Abstract
Norepinephrine, acting through β-adrenergic receptors, is implicated in mammalian memory. In in vitro and in vivo studies, norepinephrine produces potentiation of the perforant path-dentate gyrus evoked potential; however, the duration and dynamics of norepinephrine-induced potentiation have not been explored over extended time periods. To characterize the long-term effects of norepinephrine on granule cell plasticity, the present study uses glutamatergic activation of the locus ceruleus (LC) to induce release of norepinephrine in the hippocampus of the awake rat and examines the subsequent modulation of the dentate gyrus evoked potential for 3 hr (short term) and 24 hr (long term) after LC activation. LC activation initiates a potentiation of the field EPSP slope observed 24 hr later. This late-phase potentiation of the synaptic potential is not preceded by early phase potentiation, although spike potentiation can be seen both immediately after, and 24 hr after, LC activation. Intracerebroventricular infusion of the β-adrenergic antagonist, propranolol, or the protein synthesis inhibitor, anisomycin, before LC activation blocks the potentiation of perforant path input observed at 24 hr. The initiation of late-phase synaptic potentiation observed at 24 hr but not at the 3 hr after LC activation parallels the observation of a cAMP- and protein synthesis-dependent long-lasting synaptic facilitation in Aplysia that is not preceded by short-term synaptic facilitation. Locus ceruleus-initiated synaptic potentiation may selectively support long-term, rather than short-term, memory. The observation of selective initiation of long-term synaptic facilitation in a mammalian brain, as in invertebrates, is additional evidence that these two forms of memory depend on separable biological mechanisms.
Keywords: norepinephrine, hippocampus, LTP, cAMP, dentate, locus ceruleus
Introduction
In 1970, Seymour Kety proposed that “the state of arousal by means of adrenergic input to each (cerebral, hippocampal and cerebellar cortices) may serve to concurrently reinforce and to consolidate the significant sensory patterns, the affective associations and the motor programs necessary in the learning of a new adaptive response.”
The diffuse anatomical distribution of noradrenergic fibers and pharmacological data underpinned Kety's proposal that locus ceruleus (LC) activation initiates memory storage (Kety, 1970). Kety envisaged persistent synaptic facilitation linked to norepinephrine's (NE) elevation of cAMP by β-adrenergic receptor activation and to the consequent recruitment of protein synthesis. He hypothesized that novelty and affective events triggered noradrenergic activity and that inputs occurring in association with such events would be incorporated into memory. LC neurons do respond to novelty (Vankov et al., 1995) and to affective events (Berridge and Waterhouse, 2003).
Pharmacological data since Kety's proposal continue to support a role for norepinephrine in memory, particularly long-term memory, in rodents (Izquierdo et al., 1979; Roullet and Sara, 1998; Sara et al., 1999; Berman et al., 2000; Clayton and Williams, 2000; Berman and Dudai, 2001) and humans (Cahill et al., 1994; Nielson and Jensen, 1994; van Stegeren et al., 1998; Quevedo et al., 2003). However, physiological studies support an attentional, rather than a memory, role for the LC. As reviewed recently, LC activation enhances the efficacy of both excitatory and inhibitory synaptic transmissions in sensory areas, increases the signal-to-noise ratio in sensory responses, promotes detection of perithreshold sensory events and the sharpening of suprathreshold events, facilitates the fidelity of sensory transmission in the thalamus, and supports arousal patterns of EEG activity in cortical structures (Berridge and Waterhouse, 2003). These effects depend on the level of LC activity and do not persist when LC activity returns to baseline.
Although in vitro studies have demonstrated norepinephrine-induced long-term potentiation (LTP) of perforant path synaptic strength in the dentate gyrus of the hippocampus, which is dependent on β-adrenergic receptors coupled to cAMP, the potentiation is limited to the medial perforant path, because the lateral perforant path is depressed by norepinephrine (Dahl and Sarvey, 1989). In in vivo studies using anesthetized rats, norepinephrine, or activation of the LC, increases granule cell excitability, as indexed by larger population spikes to perforant path stimulation, without increasing synaptic strength, as indexed by EPSP slope (Neuman and Harley, 1983; Harley and Milway, 1986; Harley et al., 1989).
