS medially, its inferior ramus laterally, and the principal sulcus (PS) rostrally (Fig. 2a, b, yellow arrowheads; Table 1, prearcuate convexity).
Four hemispheres from three experimental animals also demonstrate the strong involvement of the posterior bank of the AS in oculomotor control (Table 1). Saccade-related activations were measured in the posterior bank of the superior ramus, as well as the dorsalmost 5 mm and ventralmost 7 mm of the inferior ramus (Table 1). The posterior bank of the AS belongs to the premotor cortical area 6, which has been subdivided into areas 6D and 6V. The former encompasses the posterior bank of the superior ramus of the AS and the medial bank of its spur as well as the exposed convexity dorsal to them, and the latter includes the posterior bank of the inferior ramus of the AS, the lateral bank of its spur, and the exposed convexity ventral to it (Barbas and Pandya, 1987). Area 6D has been further subdivided into areas F2 and F7, occupying its caudal two thirds and its rostral one third, respectively (Matelli et al., 1991), whereas area 6V has been subdivided into areas F4 caudally and F5 rostrally (Matelli et al., 1985). Both areas F2 and F5 in the posterior bank of the AS were significantly activated for saccades (Fig. 3D). The most marked saccade-related activation in the posterior bank of the superior ramus was observed within area F2 in a band extending from the fundus to the crown (Fig. 3D, F). In contrast, the fixation-related activation of the posterior bank of the superior ramus was negligible (Fig. 3C, E). Although both fixation and saccade execution activated part of area F5 in the posterior bank of the inferior ramus, monkeys executing saccades always displayed higher activation than the fixating control subject (Figs. 2b, c, 3; Table 1). In animals executing saccades, increased glucose consumption extended beyond the posterior crown of the AS into the exposed convexity behind its inferior ramus and below its spur (Fig. 2a, b, green arrowhead).
In summary, our quantitative estimation of glucose consumption in the AS regions examined (Table 1), the 2-D reconstruction of metabolic activity in the AS of the monkeys executing saccades (Fig. 3D), and the map generated after subtraction of the fixation from the saccade-related effects (Fig. 3F) demonstrate that both the anterior and posterior banks of the AS are involved in the control of saccades. Consistent with a previous blood flow study in human subjects (Paus et al., 1995), the intensity of activation may be related to the number of movements executed, because the rate of glucose utilization was higher in the monkey executing sequences of horizontal saccades (at an average rate of 43 per minute) than in the monkey executing single oblique saccades (at an average rate of 12 per minute).
Involvement of both the anterior and posterior banks of the AS in oculomotor control is further supported by the distribution of retrogradely labeled cells in four hemispheres from two animals after the injection of rabies virus in their LR muscle. It should be noted that results obtained with rabies virus are entirely specific and highly reproducible because its retrograde transneuronal transfer is strictly time dependent, whereas degeneration of infected neurons and local spread (to glial cells, neighboring neurons, or fibers of passage) do not occur (Ugolini, 1995; Tang et al., 1999; Graf et al., 2002; Morcuende et al., 2002). Remarkably, spread of rabies virus does not occur even within the injected muscle itself (Ugolini et al., 2001). This specificity was confirmed by the results of the control experiments we performed, which rule out the involvement of any muscles other than the injected LR in all experiments described in the present study. In particular, the only infected motoneurons (positive for rabies and CAT, a motoneuron marker) were the LR motoneurons in the abducens nucleus, whereas neither facial motoneurons nor motoneurons of the oculomotor and trochlear nuclei were infected. Our data confirm the absence of local spread of rabies virus to neighboring neurons, as demonstrated by the lack of involvement of abducens internuclear neurons, which are CAT negative and use aspartate and glutamate as a neurotransmitter (Nguyen and Spencer, 1999), and are intermingled with the infected (and CAT-positive) LR motoneurons (Langer et al., 1986). Similarly, the absence of spread to passing fibers is confirmed by the fact that facial motoneurons were not labeled despite the fact that their axons cross the infected abducens nucleus.
No labeled neurons were found anywhere in the frontal lobes 2.5 d postinjection (LR6), when labeling involved exclusively subcortical neurons monosynaptically connected to LR motoneurons (Graf et al., 2000; Ugolini et al., 2001). Consistent with the transfer of virus through additional synapses (Ugolini et al., 2000), periarcuate labeling followed the time course of collicular labeling (Grantyn et al., 2002), i.e., it appeared at the 3.0 d (disynaptic) time point (Figs. 4, 5, LR2) and became more extensive after 3.5 d (Fig. 4, LR4), when transfer involved one more synapse (Grantyn et al., 2002). In both cases, labeling was bilateral (Fig. 4D-H), involving 704 neurons contralaterally and 693 ipsilaterally (counted in one of every eight sections through the periarcuate cortex of LR4). The bilateral transneuronal labeling of frontal lobe oculomotor-related areas reflects their primarily bilateral projections to subcortical structures (Schnyder et al., 1985; Huerta et al., 1986; Stanton et al., 1988; Shook et al., 1990), and the primarily bilateral transneuronal labeling of the latter after unilateral rabies virus injections in the LR muscle (Grantyn et al., 2002).
Figure 4.

Location of transneuronally labeled neurons in the frontal lobes of two rhesus monkeys at 3 d (left, LR2) and 3.5 d (right, LR4) after injection of rabies virus into the left lateral rectus muscle. Each dot represents one labeled neuron. A-H, Frontal sections are arranged from rostral (A) to caudal (H). arsp, Spur of the arcuate sulcus; Cd, caudate; iar, inferior ramus of the arcuate sulcus; if, intraparietal fissure; ins, insula; OPRo, orbital proisocortex; sar, superior ramus of the arcuate sulcus; spcd, superior precentral dimple.
Figure 5.

Distribution of transneuronally labeled neurons in the unfolded arcuate sulcus of two rhesus monkeys at 3 d (LR2, circles) and 3.5 d (LR4, dots) after injection of rabies virus into the left lateral rectus muscle (see Fig. 4 for corresponding cross sections). Each thin solid line was produced from the piecewise linearization of a single section outline. Each marker represents one neuron. Solid and gray lines indicate the crown and the fundus, respectively, of the illustrated sulci. Horizontal and vertical orientation arrows apply to the exposed prearcuate convexity and the banks of the arcuate sulcus, respectively (C, caudal; R, rostral). The horizontal gray and vertical dotted lines indicate the fundus of the spur of the arcuate sulcus and the anteroposterior level that corresponds to its rostral tip, respectively. Gray areas indicate regions metabolically activated for saccades.
