Abstract
Within the CNS, the normal form of cellular prion protein (PrPC) is expressed on neurons, oligodendrocytes, and astrocytes. The contribution of these cell types to prion replication and pathogenesis is unclear. To assess the role of oligodendrocytes, we expressed PrPC under the control of the myelin basic protein (MBP) promoter in mice lacking endogenous PrPC. PrPC was detected in oligodendrocytes and Schwann cells but not in neurons and astrocytes. MBP-PrP mice never developed scrapie after intracerebral, intraperitoneal, or intraocular challenge with scrapie prions. Transgenic brains did not contain protease-resistant prion protein and did not transmit scrapie when inoculated into PrPC-overexpressing indicator mice. To investigate whether prion spread within the CNS depends on oligodendrocytic PrPC, we implanted PrPC-overexpressing neuroectodermal grafts into MBP-PrP brains. After intraocular prion inoculation, none of the grafts showed spongiform encephalopathy or prion infectivity. Hence oligodendrocytes do not support cell-autonomous prion replication, establishment of subclinical disease, and neural spread of prions. Prion resistance sets oligodendrocytes aside from both neurons and astrocytes.
Keywords: prion, PrPSc, oligodendrocytes, myelin basic protein, transgenic mice, scrapie
Introduction
Prion protein (PrP) plays a central role in the pathogenesis of transmissible spongiform encephalopathies (TSEs) such as sheep scrapie, bovine spongiform encephalopathy, and Creutzfeldt-Jakob disease (Aguzzi and Weissmann, 1997; Aguzzi et al., 2001). The normal form of PrP, designated PrPC, is encoded by the Prnp gene (Basler et al., 1986), and is almost ubiquitously expressed. In the CNS, PrPC is predominantly neuronal, but expression on astrocytes and oligodendrocytes is also detectable (Moser et al., 1995). Prnpo/o mice are devoid of PrPC and develop and behave normally (Büeler et al., 1992) but are resistant to prion disease (Büeler et al., 1993) and do not support replication of prions (Sailer et al., 1994). Reintroduction of PrP transgenes into Prnpo/o mice, even when carrying significant amino-proximal deletions, restores susceptibility to scrapie (Fischer et al., 1996; Shmerling et al., 1998; Flechsig et al., 2000).
Previous transgenetic studies have revealed that expression of PrPC on either neurons or astrocytes is sufficient for scrapie replication (Race et al., 1995; Raeber et al., 1997). Surprisingly, mice expressing PrPC only within astrocytes or only within neurons develop indistinguishable pathologies after prion infection. Although oligodendrocytes express PrPC and are histogenetically highly related to astrocytes, it is not known whether they play a role in prion replication and in development of brain damage. White-matter involvement in TSEs was proposed to be a result of direct modifications of oligodendrocyte physiology (El Hachimi et al., 1998). If this is the case, oligodendrocytes may contribute to the pathogenetic process of scrapie.
The mechanisms by which prions spread within the CNS are unknown. Although neuronal trans-synaptic spread appears plausible, expression of PrPC in myelinating cells and the alleged potential of cultured Schwann cell lines to generate prion infectivity during infection (Follet et al., 2002; Archer et al., 2004) raise a possible role for oligodendrocyte-borne PrPC in the cerebral spread of prions. The latter issue may be of practical interest, because neuroinvasion can occur, for example, via the heavily myelinated visual pathway after conjunctival instillation (Scott et al., 1993), corneal grafts (Duffy et al., 1974), and intraocular injection (Fraser, 1982).
These questions would be testable in animals that express PrPC exclusively on myelinating cells. Expression would have to be sustained and highly selective, with no other cells expressing PrPC. Here we describe transgenic mice expressing PrPC under the control of the myelin basic protein (MBP) promoter. PrPC was strongly and exclusively expressed in oligodendrocytes and Schwann cells, but not in neurons or astrocytes. Using this model, we have established that expression of PrPC on myelinating cells does not support prion replication or spread of the agent along neural pathways. These results are in contrast to previous evidence that had been gathered with cultured cells and negate any cell-autonomous role for myelinating cell-borne PrPC in the pathogenesis of prion diseases.
Materials and Methods
DNA constructions and generation and identification of transgenic mice. A blunt-ended 760 bp fragment containing the entire PrP open reading frame (ORF) region (corresponding to exon 3 of the Prnp gene) (Fischer et al., 1996) was introduced and ligated into the EcoRI restriction sites of the MBP construct (see Fig. 1 A). The 1.3 kb promoter region of the mouse MBP gene contains the cap site of MBP mRNA and 5′ noncoding sequences. Although the cDNA fragment used is only approximately two-thirds as long as the normal MBP cDNA and does not contain sequences corresponding to the 3′ portion of MBP mRNA, it contains the full coding sequence of 14 kDa for the smallest mouse MBP. Poly(A) addition signals are provided from rabbit β-globin and simian virus 40 early genes, and the second intron of the rabbit β-globin gene is placed between the cap site and PrP ORF for splicing.
Figure 1.
Generation and identification of MBP-PrP transgenic mice. A, Schematic drawing of the MBP-PrP transgene used for generation of transgenic mice. Arrows and parenthetical numbers indicate the respective restriction sites. The second intron of rabbit β-globin was placed between the cap site and MBP cDNA to improve transgene expression. mMBP, Mouse MBP; mPrnp, mouse Prnp; SV40, simian virus 40. B, Genomic Southern blot analysis of MBP-PrP transgenic mice. EcoRI exactly excises the PrP ORF, confirming the presence of the transgene. (All tested mice showed the same banding pattern.) The BglII and the BamHI-ClaI digestions verify the integrity of the 5′ and 3′ ends, respectively. C, Northern blot analysis of brain RNA. The numbers above each lane indicate the various transgenic lines analyzed. RNA from wild-type (Prnp +/+) and Prnp o/o brains was used as a control. D, PrP C expression in brains and sciatic nerves of hemizygous (hem) or homozygous (hom) tg640 transgenic mice. Expression levels were estimated by comparison with serial twofold dilutions of wild-type brain homogenate. E, Brain PrP C quantification by chemiluminescence analysis. The abscissa displays the amount of protein in micrograms; the ordinate shows chemiluminescence intensity (arbitrary units). F, Anatomical dissection of the CNS in the cerebellum, brainstem, spinal cord, olfactory bulb, and gray or white matter (cortex, subcortex) revealed strongest expression of PrP C in white-matter or white-matter-rich regions of the CNS. MBP-PrP mice display a dominant unglycosylated PrP C band, whereas wild-type mice show a dominant diglycosylated band. To facilitate sample comparisons, only 25% of total protein was loaded in wild-type samples, resulting in PrP C bands of similar intensity. The uppermost bands represent actin. WT, Wild type.