In the invertebrate, Aplysia, a cAMP-dependent increase in synaptic strength has been demonstrated to occur 24 hr after pairing of sensory input and the monoamine serotonin (Brunelli et al., 1976; Schacher et al., 1988). This long-term synaptic facilitation does not require short-term synaptic facilitation (Emptage and Carew, 1993) and supports other evidence for separate short- and long-term memory processes. In mammals, a cAMP-associated plasticity that uniquely relates to long-term facilitation of synaptic strength has not been reported.
In the present study we monitor the dentate gyrus response to perforant path input in freely moving rats for 3 hr after glutamate infusion into the LC to induce NE release and also at 24 hr after LC glutamate infusion. We show that LC activation initiates a β-adrenergic and protein synthesis-dependent long-term, but not short-term, increase in the synaptic strength of concurrently activated perforant path input to the dentate gyrus.
Materials and Methods
Surgical procedures. Male Sprague Dawley rats (Memorial University of Newfoundland) weighing 250-350 gm were housed singly and allowed ad libitum access to food and water. Experimental procedures occurred within the dark phase of the animals' cycle and were performed in accordance with the Canadian Council of Animal Care guidelines following a protocol approved by the Institutional Animal Care Committee.
Rats were anesthetized with chloral hydrate (80 mg/kg, i.p.) and placed in a stereotaxic instrument in the skull-flat position. Trephine holes were drilled, and four jewelers' screws were used to anchor the recording assembly, with two serving as reference and ground electrodes. A 22 gauge stainless steel cannula (Plastics One) was angled 20° from the vertical, positioned ∼2.7 mm above the LC (12.4 mm posterior to bregma, 1.3 mm lateral from midline), and held in place with dental acrylic. Teflon-coated stainless steel wire (A-M Systems) was used to construct stimulating (bipolar, 150 μm) and recording (50 μm) electrodes. The electrodes were positioned in the perforant path (7.2 mm posterior to bregma, and 4.1 mm lateral from midline, ∼3.0 mm ventral from brain surface) and the granule cell layer of the dentate gyrus (3.5 mm posterior and 2.0 mm lateral, ∼2.5 mm ventral from brain surface), respectively. The field EPSP and the population spike were maximized by small movements of the electrodes before they were cemented into place. Electrodes were secured in a nine-hole McIntyre connector (Ginder Scientific). A second cannula was placed above the lateral ventricle (0.8 mm posterior to bregma and 1.5 mm lateral) ipsilateral to the electrodes and LC cannula in those animals receiving an intracerebroventricular injection. After an injection of chloramphenicol (10 mg in 0.2 ml, s.c.), animals were allowed to recover for 1 week.
Habituation and recording procedures. The recording chamber consisted of a Plexiglas box measuring 42 × 30 × 42 cm in which the animals could roam freely while attached to a commutator (Joseph Biela Idea Development). Bedding covered the floor, and rat chow and water were provided. During the recovery period rats were handled daily. After 5 d rats were habituated to recording conditions by being placed in the chamber and connected to the recording apparatus for a ∼30 min period on 2 successive days. On the following day recording was begun. The first recording session consisted of 10 min habituation, 10 min of input-output data collection, and 60 min of baseline recording. The second session began at the same time of day and continued as described for the first recording session, except that after the hour of baseline recording drug infusions were performed followed by an additional 3 hr of recording. The third and last recording sessions were conducted exactly as the first session had been, and recording started at the same time of day.
Stimulation to the perforant path consisted of a single, 0.2 msec square wave pulse [interstimulus interval (ISI), 30 sec] delivered by a constant current unit (Neurodata Instruments). Evoked potentials were differentially amplified at a bandwidth of 1 Hz-3 kHz using P511 polygraph amplifiers (Grass Instruments), digitized at 10 kHz, and stored on a PC for further analysis.
Each recording session began with the determination of an input-output current intensity relationship (I-O curve) followed by a baseline or test period, or both. Sessions proceeded as follows: session 1 (24 hr pre-LC activation): I-O curve and 1 hr baseline stimulation; session 2 (LC activation): I-O curve, 1 hr baseline stimulation, glutamate injection into the LC, and 3 hr test period (short-term test); and Session 3 (24 hr post-LC activation): I-O curve and 1 hr test period (long-term test). I-O stimulation of the perforant path consisted of increasing current intensities (50-1000 μA, ISI of 10 sec, 50 μA increments) collecting three samples at each current level. Baseline and test sessions consisted of stimulation of the perforant path (ISI 30 sec) at the intensity that elicited a population spike 50% of maximum during the I-O curve of session 1.