Retrograde transneuronal labeling with rabies virus allowed us to define better the borders and the extent of the oculomotor-related region of the arcuate cortex. To this end, the location of labeled cells was plotted on high-resolution unfolded maps of the AS, divided in two by its fundus (Fig. 5): (1) a plot of the anterior bank of the AS (Fig. 5, left), and (2) a plot of the posterior bank of the AS and its spur (Fig. 5, right). Because cytoarchitectonic areas of the anterior bank of the AS (8A and 45) extend into the exposed prearcuate convexity (Walker, 1940), its straightened representation was included in the reconstructions (Fig. 5, left) and aligned on the crown of the PS. The unfolded reconstructions of the posterior bank of the AS included about half of the periarcuate cells found in each side of the brain [304 on the right (R) and 365 on the left (L)] (Fig. 5, right). Cells were found in the posterior bank of the superior and inferior limbs (aligned on the crown) as well as the floor of the AS and the banks of its spur (aligned on the fundus of the spur). The majority were found in both the medial and lateral banks of the spur (R = 134; L = 109) and in the floor of the AS underlying the genu (R = 118; L = 169). The remaining cells were distributed in the posterior banks of the superior (R = 27; L = 49) and the inferior (R = 28; L = 38) ramus of the AS (Fig. 6, right). A few transneuronally labeled neurons were seen scattered on the exposed convexity behind the inferior limb of the AS and below its spur (Fig. 5, arrowhead), in the same small region of the ventral premotor cortex where increased glucose consumption was found in animals executing saccades (Fig. 2a, b, green arrowhead). In the anterior bank of the AS (Fig. 5, anterior bank), a total of 400 (R) and 328 (L) labeled cells were found in the inferior ramus (R = 148; L = 125), in the superior ramus (R = 86; L = 118), and in the exposed prearcuate convexity (R = 166; L = 85). Most of the latter cells were behind the caudal tip of the principal sulcus; only a few were found medial (R = 5; L = 19) and lateral (R = 12; L = 8) to it. The length of the region in the anterior and posterior bank of the AS densely occupied with labeled cells was equal to ∼10 mm and extended from the fundus to the crown. This region was not centered on the genu of the AS; ∼40% or less occupied the superior ramus, and the remaining extended through the inferior ramus. It continued dorsally and rostrally into the exposed prearcuate convexity and ventrocaudally into the underlying floor and both banks of the arcuate spur immediately behind it. More ventral parts of the inferior ramus also contained labeled neurons widely distributed from the fundus to the crown almost reaching its tip. More rostral and medial portions of the superior ramus contained additional labeled cells; Figure 6 shows a dense cloud, 4 mm long, near the fundus on the left. To examine how well the extent and location of the area containing transneuronally labeled cells corresponds to the region functionally activated for saccades, the isointensity curve, which corresponds to a relative increase of glucose consumption (LCGUdif) equal to 10 μmol/100 gm/min, was defined on the 2-D reconstructions of Figure 3 (E, fixation; F, saccades). It was then translated, rotated, and stretched so that the borders of the 2-D reconstructions of metabolic activity (crowns, fundi, tips) were aligned on the same borders of the unfolded plots of the transneuronally labeled cells found in and near the right AS. As shown in Figure 6, the areas containing labeled cells correspond well, in both extent and location, to the regions that are functionally activated for saccades (shaded).
Figure 6.

A-K, Photomicrographic details of typical transneuronally labeled neurons found in the ipsilateral spur of the arcuate sulcus (A), floor of the arcuate sulcus (B), posterior bank of the arcuate sulcus (C), ipsilateral (D) and contralateral dorsomedial frontal cortex (E), contralateral exposed prearcuate convexity close to the crown of the principal sulcus (F), ipsilateral (G) and contralateral (H, I) ventral bank of the cingulate sulcus, as well as the ipsilateral medial (J) and the contralateral lateral (K) bank of the principal sulcus near its crown. Scale bar: (in B) A-K, 40 μm.
In the prearcuate convexity, labeled neurons were found in infragranular layer V. Morphological details of such cells found in experiment LR-4 are shown in Figure 6F. As shown here, the apical dendrite of one intensely stained pyramidal neuron could be followed for a considerable distance in the plane of the section. Such conspicuous dendritic labeling was only rarely observed at the shorter time point (LR2). The apical dendrites of intensely labeled neurons found in the posterior bank of the AS (Fig. 6C) and its spur (Fig. 6A) could be followed only for relatively short distances, because these areas were cut almost tangentially to the surface of the cortex. Nevertheless, many of the cells found in these areas were also pyramidal neurons of layer V, sometimes clustered with nearby smaller nonpyramidal neurons (Fig. 6B).
Principal sulcus
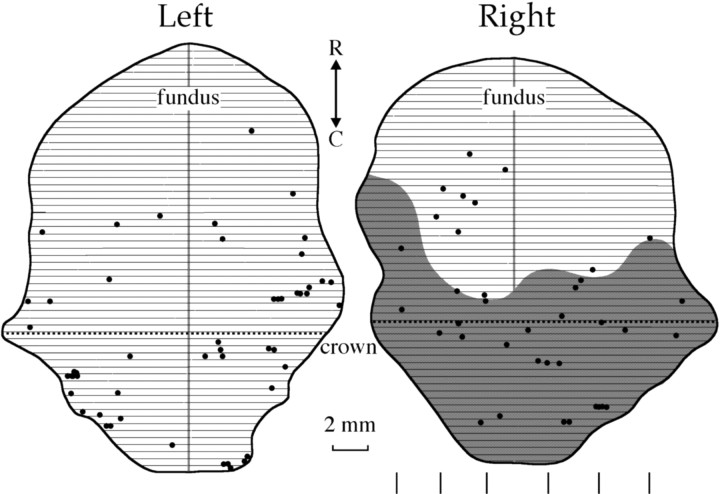
Transneuronally labeled cells were also found in the PS of one monkey (LR4), bilaterally. Their location is illustrated in Figure 7, in unfolded maps of the medial (upper) and lateral (lower) banks of the PS (occupied by area 46) (Walker, 1940) aligned on its fundus. Retrogradely labeled neurons were scattered throughout the PS with the exception of the rostral half of its lateral bank. Figure 6 illustrates typical examples of transneuronally labeled cells found near the crown of the medial (Fig. 6J) or lateral (Fig. 6K) banks of the ipsilateral and contralateral PS, respectively. As shown here, rabies immunolabeled neurons were pyramidal cells belonging to layer V. Their apical dendrites could be followed for considerable distances. Consistent with their oligosynaptic connections to extraocular muscles revealed by rabies transneuronal labeling, the execution of saccades to visual targets significantly enhanced glucose consumption in the caudal PS of two monkeys (Table 1; Fig. 2b, black arrowhead). To estimate its extent (the shaded region of Fig. 7), we measured the length of the metabolically activated area in three approximately equidistant horizontal sections through the lateral and medial banks of the right PS (their relative location is indicated by the line segments next to the scale on the right). It occupied less than the posterior 50% of the medial and lateral banks of the PS and corresponded well to the PS region containing transneuronally labeled neurons. The remaining area 46 (approximately the rostral 50% of both banks of the PS) was not activated either by fixation or by saccade execution (Table 1, Fig. 2b).
Figure 7.
Plot of the distribution of transneuronally labeled neurons in the unfolded principal sulcus. The vertical dotted line indicates the anteroposterior level that corresponds to the rostral tip of the superior ramus of the arcuate sulcus. The horizontal line segments next to the scale on the right indicate the dorsoventral location of the horizontal sections that were used to measure the rostrocaudal length of the metabolically activated area. Other symbols and abbreviations as in Figure 5.