Plasmid DNA was digested with HindIII and SalI and microinjected into homozygous Prnp o/o zygotes, as described previously (Fischer et al., 1996). Using PCR, founders were identified by the presence of Prnp o alleles and Prnp + transgenes. The primers P3 (specific for the disrupted Prnp allele), P10 (for the wild-type Prnp gene), and Mut217 (exon 3 primer) were used under standard PCR conditions (Fischer et al., 1996). Transgenic founders were mated to Prnp o/o mice, and one transgenic line, designated tg640 +/o, was established from the F1 progeny on a Prnp o/o mixed background, C57BL/6 × 129Sv. Additional breeding yielded the homozygous line tg640 +/+, or MBP-PrP.
Northern blot analyses. Mouse-brain total RNA was isolated with RNAeasy Midi kits (Qiagen, Hilden, Germany), and samples (15 μg) were electrophoresed through a 1% agarose gel in 0.02 m sodium borate, pH 8.3, 0.5 mm EDTA, and 5% formaldehyde. RNA was blotted onto Hybond-N + membranes (DuPont, Billerica, MA) using 20× SSC and cross-linked to membranes by ultraviolet radiation. Prehybridization occurred for 4 hr at 42°C in 50% formamide, 1 m NaCl, 1% SDS, and 10% dextran sulfate. Hybridization occurred overnight at 42°C in the same buffer using 32P-labeled probe A (106 cpm/ml), prepared by random priming, which is known to hybridize to transcripts produced by both wild-type and disrupted Prnp genes (Büeler et al., 1992), and 0.1 mg/ml denatured salmon-sperm DNA. Membranes were washed for 10 min at room temperature (RT) in 2× SSC, washed twice for 30 min at 60°C in 2× SSC and 1% SDS, and washed twice for 30 min at RT in 1× SSC. Autoradiography was performed for 7-24 hr at RT using Eastman Kodak (Rochester, NY) XAR-5 film and an intensifier screen.
Western blot analysis. Brain homogenates were adjusted to 5 mg/ml, and 50 μg of total protein from each sample was electrophoresed through a 12% SDS-PAGE gel. Proteins were transferred to nitrocellulose by semidry blotting. Membranes were blocked with TBS-Tween 20 and 5% nonfat milk, incubated with antibody 6H4 or POM1 (monoclonal antibody to PrP; M. Polymenidou and A. Aguzzi, unpublished data), and developed by enhanced chemiluminescence (Amersham Biosciences, Braunschweig, Germany), as described recently (Prinz et al., 2002). Quantification was performed using NIH Image software (http://rsb.info.nih.gov/nih-image/).
In situ hybridization. A 290 bp Asp718-BstEII fragment of the mouse PrP cDNA was cloned into pBluescript KS and SK vectors (Stratagene, La Jolla, CA). Digoxygenin-labeled sense and antisense probes were synthesized (Boehringer Mannheim, Mannheim, Germany) from Asp718-cleaved KS and SK constructs, respectively, using T3 RNA polymerase (Raeber et al., 1997). Sections were deparaffinized and postfixed in 4% paraformaldehyde in PBS, RNA was denatured for 10 min in 0.1 m HCl, and sections were digested with 10 μg/ml proteinase K (PK) at 37°C for 10 min. After a second fixation step in 4% paraformaldehyde for 10 min, acetylation was performed in 0.1 m triethanolamine and 0.25% acetic anhydride, and prehybridization was performed in 5× SSC, 50% formamide, 5× Denhardt's solution, and 50 μg/ml yeast tRNA for 90 min at room temperature. Hybridization was performed in the same mixture, containing the digoxygenin-labeled probe (50 ng/ml; 12 hr; 58°C). Samples were washed for 1 hr at 65°C in 0.2 and 0.1× SSC, respectively, labeled with anti-digoxigenin Fab fragments (1:5000), and developed with 5-bromo-4-chloro-3-indolyl phosphate and 4-nitroblue tetrazolium chloride. Sections were mounted in glycerol gelatin.
Transmission electron microscopy. White matter was freshly prepared from mouse brains and fixed for 4 hr at 4°C in PBS containing 4% formaldehyde and 0.1% glutaraldehyde. These small tissue fragments were immersed in 2 m sucrose containing 15% polyvinyl pyrrolidone (10 kDa; Sigma, St. Louis, MO), mounted on aluminum pins, frozen, and stored in liquid nitrogen. Grids with ultrathin sections [prepared according to Tokuyasu (1976)] were conditioned with PBS containing 0.5% milk powder and 0.02% Tween 20 for 10 min, incubated with PrP-specific mouse IgG (10 μg/ml in conditioning buffer; clone 6H4; Prionics AG, Schlieren, Switzerland) for 2 hr at room temperature, rinsed with PBS, and incubated with 8 nm gold-labeled goat anti-mouse IgG (diluted to an absorbance of 0.1 at 525 nm with conditioning buffer) for 1 hr. After rinses with PBS and distilled water, grids were embedded and stained with methylcellulose and uranyl acetate. Micrographs were taken on a Zeiss (Oberkochen, Germany) EM 910 at an original magnification of 20,000×.
Preparation of oligodendrocytes and immunofluorescence. Optic nerve oligodendrocytes were isolated from postnatal day 7-8 wild-type, transgenic, and Prnp o/o mice, as described previously for the rat (Schwab and Caroni, 1988). Four days after isolation, cells were fixed with 4% paraformaldehyde and 5% sucrose in PBS for 15 min at RT. The cultures were permeabilized and blocked in PBS supplemented with 0.1% Triton X-100 and 10% FCS and then incubated with anti-myelin-associated glycoprotein (MAG; 1:100; Roche Diagnostics, Rotkreuz, Switzerland) and anti-PrP rabbit antiserum XN (1:800; raised against full-length murine PrP) for 1 hr at RT. After washing, the cells were incubated with goat anti-mouse secondary antibodies conjugated with Alexa 488 (1:150; Molecular Probes, Eugene, OR) and goat anti-rabbit Alexa 546 (1:500). After additional washing, stained cultures were embedded in Mowiol (Calbiochem, La Jolla, CA) and mounted on slides for fluorescence microscopy.