Drug application. Monosodium-l-glutamate (Sigma, St. Louis, MO) was mixed fresh (250 mm) in sterile saline before injection into the LC. A 28 gauge internal cannula attached to a 1 μl syringe (Hamilton) by autoanalyzer tubing (Fisher Scientific, Houston, TX) was positioned in the LC. Glutamate (200-250 nl) was infused over 30 sec, and the injection cannula was left in place for 3 min.
dl-Propranolol (Sigma; 30 μg in 5 μl) was mixed in saline before each experiment and injected into the lateral ventricle over 5 min beginning 15 min before the glutamate infusion. Anisomycin (Sigma; 5 mg) was dissolved in 30 μl 1N HCl, the pH was adjusted (≅pH 7.0) with NaOH, and the solution was diluted to 100 μl with saline. Infusion of anisomycin (5 μl, 1 μl/min) began 30 min before the glutamate infusion. After the last recording session, rats were anesthetized with chloral hydrate and 250 nl of methylene blue (1%) was infused into the LC, followed by decapitation and removal of the brain. Brains were frozen in chilled methylbutane and stored at -70°C for sectioning and Nissl staining.
Experimental groups. In the first experiment, three groups of rats were used: (1) a group that received glutamate infusion into the LC (GLUT-LC; n = 7), (2) a group that received intracerebroventricular infusion of the β-adrenergic receptor antagonist, propranolol (30 μg in 5 μl) 15 min before LC activation (PROP; n = 5), and (3) a control group that only received perforant path stimulation (Control; n = 7). Control animals did not receive infusions of a vehicle into the LC because mechanical stimulation may activate LC neurons (Stone et al., 1995), presumably promoting NE release.
In the second experiment, to determine whether protein synthesis was necessary for NE-induced long-term potentiation observed at 24 hr, another three groups of rats were used: (1) a group that received glutamate infusion into the LC with previous infusion of the protein synthesis inhibitor, anisomycin (250 μg in 5 μl), into the ipsilateral lateral ventricle 30 min before LC activation (GLUT-LC + Aniso; n = 7); (2) a group that received glutamate infusion into the LC without previous infusion of anisomycin (GLUT-LC2; n = 7); and (3) a group that received intraventricular infusion of anisomycin only to assess whether inhibition of protein synthesis affects baseline responses over a 24 hr period (Aniso; n = 5).
Data analysis. Two-way (group by time) repeated measures ANOVAs were used to compare baseline and I-O data between sessions 1 and 2 for both experiments. The I-O curves were normalized to the largest mean EPSP slope or population spike of the I-O curve taken during the second session. Sessions 1 and 2 I-O field EPSP slope and population spike amplitude values were not different among groups in the same experiment. Field EPSP slope and population spike values also did not change between the two 1 hr baseline periods (sessions 1 and 2) for either the first or second set of experiments, demonstrating that I-O relationships and baselines were stable before experimental procedures were begun.
Additional repeated measures ANOVAs were performed to compare the effects of LC activation on EPSP slope and population spike measures. When significant F values were obtained, Duncan's multiple range tests were used to probe specific differences (p < 0.05).
Results
Experiment 1: norepinephrine-induced potentiation
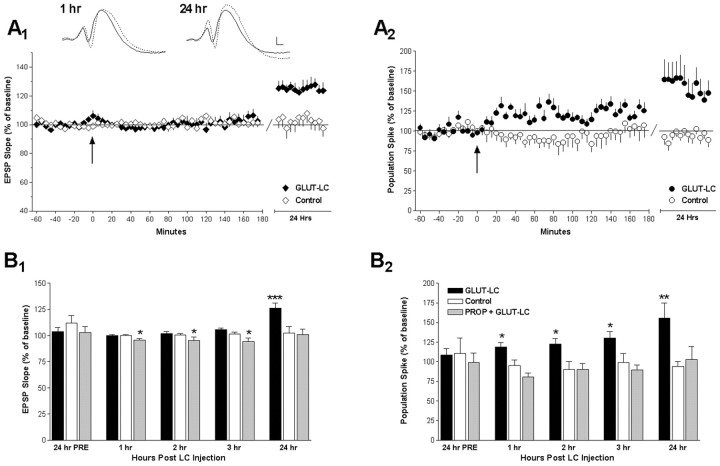
Glutamatergic activation of the LC resulted in a substantive increase in the 24 hr synaptic response to perforant path input as measured by the slope of the field EPSP over previous baseline levels (126%; p < 0.00005) (Fig. 1A1) and by an increase in the population spike amplitude (156%; p < 0.001) (Fig. 1A2). All rats in the GLUT-LC group of the first experiment exhibited these increases. β-Receptor blockade prevented the facilitation of both EPSP slope and population spike amplitude (PROP group: EPSP 101%; population spike amplitude 102%) (Fig. 1B1,B2). In the absence of LC activation in control rats, there was no change in EPSP slope or population spike amplitude over the 24 hr period (Fig. 1A,B).
