Abstract
Numerous studies have identified the roof plate as an important signaling center controlling dorsal interneuron specification and differentiation in the developing spinal cord. Currently, the molecular pathways of roof plate formation and function are poorly understood. We determined that the LIM-homeodomain transcription factor Lmx1b is sufficient to induce functional roof plate in the early chick developing spinal cord. In the chick, Lmx1b acts upstream of Lmx1a in the roof plate developmental program. Once the roof plate forms, we show that Bmp and Wnt signaling are the major components of Lmx1a/b-dependent roof plate dorsal patterning activity. The roof plate function of Lmx1b is not conserved across vertebrates because Lmx1b is not expressed in mouse roof plate progenitors. Instead, mouse caudal CNS roof plate formation relies entirely on Lmx1a. Lmx1b can, however, partially rescue roof plate development in dreher (Lmx1a-/-) mice, indicating that Lmx1b has some functional redundancy to Lmx1a. Furthermore, we demonstrate that the roof plate-inducing activity of Lmx1b can be suppressed by Mash1 (Cash1), which is normally expressed in intermediate neural tube in both chick and mouse. Our data identify Lmx1b as a key regulator of spinal cord roof plate induction and function.
Keywords: Lmx1b, roof plate, Lmx1a, dreher, Bmp, Wnt, developing spinal cord
Introduction
The roof plate of the developing CNS is a transient non-neural dorsal midline structure that controls the specification, differentiation, and axonal trajectories of the neurons at the dorsal neural tube (Lee and Jessell, 1999; Butler and Dodd, 2003). In caudal CNS, the roof plate originates from mitotically active progenitors of the neural folds in response to Bmp signaling from the adjacent epidermal ectoderm (Liem et al., 1997; Lee and Jessell, 1999). As development proceeds, roof plate progenitors exit the cell cycle, acquire typical roof plate morphology, and initiate expression of differentiated roof plate markers, including MafB. In addition, the roof plate secretes peptides of the Bmp and Wnt families, which have been proposed to play major roles in the specification of adjacent dorsal interneurons both in vitro and in vivo (Liem et al., 1997; Lee and Jessell, 1999; Muroyama et al., 2002; Timmer et al., 2002; Helms and Johnson, 2003). Although numerous studies have firmly established the importance of the roof plate as a signaling center controlling dorsal CNS patterning, little is understood regarding the molecular pathways driving roof plate induction or function.
We established previously that the LIM-homeodomain protein Lmx1a is required for roof plate formation. Lmx1a is expressed in the neural folds and in the developing roof plate (Millonig et al., 2000; Failli et al., 2002). Loss of Lmx1a function in dreher mutant mice leads to the failure of roof plate formation in the caudal CNS and subsequent disruption of dorsal neural patterning (Millonig et al., 2000). Lmx1a is a member of a large family of LIM-homeobox-encoding genes. Within this large, diverse family, Lmx1a is most highly related to Lmx1b, a protein with 61% overall amino acid identity (Hobert and Westphal, 2000).
Lmx1b is widely expressed during embryogenesis, and its function is required for normal limb, kidney, eye, and skull development (Riddle et al., 1995; Vogel et al., 1995; Chen et al., 1998; Dreyer et al., 1998). In the CNS, Lmx1b is expressed in the isthmus, developing serotonergic and dopaminergic neurons of the hindbrain and midbrain, and a subset of dorsal interneurons of the spinal cord. Lmx1b is also expressed in the roof plate and floor plate of the neural tube (Yuan and Schoenwolf, 1999; Adams et al., 2000; Smidt et al., 2000; Gross et al., 2002; Matsunaga et al., 2002; Muller et al., 2002; Ding et al., 2003). Although the role of Lmx1b in the isthmus (Adams et al., 2000; Matsunaga et al., 2002) and serotonergic and dopaminergic neurons (Smidt et al., 2000; Ding et al., 2003) is clearly established, its function in dorsal spinal cord development is unclear.
Using chick in ovo electroporation technology in conjunction with mutant mouse analysis, we determined that roof plate induction is the primary activity of Lmx1b in the early developing dorsal spinal cord. Lmx1b expression in the developing roof plate is activated by Bmp expression, and its roof plate-inducing activity can be inhibited by Mash1 (Cash1), normally expressed in intermediate neural tube. Strikingly, Lmx1b is not expressed in the roof plate of the mouse caudal CNS. Mouse spinal cord roof plate development is entirely dependent on Lmx1a. Lmx1b acts upstream of Lmx1a in the chick roof plate differentiation program and can partially rescue roof plate development in the dreher mouse. Using both gain-of-function and loss-of-function approaches, we identified Bmp signaling as the major component of Lmx1a/b-dependent roof plate signaling in chick developing spinal cord. Lmx1a/b also activates dorsal midline Wnt1 expression. The role of Lmx1a/b-dependent Wnt1 expression, however, is limited, regulating the numbers of only the most dorsal neuronal population, the dI1 interneurons.
Materials and Methods
Embryos. Fertilized White Leghorn eggs were incubated and staged according to Hamburger and Hamilton (1951). dreher (Lmx1a-/-) and Lmx1b mutant mice were maintained and genotyped as described previously (Chen et al., 1998; Millonig et al., 2000).
Chick embryo manipulations. In ovo electroporation was used to express proteins of interest in chick developing spinal cord as described previously (Megason and McMahon, 2002). Full-length mouse Lmx1b was cloned upstream of internal ribosomal entry site followed by enhanced green fluorescent protein (IRES-EGFP) in the pCIG expression vector (Megason and McMahon, 2002). Chick Lmx1b was expressed using chick Lmx1b/RCAS(A) virus (Riddle et al., 1995). Mouse Lmx1a, Bmp4, Wnt1, dominant-negative (dn) Wnt1, chick Noggin, and Xenopus Follistatin were expressed from pCIG-Lmx1a (Chizhikov and Millen, 2004), pMiwII-BMP4 (Kishimoto et al., 2002), pCIG-Wnt1 (Megason and McMahon, 2002), pC1-neo-dnWn1 (Garcia-Castro et al., 2002), pMT-Noggin, and pCMV-Follistatin (Liem et al., 2000), respectively. In all experiments, an appropriate empty vector was used as a control. In cell cycle experiments, 150 μl of different concentrations of olomoucin or aphidicolin (Sigma, St. Louis, MO) were applied on top of embryos in ovo 5 hr after electroporaion (h.a.e.), corresponding to the approximate initial expression of the exogenous dnWnt1. Chick intermediate explants from electroporated and control neural plates were isolated according to Liem et al. (1995) and cultured in serum-free medium as described previously (Garcia-Castro et al., 2002).
Mouse embryo manipulations. Embryonic day 9.25 (E9.25) whole dreher embryos or their subdissected neural tubes (Lee et al., 1998) were embedded into collagen and electroporated as described previously (Akamatsu et al., 1999). They were then cultured for 24 hr using previously published conditions (Lee et al., 1998). All cultured embryos and some neural tube explants were processed for immunohistochemistry. Some neural tube explants were used for RNA isolation and reverse transcription (RT)-PCR analysis.