Scrapie infection and determination of infectivity. Mice were inoculated intraperitoneally with 100 μl of brain homogenate containing 6 logLD50, intracerebrally with 30 μl (3 × 105 LD50) of the Rocky Mountain Laboratory (RML) scrapie strain (passage 5), or intraocularly with 10 μl(1 × 105 LD50) of the same RML scrapie strain, prepared as described previously (Büeler et al., 1993). Mice were monitored every second day, and scrapie was diagnosed according to standard clinical criteria. Mice were killed on the day of onset of terminal clinical signs of scrapie. Infectivity of tissues was determined on 1% spleen or brain homogenates. Tissues were homogenized (10% w/v) in 320 mm sucrose with a microhomogenizer, passed several times through 18 and 22 gauge needles, and diluted in PBS with 5% BSA. When the solution appeared homogenous, it was spun for 5 min at 500 × g. Supernatants (30 μl) were inoculated intracerebrally into groups of four tga20 mice (Fischer et al., 1996). Indicator mice were killed after development of terminal scrapie and the relationship y = 11.45 - 0.088 x, where y is logLD50/ml homogenate and x is incubation time in days to terminal disease, was used to calculate infectivity titers (Prusiner et al., 1982). The presence of a protease-resistant isoform of PrP (PrP Sc) in the infected brains was investigated on proteinase K-treated (50 μg/ml; 30 min; 37°C) homogenates by Western blot analysis, as described above.
Histoblots. Histoblots were performed as described previously (Prusiner et al., 1982). Frozen brains that were cut into 12-μm-thick slices were mounted on nitrocellulose membranes. Total PrP, as well as PrP Sc after digestion with 50 μg/ml proteinase K for 4 hr at 37°C, were detected with PrP antibody 6H4 (1:2000 in 1% nonfat milk, overnight at 4°C) and alkaline phosphatase immunoconjugates. Visualization was performed using 5-bromo-4-chloro-3-indolyl phosphate and 4-nitroblue tetrazolium chloride (Boehringer Mannheim).
Establishment and analysis of intracerebral neuronal grafts. Embryos were obtained at day 12.5 after conception from homozygous matings of tga20 transgenic mice and transferred into modified HBSS that was supplemented with 10% FCS and 2% glucose at 4°C. The neuroectodermal anlage was prepared, homogenized in a volume of 30 μl, and grafted into the caudoputamen of adult wild-type mice, as described previously (Brandner et al., 1996a; Isenmann et al., 1996a,b). For histological analysis, mice were killed while under deep anesthesia. For paraffin histology, whole mouse brains were fixed for at least 72 hr in 4% paraformaldehyde and PBS and processed for paraffin embedding. Hematoxylin and eosin staining and immunohistochemistry for GFAP (anti-GFAP antiserum; 1:300; Dako, High Wycombe, UK) and synaptophysin (synaptophysin antiserum; 1:40; Dako) were all performed on sections of paraffin embedded tissues. Biotinylated secondary antibodies (goat anti-rabbit and rabbit-anti mouse; Dako) were used at a 1:200-1:300 dilution. Visualization was achieved using biotin-avidin-peroxidase (Dako) and diaminobenzidine as a chromogen, according to the protocols suggested by the manufacturer.
Results
MBP-PrP transgenic mice express PrPC exclusively on myelinating cells
To drive expression of PrPC in myelinating cells, we prepared a DNA construct in which the entire coding region of the murine Prnp gene was placed under the transcriptional control of a murine MBP promoter fragment. A similar strategy has been used for expressing myelin basic protein antisense transcripts to produce a shiverer-like hypomyelinating mouse model (Katsuki et al., 1988). The construct, consisting of the 1.3 kb promoter region of the mouse MBP gene and two 0.6 kb rabbit β-globin introns that flank the 0.76 kb PrP open reading frame region (Fig. 1A), was excised from its prokaryotic backbone and injected into the pronuclei of Prnpo/o zygotes.
Transgenic founders were identified by PCR analysis and Southern blotting (Fig. 1B). Nine of 11 PCR-positive founder (F0) mice transmitted the transgene to their offspring. Transgene-carrying animals were bred to Prnpo/o mice. Transgene expression in the resulting mouse colony (mixed C57BL/6 × 129Sv) was analyzed in organs of 12-week-old F1 offspring. Unless otherwise specified, all subsequent analyses were performed with homozygous offspring of one line (designated tg640) that showed sustained transcription of the transgene (Fig. 1C). Northern blot analysis failed to detect expression of the transgene in spleen, kidney, thymus, liver, testes, lung, heart, and muscle (data not shown).
We amplified and sequenced genomic DNA of six randomly chosen mice, as well as RNA from brain samples of four randomly chosen mice, after retrotranscription, with primers encompassing the Prnp open reading frame, which is contained in one single exon. We only detected sequences corresponding to the wild-type Prnp open reading frame, thereby excluding mutations in the transgene or RNA editing leading to a mutated protein (data not shown).
We then assessed expression of PrPC by Western blot analysis. Homogenates of whole tg640 brains, as well as of subregions thereof, yielded a characteristic three-band pattern of unglycosylated, monoglycosylated, and diglycosylated PrP (Fig. 1D,F). Western blots of spleen, heart, lung, thymus, muscle, liver, and kidney did not reveal any PrPC expression (data not shown). Tg640 mice developed normally and remained healthy, without any abnormal clinical signs, for >670 d.
To quantitate total PrP levels in transgenic brains (Fig. 1D), serial twofold dilutions of tg640 and wild-type brain homogenates in Prnpo/o homogenates were blotted, and the relative expression levels were calculated from calibration curves obtained by direct acquisition of chemiluminescence (Fig. 1E), as described previously (Heppner et al., 2001). The PrPC content in tg640 brains was ∼35% of that found in wild-type mice. Immunoblotting of sciatic nerves of MBP-PrP, Prnpo/o, and Prnp+/+ mice (Fig. 1D) revealed expression of PrPC in sciatic nerves of MBP-PrP mice. However, the amount of PrPC was only 7% of that found in wild-type mice.
We then analyzed the details of intracerebral PrPC distribution in tg640 mice (Fig. 1F). Western blot analysis of anatomically dissected cortical and subcortical forebrain compartments indicated strong PrPC signals in the white matter (subcortex), yet no PrPC was detected in the gray matter (cortex). Instead, in wild-type (Prnp+/+) mice, both compartments contained sizeable levels of PrPC (data not shown). MBP-PrP mice displayed a predominant unglycosylated PrPC band, whereas wild-type mice show a dominant diglycosylated band, suggesting that posttranslational processing of PrPC differs between oligodendrocytes and other CNS cells.