Figure 1.
NE-induced late-phase LTP after LC activation requires β-adrenergic receptor activation. A1, Inset, Examples of the evoked potential waveforms from a GLUT-LC rat during the baseline period of session 2 (solid line), 1 and 24 hr after injection (dotted lines). Calibration: 2 mV, 2 msec. A1, Graph, EPSP slope for the GLUT-LC group (n = 7) receiving glutamate at the arrow and the control group (n = 7) during session 2. Data are normalized to the baseline of session 2, and 5 min averages with SEMs are shown for both the 3 hr test and long-term test (24 hr) periods. EPSP slope of the GLUT-LC group did not increase during the 3 hr test period, but an increase was observed at 24 hr. A2, Population spike data as outlined for EPSP slope in A1. The population spike amplitude in the GLUT-LC group increased over baseline after the glutamate injection and was elevated at 24 hr. B, Bar graphs of data from A representing 1 hr means for the baseline period of session 1 (24 hr previously), the 3 hr test session, and the long-term test period (24 hr after) for GLUT-LC, control, and PROP (n = 5) groups. The 1 hr baseline period of session 2 is taken as 100%. B1, The GLUT-LC group showed a significant EPSP slope increase at 24 hr. The PROP group demonstrated a small decrease in EPSP slope over the 3 hr test period but had returned to baseline at 24 hr. Controls showed no change in EPSP slope at any time point. B2, The GLUT-LC group showed significant increases in spike amplitude in all sessions after glutamate injection. Neither the control nor PROP group showed any change in spike amplitude. Data are normalized to the baseline taken during the second session and are shown with the SEM. Duncan's multiple range tests were performed on raw data but are shown as percentage change. *p < 0.05; **p < 0.001; ***p < 0.00005.
During the immediate 3 hr period after LC activation, EPSP slope did not vary; however, an overall long-lasting increase in the amplitude of the population spike was observed (118, 122, and 130%, respectively, for the each of the 3 hr) (Fig. 1A,B). Propranolol prevented the increase in population spike amplitude, and there was a small reduction in the synaptic component of the evoked potential lasting for the 3 hr (p < 0.05) (Fig. 1B1,B2). Individual animals in the GLUT-LC group varied in their profiles of initial spike potentiation. Five rats exhibited elevated spike amplitude throughout the 3 hr period, whereas in the other two rats, population spikes were elevated in the first 2 hr but decreased at 3 hr.