Immunohistochemistry, in situ hybridization, bromodeoxyuridine, and terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling. Immunohistochemistry was performed on frozen sections as described previously (Helms and Johnson, 1998). The following primary antibodies were used: anti-MafB (Pouponnot et al. 1995), anti-LH2A/B (Lee et al., 1998), anti-Math1 (Helms and Johnson, 1998), anti-Lbx1 (Muller et al., 2002), anti-Lmx1a (M. German, unpublished observation; a gift from M. German, University of California San Francisco, San Francisco, CA), anti-Brn3a (Fedtsova and Turner, 1997), anti-Lmx1b, anti-Islet1 (51.4H9), anti-Lim1/2 (4F2), anti-Msx1/2 (4G1), anti-Pax7, and anti-Pax6 [all obtained from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA), developed under the auspices of the National Institute of Child Health and Human Development]. Secondary species-appropriate antibodies with Texas Red or FITC conjugates were obtained from Jackson ImmunoResearch (West Grove, PA). In situ hybridization was performed as described previously (Timmer et al., 2002) using digoxigenin-labeled riboprobes to chick Gdf7, Bmp4, Wnt1, Cash1, and mouse Lmx1b provided by T. Jessell (Columbia University, New York, NY) (Gdf7), P. Brickell (Institute of Child Health, London, UK) (Bmp4), A. McMahon (Harvard University, Cambridge, MA) (Wnt1), J. Johnson (University of Texas Southwestern Medical Center, Dallas, TX) and D. Anderson (California Institute of Technology, Pasadena, CA) (Cash1), and R. Johnson (University of Texas, M.D. Anderson Cancer Center, Houston, TX) (Lmx1b). Bromodeoxyuridine (BrdU) and terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling were performed as described previously (Megason and McMahon, 2002). Sections were digitally photographed with an AxioCam on a Zeiss (Oberkochen, Germany) AxioPlan 2 microscope and processed using Adobe Photoshop (Adobe Systems, San Jose, CA).
RT-PCR. Each explant was collected into 200 μl of extraction buffer, and RNA was extracted using PicoPure RNA extraction kit (Arcturus, Mountain View, CA). Total RNA was reverse transcribed by use of random primers. cDNA was amplified by PCR for 30 cycles. Primer pairs to amplify mouse transcripts were 5′-TCACTGCACGTGGACTTTAAGGAG-3′ and 5′-TGATGGGACTGAGCCTTGCG-3′ for Gdf7, and 5′-ACTGCCGTCGCCGTCGCCATTCACTA-3′ and 5′-CACCACCTTGTCATACTCATCCAG-3′ for Bmp4. Amplification of a fragment of GAPDH was also performed as a control with primers 5′-TGACGTGCCGCCTGGAGAAA-3′ and 5′-GGTCCACCACCCTGTTGCTGTA-3′. PCR products were fractionated in 2% agarose gels and visualized by staining with ethidium bromide.
Cell count and statistical analysis. All sections were taken from the region between forelimbs and hindlimbs. Only sections with sufficient levels of GFP expression (10-15 representative sections per embryo) were used for analysis. All results were replicated in at least four embryos or explants. For quantification of roof plate cells, the total number of positive cells was counted on each section. For quantification of dorsal interneurons, only positive cells on the electroporated side were counted on each section. These numbers were compared with numbers of cells of interest in GFP-electroporated control embryos and not to the numbers of on the non-electroporated slides of the experimental embryos, because we sometimes observed that Lmx1b-induced roof plate non-autonomously affected not only the electroporated but also the control side of the neural tube. All quantitative data are expressed as the mean ± SEM. Statistical significance was determined by two-tailed t test. * indicates p < 0.01 in all figures.
Results
Roof plate induction by Lmx1b in chick developing dorsal spinal cord
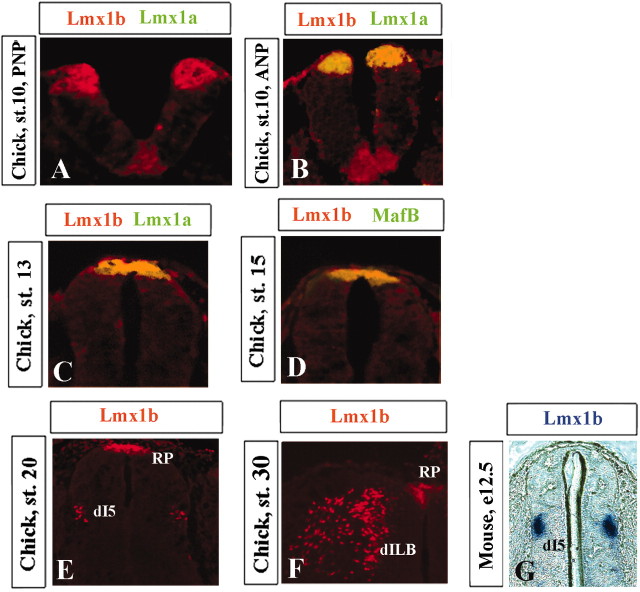
In the chick developing dorsal spinal cord, Lmx1b is expressed in the dorsal midline, and dorsal dI5 and dILB interneurons (Fig. 1A-F) (Yuan and Schoenwolf, 1999; Gross et al., 2002; Muller et al., 2002). In the chick caudal CNS, we first detected Lmx1b expression at the beginning of neurulation, specifically in the elevating and converging neural folds (Fig. 1A). Just before neural tube closure, expression of the roof plate marker Lmx1a was then initiated. In both the neural folds and dorsal midline of the formed neural tube at later stages, the Lmx1b expression completely overlapped with the Lmx1a expression domain (as detected by double immunolabeling) (Fig. 1B, C). Lmx1b expression also completely overlapped with MafB, a specific marker of differentiated roof plate that is first expressed at stage 15 (Fig. 1D). By electroporating stage 10 chick neural plates with a Bmp4 expression vector, we showed that, similar to other roof plate markers (MafB, Bmp4, Wnt1), roof plate Lmx1b expression is positively regulated by Bmp signaling (data not shown) (Liem et al., 1997; Liu et al., 2004). Strikingly, Lmx1b expression was not detected in the neural folds or in the dorsal midline of the developing spinal cord in the mouse at any stage investigated (Fig. 1G), although, in the mouse, Lmx1b is clearly expressed in the dI5 and dILB dorsal interneurons (Fig. 1G and data not shown) (Gross et al., 2002; Muller et al., 2002).
Figure 1.
Expression of Lmx1b in chick and mouse developing spinal cord. Immunofluorescence labeling of Lmx1b (red) alone (E, F) or together with Lmx1a (green) (A-C), and MafB (green) (D) in chick developing spinal cord at the stages (st.) indicated. PNP, Posterior neural plate; ANP, anterior neural plate. Cells that coexpress two markers appear yellow. Expression of Lmx1b (blue) detected by in situ hybridization in E12.5 mouse developing spinal cord. RP, Roof plate.
Because Lmx1b is highly expressed in roof plate progenitors and differentiated roof plate cells in the chick developing spinal cord, we investigated whether Lmx1b could induce roof plate fate. We overexpressed chick and mouse Lmx1b in caudal neural plate shortly before neural tube closure using in ovo electroporation of chick embryos at stage 10, the time of initiation of endogenous Lmx1b expression in the neural folds. In all experiments described below, overexpression of chick and mouse Lmx1b resulted in identical phenotypes (all figures show overexpression of chick Lmx1b).
Roof plate marker analysis of embryos electroporated with Lmx1b revealed an approximate fourfold increase in the number of dorsal MafB-positive cells compared with embryos electroporated with EGFP alone (Fig. 2A-E). In addition, the expression domains of secreted roof plate markers Gdf7, Bmp4, and Wnt1 were also ectopically expanded in embryos electroporated with Lmx1b (10 of 14 embryos) but not EGFP alone (n = 6 embryos) (Fig. 2F-I and data not shown). The ectopic expression of all roof plate markers was limited to the most dorsal region of the neural tube, despite widespread electroporation of exogenous Lmx1b (n = 14) (Fig. 2A, B and data not shown), suggesting a regionally restricted competence to produce roof plate cells in response to Lmx1b activity. An alternative hypothesis is that Lmx1b can only expand already existing roof plate and is not sufficient to induce it de novo. To distinguish these two possibilities, in vitro explant experiments were performed. Control stage 10 intermediate neural tube naive explants (n = 7) (Liem et al., 1997) or those electroporated with EGFP alone (n = 5) failed to generate any roof plate cells, as assessed by Pax7/MafB double staining (Fig. 2L-N). After 30 hr in culture, however, these control explants initiated coexpression of endogenous Lmx1b and Lbx1, the generic marker of dI4-dI6 interneurons (data not shown). Because in developing spinal cord this marker combination is unique to dI5 dorsal interneurons (Gross et al., 2002; Muller et al., 2002), we conclude that some explants cells adopted dI5 interneuron fate in the absence of dorsalizing or ventralizing signals. In contrast, intermediate explants electroporated with Lmx1b produced numerous roof plate cells (n = 4 explants) (Fig. 2J, K, N), indicating that Lmx1b can induce roof plate de novo. No expression of Lbx1 was detected in these explants at any time investigated (data not shown). Thus, Lmx1b can induce roof plate de novo in stage 10 naive intermediate neural plate explants and can either directly or indirectly override the dI5 cell fate when it is expressed at this early stage.