We performed in situ hybridizations on brain and sciatic nerve sections with digoxigenin-labeled riboprobes for PrP mRNA (Fig. 2). In Prnp+/+ mice, PrP mRNA was found mainly in neuronal cell bodies but was not detectable in glial cell bodies within fiber tracts, such as in the corpus callosum (Fig. 2A). No Prnp transcripts were detected in the corpus callosum of Prnpo/o mice (Fig. 2B). In contrast, strong mRNA signals were observed in the corpus callosum and, to a lesser extent, in the cerebral cortex (data not shown) of adult tg640 brains, indicative of strong PrP expression in oligodendrocytes (Fig. 2C). Control hybridization with digoxigenin-labeled sense transcripts did not yield any signal (Fig. 2D-F). In situ hybridizations of sciatic nerves yielded PrP signals in both Prnp+/+ (tga20) and MBP-PrP mice but not in Prnpo/o mice (Fig. 2J-L). Hybridization signal intensity was less prominent in MBP-PrP mice than in wild-type mice, which is in line with the weaker protein expression. Again, sense probes did not hybridize (data not shown). Oligodendrocyte-restricted expression of the PrP transgene in brains of tg640 mice was demonstrated by colocalization of mRNA for Prnp and the oligodendrocyte-specific proteolipid protein (PLP) (Fig. 2G-I).
Figure 2.

Localization of PrP mRNA in MBP-PrP transgenic mice. In situ hybridization of forebrain sections (A-I) with corpus callosum and of sciatic nerve sections (J-L) of adult Prnp +/+, Prnp o/o, and MBP-PrP transgenic mice is shown. Expression of PrP mRNA occurs in neurons of wild-type (Prnp +/+) forebrains (A) and in Prnp +/+ Schwann cells (J), whereas Prnp o/o brains (B) and nerves (K) lack any signal. In contrast, strong PrP signals are visible in callosal oligodendrocytes (C) and sciatic Schwann cells (I-L) of transgenic mice. D-F, Sense probes did not reveal any signals in brains and sciatic nerves. G-I, The oligodendrocyte-specific PLP antisense probe revealed strong mRNA signals in the forebrains of all genotypes investigated. Scale bars, 50 μm.
Transmission electron microscopy was used to trace the subcellular distribution of PrPC within the white matter of adult mice (Fig. 3A-C). Punctuate distribution of colloidal gold-labeled antibodies to PrPC (arrows) was found in axons as well as in surrounding myelin sheaths of wild-type mice (Fig. 3A), whereas only occasional randomly distributed gold particles were found in Prnpo/o mice. In contrast, tg640 mice displayed the largest number of gold particles within myelinating oligodendrocyte processes (Fig. 3C).
Figure 3.
Localization of PrP C in MBP-PrP mice. A-C, Cellular distribution of PrP C with in the white matter of adult mice revealed by immunoelectron microscopy of gold-labeled anti-PrP antibody. Arrows indicate the location of the black gold particles. MBP-PrP mice display abundant decoration of myelin with gold particles. Scale bar, 5 nm. D-F, Immunofluorescence of primary optic nerve oligodendrocytes cultured from 7- to 8-d-old mice. Top row, expression of PrP is detectable only in transgenic oligodendrocytes (F), whereas the early oligodendrocyte marker MAG is expressed on all oligodendrocytes (bottom row). Scale bar, 5 μm.
Expression of PrP was also studied by fluorescence microscopy in optic nerve ex-plants from 7- to 8-d-old mice. Strong PrPC labeling was observed only in oligodendrocytes derived from tg640 mice, but not from Prnp+/+ or Prnpo/o mice (Fig. 3D-F, top row). Costaining with the early oligodendrocyte marker MAG (Fig. 3D-F, bottom row) confirmed the assignment of signals to oligodendrocytes, as well as the purity of the cultured cells. The majority (but not all) of MAG+-cultured cells also expressed PrPC. Thus, only more mature cells that had formed membrane sheets between their processes were PrP positive, which correlates with late MBP expression. Ectopic PrP expression did not affect oligodendrocyte density, morphology, or MAG expression.
Resistance of tg640 mice to scrapie
To test whether oligodendrocyte-restricted expression of PrPC suffices to render Prnpo/o mice susceptible to scrapie, tg640 mice were challenged with scrapie prions (RML strain, passage 5) intracerebrally, intraperitoneally, or intraocularly. However, challenge of homozygous (intraperitoneally, n = 12; intracerebrally, n = 10; intraocularly, n = 7) or hemizygous (intraperitoneally, n = 11; intracerebrally, n = 10; intraocularly, n = 6) tg640 mice and, as a control, Prnpo/o mice (intraperitoneally, n = 4; intracerebrally, n = 5) did not result in clinical signs of scrapie in any of the mice as late as 641 d postinoculation (dpi). This period of time approaches the natural life span of laboratory mice. Instead, wild-type mice that had been challenged with an inoculum of the same size died of scrapie at 195 ± 3 d after intraperitoneal (n = 6), 163 ± 4 d after intracerebral (n = 6), and 194 ± 11 d after intraocular (n = 7) inoculation (Table 1). All of these mice developed typical clinical symptoms of scrapie, such as ataxia, paralysis, kyphosis, foot-clasp reflex, and mincing gait. Thus, expression of full-length PrPC by oligodendrocytes of Prnpo/o mice does not restore clinical susceptibility to scrapie.
Table 1.
Resistance to scrapie of MBP-PrP mice
|
|
Mouse genotypes |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MBP-PrP+/o
|
MBP-PrP+/+
|
Prnp+/+
|
Prnpo/o
|
||||||||||||
| Type of prion challenge (dose) |
Attack rate |
Disease latencya
|
Attack rate |
Disease latencya
|
Attack rate |
Disease latencya
|
Attack rate |
Disease latencya
|
|||||||
| Intraperitoneal (6 logLD50) | 0/11 | >641 | 0/12 | >467 | 6/6 | 195 ± 3 | 0/4 | >641 | |||||||
| Intracerebral (3 × 105 LD50) | 0/10 | >641, 2 × >210b | 0/10 | >467, 1 × >197b | 6/6 | 163 ± 4 | 0/5 | >467 1 × >243b | |||||||
| Intraocular (1 × 105 LD50) |
0/6 |
>531c
|
0/7 |
>410, 1 × >376d
|
7/7 |
194 ± 11 |
ND |
|
|||||||
Only control wild-type mice (Prnp+/+) developed scrapie after intraperitoneal, intracerebral, and intraocular prion challenge, whereas mice carrying the MBP-PrP transgene hemizygously or homozygously and Prnpo/o mice never developed clinical signs of scrapie. Average incubation times and SDs were calculated for the groups of mice that developed scrapie. For mice that remained free of disease, the total observation time is reported. All mice were bred to a similar mixed genetic background (C57BL/6 × 129Sv). ND, Not determined.
Average ± SD.
Clinically healthy mice were killed at the time points indicated, and organs were used for infectivity analysis.
One mouse died 24 hr after inoculation.
Death of one mouse 376 d after inoculation resulting from abdominal tumor growth.