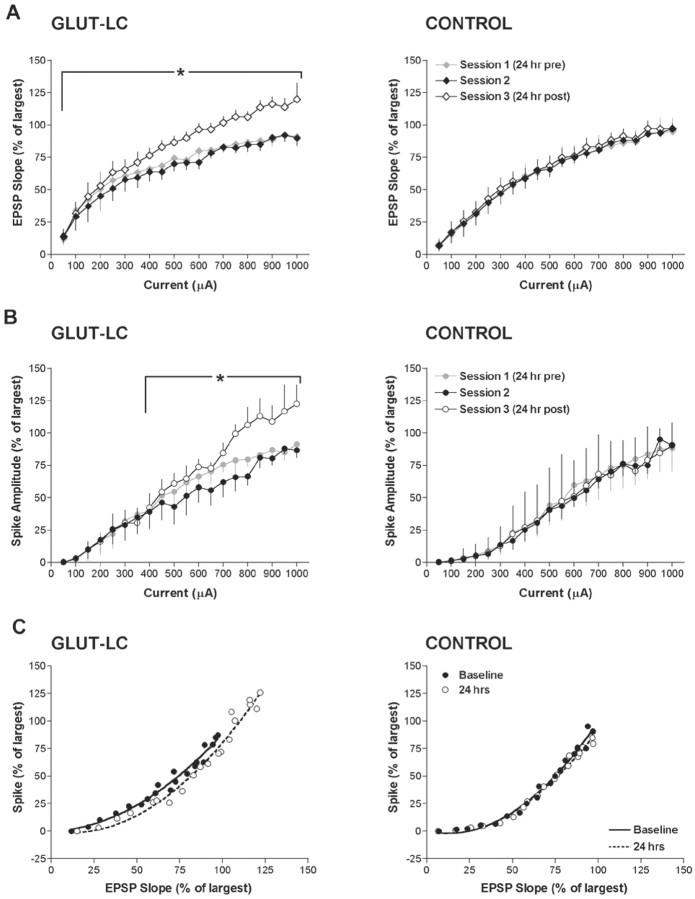
The I-O curve analyses revealed that the EPSP slope (Fig. 2A) increased at all but the lowest current intensity 24 hr after LC activation in the GLUT-LC group. The population spike (Fig. 2B) was increased at higher current intensities. Control animals showed no change in the I-O curves of the EPSP slope (Fig. 2A) or population spike amplitude (Fig. 2B) over the three recording sessions. The effect of late-phase NE-induced LTP on EPSP slope to population spike (E-S) coupling was probed by plotting the mean percentage EPSP slope versus spike amplitude in GLUT-LC and control animals (Fig. 2C). There was no leftward shift in the EPSP-population spike relationship at 24 hr, suggesting that EPSP slope potentiation at 24 hr accounted for the population spike potentiation seen at the same time.
Figure 2.
I-O analysis. I-O curves for the three recording sessions. The mean population spike amplitude or EPSP slope for each intensity was converted to a percentage of the largest mean spike amplitude or EPSP slope obtained during the (I-O) curve of the second session. All data represent the mean across groups ± SEMs. A, I-O analysis of the EPSP slope. No differences were observed in the population spike during the baseline periods in GLUT-LC and control groups. In contrast, significant differences were observed in the GLUT-LC group 24 hr after the glutamate injection. B, I-O analysis of the population spike amplitude. No differences were observed in the population spike during the baseline periods in GLUT-LC and control groups. As with the EPSP slope data, significant differences were observed 24 after the injection of glutamate (repeated measures ANOVA; Duncan's range; *p < 0.05). C, E-S coupling ratio for GLUT-LC and control groups. At 24 hr after glutamate injection, there is no leftward shift in E-S coupling as occurs in short-term NE potentiation. There is no change in the control group.
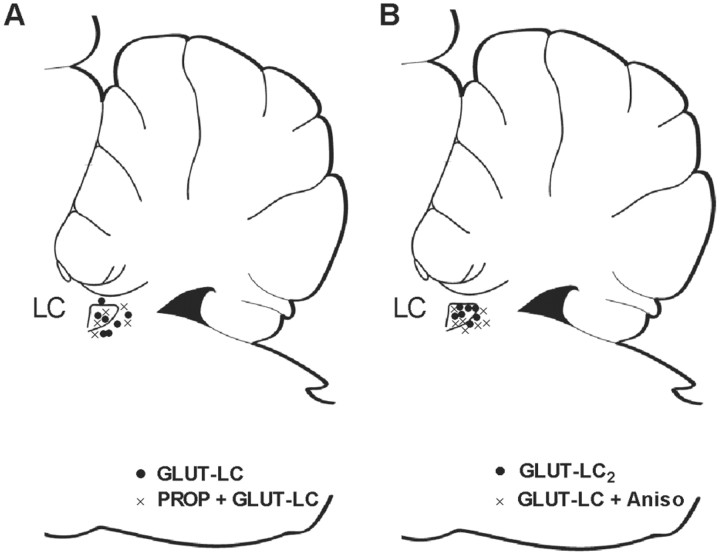
Histological analysis of LC activation sites for both GLUT-LC and PROP + GLUT-LC animals revealed a mixture of intranuclear and pericerulear locations, although pericerulear locations predominated.