Figure 2.
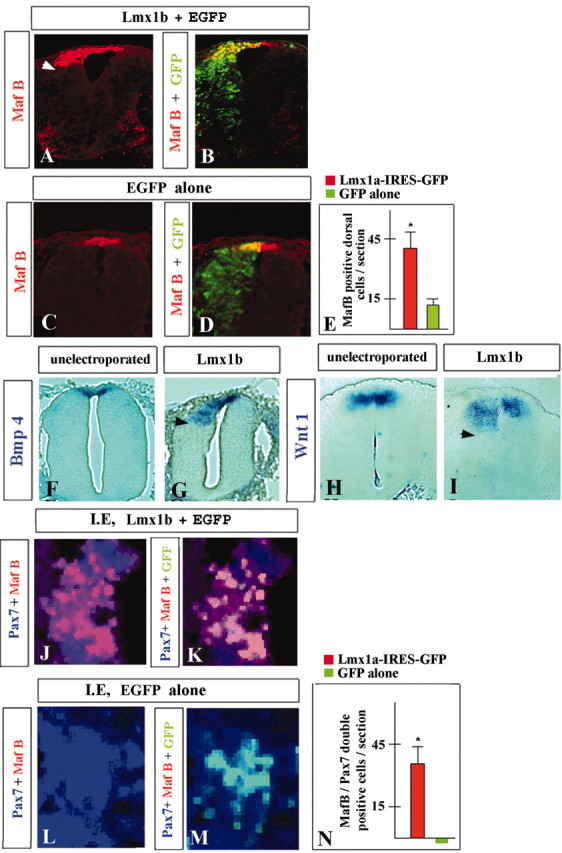
Lmx1b induces roof plate in chick developing spinal cord. Expression of MafB (A-D) (red, detected by immunofluorescence), Bmp4 (F, G), and Wnt1 (H, I)(both blue, detected by in situ hybridization) in neural tubes of unelectroporated embryos (F, H) and embryos electroporated with either Lmx1b-EGFP (A, B, G, I) or EGFP alone (C, D). All pictures are from stage 24 embryos, 48 h.a.e. Arrowheads point to the ventral boundary of ectopic domains of expression of roof plate markers induced by exogenous Lmx1b. E, Quantitative analysis of dorsal MafB-positive cells in embryos electroporated with Lmx1b and EGFP alone. J-M, Expression of MafB (red) and Pax7 (blue) (J, L) or MafB and Pax7 together with GFP (green) (K, M) and quantitative analysis of roof plate cells (N) in chick intermediate neural plate explants (I.E.) electroporated with Lmx1b-EGFP (J, K) or EGFP alone (L, M).
Because endogenous roof plate consists of non-dividing differentiated cells, we investigated whether Lmx1b expression was sufficient to withdraw neural progenitors from the cell cycle. Surprisingly, many cells expressing exogenous Lmx1b were still BrdU positive as late as 48 h.a.e., and no differences in numbers of BrdU-incorporating cells were detected between Lmx1b- electroporated and control sides of the neural tubes (supplemental Fig. 1, available at www.jneurosci.org). Additionally, no changes in BrdU incorporation were detected when Lmx1b was overexpressed in subdissected E9.25 mouse neural tubes in vitro (data not shown). Thus, although Lmx1b can induce expression of all roof plate markers investigated, we found no evidence that Lmx1b alters the cell cycle of electroporated cells.
Functional activity of Lmx1b-induced roof plate
The primary function of roof plate in developing spinal cord is to pattern adjacent dorsal tissue (Lee and Jessell, 1999). To investigate whether the ectopic roof plate induced by exogenous Lmx1b in vivo was functional, we first examined the expression of Pax and Msx family members as early indicators of regional identity in developing spinal cord (Liem et al., 1995; Ericson et al., 1997; Timmer et al., 2002). Additionally, we assessed the patterning of dI1, dI2, dI3, and dI4-dI6 dorsal spinal cord interneurons (Fig. 3Q). These were identified by their expression of the LIM-homeodomain proteins LH2A/B, Lim1/2, Islet1, and Lbx1, respectively (Gross et al., 2002; Muller et al., 2002) (Fig. 3). To avoid the complications of any potential cell-autonomous effects of Lmx1b on dorsal interneuron cell fate, we limited our analysis to sections in which exogenous Lmx1b expression was restricted to only the most dorsal regions of the neural tube (as detected by GFP fluorescence and confirmed by anti-GFP antibody staining) (Fig. 3B, D, F, I, L, O and data not shown).
Figure 3.
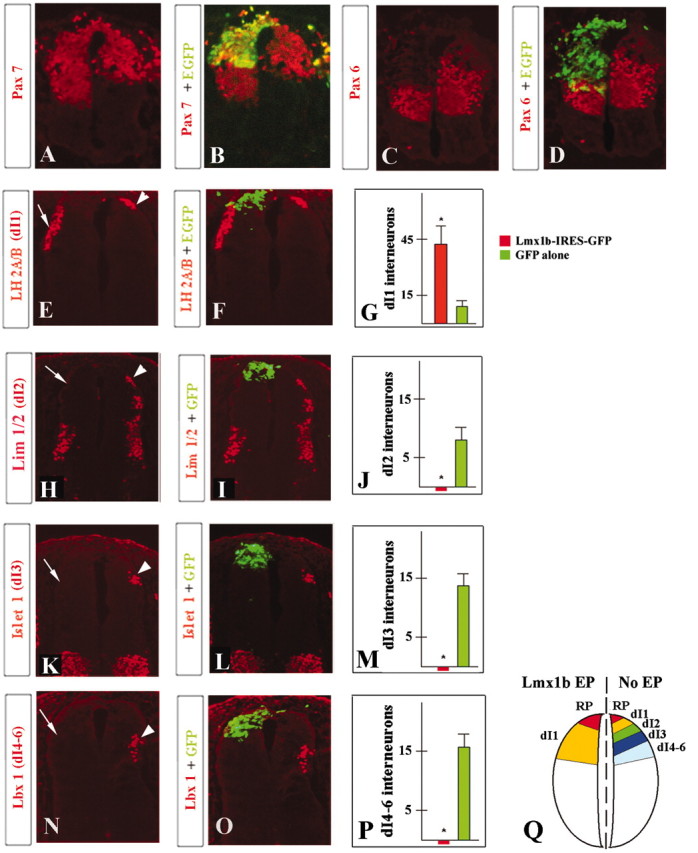
Effects of ectopic Lmx1b-generated roof plate on dorsal and intermediate spinal cord specification. Expression of Pax7 (A, B), Pax6 (C, D), LH2A/B (E, F), Lim1/2 (H, I), Islet1 (K, L), and Lbx1 (N, O) in neural tubes of chick embryos electroporated with Lmx1b-EGFP. All pictures are taken at stage 24, 48 h.a.e. Arrowheads point to domains of non-affected neuronal populations. Arrows point to neuronal populations affected by non-cell autonomous Lmx1b ectopic expression (as evident from F, I, L, O). G, J, M, P, Quantitative analysis of dI1-dI6 interneurons in embryos electroporated with Lmx1b and EGFP alone. Q, A diagram showing location of dorsal interneurons in non-electroporated and Lmx1b-electroporated chick spinal cord. RP, Roof plate; EP, electroporation.