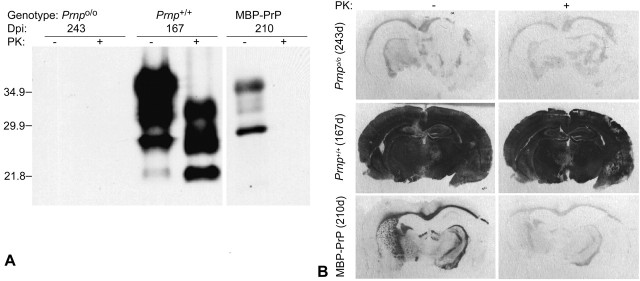
A major hallmark of TSE diseases is brain deposition of PrPSc (McKinley et al., 1983). We assessed formation of PrPSc in scrapie-infected MBP-PrP mice by Western blot analysis of brain extracts (Fig. 4A). Terminally scrapie-sick, intracerebrally scrapie-inoculated wild-type mice (Prnp+/+) showed high levels of PrPSc. However, clinically asymptomatic hemizygous tg640 mice killed 210 d after intracerebral inoculation did not exhibit any detectable PrPSc in their brains. All brains of scrapieinoculated mice surviving until termination of the experiments (641 d postinoculation) were analyzed by histology. This analysis failed to indicate any morphological signs of scrapie, such as spongiosis or gliosis (data not shown).
Figure 4.
Absence of conversion of PrP C into PrP Sc after scrapie challenge of MBP-PrP mice. A, Western blots of homogenized brain material electrophoresed natively (-) or after digestion with proteinase K (+). Large amounts of PK-resistant prion protein (PrP Sc) are detectable in brains of wild-type mice (Prnp +/+) that developed terminal scrapie 167 d postinoculation. No PrP Sc is visible in Prnp o/o or MBP-PrP mice that had been challenged intracerebrally with a high dose of the RML strain. Molecular weight markers are indicated on the left. B, Histoblot analysis of PrP Sc expression. Protease-resistant PrP was detectable only in intracerebrally infected Prnp +/+ mice. MBP-PrP and Prnp o/o mice did not accumulate PrP Sc. As reported previously (Brandner et al., 1996b), myelinated structures show faint homogeneous background staining in inoculated Prnp o/o mice.
Next, we investigated whether myelin-derived PrPC is required for the accumulation of PrPSc in situ by histoblotting. Mice were killed at the indicated time points and analyzed for the presence of disease-associated PrPSc (Fig. 4B). Large amounts of proteinase K-resistant prion protein were found in infected Prnp+/+ mice, whereas MBP-PrP and Prnpo/o mice lacked any detectable PK-resistant PrPSc. As reported previously (Brandner et al., 1996a), myelinated structures show faint homogeneous background staining in inoculated Prnpo/o mice. However, these myelinated structures were much less intensive than the PrPC-containing white-matter tracts in MBP-PrP mice.
To determine whether scrapie infectivity might subclinically propagate in brains or spleens of asymptomatic tg640 mice, tissue homogenates from animals killed at various time points were injected intracerebrally into indicator tga20 mice (Fischer et al., 1996), which overexpress Prnp under transcriptional control of orthologous regulatory elements (Table 2). Brain homogenates of Prnp+/+ mice 140 d after intraperitoneal inoculation or 167 d after intracerebral inoculation elicited disease in tga20 mice after 58-71 d. This result was compared with a calibration curve and found to correspond to a prion titer of ∼4.9-6.3 logLD50. In contrast, brains of tg640 transgenic mice never induced scrapie after transmission to tga20 mice. We conclude that tg640 brains do not replicate prion infectivity despite PrPC expression in oligodendrocytes. The detection threshold of the mouse bioassay with tga20 mice under the conditions used here is ∼1.5 logLD50 per gram of tissue.
Table 2.
Prion load in brains and spleens of individual tg640 mice
|
|
|
Transmission of brain to indicator mice |
Transmission of spleen to indicator mice |
||||
|---|---|---|---|---|---|---|---|
| Mouse genotypes |
Days after inoculation; route of infection |
Brain infectivity (logLD50 per gram) |
Attack rate (mean ± SD days) |
Spleen infectivity (logLD50 per gram) |
Attack rate (mean ± SD days) |
||
| MBP-PrP | 35 d; intraperitoneal | <1.5 | 0/4 | <1.5 | 0/4 | ||
| <1.5 | 0/4 | <1.5 | 3/4 (99,148,150)a | ||||
| 140 d; intraperitoneal | <1.5 | 0/4 | <1.5 | 0/4 | |||
| <1.5 | 0/4 | <1.5 | 0/4 | ||||
| 210 d; intracerebral | <1.5 | 0/4 | <1.5 | 0/4 | |||
| <1.5 | 0/3 | <1.5 | 0/4 | ||||
| Prnp+/+ | 35 d; intraperitoneal | <1.5 | 0/4 | 5.0 | 4/4 (73 ± 5) | ||
| <1.5 | 0/4 | 5.1 | 4/4 (72 ± 4) | ||||
| 140 d; intraperitoneal | 5.3 | 4/4 (68 ± 6) | 4.0 | 3/3 (84 ± 4) | |||
| 4.9 | 4/4 (74 ± 7) | 5.1 | 4/4 (72 ± 8) | ||||
| 167 d; intracerebral | 6.3 | 4/4 (58 ± 6) | 5.1 | 4/4 (72 ± 5) | |||
| 6.1 | 4/4 (60 ± 7) | 5.3 | 4/4 (70 ± 2) | ||||
| Prnpo/o | 42 d; intraperitoneal | <1.5 | 0/4 | <1.5 | 0/4 | ||
|
|
|
<1.5 |
0/4 |
<1.5 |
1/4 (99) |
||
Wild-type (Prnp+/+) and tg640 mice homozygous for the MBP-PrP transgenic cluster were inoculated intraperitoneally and intracerebrally and killed for analysis at the time points indicated.
Prion disease in three of four tga20 indicator mice that had received spleen extracts from a 35 dpi-challenged tg640 mouse is most likely a result of prions persisting from the inoculum.
Low levels of scrapie infectivity were detected in one spleen of a tg640 mouse killed 35 d after intraperitoneal inoculation, whereas three of four recipient tga20 mice died. This finding is most likely a result of the presence of residual inoculum in this investigated tg640 mouse. Similar findings have long been known to occur, even after inoculation of PrPC-deficient mice (Büeler et al., 1993; Sailer et al., 1994). Neuropathological investigations of brains from clinically sick wild-type mice revealed typical signs of scrapie (e.g., spongiform changes and pronounced astrogliosis predominantly in the neocortex and hippocampus; data not shown). In contrast, no typical histopathological signs of scrapie were visible in any of the examined clinically healthy tg640 mice (n = 9; killed at 641 dpi; data not shown). These observations virtually exclude the possibility that scrapie prions replicate sub-clinically in MBP-PrP mice.