Experiment 2: norepinephrine-induced potentiation is dependent on protein synthesis
One condition, common across memory models, is that long-term changes in neural responses are dependent on the synthesis of new proteins. Both long-term facilitation in Aplysia (Dale et al., 1987) and late-phase frequency-induced long-term potentiation in the mammalian hippocampus (Sarvey et al., 1989) rely on the products of protein synthesis.
An analysis of the results of experiment 2 comparing the mean 24 hr after LC activation with that of session 2 baseline again revealed that all animals receiving LC activation (GLUT-LC2; n = 7) show a significant increase in EPSP slope (110%; repeated measures ANOVA; F(1,6) = 19.20; p < 0.005) and population spike amplitude (130%; repeated measures ANOVA; F(1,6) = 9.31; p < 0.02) at 24 hr (Fig. 3). Animals receiving intraventricular anisomycin to inhibit protein synthesis before LC activation (GLUT-LC + Aniso) showed no change in EPSP slope (F(1,6) = 3.80; p < 0.10) or in the amplitude of the population spike (F(1,6) = 1.12; p < 0.33). Animals receiving intraventricular application of anisomycin alone did not vary from baseline levels, indicating that inhibition of protein synthesis in the absence of LC activation does not affect baseline synaptic efficacy.
Figure 3.
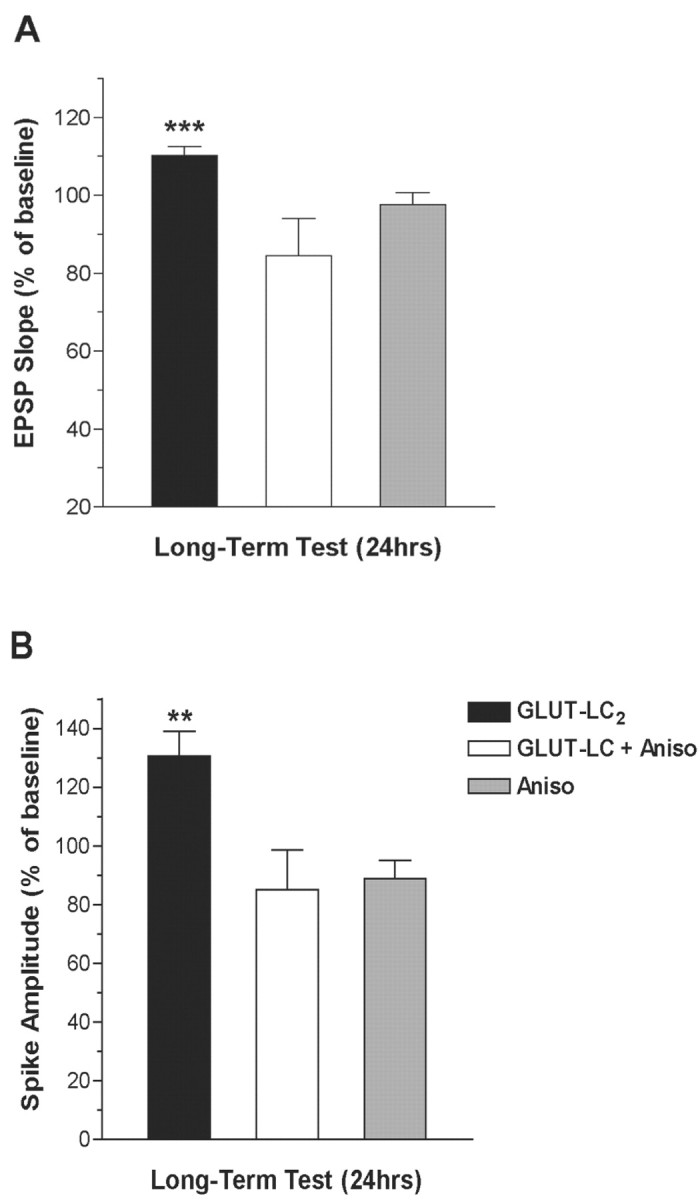
Late-phase NE-LTP is protein synthesis dependent. A, EPSP slope amplitude during the long-term test (24 hr). Infusion of anisomycin, an inhibitor of protein synthesis (GLUT-LC + Aniso), blocks the increase in EPSP slope induced by injection of glutamate into the LC (GLUT-LC2; repeated measures ANOVA; ***p < 0.005). Responses in animals receiving anisomycin alone (Aniso) did not vary from baseline response. B, Population spike amplitude during the long-term test (24 hr). As with the EPSP slope, anisomycin prevented the increase in the population spike observed after glutamate injection into the LC (repeated measures ANOVA; **p < 0.02). Anisomycin alone did not alter the population spike.