Dorsomedial frontal cortex
All eight hemispheres from all five experimental animals studied with both methods indicate the involvement of the DMFC in oculomotor control. Fixation and saccadic eye movements induced significant enhancement of glucose consumption within a circumscribed region of the DMFC. The activated region straddled the ridge of the cortex (it appears as the rostral tip in the fairly dorsal horizontal sections illustrated in Fig. 8), extending both medially and laterally to it. Starting from the rostral tip of the superior ramus of the arcuate sulcus, the strongest activation in the contralateral hemisphere of the two monkeys executing saccades encompassed a strip of tissue extending for ∼10 mm rostrocaudally and 2 mm mediolaterally. Both monkeys executing saccades displayed higher DMFC activity than the fixating subject (Fig. 8, yellow arrowhead; Table 1). The intensity of activation could be related to the number of movements executed, because the rate of glucose utilization was higher (Table 1) in the monkey executing sequences of horizontal saccades (at an average rate of 43 per minute) than in the monkey performing single oblique saccades (at an average rate of 12 per minute).
Figure 8.
Quantitative, color-coded, 14C-DG glucograms from horizontal sections (a, b) through the dorsomedial frontal cortex at levels indicated in the diagrammatic representation of the brain (bottom left). Left column, Glucograms of sections from the fixating monkey. Right column, Glucograms of sections from the monkey executing leftward horizontal, visually guided saccades. Yellow arrowheads point to areas of increased metabolic activation in the dorsomedial frontal cortex of the monkey executing saccades. spd, Superior precentral dimple. Other abbreviations as in Figure 2.
Activation of the DMFC for saccades is consistent with the fact that it houses the SEF (Schlag and Schlag-Rey, 1987; Schall, 1991; Bon and Lucchetti, 1992; Russo and Bruce, 1993; Tehovnik and Lee, 1993; Fujii et al., 1995). The location and extent of the region occupied by neurons labeled transneuronally after injections of rabies virus in the LR muscle (Fig. 4B-G) could help us better define its borders, which, as outlined in the introductory paragraphs, has been a matter of considerable debate. To this end, part of the DMFC bounded by the crown of the cingulate sulcus (CgS) medially, its rostral tip rostrally, the PS, AS, and superior precentral dimple laterally, and the primary motor cortex caudally was unfolded and the location of the transneuronally labeled neurons of monkey LR4 was plotted on it (Fig. 9). A fairly compact cluster of cells was found to straddle the ridge of both hemispheres but was better demarcated on the contralateral side. It contained a relatively high concentration of cells (two cells per square millimeter on the right and three cells per square millimeter on the left) when compared with the average cell density in other DMFC regions of similar surface area (0.25 cells per square millimeter). It occupied an area of ∼20 mm2 extending from ∼2 mm behind the rostral tip of the AS to ∼3 mm in front of its genu and from 2.5 mm lateral to the ridge to ∼1.5 mm medial to it. This fits well the location and extent of the DMFC area activated for saccades to visual targets in our 2-DG experiments (the shaded region of the plot devoted to the right DMFC in Fig. 9; drawn so that its rostrocaudal and mediolateral lengths fit the measurements obtained from the autoradiograms described above and placed so that it straddles the ridge of the brain and that its rostral edge coincides with the rostral tip of the AS). Neurons recovered in the DMFC were pyramidal cells of layer V (see Fig. 5, D and E, for examples of labeled neurons with apical dendrites, which could be followed for up to half of 1 mm or more in the plane of the section). It should be noted that cells found in the DMFC were seen not only in area F7 (Luppino et al., 1991) but also in area F6. A few scattered neurons were found in the premotor cortex on the exposed convexity behind the genu of the AS within F2, bilaterally (Fig. 9). Others were found in the mesial surface of the brain, also bilaterally, as far ventrally as the CgS and as far rostrally as its rostral tip (Fig. 9). Finally, a few scattered neurons were found in front of the rostral tip of the superior ramus of the AS (Fig. 4B), throughout areas 8B and 9, and sometimes as far laterally as the crown of the medial bank of the PS (Fig. 9).
Figure 9.
Distribution of transneuronally labeled neurons on a plot of the unfolded dorsomedial frontal cortex aligned on the ridge of the brain (horizontal gray line). Solid lines indicate the crown of the sulci illustrated. The vertical dotted line indicates the anteroposterior level that corresponds to the genu of the arcuate sulcus. ASsr, Superior ramus of the arcuate sulcus. Other symbols and abbreviations as in Figures 5 and 8.
Dorsolateral prefrontal and orbitofrontal cortex
The cortex of the dorsal periprincipal convexity (dorsolateral prefrontal) was moderately activated for saccades (two animals) and not by fixation (two hemispheres of one animal) (Table 1). The cortex of the periprincipal convexity ventral to the PS and rostral to the inferior ramus of the AS (ventrolateral prefrontal areas 46 and 12) was significantly activated both for fixation and saccades (Table 1, ventrolateral prefrontal). Moreover, circumscribed regions within the orbitofrontal part of area 12 (Walker, 1940; Romanski et al., 1997), posterior and lateral to the lateral orbital sulcus (LOS), were activated by fixation and saccades (Table 1, orbitofrontal 12 max effect; Fig. 2c, red arrowhead). The same area was also seen to contain transneuronally labeled neurons after rabies virus injections in the LR muscle (Fig. 4, LR4). Their distribution is documented in our plot of the unfolded orbitofrontal cortex (ObFC) (Fig. 10). Besides orbitofrontal area 12, recovered neurons also occupied its continuation through the orbital margin of the frontal lobe and the exposed convexity ventral to the PS (Figs. 4C, D, 10). The same plot also includes area 14 on the gyrus rectus (Walker, 1940; Romanski et al., 1997) as well as its continuation on the mesial surface of the brain ventral to the rostral sulcus (RS). To examine the overlap between the orbitofrontal regions containing transneuronally labeled cells and those activated for saccades, the isointensity curve, which corresponds to a relative increase of glucose consumption equal to 10 μmol/100 gm/min was drawn on unfolded plots the right orbitofrontal cortex (Fig. 10, shaded) as described for the arcuate sulcus above. Neither the mesial (between the anterior tip of the brain and the RS) nor the orbital (between the anterior tip of the brain and the MOS) portions of prefrontal cortical areas 10 and 14 were significantly affected by fixation and saccade execution (Table 1) and except for a few scattered cells they were not seen to contain transneuronally labeled neurons either (Fig. 10). The orbital gyrus between the medial orbital sulcus (MOS) and the LOS encompassing areas 11 (rostrally) and 13 (caudally) (Walker, 1940; Romanski et al., 1997) displayed significant focal metabolic activation during fixation and execution of saccades (Table 1; Fig. 11, yellow arrowhead) but contains hardly any neurons oligosynaptically connected to extraocular muscles (Fig. 10).
Figure 10.