Oligodendrocyte-borne PrPC does not support oculocerebral prion spread
Intraocular prion inoculation leads to progressive scrapie pathology along the optic nerve and optic tract to the contralateral superior colliculus and lateral geniculate nucleus, followed by generalized encephalopathy (Fraser, 1982; Brandner et al., 1996b). These results suggest that the infectious agent travels along fiber tracts of the CNS, such as the retinotectal projection.
To determine whether oligodendrocyte-restricted PrPC is sufficient for centripetal scrapie spread, prions were inoculated intraocularly, intraperitoneally, and intracerebrally into either tg640 or Prnpo/o mice containing a PrP-overexpressing tga20 neurograft (Fig. 5) (Brandner et al., 1996a). Prion injections were performed ≥62 d after transplantation to ensure full differentiation of embryonic tissue into normal neuronal and glial components and its integration into the host brain (Isenmann et al., 1996a).
Figure 5.

Oligodendrocytic PrP C does not support intraneural prion transport. Embryonal PrP-overexpressing tga20 neural tissue was transplanted into Prnp o/o (left) or MBP-PrP (right) mice. Hosts were inoculated with scrapie prions intraocularly (i.o.), intraperitoneally (i.p.), or intracerebrally (i.c.). Neuropathological signs of scrapie (gliosis visualized by overexpression of glial fibrillary acid protein, synaptic loss shown by loss of synaptophysin, and deposition of PrP) were only visible in grafts with hosts that had been inoculated intracerebrally. In contrast, neural grafts remained free of disease after intraocular or intraperitoneal inoculation. The trauma deriving from transplantation procedure induced a slight gliosis in all grafts. HE, Hematoxylin and eosin. Scale bars, 100 μm.
Tg640 or Prnpo/o mice grafted with tga20 neuroectoderm were killed at ≥247 dpi. By this time, all intracerebrally infected grafts in both tg640 (n = 8) and Prnpo/o (n = 7) hosts had developed severe scrapie encephalopathy, as diagnosed by the presence of typical histopathological features, including spongiosis. Pronounced gliosis typical of scrapie was visualized by immunocytochemistry for GFAP, and synaptic loss was illustrated by coarse granular deposits and patchy staining for synaptophysin. Predominantly synaptic deposits of PrPSc were apparent with the anti-PrP antibody SAF84. In contrast, none of nine tg640 and four Prnpo/o mice that were inoculated intraocularly showed typical histopathological features of scrapie encephalopathy. Identical results were obtained with five intraperitoneally inoculated tg640 and five Prnpo/o hosts. In two instances, brain grafts of intraocularly inoculated tg640 mice were assayed by transmission of tissue homogenates to groups of four tga20 mice. However, none of the recipient mice developed disease, indicating that the grafts were devoid of prion infectivity. We therefore exclude the possibility that PrPC, when expressed solely on myelin components, supports prion spread within the CNS.
Discussion
Spongiosis, neuronal loss, astroglial and microglial activation, and accumulation of PrPSc are the most prominent neuropathological features of TSEs. Although neurons are clearly affected in the disease process, it is still unclear whether other brain cells also play a role. Although it is undisputed that during the course of prion diseases neurons can undergo pathological changes culminating in cell death, it is by no means clear whether neurons are the sole or even the primary target of prions. Previous studies have shown that astrocyte-specific expression of PrP driven by the GFAP promoter is sufficient to mediate susceptibility to scrapie in Prnpo/o mice (Raeber et al., 1997) and, most surprisingly, to produce a pathology similar to that of wild-type mice. Furthermore, it was speculated that oligodendrocytes may also participate in scrapie pathogenesis, because PrPSc accumulated most strongly in the white-matter areas, such as the corpus callosum or the fiber tracts of the striatum, during scrapie disease (Bendheim et al., 1992; Taraboulos et al., 1992). In addition, substantial PrP mRNA expression in the white-matter tracts, as shown previously (Moser et al., 1995), implied that oligodendrocytes might be the primary producers of PrPSc and may be the primary target for prions as well.
Here we describe transgenic mice expressing full-length PrP C under the control of the MBP promoter. The MBP expression vector was shown to drive expression of the smallest isotype of myelin basic protein and cure the hypomyelinating mouse model shiverer (Kimura et al., 1989). Only one of nine transgenic lines expressed sustained levels of PrPC. Inherent toxicity of the transgene is unlikely, because the incidence of transgene-positive founder mice was normal and line tg640 exhibited undistorted Mendelian transmission of the MBP-PrP transgene. The relative inefficiency of expression may be specific to the MBP-PrP minigene with a coding exon of 1.3 kb flanked by two small artificial introns. In tg640 mice, full-length PrPC was found exclusively on oligodendrocytes and on Schwann cells but was absent from other tissues and cell types. Although the constitutive level of PrPC in the total brains of transgenic mice was only ∼30-35% of that observed in the normal mouse brain, PrP C protein was clearly overexpressed on transgenic oligodendrocytes compared with wild-type oligodendrocytes.
There was no evidence for PrPC expression in either neurons or astrocytes. If any expression occurred, it would be under the detection limit of the methods used. Perhaps the strongest argument for the strict specificity of expression in oligodendrocytes is that we could not detect replication of prions in transgenic mice, whereas we know from studies published previously that even very low levels of expression in astrocytes or neurons would restore cerebral prion replication (Büeler et al., 1994; Race et al., 1995; Raeber et al., 1997). Inoculation of tg640 mice with scrapie prions by different routes did not induce clinical disease or cerebral PrPSc deposition. This was unexpected in view of the proposed role of oligodendrocytes during disease, as described above.
The fact that PrPC expression was genuinely restricted to oligodendrocytes allowed us to ask questions related to the spread of prions within the CNS. Intraocular inoculation into Prnpo/o mice carrying PrPC-overexpressing grafts did not provoke scrapie in grafts, supporting the conjecture that lack of PrPC on cells belonging to the visual pathway prevents prion spread within the CNS (Brandner et al., 1996b). However, it is unclear which mechanisms, be they axonal or nonaxonal, may be involved. Nonneuronal cells, including oligodendrocytes in the CNS and Schwann cells, may play a supportive or obligate role. Within the framework of the prion-only hypothesis, one might hypothesize a “domino” mechanism, by which incoming PrPSc converts resident PrPC on the axolemmal or myelin surface, thereby spatially propagating the infection (Aguzzi and Weissmann, 1997). Indeed, one study showed that the velocity of neuronal prion spread is extremely slow (Kimberlin et al., 1983) and may not follow the canonical mechanisms of fast axonal transport. Additional findings support nonaxonal transport mechanisms that result in periaxonal deposition of PrPSc (Hainfellner and Budka, 1999; Glatzel and Aguzzi, 2000).