During the 3 hr post-LC activation in this experiment, there was a 5-10 min increase in EPSP slope in five of seven rats but no significant EPSP slope effect over the three 1 hr blocks. There was also no overall population spike potentiation; however, individual animals varied. Two rats in the GLUT-LC group and in the GLUT-LC + Aniso group exhibited spike potentiation throughout the 3 hr block; the remainder showed no change or a decrease in spike amplitude.
Locations of the glutamate infusion sites were predominantly in the body of the LC, as revealed by histological analysis. A separate analysis of LC activation effects in rats with pericerulear versus direct cerulear locations of the cannula over the two sets of experiments (Fig. 4) revealed that pericerulear sites were associated more often with early potentiation of spike amplitude and no change in EPSP slope (five of seven), whereas cerulear sites were associated with a brief 5-10 min increase in EPSP slope without early potentiation of spike amplitude (four of seven). One cerulear-targeted rat showed both effects. Both locations produced a protein synthesis and β-adrenergic receptor-dependent potentiation of EPSP slope and spike amplitude at 24 hr.
Figure 4.
Glutamate infusion sites in experiments 1 and 2. Intracerulear sites are within the outlined LC boundary; pericerulear sites are adjacent. Sites for experimental and control groups in experiment 1 (A) and experiment 2 (B) overlapped. Pericerulear sites in both experiments were associated with potentiation of population spike amplitude in the first 3 hr after glutamate infusion.
Discussion
To our knowledge, this is the first study to demonstrate persistent facilitation of synaptic strength after activation of the LC, as predicted originally by Kety (1970), confirming the hypothesis that the LC provides a mechanism for memory. Two important features of these observations are the occurrence of the increase in synaptic strength at 24 hr and a failure to see the increase in synaptic strength during the immediate 3 hr after LC activation. This suggests the LC mechanism supports long-term, but not short-term, memory. Thus, glutamate infusion in the LC, known to produce a burst of LC cell activity (Harley and Sara, 1992), may initially promote spike potentiation, as seen here and in previous studies (Harley and Milway, 1986; Harley et al., 1989; Washburn and Moises, 1989); however, a significant increase in EPSP slope is observed 24 hr later. The increase in EPSP slope at 24 hr predicts the increase in population spike amplitude at that time. The initial increase in spike amplitude after glutamate infusion in the LC may reflect an increase in cell excitability [but see Lacaille and Harley (1985)]. Both the 24 hr EPSP slope and population spike amplitude increase induced by LC activation depend on β-adrenergic receptor mediation and protein synthesis as predicted by Kety (1970).
In Aplysia, repeated 5-HT application produces long-term facilitation of the sensorimotor neuron synaptic response (Montarolo et al., 1986; Mauelshagen et al., 1998). This depends on activation of a receptor coupled to the cAMP cascade (Schacher et al., 1988), is blocked by anisomycin (Montarolo et al., 1986; Schacher et al., 1988; Sherff and Carew, 1999), and can occur in the absence of short-term facilitation (Emptage and Carew, 1993). Thus, the noradrenergic promotion of long-term synaptic potentiation observed in the rat parallels serotonergic promotion of a long-term synaptic facilitation in Aplysia. The ability to induce LTP, without an initial short-term potentiation, is consistent with other evidence for separate mechanisms supporting short-term and long-term memory processes (Warrington, 1979; Sullivan and Sagar, 1991; Izquierdo et al., 2002).