In unelectroporated embryos, Pax7 and Msx1/2 expression was restricted to dorsal cells of the neural tube (Liem et al., 1995; Timmer et al., 2002). Pax6 was expressed at high levels in the intermediate regions of the neural tube and at reduced levels dorsally (Ericson et al., 1997; Timmer et al., 2002). In the neural tubes of Lmx1b-electroporated embryos, however, the expression domains of Pax7 and Msx1/2 were significantly extended ventrally (Fig. 3A, B and data not shown). The Pax6 expression domain was reduced and also shifted ventrally (Fig. 3C, D). There was an approximate fourfold increase in numbers of dI1 interneurons in samples expressing exogenous Lmx1b. Additionally, there was a marked shift of these interneurons to more ventral regions of the neural tube (eight of nine embryos), areas normally populated by dI2 and dI3 interneurons (Fig. 3E-G). No dI2, dI3, or dI4-dI6 interneurons were detected in neural tubes with high levels of exogenous Lmx1b expression in the dorsal midline region (Fig. 3H-P). We conclude that the ectopic roof plate induced by exogenous Lmx1b is functional because it repatterns the regional identity of the developing neural tube (summarized in Fig. 3Q), non-autonomously altering dorsal neuronal specification.
Lmx1b acts upstream of Lmx1a in the chick roof plate differentiation program and can partially rescue roof plate development in dreher mouse embryos
Lmx1b can induce Lmx1a expression when ectopically expressed in both developing spinal cord in vivo (five of seven embryos) (Fig. 4A-C) and in chick naive intermediate neural plate explants in vitro (40 ± 7 Lmx1a-positive cells per section; five of six explants) (data not shown). In contrast, Lmx1a could not induce Lmx1b expression either in vivo (n = 5 embryos) (Fig. 4D-F) or in vitro (n = 4 explants) (data not shown), indicating that Lmx1b acts upstream of Lmx1a in the roof plate differentiation program. We determined previously that Lmx1a is sufficient to induce the entire roof plate differentiation program in chick developing spinal cord (our published observations). Thus, the induction of the roof plate by Lmx1b may be explained by the activation of Lmx1a expression. To determine whether the Lmx1b roof plate-inducing activity relies entirely on Lmx1a, we performed mouse electroporation experiments. We electroporated E9.25 dreher (Lmx1a-/-) embryos or their subdissected neural tubes with either chick or mouse Lmx1b-expressing or control vectors, cultured them in vitro for 24 hr, and then stained them with antibodies or processed for gene expression analysis by RT-PCR. As expected, unelectroporated dreher embryos or those electroporated with EGFP did not express MafB (Fig. 4H, J, K), Gdf7, or Bmp4, as detected by RT-PCR (Fig. 4L). In contrast, dreher embryos electroporated with either chick or mouse Lmx1b had numerous MafB-positive cells in the dorsal midline domain of their developing spinal cord (Fig. 4I-K). Strong Gdf7 and Bmp4 expression was detected in their neural tubes (Fig. 4L). These data indicate that, although Lmx1b is not expressed in mouse caudal roof plate, both chick and mouse Lmx1b can at least partially rescue roof plate development in dreher mouse embryos. These assays, however, cannot distinguish between the possibility that Lmx1b has similar functional capacity to Lmx1a or whether Lmx1b can induce roof plate via an independent pathway.
Figure 4.
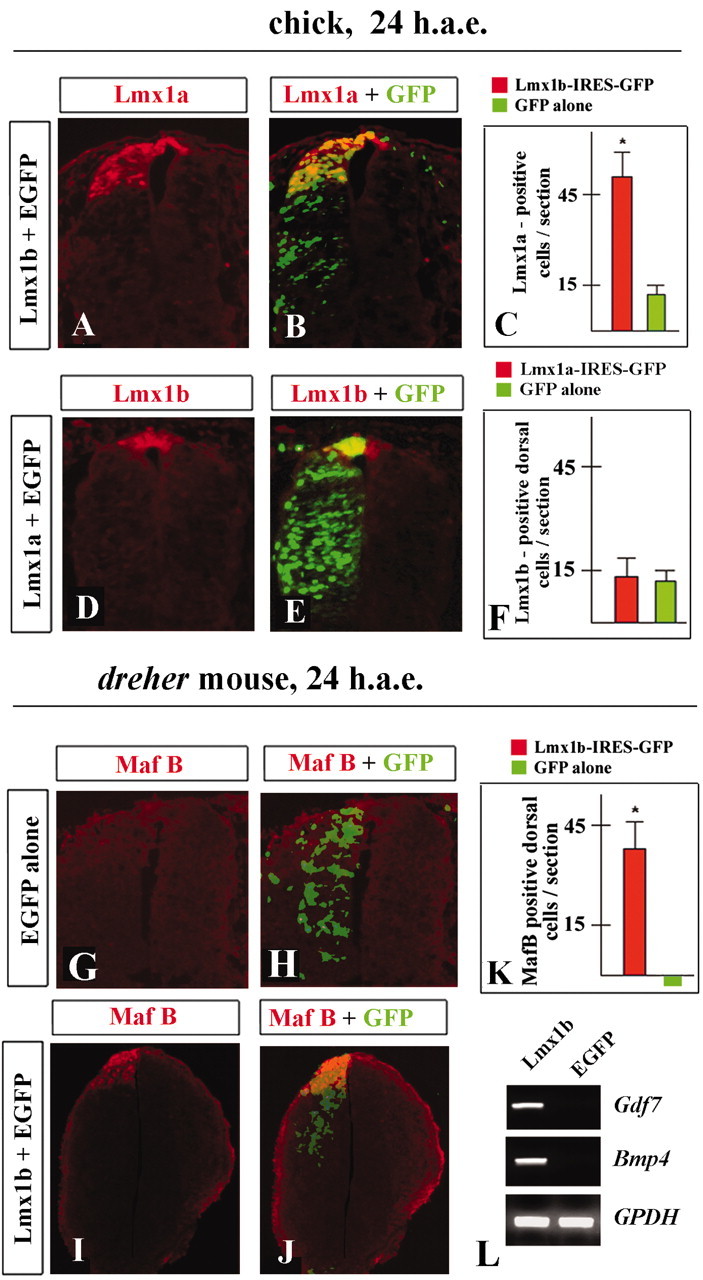
Lmx1b acts upstream of Lmx1a and can partially rescue roof plate phenotype in dreher mouse embryos. A-C, Expression of Lmx1a (A, B) and quantitative analysis of numbers of Lmx1a-positive cells (C) in chick neural tubes electroporated with Lmx1b 24 h.a.e. D-F, Expression of Lmx1b (D, E) and quantitative analysis of numbers of Lmx1b-positive cells (F) in chick neural tubes electroporated with Lmx1a 24 h.a.e. G-K, Expression of MafB (G-J) and quantitative analysis of number of MafB-positive cells (K) in developing spinal cords of dreher mouse embryos electroporated with Lmx1b (I, J) or EGFP alone (G, H) 24 h.a.e. L, RT-PCR analysis of expression of Gdf7 and Bmp4 in dreher developing spinal cords electroporated with Lmx1b and EGFP.
The molecular nature of Lmx1-dependent roof plate signaling
Bmps and Wnts have been implicated in the development of several classes of dorsal interneurons (Liem et al., 1997; Muroyama et al., 2002; Timmer et al., 2002; Liu et al., 2004), although many details of their specific roles remain unclear. Because Lmx1a/b can induce functional roof plate, manipulation of Lmx1a/b expression provides an opportunity to investigate the molecular nature of Lmx1-dependent roof plate signaling. We first established that overexpression of Bmp4 in stage 15 chick caudal neural tubes resulted in a phenotype that was indistinguishable from the Lmx1b overexpression phenotype (compare supplemental Fig. 2, available at www.jneurosci.org, with Fig. 3), suggesting that Bmp signaling likely represents a major component of Lmx1b-dependent roof plate signaling. In contrast, ectopic expression of Wnt1 had a very limited effect on dorsal spinal cord development, affecting only dI1 interneuron development (supplemental Fig. 2, available at www.jneurosci.org). Wnt1 overexpression led to a threefold increase in dI1 population (supplemental Fig. 2H, J, available at www.jneurosci.org). However, unlike the phenotype caused by Lmx1b overexpression, these dI1 interneurons were still located in their endogenous domain and not significantly expanded ventrally (supplemental Fig. 2H, available at www.jneurosci.org). Although previous studies have found that Wnt1 has a mitogenic effect (Megason and McMahon, 2002), the observed increase in dI1 numbers in Wnt1-electroporated embryos cannot be completely explained by the mitogenic activity of Wnt1, because the numbers of other dorsal interneurons (such as dI2, dI3, and dI4-dI6) in Wnt1-electroporated neural tubes were only moderately increased (supplemental Fig. 2I, J, available at www.jneurosci.org, and data not shown).