In a neurograft paradigm, we find that oligodendrocyte-borne PrPC does not support transfer of prion infectivity from the eye to intracerebral compartments. Could the negative outcome of the grafting experiment be caused by technical problems of grafting or of intraocular inoculation? This is unlikely, because tga20 neurografts are easily infectible via the optic route when implanted into Prnp+/o hosts, but not when grafted into Prnpo/o hosts (Brandner et al., 1996b).
Our findings on the ineffective spread of prions along the visual pathway of tg640 mice do not favor the nonaxonal theory. The hypothesis that spread of prions occurs axonally rests mainly on the demonstration of progressive spongiform changes along the retinal pathway after intraocular infection (Fraser, 1982). Within these experiments, the spread of scrapie pathology was observed first in the contralateral superior colliculus followed by the lateral geniculate nucleus and visual cortex.
The negative outcome of all prion-related tests prompted us to verify that MBP-PrP mice express intact PrPC on myelinating cells. Northern and Western blot analyses, as well as in situ hybridization, confirmed correct transcription of the transgene and translation into PrP-immunoreactive protein with the expected molecular characteristics. Because prion replication may be compromised even by immunochemically silent point mutations, we sequenced the integrated transgene from PCR-amplified genomic DNA. Again, no deviations from the expected sequence were identified. Finally, we verified MBP-PrP transcripts by sequencing reverse transcription-PCR amplification products from brain homogenates. The results of the latter assay rule out RNA editing, which might lead to expression of mutated proteins despite correct genomic sequences.
We still do not know how prions propagate within the brain. The current study excludes any oligodendrocytic contribution to prion pathogenesis by a PrPC-dependent cell-autonomous mechanism. It is still possible, however, that oligodendrocytic PrPC modulates the speed of prion neuroinvasion in a non-cell-autonomous manner, perhaps by transcellular “painting” of glycosylphosphatidylinositol-linked proteins to adjacent axolemmal surfaces. Conversely, other experiments suggest that PrPSc of neuronal origin is unlikely to disturb oligodendrocyte physiology to a clinically significant degree (Mallucci et al., 2003). The mouse model described here will make it possible to address this and related questions in a direct way.
Footnotes
Correspondence should be addressed to Dr. Adriano Aguzzi, Institute of Neuropathology, University Hospital of Zürich, Schmelzbergstrasse 12, CH-8091 Zürich, Switzerland. E-mail: adriano@pathol.unizh.ch.
This work was supported by grants from the Swiss National Research Fund, the National Center of Competence in Research, Neuronal Plasticity and Repair, and the European Union (Bundesamt für Bildung und Wissenschaft)(A.A.). M.P. was a postdoctoral fellow of the Deutsche Forschungsgemeinschaft (Grant Pr 577/2-1). We thank Jürgen Roth, Marius Lötscher, and Maria Grazia Barenco for their help. We also thank Dr. M. Kimura (Tokai University, Isehara, Japan) for providing the pMP302 vector.
M. Prinz's present address: Institute of Neuropathology, Georg-August-University Göttingen, Robert-Koch-Strasse 40, D-37075 Göttingen, Germany.
F. Montrasio's present address: Prion Research Group, Paul-Ehrlich-Institute, Paul-Ehrlich-Strasse 51-59, D-63225 Langen, Germany.
H. Furukawa's present address: Department of Pharmacology 1, Nagasaki University Graduate School of Biomedical Sciences, 1-12-4 Sakamoto, 852-8523 Nagasaki, Japan.
M. E. van der Haar's present address: Prionics AG, Wagistrasse 27a, CH-8952 Schlieren, Switzerland.
Copyright © 2004 Society for Neuroscience 0270-6474/04/245974-08$15.00/0
M.P. and F.M. contributed equally to this work
References
- Aguzzi A, Weissmann C (1997) Prion research: the next frontiers. Nature 389: 795-798. [DOI] [PubMed] [Google Scholar]
- Aguzzi A, Montrasio F, Kaeser PS (2001) Prions: health scare and biological challenge. Nat Rev Mol Cell Biol 2: 118-126. [DOI] [PubMed] [Google Scholar]
- Archer F, Bachelin C, Andreoletti O, Besnard N, Perrot G, Langevin C, Le Dur A, Vilette D, Baron-Van Evercooren A, Vilotte JL, Laude H (2004) Cultured peripheral neuroglial cells are highly permissive to sheep prion infection. J Virol 78: 482-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basler K, Oesch B, Scott M, Westaway D, Walchli M, Groth DF, McKinley MP, Prusiner SB, Weissmann C (1986) Scrapie and cellular PrP isoforms are encoded by the same chromosomal gene. Cell 46: 417-428. [DOI] [PubMed] [Google Scholar]
- Bendheim PE, Brown HR, Rudelli RD, Scala LJ, Goller NL, Wen GY, Kascsak RJ, Cashman NR, Bolton DC (1992) Nearly ubiquitous tissue distribution of the scrapie agent precursor protein. Neurology 42: 149-156. [DOI] [PubMed] [Google Scholar]
- Brandner S, Isenmann S, Raeber A, Fischer M, Sailer A, Kobayashi Y, Marino S, Weissmann C, Aguzzi A (1996a) Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature 379: 339-343. [DOI] [PubMed] [Google Scholar]
- Brandner S, Raeber A, Sailer A, Blattler T, Fischer M, Weissmann C, Aguzzi A (1996b) Normal host prion protein (PrPC) is required for scrapie spread within the central nervous system. Proc Natl Acad Sci USA 93: 13148-13151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büeler H, Raeber A, Sailer A, Fischer M, Aguzzi A, Weissmann C (1994) High prion and PrPSc levels but delayed onset of disease in scrapieinoculated mice heterozygous for a disrupted PrP gene. Mol Med 1: 19-30. [PMC free article] [PubMed] [Google Scholar]
- Büeler HR, Fischer M, Lang Y, Bluethmann H, Lipp HP, DeArmond SJ, Prusiner SB, Aguet M, Weissmann C (1992) Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature 356: 577-582. [DOI] [PubMed] [Google Scholar]
- Büeler HR, Aguzzi A, Sailer A, Greiner RA, Autenried P, Aguet M, Weissmann C (1993) Mice devoid of PrP are resistant to scrapie. Cell 73: 1339-1347. [DOI] [PubMed] [Google Scholar]