Distribution of transneuronally labeled neurons on a plot of the unfolded ObFC. Thick solid lines indicate the borders of the ObFC (i.e., the lateral edge of the ventral surface of the brain) and the bottom of its mesial surface (straight horizontal; also used for section alignment). Thin solid and gray lines indicate the crown and the fundus, respectively, of the MOS and LOS, the crown of the ventral bank of the RS, and the crown of the lateral bank of the PS. The vertical dotted line indicates the anteroposterior level that corresponds to the rostral tip of the inferior ramus of the arcuate sulcus. OPro, Orbital proisocortex. Other symbols and abbreviations as in Figure 5.
Figure 11.
Quantitative, color-coded, 14C-DG glucograms from horizontal sections through the orbitofrontal cortex at the level indicated in the diagrammatic representation of the brain (bottom left). Left, Fixating monkey. Right, Monkey executing leftward horizontal, visually guided saccades. Yellow arrowhead points to an area of increased metabolic activation between the MOS and the LOS in the orbitofrontal cortex of the monkey executing saccades. ipd, Inferior precentral dimple. Other abbreviations as in Figure 2.
Limbic-paralimbic cortex
To better examine the distribution of transneuronally labeled cells in the limbic-paralimbic cortex (Fig. 4), we prepared (see Materials and Methods) unfolded reconstructions of their distribution (Fig. 12), which comprise part of the mesial surface bounded by the crown of the dorsal bank of the CgS dorsally, its rostral tip rostrally, the crown of the ventral bank of the RS, the ventral tip of the mesial surface of the brain and the dorsal border of the corpus callosum ventrally, and its caudal tip caudally. The reconstruction includes part of area 6 and areas 24 and 23. The border between areas 24 and 6 runs along the dorsal bank of the CgS close to its dorsal lip (Matelli et al., 1991). The border between areas 24 and 23 can span several millimeters of transitional cortex (Bates and Goldman-Rakic, 1993). Traditionally, it has been placed at the anteroposterior level of the caudal tip of the spur of the AS (Ghosh and Gattera, 1995), a tradition we followed in Figure 12. This is probably conservative because the area 24d extends 2-3 mm further caudally (Matelli et al., 1991). Our plot of the mesial surface of the hemispheres ventral to the CgS also includes part of area 25 (ventral to the corpus callosum) (Walker, 1940) and area 32 (between the CgS and the RS) (Yeterian and Pandya, 1988; Romanski et al., 1997). It also includes the outlines of the regions enclosed by isointensity curves, which correspond to a relative increase of glucose consumption equal to 10 μmol/100 gm/min obtained from two monkeys executing saccades and drawn on the unfolded plots of the right mesial cortex (Fig. 12, shaded) as described for the arcuate sulcus above. A considerable number of transneuronally labeled neurons were found in both the dorsal and the ventral banks of the rostral CgS bilaterally (Figs. 4A-H, 12), a finding consistent with the metabolic activation of the rostral half of the anterior cingulate induced by saccades (Table 1). The caudal CgS was mostly devoid of labeled cells (Fig. 12). Figure 5 illustrates typical examples of neurons found in the ipsilateral (Fig. 6G) and contralateral (Fig. 6I) area 24. Most recovered neurons were pyramidal cells of layer V; however, more superficial faintly labeled neurons (Fig. 6H) were sometimes observed. The caudal CgS was not oligosynaptically connected to extraocular muscles, and its involvement in saccades is uncertain because it was significantly activated for the sequences of horizontal saccades executed by one animal but not for single 20° oblique saccades executed by another (Table 1). In our imaging study, fixation and execution of saccades induced significant, albeit not pronounced, activation of mesial prefrontal cortical areas 25 and 32 behind the RS (Table 1). The same regions were also seen to contain a considerable number of labeled cells after rabies virus injections in the LR muscle (Fig. 12).
Figure 12.
Distribution of transneuronally labeled neurons on a plot of the unfolded CgS. Thin solid and gray lines indicate the crown and the fundus, respectively, of the RS and of the CgS (its fundus is indicated by the straight horizontal line that was used for the alignment of adjacent sections). The thick solid lines indicate the dorsal edge of the corpus callosum (CC) and the ventral edge of the mesial surface of the brain. The rostral and caudal vertical dotted lines indicate anteroposterior levels corresponding to the rostral tip of the superior ramus and the caudal tip of the spur of the arcuate sulcus, respectively. Other symbols and abbreviations as in Figure 5.
Discussion
Our study is the first to provide high-resolution functional images of the frontal lobes of rhesus monkeys during saccades to visual targets. Activated areas include the anterior and posterior banks of the AS, prearcuate convexity, dorsomedial frontal, caudal principal and periprincipal, anterior cingulate, and parts of the orbitofrontal cortex. All of these areas are oligosynaptically connected to oculomotoneurons, as demonstrated here by retrograde transneuronal labeling after rabies virus injections into the LR muscle. In view of their confinement to layer V, recovered neurons must have been labeled via corticofugal efferents targeting subcortical structures. Here, we discuss our major findings in the light of existing evidence implicating several frontal lobe areas in oculomotor control.
The arcuate sulcus
The extent of the densest cloud of transneuronally labeled cells in the anterior bank of the AS (10 mm along the sulcus by 6 mm, distance from crown to fundus) agrees remarkably well with the surface area (80 mm2) and the extent (10 mm along the AS) of the electrophysiologically defined low-threshold FEF (Bruce et al., 1985). However, our transneuronal and imaging data demonstrate that a much larger cortical region of the frontal lobes in and near the AS is oligosynaptically connected to extraocular motoneurons and is metabolically activated when the rhesus monkey participates in oculomotor tasks. Its surface area is larger than 150 mm2 and encompasses the exposed prearcuate convexity, both banks of the AS and spur, and a small part of the neighboring postarcuate convexity. This topography is consistent with the earlier description of Robinson and Fuchs (1969) and with more recent electrophysiological reports demonstrating that the oculomotor-related part of the AS extends beyond the confines of the low-threshold FEF. Indeed, the prearcuate convexity has been implicated in saccades (Robinson and Fuchs, 1969) vergence, divergence, and ocular accommodation (Gamlin and Yoon, 2000). More caudally, the oculomotor-related AS has been shown to invade premotor area 6. Near the fundus of the posterior bank of the AS, a small region sandwiched between the small saccade area of the FEF and the somatic premotor cortex is involved in smooth pursuit (Bruce et al., 1985; MacAvoy et al., 1991). Behind it, a region near the arcuate spur contains cells discharging in relation to saccades, eye position, and smooth pursuit (Fukushima et al., 2002; Tanaka and Lisberger, 2002), whereas microstimulation of a restricted part of the ventral premotor cortex in the postarcuate convexity has been shown to evoke saccades (Fujii et al., 1998).