Next, we used an inhibitor approach to directly test the role of Bmp and Wnt signaling as components of Lmx1a/b-dependent roof plate signaling. Coelectroporation of Lmx1b or Lmx1a with either the Bmp inhibitors noggin and follistatin (Liem et al., 1997) or the Wnt inhibitor dnWnt1 (Garcia-Castro et al., 2002) did not affect the ability of Lmx1 genes to induce MafB-positive cells both in vivo and in intermediate neural plate explants in vitro (data not shown). These data indicate that the Bmps and Wnts are not required downstream of Lmx1a/b to form roof plate. In contrast, however, Bmp signaling from the Lmx1a/b-induced roof plate is required for roof plate function, because coelectroporation of Lmx1a/b together with noggin and follistatin blocked the ability of Lmx1 genes to cause ventral expansion of Pax7 and Msx1/2 expression domains. Instead, these electroporations caused a decrease in Pax7 and Msx1/2 expression in dorsal neural tube and a dorsal shift in the Pax6 expression domain (Fig. 5A, B and data not shown). Coelectroporation of Lmx1a/b with noggin and follistatin also blocked the Lmx1-dependent loss of dI4-dI6 interneurons and, instead, expanded their domain dorsally (Fig. 5D, F and data not shown). Additionally, the Bmp inhibitors prevented the ventral extension of dI1 interneurons (Fig. 5C) that was always observed when Lmx1b or Lmx1a was overexpressed alone. Importantly, however, a slight increase in dI1 interneuron numbers within their endogenous domain was still observed (Fig. 5C, E). These data indicate that Bmps are required to allow Lmx1a/b-induced roof plate to repattern adjacent neural tube tissue in our overexpression assays. These data also suggest that there are other Lmx1-dependent factors that specifically positively regulate dI1 interneuron development, even when Bmp signaling is downregulated.
Figure 5.
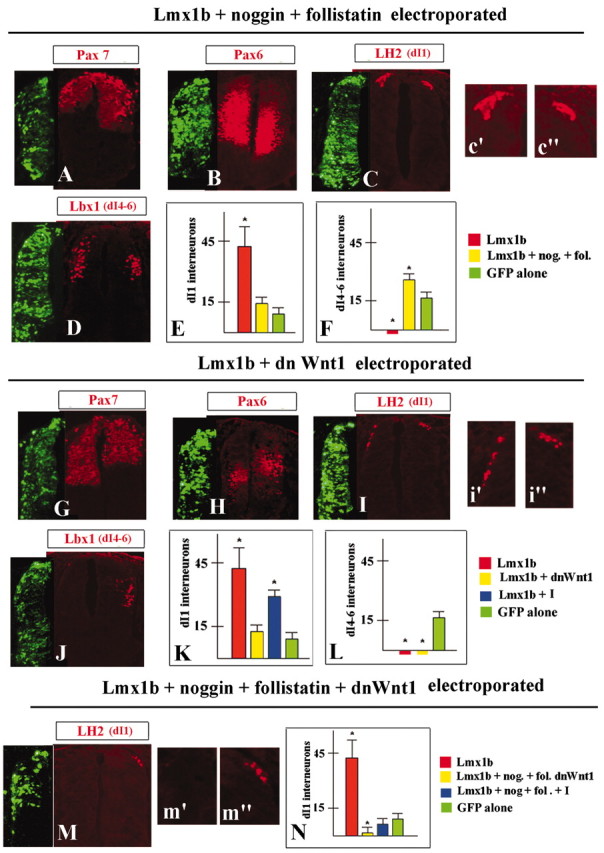
Blocking of Lmx1b-dependent roof plate signaling by Bmps and Wnts inhibitors. Effect of overexpression of Lmx1b together with noggin (nog.) and follistatin (fol.) (A-F), Lmx1b together with dnWnt1 (G-L), and Lmx1b together with noggin and follistatin-dnWnt1 (M, N) on expression of Pax7 (A, G), Pax6 (B, H), and dI1 (C, I) and dI4-dI6 (D, J) interneuron development. Insets show higher magnifications of selected neuronal populations. Quantitative analysis of numbers of selected classes of interneurons in neural tubes electroporated with Lmx1b together with noggin and follistatin (E, F), Lmx1b together with dnWnt1 (K, L), and Lmx1b and together with noggin and follistatin-dnWnt1 (N). The label I indicates cell cycle inhibitors amphidicolin and olomoucin.
In contrast to Bmp inhibition, most Lmx1a/b-overexpression phenotypes were unaffected when either gene was coelectroporated with the Wnt inhibitor dnWnt1. The ventral extension of Pax7 and Msx1/2 expression domain and strong ventral shift of Pax6 expression were still observed (Fig. 5G, H and data not shown). Additionally, no dI4-dI6 interneurons were detected on the electroporated sides of the neural tubes (Fig. 5J, L and data not shown). Downregulation of Wnt signaling when Lmx1a/b was overexpressed, however, had a very specific effect on the development of dI1 interneurons. Although dI1 interneurons were significantly expanded ventrally in neural tubes coelectroporated with Lmx1b and dnWnt1, their increase in number was only moderate (25% increase) (Fig. 5I, K). This increase was much lower than that in neural tubes expressing exogenous Lmx1b alone (fourfold increase), indicating that downregulation of Wnt signaling partially suppressed the activity of Lmx 1a/b to induce dI1 interneurons. Because Wnts have been reported previously to be mitogenic signals for dorsal neural tube cells (Megason and McMahon, 2002), it was possible that the negative effect of dnWnt1 on Lmx1a/b-dependent dI1 interneuron induction is attributable to an inhibition of cellular proliferation. Alternatively, Wnt signaling may regulate Lmx1-dependent dI1 interneuron specification more directly. To distinguish these possibilities, we evaluated the effects of dnWnt1 on the proliferation of dorsal neural tube cells. When the Lmx1b and dnWnt1 expression vectors (1 μg/μl each) were coelectroporated, we usually observed a 70% decrease of BrdU incorporation in dorsal neural tubes compared with overexpression of Lmx1b alone. We duplicated this 70% reduction of BrdU incorporation by applying the cell cycle inhibitors olomoucin (200 μM) or aphidicolin (10 μM) on the top of Lmx1b (1 μg/μl)-electroporated chick embryos (data not shown). Despite the presence of these inhibitors, Lmx1b overexpression still caused a twofold increase in the number of dI1 interneurons (Fig. 5K). Thus, a decrease of cell proliferation cannot fully account for the entire decrease in the number of Lmx1b-induced dI1 interneurons when Wnt signaling is downregulated. Instead, we conclude that Wnt1 has a more specific role in Lmx1a/b-dependent dI1 interneuron specification. To determine whether other signaling pathways have discernable roles in roof plate function, we coelectroporated Lmx1b or Lmx1a together with noggin, follistatin, and dnWnt1. No dI1 interneurons were detected in the majority of sections of these embryos. We therefore conclude that, in our overexpression assays, Bmps and Wnts act as the major components of Lmx1-dependent roof plate signaling. Bmp signaling influences the specification of numerous dorsal cellular populations, whereas Wnt signaling specifically regulates dI1 specification.