- Duffy P, Wolf J, Collins G, DeVoe AG, Streeten B, Cowen D (1974) Possible person-to-person transmission of Creutzfeldt-Jakob disease. N Engl J Med 290: 692-693. [PubMed] [Google Scholar]
- El Hachimi KH, Chaunu MP, Brown P, Foncin JF (1998) Modifications of oligodendroglial cells in spongiform encephalopathies. Exp Neurol 154: 23-30. [DOI] [PubMed] [Google Scholar]
- Fischer M, Rülicke T, Raeber A, Sailer A, Moser M, Oesch B, Brandner S, Aguzzi A, Weissmann C (1996) Prion protein (PrP) with aminoproximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J 15: 1255-1264. [PMC free article] [PubMed] [Google Scholar]
- Flechsig E, Shmerling D, Hegyi I, Raeber AJ, Fischer M, Cozzio A, von Mering C, Aguzzi A, Weissmann C (2000) Prion protein devoid of the octapeptide repeat region restores susceptibility to scrapie in PrP knockout mice. Neuron 27: 399-408. [DOI] [PubMed] [Google Scholar]
- Follet J, Lemaire-Vieille C, Blanquet-Grossard F, Podevin-Dimster V, Lehmann S, Chauvin JP, Decavel JP, Varea R, Grassi J, Fontes M, Cesbron JY (2002) PrP expression and replication by Schwann cells: implications in prion spreading. J Virol 76: 2434-2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser H (1982) Neuronal spread of scrapie agent and targeting of lesions within the retino-tectal pathway. Nature 295: 149-150. [DOI] [PubMed] [Google Scholar]
- Glatzel M, Aguzzi A (2000) PrP(C) expression in the peripheral nervous system is a determinant of prion neuroinvasion. J Gen Virol 81: 2813-2821. [DOI] [PubMed] [Google Scholar]
- Hainfellner JA, Budka H (1999) Disease associated prion protein may deposit in the peripheral nervous system in human transmissible spongiform encephalopathies. Acta Neuropathol (Berl) 98: 458-460. [DOI] [PubMed] [Google Scholar]
- Heppner FL, Musahl C, Arrighi I, Klein MA, Rulicke T, Oesch B, Zinkernagel RM, Kalinke U, Aguzzi A (2001) Prevention of scrapie pathogenesis by transgenic expression of anti-prion protein antibodies. Science 294: 178-182. [DOI] [PubMed] [Google Scholar]
- Isenmann S, Brandner S, Sure U, Aguzzi A (1996a) Telencephalic transplants in mice: characterization of growth and differentiation patterns. Neuropathol Appl Neurobiol 22: 108-117. [PubMed] [Google Scholar]
- Isenmann S, Brandner S, Kuhne G, Boner J, Aguzzi A (1996b) Comparative in vivo and pathological analysis of the blood-brain barrier in mouse telencephalic transplants. Neuropathol Appl Neurobiol 22: 118-128. [PubMed] [Google Scholar]
- Katsuki M, Sato M, Kimura M, Yokoyama M, Kobayashi K, Nomura T (1988) Conversion of normal behavior to shiverer by myelin basic protein antisense cDNA in transgenic mice. Science 241: 593-595. [DOI] [PubMed] [Google Scholar]
- Kimberlin RH, Hall SM, Walker CA (1983) Pathogenesis of mouse scrapie. Evidence for direct neural spread of infection to the CNS after injection of sciatic nerve. J Neurol Sci 61: 315-325. [DOI] [PubMed] [Google Scholar]
- Kimura M, Sato M, Akatsuka A, Nozawa-Kimura S, Takahashi R, Yokoyama M, Nomura T, Katsuki M (1989) Restoration of myelin formation by a single type of myelin basic protein in transgenic shiverer mice. Proc Natl Acad Sci USA 86: 5661-5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallucci G, Dickinson A, Linehan J, Klohn PC, Brandner S, Collinge J (2003) Depleting neuronal PrP in prion infection prevents disease and reverses spongiosis. Science 302: 871-874. [DOI] [PubMed] [Google Scholar]
- McKinley MP, Bolton DC, Prusiner SB (1983) A protease-resistant protein is a structural component of the scrapie prion. Cell 35: 57-62. [DOI] [PubMed] [Google Scholar]
- Moser M, Colello RJ, Pott U, Oesch B (1995) Developmental expression of the prion protein gene in glial cells. Neuron 14: 509-517. [DOI] [PubMed] [Google Scholar]
- Prinz M, Montrasio F, Klein MA, Schwarz P, Priller J, Odermatt B, Pfeffer K, Aguzzi A (2002) Lymph nodal prion replication and neuroinvasion in mice devoid of follicular dendritic cells. Proc Natl Acad Sci USA 99: 919-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner SB, Cochran SP, Groth DF, Downey DE, Bowman KA, Martinez HM (1982) Measurement of the scrapie agent using an incubation time interval assay. Ann Neurol 11: 353-358. [DOI] [PubMed] [Google Scholar]
- Race RE, Priola SA, Bessen RA, Ernst D, Dockter J, Rall GF, Mucke L, Chesebro B, Oldstone MB (1995) Neuron-specific expression of a hamster prion protein minigene in transgenic mice induces susceptibility to hamster scrapie agent. Neuron 15: 1183-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raeber AJ, Race RE, Brandner S, Priola SA, Sailer A, Bessen RA, Mucke L, Manson J, Aguzzi A, Oldstone MB, Weissmann C, Chesebro B (1997) Astrocyte-specific expression of hamster prion protein (PrP) renders PrP knockout mice susceptible to hamster scrapie. EMBO J 16: 6057-6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailer A, Büeler H, Fischer M, Aguzzi A, Weissmann C (1994) No propagation of prions in mice devoid of PrP. Cell 77: 967-968. [DOI] [PubMed] [Google Scholar]
- Schwab ME, Caroni P (1988) Oligodendrocytes and CNS myelin are non-permissive substrates for neurite growth and fibroblast spreading in vitro J Neurosci 8: 2381-2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JR, Foster JD, Fraser H (1993) Conjunctival instillation of scrapie in mice can produce disease. Vet Microbiol 34: 305-309. [DOI] [PubMed] [Google Scholar]
- Shmerling D, Hegyi I, Fischer M, Blattler T, Brandner S, Gotz J, Rulicke T, Flechsig E, Cozzio A, von Mering C, Hangartner C, Aguzzi A, Weissmann C (1998) Expression of amino-terminally truncated PrP in the mouse leading to ataxia and specific cerebellar lesions. Cell 93: 203-214. [DOI] [PubMed] [Google Scholar]
- Taraboulos A, Jendroska K, Serban D, Yang SL, DeArmond SJ, Prusiner SB (1992) Regional mapping of prion proteins in brain. Proc Natl Acad Sci USA 89: 7620-7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu KT (1976) Membranes as observed in frozen sections. J Ultrastruct Res 55: 281-287. [DOI] [PubMed] [Google Scholar]