The high-resolution functional images of our 14C-DG study emphasizes the involvement of the premotor area 6, within the posterior bank of the AS, in the control of saccades to visual targets. To date, area F2 in the posterior bank of the superior limb of the AS has been implicated in forelimb movements (Raos et al., 2003; Gregoriou and Savaki, 2003). Similarly, area F5 in the posterior bank of the inferior limb is thought to participate in the visual guidance of reaching (Gregoriou and Savaki, 2003) and the representation of arm, hand, and mouth movements (Rizzolatti et al., 1988). It might be argued that the increased glucose consumption that we observed in the caudal bank of the AS reflects its participation in movements other than saccades (e.g., blinks or attempted head turns). In our experiments, the method used to record eye position also informed us of blinks. Because our monkeys generated approximatley the same number of blinks during fixation and saccades, the increased activation of the frontal lobes of animals executing saccades cannot reflect blinks. In contrast, we cannot rule out the possibility that they partly reflect the contribution of eye-head gaze shifts. The pronounced metabolic activation of the posterior bank of the AS observed in our 14C-DG study indicates a strong involvement of areas F2 and F5 in shifts of the line of sight, and our rabies virus results imply that, at least in part, these are accomplished through the engagement of extraocular muscles.
Our study is also the first to provide high-resolution functional maps of the fixation-related area in the AS of the rhesus monkey. The highest metabolic activation induced by fixation is located in the dorsalmost 5 mm of the inferior ramus of the AS, near its genu. It occupies portions of both banks of the AS, particularly around its fundus. Involvement of the primate FEF in fixation is supported by the finding that its removal interferes with it (Dias et al., 1995). Moreover, the FEF contains neurons that discharge in response to foveal visual stimulation and in relation to maintenance and disengagement of fixation (Bizzi, 1968; Suzuki and Azuma, 1977; Bruce and Goldberg, 1985).
The dorsomedial frontal cortex
Our data demonstrate that a large part of the superior frontal gyrus is also oligosynaptically connected to extraocular motoneurons and is metabolically activated during oculomotor tasks. The activated region measures ∼10 by 2 mm (rostrocaudal by mediolateral length) and straddles the ridge of the cortex. Its metabolic activation for saccades is consistent with the numerous saccade-related cells found in the DMFC (Schlag and Schlag-Rey, 1987; Bon and Lucchetti, 1992; Fujii et al., 1995; Russo and Bruce, 2000). Consistent with the fact that it also contains neurons that discharge for as long as the animal fixates a region of space (Schall, 1991), fixation also induced significant enhancement of glucose consumption within the DMFC.
In agreement with our imaging data, a somewhat dense and compact cloud of cells was recovered in a portion of the DMFC occupying ∼20 mm2. This corresponds to the region of the superior frontal gyrus projecting to the FEF (24 mm2) (Schall et al., 1993). The territory occupied by labeled neurons is slightly bigger than the SEF described by Russo and Bruce (2000) but considerably smaller than that described by Tehovnik et al. (1993). Moreover, it is more posterior and medial than in Russo and Bruce (2000), its borders agreeing remarkably well to those of Fujii et al. (1995). The small region with a high concentration of saccade-related neurons described by these authors is surrounded by a much larger area also containing saccade-related neurons, albeit less frequently, which encompasses the supplementary motor area (SMA), the pre-SMA, and areas of the exposed surface lateral, caudal, and rostral to the SEF (Fujii et al., 1995). Consistent with this, the fact that saccades are evoked in response to electrical stimulation of areas 6 (Shook et al., 1988) and 8B (Bon and Lucchetti, 1994) and the fact that saccade-related cells are encountered in areas 8B (Bon and Lucchetti, 1994) and 9 (Joseph and Barone, 1987), we found additional transneuronally labeled neurons in areas 6, 8B, 9, and corresponding mesial cortical regions.
The principal sulcus
Our data demonstrate that the caudal part of the PS is oligosynaptically connected to extraocular motoneurons and is metabolically activated during oculomotor tasks. Previous electrical stimulation and blood flow studies of this region produced equivocal results. Saccades were evoked in response to its electrical stimulation in one study (Azuma et al., 1988) but not in another (Boch and Goldberg, 1989). Moreover, saccade-related activation of regions homologous to the primate PS has been found in some blood flow studies in humans (Sweeney et al., 1996; Doricchi et al., 1997) but not in others (Fox et al., 1985; Petit et al., 1993; O'Driscoll et al., 1995). In contrast, our data are consistent with previous extracellular recording and lesion findings. First, cells discharging in relation to saccades have been found in the caudal half of the PS (Boch and Goldberg, 1989; Funahashi et al., 1991). Second, unilateral lesions in and around the PS disrupt the performance of memory guided eye movements to spatial cues in the visual field contralateral to the lesioned hemisphere (Funahashi et al., 1993).
The cingulate sulcus
Our study is the first to demonstrate that an extensive portion of the rostral cingulate cortex of rhesus monkeys (including areas 24, 32, and 25) is oligosynaptically connected to extraocular motoneurons and is activated during saccades toward, and fixation of, visual targets. This is consistent with reports that electrical stimulation of the anterior cingulate cortex in humans evokes saccades (Talairach et al., 1973) and that antisaccades, memory guided saccades, and sequences of visually guided saccades are impaired in patients with anterior cingulate lesions (Gaymard et al., 1998). It is also consistent with the results of blood flow studies in humans, showing activation of a cingulate region underlying the SEF during execution of prelearned sequences or self-paced horizontal saccades (Petit et al., 1996). Moreover, areas 24 and 32 are activated during saccades toward or away from visual targets and toward memorized targets (Anderson et al., 1994; Doricchi et al., 1997). In agreement with our data, the anterior cingulate (including areas 32 and 24) is activated by fixation of visual (Anderson et al., 1994) or imaginary (Petit et al., 1995) targets, and its lesion impairs suppression of reflexive saccades in humans (Paus, 1991).
The orbitofrontal cortex
Our data demonstrate that neurons occupying a portion of the lateral ObFC, which includes area 12, are oligosynaptically connected to extraocular motoneurons. This region contains cells that increase their discharge when monkeys gaze at a spot of light (Suzuki and Azuma, 1977). Our data show that this area is activated for saccades toward and fixation of visual targets, as are areas 11 and 13. This is partly consistent with the fact that area 11 is activated during active fixation of visual targets as shown by blood flow studies in humans (Anderson et al., 1994). Because we could not establish their oligosynaptic connections to extraocular motoneurons and because their metabolic activity increased both for saccades and fixation, activation of these orbitofrontal areas could reflect reward-related polysynaptic processes participating in the behavioral tasks we used (Barbas et al., 1999).