Lmx1b is not involved in dI5 and dILB interneuron specification
Lmx1b is expressed not only in roof plate but also in other cellular populations of the spinal cord, including dI5 and dILB interneurons in both chick and mouse (Fig. 1E-G). To test whether Lmx1b is involved in specification and early differentiation of these neurons, we electroporated Lmx1b into stage 15 chick neural tubes, at the time of initiation of normal exogenous Lmx1b dI5 expression. To avoid the complications of any non-autonomous effects of ectopic Lmx1b-induced roof plate on dorsal interneuron cell fate, we limited our analysis to sections in which the expression of exogenous Lmx1b was restricted to intermediate regions of the neural tube (supplemental Fig. 3B, D, available at www.neurosci.org, and data not shown). Using LH2, Isl1, Lim1/2, Brn3a, and Lbx1 immunostaining, we found that Lmx1b overexpression neither induced nor repressed expression of any of these markers (supplemental Fig. 3A-D, available at www.jneurosci.org, and data not shown). dI5 and dILB cell fates were also assessed in Lmx1b targeted null mutant mice (Chen et al., 1998). Using an Lmx1b in situ probe that detected both wild-type and mutant transcripts, we found no difference between wild-type and Lmx1b-/- embryos in either the number or distribution of dorsal Lmx1b-positive cells at E11.5 or E12.5 (data not shown). Immunohistochemical analysis revealed that dI5 and dILB neurons (and flanking dI1-dI4 and dI6 interneurons) were normally specified in Lmx1b-/- embryos (supplemental Fig. 3E-H, available at www.jneurosci.org, and data not shown). Furthermore, there was no difference in the numbers of proliferating and apoptotic cells in dorsal and intermediate developing spinal cord of wild-type and Lmx1b-/- embryos at E10.5-E12.5. (data not shown). We conclude that Lmx1b has no role in the specification or early differentiation of dI5 and dILB interneurons.
Mash1, normally expressed in intermediate neural tube progenitors, can suppress roof plate-inducing activity of Lmx1b
Our data indicate that, because overexpression of Lmx1b in stage 10 naive intermediate neural plate explants induces roof plate and prevents expression of dI5 interneuron markers, roof plate induction is the primary role of Lmx1b in the early developing chick spinal cord. At later stages, however, dI5 and dILB interneurons express endogenous Lmx1b in both chick and mouse without acquiring roof plate properties. This suggests that, during normal development, the roof plate-inducing activity of Lmx1b must become suppressed in intermediate cells and limited to the dorsal midline. Evidence of repression is also provided by our electroporation of Lmx1b into chick stage 10 neural plates. In these experiments, ectopic Lmx1b-induced roof plate was always limited to the most dorsal region of the neural tube and was never observed in intermediate spinal cord (Fig. 2A, B and data not shown). One factor that may suppress the roof plate-inducing activity of Lmx1b is Mash1. Mash1 (and its chick homolog Cash1) initiates its expression in the intermediate neural tube shortly after neural tube closure (Gowan et al., 2001) (data not shown). It has been implicated in the development of several neuronal lineages and is expressed in both dI5 and dILB progenitors before initiation of Lmx1b expression in these differentiating neurons (Gross et al., 2002; Muller et al., 2002; Qian et al., 2002). To test whether Mash1 could suppress the roof plate-inducing activity of Lmx1b, we coelectroporated Mash1 and Lmx1b in caudal developing chick spinal cord just before neural tube closure at stage 10. In contrast to electroporation of Lmx1b alone, coelectroporation of Lmx1b with Mash1 failed to induce ectopic roof plate, as detected by MafB, Bmp4, or Gdf7 staining (Fig. 6 and data not shown). In addition, we observed an inhibition of endogenous roof plate formation when Mash1 alone was electroporated into chick stage 10 neural plates (Fig. 6C and data not shown). Together, these data indicate that Mash1 can suppress roof plate-inducing activity of Lmx1b when overexpressed in chick developing spinal cord.
Figure 6.
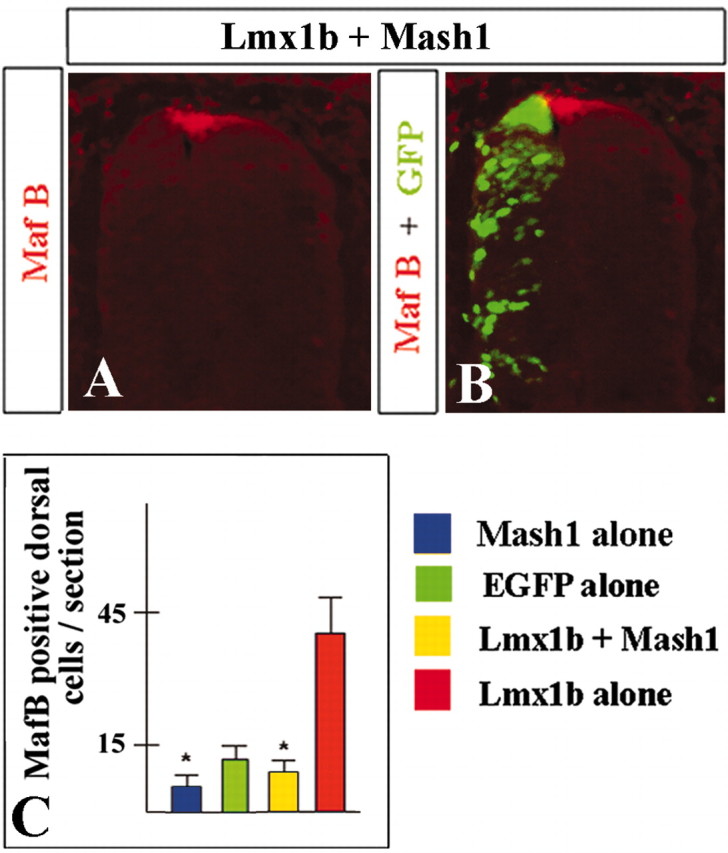
Mash1 suppresses roof plate-inducing activity of Lmx1b. Expression of MafB (red) (A) alone and together with GFP (green) (B) in chick developing spinal cord coelectroporated with Lmx1b and Mash1, 24 h.a.e. Quantitative analysis of roof plate cells in embryos electroporated with Mash1 alone versus EGFP alone and Lmx1b together with Mash1 versus Lmx1b alone.
Discussion
The roof plate is an important signaling center that regulates dorsal CNS patterning during vertebrate development, yet very little is understood about the molecular pathways that drive its induction and function. In this study, we show that Lmx1b can drive ectopic roof plate formation in the developing chick spinal cord and that Bmp and Wnt signaling are major components of Lmx1-dependent roof plate signaling. This function is not conserved across vertebrate evolution because Lmx1b is not expressed in the roof plate of mouse spinal cord. In mouse, caudal CNS roof plate formation relies entirely on Lmx1a. Lmx1b acts upstream of Lmx1a in the chick roof plate differentiation program and can partially rescue roof plate development in the dreher (Lmx1a-/-) mouse. Although Lmx1b is also normally expressed in dI5 and dILB interneurons in intermediate regions of the neural tube, loss-of-function or ectopic expression of Lmx1b does not alter the specification of these neurons. We therefore conclude that Lmx1b is not critically involved in dI5 and dILB interneuron specification and that roof plate induction is the primary activity of Lmx1b in early dorsal spinal cord development in chick. This activity may be suppressed by Mash1 (Cash1), normally expressed in intermediate regions of the mouse and chick neural tube.