Sources of cortical labeling
Brainstem nuclei containing neurons labeled transneuronally after rabies virus injections in the LR muscle (Graf et al., 1999, 2000; Ugolini et al., 2000, 2001; Grantyn et al., 2002) include the paramedian pontine reticular formation (PPRF), supraoculomotor area (Soc), mesencephalic reticular formation (MRF), vestibular nuclei as well as the nuclei reticularis tegmenti pontis (NRTP), prepositus hypoglossi (NPH), rostral interstitial nucleus of the medial longitudinal fasciculus (riMLF), and interstitial of Cajal (NIC) labeled within 2.5 d (LR6), as well as the intermediate gray layer of the superior colliculus (SGI) and the nucleus raphe interpositus (rip) labeled at 3 d (LR2). Several of these nuclei are known to receive direct projections from the cortical areas containing labeled neurons. For example, the anterior bank of the AS projects to the MRF, SGI, NPH, Soc, NRTP, PPRF, NIC, and rip (Schnyder et al., 1985; Huerta et al., 1986; Stanton et al., 1988; Shook et al., 1990). Taking into consideration previous descriptions regarding the relative strength of these projections and the time course of labeling in cortical and subcortical structures in our experiments, the more likely sources of the labeling in the anterior bank of the AS are the Soc, PPRF, NRTP, and MRF at 3 d postinoculation (LR2) and the SGI at 3.5 d postinoculation (LR4). Similarly, the PPRF (in LR2) and the SGI (in LR4) are the most likely sources of labeling in the posterior bank of the AS (Leichnetz et al., 1981; Fries, 1984; Schnyder et al., 1985). The DMFC shares many of the projections of the AS such as to the MRF, NIC, SGI, NPH, Soc, NRTP, PPRF, and rip (Leichnetz et al., 1981; Schnyder et al., 1985; Shook et al., 1988, 1990; Huerta and Kaas, 1990). Again, taking into consideration previous descriptions regarding the relative strength of these projections, the most likely sources of labeling in the DMFC are the rip, NRTP, Soc, and SGI. Labeling in areas 8B and 9 is consistent with their projections to the SC (Fries, 1984). Finally, the PPRF, Soc, and SGI are the most likely sources of labeling in the PS (Leichnetz et al., 1981; Fries, 1984; Schnyder et al., 1985), whereas labeling of the rostral CgS and area 12 of the ObFC is consistent with projections to the SC (Leichnetz et al., 1981; Fries, 1984).
In conclusion, retrograde transneuronal transfer of rabies virus from an extraocular muscle demonstrates that several frontal lobe areas are oligosynaptically connected to eye muscles. These include the anterior and posterior banks of the AS, DMFC, caudal principal, and periprincipal cortex as well as cingulate and orbitofrontal areas. In addition, our imaging study demonstrates that all of these areas display significantly increased metabolic activity and, in view of their simultaneous transneuronal labeling, they may work in tandem during saccades to visual targets.
Footnotes
This work was supported by European Union Grant BIO4-CT98-0546 and Centre National de la Recherche Scientifique Grant UMR9950. We gratefully acknowledge the help from M. Kefaloyianni and M. Koumaki with the autoradiograms and from M. A. Thomas and S. Brasiles with the histochemistry.
Correspondence should be addressed to A. K. Moschovakis, Department of Basic Sciences, Faculty of Medicine, University of Crete, P.O. Box 1393, Crete, Greece. E-mail: moschov@med.uoc.gr.
Copyright © 2004 Society for Neuroscience 0270-6474/04/245726-15$15.00/0
References
- Anderson TJ, Jenkins IH, Brooks DJ, Hawken MB, Frackowiak RSJ, Kennard C (1994) Cortical control of saccades and fixation in man. A PET study. Brain 117: 1073-1084. [DOI] [PubMed] [Google Scholar]
- Azuma M, Nakayama H, Sasaki Y, Suzuki H (1988) Relation between visual input and motor outflow for eye movements in monkey visual field. Behav Brain Res 27: 93-98. [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN (1987) Architecture and frontal cortical connections of the premotor cortex (area 6) in the rhesus monkey. J Comp Neurol 256: 211-228. [DOI] [PubMed] [Google Scholar]
- Barbas H, Ghashghaei H, Dombrowski SM, Rempel-Clower NL (1999) Medial prefrontal cortices are unified by common connections with superior temporal cortices and distinguished by input from memory-related areas in the rhesus monkey. J Comp Neurol 410: 343-367. [DOI] [PubMed] [Google Scholar]
- Bates JF, Goldman-Rakic PS (1993) Prefrontal connections of medial motor areas in the rhesus monkey. J Comp Neurol 311: 211-228. [DOI] [PubMed] [Google Scholar]
- Bizzi E (1968) Discharge of frontal eye field neurons during saccadic and following eye movements in unanesthetized monkeys. Exp Brain Res 6: 69-80. [DOI] [PubMed] [Google Scholar]
- Boch R, Goldberg ME (1989) Participation of prefrontal neurons in the preparation of visually guided eye movements in the rhesus monkey. J Neurophysiol 61: 1064-1084. [DOI] [PubMed] [Google Scholar]
- Bon L, Lucchetti C (1992) The dorsomedial frontal cortex of the macaca monkey: fixation and saccade related activity. Exp Brain Res 89: 571-580. [DOI] [PubMed] [Google Scholar]
- Bon L, Lucchetti C (1994) Ear and eye representation in the frontal cortex, area 8b, of the macaque monkey: an electrophysiological study. Exp Brain Res 102: 259-271. [DOI] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME (1985) Primate frontal eye fields. I. Single neurons discharging before saccades. J Neurophysiol 53: 603-635. [DOI] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME, Stanton GB, Bushnell MC (1985) Primate frontal eye fields. II. Physiological and anatomical correlates of electrically evoked eye movements. J Neurophysiol 54: 714-734. [DOI] [PubMed] [Google Scholar]
- Dalezios Y, Raos VC, Savaki HE (1996) Metabolic activity pattern in the motor and somatosensory cortex of monkeys performing a visually guided reaching task with one forelimb. Neuroscience 72: 325-333. [DOI] [PubMed] [Google Scholar]
- Dias EC, Kiesau M, Segraves MA (1995) Acute activation and inactivation of macaque frontal eye field with GABA-related drugs. J Neurophysiol 74: 2744-2748. [DOI] [PubMed] [Google Scholar]
- Doricchi F, Perani D, Incoccia C, Grassi F, Cappa SF, Bettinardi V, Galati G, Pizzamiglio L, Fazio F (1997) Neural control of fast-regular saccades and antisaccades: an investigation using positron emission tomography. Exp Brain Res 116: 50-62. [DOI] [PubMed] [Google Scholar]
- Fox PT, Fox JM, Raichle ME, Burde RM (1985) The role of cerebral cortex in the generation of voluntary saccades: a positron emission tomographic study. J Neurophysiol 54: 348-369. [DOI] [PubMed] [Google Scholar]
- Fries W (1984) Cortical projections to the superior colliculus in the macaque monkey: a retrograde study using horseradish peroxidase. J Comp Neurol 230: 55-76. [DOI] [PubMed] [Google Scholar]
- Fujii N, Mushiake H, Tamai M, Tanji J (1995) Microstimulation of the supplementary eye field during saccade preparation. NeuroReport 18: 2565-2568. [DOI] [PubMed] [Google Scholar]
- Fujii N, Mushiake H, Tanji J (1998) An oculomotor representation area within the ventral premotor cortex. Proc Natl Acad Sci USA 95: 12034-12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima K, Yamanobe T, Shinmei Y, Fukushima J, Kurkin S, Peterson BW (2002) Coding of smooth eye movements in three-dimensional space by frontal cortex. Nature 419: 157-162. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS (1991) Neuronal activity related to saccadic eye movements in the monkey's dorsolateral prefrontal cortex. J Neurophysiol 65: 1464-1483. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS (1993) Dorsolateral prefrontal lesions and oculomotor delayed-response performance: evidence for mnemonic “scotomas.” J Neurosci 13: 1479-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamlin PD, Yoon K (2000) An area for vergence eye movement in primate frontal cortex. Nature 407: 1003-1007. [DOI] [PubMed] [Google Scholar]
- Gaymard B, Rivaud S, Cassarini JF, Dubard T, Rancurel G, Agid Y, Pierrot-Deseilligny C (1998) Effects of anterior cingulate cortex lesions on ocular saccades in humans. Exp Brain Res 120: 173-183. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Gattera R (1995) A comparison of the ipsilateral cortical projections to the dorsal and ventral subdivisions of the macaque premotor cortex. Somatosens Mot Res 12: 359-378. [DOI] [PubMed] [Google Scholar]
- Graf W, Klam F, Ugolini G (1999) Retrograde transneuronal labeling with rabies virus of horizontal eye movement circuits in primates. Soc Neurosci Abstr 25: 1651. [Google Scholar]
- Graf W, Dubayle D, Klam F, Biarnais T, Büttner-Ennever J, Ugolini G (2000) Horizontal eye movement network in primates. I. Monosynaptic input. Soc Neurosci Abstr 26: 969. [DOI] [PubMed] [Google Scholar]
- Graf W, Gerrits N, Yatim-Dhiba N, Ugolini G (2002) Mapping the oculomotor system: the power of transneuronal labelling with rabies virus. Eur J Neurosci 15: 1557-1562. [DOI] [PubMed] [Google Scholar]
- Grantyn A, Brandi A-M, Dubayle D, Graf W, Ugolini G, Hadjidimitrakis K, Moschovakis A (2002) Density gradients of trans-synaptically labeled collicular neurons after injections of rabies virus in the lateral rectus muscle of the rhesus monkey. J Comp Neurol 451: 346-361. [DOI] [PubMed] [Google Scholar]
- Gregoriou GG, Savaki HE (2001) The intraparietal cortex: subregions involved in fixation, saccades and in the visual and somatosensory guidance of reaching. J Cereb Blood Flow Metab 21: 671-682. [DOI] [PubMed] [Google Scholar]
- Gregoriou GG, Savaki HE (2003) When vision guides movement: a functional imaging study of the monkey brain. NeuroImage 19: 959-967. [DOI] [PubMed] [Google Scholar]
- Guldin W, Dahrmann G, Doldan M, Graf W, Bäurle J, Moschovakis A, Ugolini G (2000) Horizontal eye movement network in primates. III. Cortical relays. Soc Neurosci Abstr 26: 969. [Google Scholar]
- Hubel DH, Wiesel TN (1972) Laminar and columnar distribution of geniculo-cortical fibers in the macaque monkey. J Comp Neurol 146: 421-450. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Kaas JH (1990) Supplementary eye field as defined by intracortical microstimulation: connections in macaques. J Comp Neurol 293: 299-330. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Krubitzer LA, Kaas JH (1986) Frontal eye field as defined by intracortical microstimulation in squirrel monkeys, owl monkeys, and macaque monkey. I. Subcortical connections. J Comp Neurol 253: 415-439. [DOI] [PubMed] [Google Scholar]
- Joseph JP, Barone P (1987) Prefrontal unit activity during a delayed oculomotor task in the monkey. Exp Brain Res 67: 460-468. [DOI] [PubMed] [Google Scholar]
- Kennedy C, Sakurada O, Shinohara M, Jehle J, Sokoloff L (1978) Local cerebral glucose utilization in the normal conscious macaque monkey. Ann Neurol 4: 293-301. [DOI] [PubMed] [Google Scholar]
- Langer T, Kaneko CRS, Scudder CA, Fuchs AF (1986) Afferents to the abducens nucleus in the monkey and cat. J Comp Neurol 245: 379-400. [DOI] [PubMed] [Google Scholar]
- Leichnetz GR, Spencer RF, Hardy SGP, Astruc J (1981) The prefrontal corticotectal projection in the monkey: an anterograde and retrograde horseradish peroxidase study. Neuroscience 6: 1023-1041. [DOI] [PubMed] [Google Scholar]
- Luppino G, Matelli M, Camarda RM, Gallese V, Rizzolatti G (1991) Multiple representations of body movements in mesial area 6 and the adjacent cingulate cortex: an intracortical microstimulation study in the macaque monkey. J Comp Neurol 311: 463-482. [DOI] [PubMed] [Google Scholar]
- MacAvoy MG, Gottleib JP, Bruce CJ (1991) Smooth-pursuit eye movement representation in the primate frontal eye field. Cereb Cortex 1: 95-102. [DOI] [PubMed] [Google Scholar]
- Matelli M, Luppino G, Rizzolatti G (1985) Patterns of cytochrome oxidase activity in the frontal agranular cortex of the macaque monkey. Behav Brain Res 18: 125-136. [DOI] [PubMed] [Google Scholar]
- Matelli M, Luppino G, Rizzolatti G (1991) Architecture of superior and mesial area 6 and the adjacent cingulate cortex in the macaque monkey. J Comp Neurol 311: 445-462. [DOI] [PubMed] [Google Scholar]
- Morcuende S, Delgado-García JM, Ugolini G (2002) Neuronal premotor networks involved in eyelid responses: retrograde transneuronal tracing with rabies virus from the orbicularis oculi muscle in the rat. J Neurosci 22: 8808-8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschovakis AK, Scudder CA, Highstein SM (1996) The microscopic anatomy and physiology of the mammalian saccadic system. Progr Neurobiol 50: 133-254. [DOI] [PubMed] [Google Scholar]
- Moschovakis AK, Gregoriou GG, Savaki HE (2001) Functional imaging of the primate superior colliculus during saccades to visual targets. Nat Neurosci 4: 1026-1031. [DOI] [PubMed] [Google Scholar]
- Nguyen LT, Spencer RF (1999) Abducens internuclear and ascending tract of Deiters inputs to medial rectus motoneurons in the cat oculomotor nucleus: neurotransmitters. J Comp Neurol 411: 73-86. [PubMed] [Google Scholar]
- O'Driscoll GA, Alpert NM, Matthysse SW, Levy DL, Rauch SL, Holzman PS (1995) Functional neuroanatomy of antisaccade eye movements investigated with positron emission tomography. Proc Natl Acad Sci USA 92: 925-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T (1991) Medial versus lateral frontal lobe lesions and differential impairment of central gaze fixation maintenance in man. Brain 114: 2051-2067. [DOI] [PubMed] [Google Scholar]