Roof plate induction mechanisms are different in chick and mouse caudal CNS
Roof plate is an embryonic signaling center that consists of non-dividing differentiated cells. In the developing vertebrate spinal cord, the roof plate forms from mitotically active progenitors at the lateral edges of the neural plate in response to Bmp signaling from adjacent ectodermal ectoderm (Lee and Jessell, 1999). Here we provide evidence that Lmx1b can induce ectopic expression of numerous roof plate markers, including Lmx1a, MafB, Bmp4, Gdf7, and Wnt1, when overexpressed in stage 10 chick neural plate. Surprisingly, Lmx1b was not sufficient to withdraw progenitor cells from the cell cycle, showing that it does not control this aspect of roof plate differentiation program. Nevertheless, we consider that Lmx1b can induce “roof plate,” because the induced structure non-autonomously affects the specification of adjacent neurons in the dorsal spinal cord. The results of our electroporation studies do not allow us to directly conclude that roof plate induction is the normal function of Lmx1b in chick developing spinal cord. However, together with the fact that Lmx1b is expressed in the chick roof plate progenitors and differentiated roof plate cells and that, similar to other roof plate markers, Lmx1b expression is responsive to Bmp signaling, our data strongly suggest that Lmx1b contributes to roof plate formation during normal chick spinal cord development.
We showed recently that Lmx1a also has roof plate-inducing activities in the chick neural plate and is regulated by Bmp signaling (Chizhikov and Millen, 2004). We conclude that, in the chick, Lmx1b and Lmx1a have at least partially redundant roof plate-inducing properties. However, because Lmx1b acts upstream of Lmx1a, at least some of the roof plate-inducing activity of Lmx1b may be mediated by Lmx1a.
In mouse caudal CNS, roof plate induction relies entirely on the action of Lmx1a, because Lmx1b is never expressed in roof plate progenitors or differentiated roof plate in the mouse spinal cord. Loss of Lmx1a function in the dreher (Lmx1a-/-) mutant mouse results in failure of roof plate development, which in turn, causes subsequent disruptions in the specification, patterning, and differentiation of adjacent dorsal sensory interneurons in the dreher spinal cord (Millonig et al., 2000). Interestingly, both chick and mouse Lmx1b can induce roof plate gene expression in the dreher spinal cord, partially rescuing the Lmx1a mutant phenotype, indicating that mouse Lmx1b still has roof plate-inducing properties. This finding is also supported by the observation that chick and mouse Lmx1b have equivalent properties in chick electroporation experiments. Importantly, however, mouse Lmx1b is not expressed in roof plate progenitors or developing roof plate, despite high levels of endogenous Bmp signals from the adjacent epidermal ectoderm, levels that are sufficient to induce Lmx1a. This indicates that the primary difference between chick and mouse Lmx1b is not its functional properties but rather the regulation of its expression. Cross-species and inter-loci comparative sequence analyses are likely to reveal regulatory differences contributing to this fundamental difference between mouse and chick roof plate-inductive mechanisms.
Although the roof plate-inducing activities of Lmx1a and Lmx1b are similar, they are not entirely overlapping. In contrast to Lmx1a (Chizhikov and Millen, 2004), Lmx1b overexpression cannot cause progenitor cell cycle withdrawal in either mouse explants or chick spinal cord. Thus, the cell cycle withdrawal activity is unique to Lmx1a. Whether these differences are attributable to actual sequence differences between Lmx1a and Lmx1b proteins or whether it is attributable to the requirement for different cofactors remains to be investigated.
The molecular nature of Lmx1-dependent roof plate signaling
The ability of Lmx1b and Lmx1a to induce functional roof plate provided an opportunity to asses the molecular nature of Lmx1-dependent roof plate signaling. By coexpression of Lmx1b or Lmx1a together with inhibitors of Bmp and Wnt signaling in chick developing spinal cord, we showed that Bmps are major components of Lmx1a/b-dependent roof plate signaling in these experimental conditions, regulating the development of many dorsal interneurons. Interestingly, loss of roof plate Bmp signaling (but not Wnt signaling) observed in the dreher mouse spinal cord is also associated with decrease of dI1 interneuron numbers (Millen et al., 2004), suggesting that Bmps may be important mediators of Lmx1 signaling not only in the chick but also in the mouse. Lmx1a/b expression also activates Wnt signaling. In contrast to widespread actions of Bmps, the action of Lmx1-dependent Wnt signaling is limited to the positive regulation of dI1 interneuron development. Previous studies have identified Wnt1 as a mitogen for the neural cells (Megason and McMahon, 2002). We showed that the effects of cell cycle inhibitors on dI1 development in the presence of ectopic Lmx1b are not equivalent to coexpression of Lmx1b and Wnt1 inhibitors. These results indicate that Wnt1 plays a more direct role in patterning dI1 specification. Interestingly, overexpression of Wnt1 in chick had no significant effect on the specification of any group of dorsal or intermediate cells except dI1 interneurons, suggesting that Wnt1 is unlikely a major regulator of dorsal cell identity in chick caudal CNS. This is in contrast to mouse, in which knock-out studies identified Wnt1 and Wnt3a as major regulators of dorsal cell identity in developing neural tube (Muroyama et al., 2002). Together, this suggests that there may be differences in the role of Wnt signaling in dorsal neuronal specification between chick and mouse.
Lmx1b and dI5/dILB dorsal interneuron development
Our stage 10 neural plate electroporation data indicate that the primary function of Lmx1b in the early developing spinal cord is roof plate induction. At later stages, however, differentiating dI5 and dILB interneurons express endogenous Lmx1b in both chick and mouse without acquiring roof plate properties. By coelectroporating chick stage 15 neural tubes with Lmx1b and Mash1, which are normally expressed in intermediate neural tube including dI5 and dILB progenitors, we showed that Mash1 can inhibit the roof plate-inducing activity of Lmx1b. Thus, Mash1 (Cash1) may be one factor that prevents dI5 and dILB progenitors from adopting roof plate properties. Analysis of Mash1-/- mice is required to further support this hypothesis.
Interestingly, our loss-of-function studies in mouse revealed no role for Lmx1b in dI5 and dILB interneuron specification. This conclusion was further supported by gain-of-function experiments, performed on stage 15 chick developing spinal cord, at the time of initiation of expression of endogenous Lmx1b in dI5 interneurons. These data show that, at this stage, exogenous Lmx1b can neither induce additional dI5 interneurons nor block normal specification of other dorsal interneurons. This is in contrast to other Lmx1b-expressing neuronal populations in the CNS in which Lmx1b regulates cell fate [e.g., dopaminergic and serotonergic neurons (Smidt et al., 2000; Ding et al., 2003)]. Additional analysis of dI5/dILB neuronal migration and their axon development is required to determine whether Lmx1b is involved in later steps of development of dI5 and dILB neurons.
Footnotes
This work was supported by a Seed Grant from the Brain Research Foundation and a grant-in-aid from the Whitehall Foundation (to K.J.M.). We thank K. Sharma for his generous assistance with chick electroporation; R. Johnson, A. McMahon, M. German, T. Jessell, J. Briscoe, J. Timmer, L. Niswander, J. Johnson, D. Anderson, K. Kishimoto, T. Muller, C. Birchmeier, P. Brickell, M. Takeichi, E. Turner, and M. Bronner-Fraser for Lmx1b-/- mice, expression constructs, in situ probes, or antibodies; and A. Lindgren, I. Grinberg, and E. Steshina for technical assistance and/or valuable comments on this manuscript.
Correspondence should be addressed to Kathleen J. Millen, Department of Human Genetics, University of Chicago, 920 East 58th Street, Cummings Life Sciences Center 319, Chicago, IL 60637. E-mail: kmillen@genetics.bsd.uchicago.edu.
Copyright © 2004 Society for Neuroscience 0270-6474/04/245694-10$15.00/0
References
- Adams KA, Maida JM, Golden JA, Riddle RD (2000) The transcription factor Lmx1b maintains Wnt1 expression within the isthmic organizer. Development 127: 1857-1867. [DOI] [PubMed] [Google Scholar]
- Akamatsu W, Okano HJ, Osumi N, Inoue T, Nakamura S, Sakakibara S, Miura M, Matsuo N, Darnell RB, Okano H (1999) Mammalian ELAV-like neuronal RNA-binding proteins HuB and HuC promote neuronal development in both the central and the peripheral nervous systems. Proc Natl Acad Sci USA 96: 9885-9890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SJ, Dodd J (2003) A role for BMP heterodimers in roof plate-mediated repulsion of commissural axons. Neuron 38: 389-401. [DOI] [PubMed] [Google Scholar]
- Chen H, Lun Y, Ovchinnikov D, Kokubo H, Oberg KC, Pepicelli CV, Gan L, Lee B, Johnson RL (1998) Limb and kidney defects in Lmx1b mutant mice suggest an involvement of LMX1B in human nail patella syndrome. Nat Genet 19: 51-55. [DOI] [PubMed] [Google Scholar]
- Chizhikov VV, Millen KJ (2004) Control of roof plate formation by Lmx1a in the developing spinal cord. Development 131: 2693-2705. [DOI] [PubMed] [Google Scholar]
- Ding YQ, Marklund U, Yuan W, Yin J, Wegman L, Ericson J, Deneris E, Johnson RL, Chen ZF (2003) Lmx1b is essential for the development of serotonergic neurons. Nat Neurosci 6: 933-938. [DOI] [PubMed] [Google Scholar]
- Dreyer SD, Zhou G, Baldini A, Winterpacht A, Zabel B, Cole W, Johnson RL, Lee B (1998) Mutations in LMX1B cause abnormal skeletal patterning and renal dysplasia in nail patella syndrome. Nat Genet 19: 47-50. [DOI] [PubMed] [Google Scholar]
- Ericson J, Rashbass P, Schedl A, Brenner-Morton S, Kawakami A, van Heyningen V, Jessell TM, Briscoe J (1997) Pax6 controls progenitor cell identity and neuronal fate in response to graded Shh signaling. Cell 90: 169-180. [DOI] [PubMed] [Google Scholar]
- Failli V, Bachy I, Retaux S (2002) Expression of the LIM-homeodomain gene Lmx1a (dreher) during development of the mouse nervous system. Mech Dev 118: 225-228. [DOI] [PubMed] [Google Scholar]
- Fedtsova N, Turner EE (1997) Inhibitory effects of ventral signals on the development of Brn-3.0-expressing neurons in the dorsal spinal cord. Dev Biol 190: 18-31. [DOI] [PubMed] [Google Scholar]
- Garcia-Castro MI, Marcelle C, Bronner-Fraser M (2002) Ectodermal Wnt function as a neural crest inducer. Science 297: 848-851. [DOI] [PubMed] [Google Scholar]
- Gowan K, Helms AW, Hunsaker TL, Collisson T, Ebert PJ, Odom R, Johnson JE (2001) Crossinhibitory activities of Ngn1 and Math1 allow specification of distinct dorsal interneurons. Neuron 31: 219-232. [DOI] [PubMed] [Google Scholar]
- Gross MK, Dottori M, Goulding M (2002) Lbx1 specifies somatosensory association interneurons in the dorsal spinal cord. Neuron 34: 535-549. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL (1951) A series of normal stages in the development of the chick embryo. J Morphol 88: 49-92. [PubMed] [Google Scholar]
- Helms AW, Johnson JE (1998) Progenitors of dorsal commissural interneurons are defined by MATH1 expression. Development 125: 919-928. [DOI] [PubMed] [Google Scholar]
- Helms AW, Johnson JE (2003) Specification of dorsal spinal cord interneurons. Curr Opin Neurobiol 13: 42-49. [DOI] [PubMed] [Google Scholar]
- Hobert O, Westphal H (2000) Functions of LIM-homeobox genes. Trends Genet 16: 75-83. [DOI] [PubMed] [Google Scholar]
- Kishimoto KN, Watanabe Y, Nakamura H, Kokubun S (2002) Ectopic bone formation by electroporatic transfer of bone morphogenetic protein-4 gene. Bone 31: 340-347. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Jessell TM (1999) The specification of dorsal cell fates in the vertebrate central nervous system. Annu Rev Neurosci 22: 261-294. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Mendelsohn M, Jessell TM (1998) Neuronal patterning by BMPs: a requirement for GDF7 in the generation of a discrete class of commissural interneurons in the mouse spinal cord. Genes Dev 12: 3394-3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem KF, Tremml G, Roelink H, Jessell TM (1995) Dorsal differentiation of neural plate cells induced by Bmp-mediated signals from epidermal ectoderm. Cell 82: 969-979. [DOI] [PubMed] [Google Scholar]
- Liem KF, Tremml G, Jessell TM (1997) A role for the roof plate and its resident TGFbeta-related proteins in neuronal patterning in the dorsal spinal cord. Cell 91: 127-138. [DOI] [PubMed] [Google Scholar]
- Liem KF, Jessell TM, Briscoe J (2000) Regulation of the neural patterning activity of sonic hedgehog by secreted BMP inhibitors expressed by onto-chord and somites. Development 127: 4855-4866. [DOI] [PubMed] [Google Scholar]
- Liu Y, Helms AW, Johnson JE (2004) Distinct activities of Msx1 and Msx3 in dorsal neural tube development. Development 131: 1017-1028. [DOI] [PubMed] [Google Scholar]
- Matsunaga E, Katahira T, Nakamura H (2002) Role of Lmx1b and Wnt1 in mesencephalon and metencephalon development. Development 129: 5269-5277. [DOI] [PubMed] [Google Scholar]
- Megason SG, McMahon AP (2002) A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development 129: 2087-2098. [DOI] [PubMed] [Google Scholar]
- Millen KJ, Millonig JH, Matten ME (2004) Roof plate and dorsal spinal cord dI1 interneuron development in the dreher mutant mouse. Dev Biol, in press. [DOI] [PubMed]
- Millonig JH, Millen KJ, Hatten ME (2000) The mouse Dreher gene Lmx1a controls formation of the roof plate in the vertebrate CNS. Nature 403: 764-769. [DOI] [PubMed] [Google Scholar]
- Muller T, Brohmann H, Pierani A, Heppenstall PA, Lewin GR, Jessell TM, Birchmeier C (2002) The homeodomain factor lbx1 distinguishes two major programs of neuronal differentiation in the dorsal spinal cord. Neuron 34: 551-562. [DOI] [PubMed] [Google Scholar]
- Muroyama Y, Fujihara M, Ikeya M, Kondoh H, Takada S (2002) Wnt signaling plays an essential role in neuronal specification of the dorsal spinal cord. Genes Dev 16: 548-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouponnot C, Nishizawa M, Calothy G, Pierani A (1995) Transcriptional stimulation of the retina-specific QR1 gene upon growth arrest involves a Maf-related protein. Mol Cell Biol 15: 5563-5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Shirasawa S, Chen CL, Cheng L, Ma Q (2002) Proper development of relay somatic sensory neurons and D2/D4 interneurons requires homeobox genes Rnx/Tlx-3 and Tlx-1. Genes Dev 16: 1220-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle RD, Ensini M, Nelson C, Tsuchida T, Jessell TM, Tabin C (1995) Induction of the LIM homeobox gene Lmx1 by WNT7a establishes dorsoventral pattern in the vertebrate limb. Cell 83: 631-640. [DOI] [PubMed] [Google Scholar]
- Smidt MP, Asbreuk CH, Cox JJ, Chen H, Johnson RL, Burbach JP (2000) A second independent pathway for development of mesencephalic dopaminergic neurons requires Lmx1b. Nat Neurosci 3: 337-341. [DOI] [PubMed] [Google Scholar]
- Timmer JR, Wang C, Niswander L (2002) BMP signaling patterns the dorsal and intermediate neural tube via regulation of homeobox and helix-loop-helix transcription factors. Development 129: 2459-2472. [DOI] [PubMed] [Google Scholar]
- Vogel A, Rodriguez C, Warnken W, Izpisua Belmonte JC (1995) Dorsal cell fate specified by chick Lmx1 during vertebrate limb development. Nature 378: 716-720. [DOI] [PubMed] [Google Scholar]
- Yuan S, Schoenwolf GC (1999) The spatial and temporal pattern of C-Lmx1 expression in the neuroectoderm during chick neurulation. Mech Dev 88: 243-247. [DOI] [PubMed] [Google Scholar]