- Paus T, Marrett S, Worsley KJ, Evans AC (1995) Extraretinal modulation of cerebral blood flow in the human visual cortex: implications for saccadic suppression. J Neurophysiol 74: 2179-2183. [DOI] [PubMed] [Google Scholar]
- Petit L, Orssaud C, Tzourio N, Salamon G, Mazoyer B, Berthoz A (1993) PET study of voluntary saccadic eye movements in humans: basal ganglia-thalamocortical system and cingulate cortex involvement. J Neurophysiol 69: 1009-1017. [DOI] [PubMed] [Google Scholar]
- Petit L, Tzourio N, Orssaud C, Pietrzyk U, Berthoz A, Mazoyer B (1995) Functional neuroanatomy of the human visual fixation system. Eur J Neurosci 7: 169-174. [DOI] [PubMed] [Google Scholar]
- Petit L, Orssaud C, Tzourio N, Crivello F, Berthoz A, Mazoyer B (1996) Functional anatomy of a prelearned sequence of horizontal saccades in humans. J Neurosci 16: 3714-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raos V, Franchi G, Galesse V, Fagassi L (2003) Somatotopic organization of the lateral part of area F2 (dorsal premotor cortex) of the macaque monkey. J Neurophysiol 89: 1503-1518. [DOI] [PubMed] [Google Scholar]
- Remmel RS (1984) An inexpensive eye movement monitor using the sclera coil technique. IEEE Trans Biomed Engin 4: 388-390. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Camadra R, Fogassi L, Gentilucci M, Luppino G, Matelli M (1988) Functional organization of inferior area 6 in the macaque monkey. II. Area F5 and the control of distal movements. Exp Brain Res 71: 491-507. [DOI] [PubMed] [Google Scholar]
- Robinson DA, Fuchs AF (1969) Eye movements evoked by stimulation of frontal eye fields. J Neurophysiol 32: 637-648. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Giguere M, Bates JF, Goldman-Rakic PS (1997) Topographic organization of medial pulvinar connections with the prefrontal cortex in the rhesus monkey. J Comp Neurol 379: 313-332. [PubMed] [Google Scholar]
- Russo GS, Bruce CJ (1993) Effect of eye position within the orbit on electrically elicited saccadic eye movements: a comparison of the macaque monkey's frontal and supplementary eye fields. J Neurophysiol 69: 800-818. [DOI] [PubMed] [Google Scholar]
- Russo GS, Bruce CJ (2000) Supplementary eye field: representation of saccades and relationship between neural response fields and elicited eye movements. J Neurophysiol 84: 2605-2621. [DOI] [PubMed] [Google Scholar]
- Savaki HE, Kennedy C, Sokoloff L, Mishkin M (1993) Visually guided reaching with the forelimb contralateral to a “blind” hemisphere: a metabolic mapping study in monkeys. J Neurosci 13: 2772-2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savaki HE, Raos VC, Dalezios Y (1997) Spatial cortical patterns of metabolic activity in monkeys performing a visually guided reaching task with one forelimb. Neuroscience 76: 1007-1034. [DOI] [PubMed] [Google Scholar]
- Schall JD (1991) Neuronal activity related to visually guided saccadic eye movements in the supplementary motor area of rhesus monkeys. J Neurophysiol 66: 530-558. [DOI] [PubMed] [Google Scholar]
- Schall JD, Morel A, Kaas JH (1993) Topography of supplementary eye field afferents to frontal eye field in macaque: implications for mapping between saccade coordinate systems. Vis Neurosci 10: 385-393. [DOI] [PubMed] [Google Scholar]
- Schlag J, Schlag-Rey M (1987) Evidence for a supplementary eye field. J Neurophysiol 57: 179-200. [DOI] [PubMed] [Google Scholar]
- Schnyder H, Reisine H, Hepp K, Henn V (1985) Frontal eye field projection to the paramedian pontine reticular formation traced with wheat germ aggluttinin in the monkey. Brain Res 329: 151-160. [DOI] [PubMed] [Google Scholar]
- Shook BL, Schlag-Rey M, Schlag J (1988) Direct projections from the supplementary eye field to the nucleus raphe interpositus. Exp Brain Res 73: 215-218. [DOI] [PubMed] [Google Scholar]
- Shook BL, Schlag-Rey M, Schlag J (1990) Primate supplementary eye field. I. Comparative aspects of mesencephalic and pontine connections. J Comp Neurol 301: 618-642. [DOI] [PubMed] [Google Scholar]
- Sokoloff L (1977) Relation between physiological function and energy metabolism in the central nervous system. J Neurochem 29: 13-26. [DOI] [PubMed] [Google Scholar]
- Stanton GB, Goldberg ME, Bruce CJ (1988) Frontal eye field efferents in the macaque monkey. II. Topography of terminal fields in midbrain and pons. J Comp Neurol 271: 493-506. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Azuma M (1977) Prefrontal neuronal activity during gazing at a light spot in the monkey. Brain Res 126: 497-508. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Mintun MA, Kwee S, Wiseman MB, Brown DL, Rosenberg DR, Carl JR (1996) Positron emission tomography study of voluntary saccadic eye movements and spatial working memory. J Neurophysiol 75: 454-468. [DOI] [PubMed] [Google Scholar]
- Talairach J, Bancaud J, Geier S, Bordas-Ferrer M, Bonis A, Szikla G, Rusu M (1973) The cingulate cortex and human behavior. Electroencephalogr Clin Neurophysiol 34: 45-52. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Lisberger SG (2002) Role of arcuate frontal cortex of monkeys in smooth pursuit eye movements. I. Basic response properties to retinal image motion and position. J Neurophysiol 87: 2684-2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Rampin O, Giuliano F, Ugolini G (1999) Spinal and brain circuits to motoneurons of the bulbospongiosus muscle: retrograde transneuronal tracing with rabies virus. J Comp Neurol 414: 167-192. [PubMed] [Google Scholar]
- Tehovnik EJ, Lee K (1993) The dorsomedial frontal cortex of the rhesus monkey: topographic representation of saccades evoked by electrical stimulation. Exp Brain Res 96: 430-442. [DOI] [PubMed] [Google Scholar]
- Ugolini G (1995) Specificity of rabies virus as a transneuronal tracer of motor networks: transfer from hypoglossal motoneurons to connected second-order and higher order central nervous system cell groups. J Comp Neurol 356: 457-480. [DOI] [PubMed] [Google Scholar]
- Ugolini G, Dubayle D, Grantyn A, Brandi A, Berthoz A, Büttner-Ennever J, Moschovakis A, Graf W (2000) Horizontal eye movement network in primates. II. Polysynaptic input. Soc Neurosci Abstr 26: 969. [Google Scholar]
- Ugolini G, Büttner-Ennever J, Doldan M, Dubayle D, Klam F, Graf W (2001) Horizontal eye movement networks in primates: differences in monosynaptic input to slow and fast abducens motoneurons. Soc Neurosci Abstr 27: 403-13. [DOI] [PubMed] [Google Scholar]
- Walker AE (1940) A cytoarchitectonic study of the prefrontal area of the macaque monkey. J Comp Neurol 73: 59-86. [Google Scholar]
- Yeterian EH, Pandya DN (1988) Corticothalamic connections of paralimbic regions in the rhesus monkey. J Comp Neurol 269: 130-146. [DOI] [PubMed] [Google Scholar]