NE-induced LTP at 24 hr also shares important similarities with late-phase tetanic-induced LTP. The latter has been examined at all synapses in the tri-synaptic hippocampal pathway (Huang et al., 1996). This form of LTP also depends on the cAMP-protein kinase A cascade (Nguyen and Kandel, 1996; Abel et al., 1997) and protein synthesis (Krug et al., 1984; Frey et al., 1988; Barea-Rodriguez et al., 2000). β-Adrenergic receptor activation is a requirement for tetanus-induced late-phase LTP at both the lateral and medial perforant path-dentate gyrus synapses (Bramham et al., 1997), at the mossy fiber-CA3 synapse (Huang and Kandel, 1996), and for late-phase LTP in the lateral amygdala (Huang et al., 2000). In a recent study, using the population spike as the index of potentiation, Straube and Frey (2003) have shown that LTP induced by moderate or strong protocols in the perforant path requires β-adrenergic receptor activation for late-phase maintenance, although the strongest protocol was independent of β-adrenergic receptor activation. Most recently, Straube et al. (2003) have shown that novelty exploration, up to 30 min after an early LTP protocol, transforms early LTP in the dentate gyrus into late LTP. Both propranolol and anisomycin prevent this transformation. Positive and negative reinforcers have also been reported to produce a β-adrenergic-dependent enhancement of LTP duration in dentate gyrus (Seidenbecher et al., 1997). Thus, multiple mechanisms recruit persistent facilitation at this synapse, and tetanic LTP protocols synergize with β-adrenergic receptor activation-supported heterosynaptic facilitation.
Although repeated presentations of serotonin and tetani are typically used to produce long-term facilitation and late-phase LTP, respectively, a 3 sec theta-burst tetanus will trigger late-phase LTP in the hippocampus (Nguyen and Kandel, 1997). LC activation initiates theta rhythm in the hippocampus (Berridge and Foote, 1991). We suggest that the combination of convergent glutamatergic and strong phasic noradrenergic input coincident with theta recruitment may be initiating conditions for the late-phase NE-induced LTP seen here. Late-phase LTP of EPSP slope can also be induced with repeated, spaced application of a low concentration of the β-adrenergic agonist, isoproterenol, in vitro. (Dahl and Li, 1994).
The early effects of LC activation parallel those reported in other studies (Lacaille and Harley, 1985; Harley et al., 1989; Washburn and Moises, 1989; Klukowski and Harley, 1994) and suggest that there may be both short-term and intermediate forms of NE potentiation. There is some evidence presented here that the site of LC activation (cerulear vs pericerulear) influences short- and intermediate-term effects on the evoked activity. It is possible that differences in NE release patterns between the two sites of activation could differentially affect early potentiation, although both sites elicit a β-adrenergic receptor- and protein synthesis-dependent increase in the field EPSP and spike amplitude at 24 hr. The early effects of LC activation are either predominantly on cell excitability or on medial perforant path input (Dahl and Sarvey, 1989). The critical conditions for their appearance, such as differing sites of LC activation, and the details of their properties require further investigation. The early effect of spike potentiation alone is consistent with other evidence implicating activation of LC in attention (Berridge and Waterhouse, 2003) rather than memory.
The 24 hr data suggest, however, that LC also acts to selectively promote synaptic LTP, a putative mediator of long-term memory. LC activation paired with sensory input does produce long-term memory in some paradigms (Sullivan et al., 1989). In rat pup olfactory preference learning, β-adrenoceptor stimulation in the olfactory bulb paired with glutamatergic input from olfactory neurons produces an odor preference 24 hr later (Sullivan et al., 2000). This olfactory memory depends on cAMP elevation (Yuan et al., 2003) and the phosphorylation of cAMP response element-binding protein (CREB) at the time of acquisition (Yuan et al., 2000). Both cAMP and phosphorylation of CREB are widely implicated in memory formation (Bernabeu et al., 1996; Silva et al., 1998). Delayed long-lasting CREB phosphorylation is also associated with nondecremental LTP induced by tetani to the perforant path of awake rats (Schulz et al., 1999).
Although few behavioral studies have investigated the effects of NE on memory using designs that assess both short-term and long-term memory, there are data suggesting that NE may have a more important role in long-term than short-term memory. Infusing NE into the hippocampus immediately after training, Izquierdo and colleagues (1998) found no difference in the retention of an inhibitory avoidance task measured 1.5 hr after NE infusion, but when tested 24 hr after NE infusion, retention of the memory task was greater than that of controls. In an active avoidance task, the level of NE release at training correlated positively with the strength of memory at 24 hr (McIntyre et al., 2002). In a taste aversion task, activation of the protein kinase A pathway is necessary for long-term but not short-term memory (Koh et al., 2002). With genetic reduction of NE synthesis, long-term memory, but not short-term memory, for three forms of associative learning is selectively impaired (Kobayashi et al., 2000). Other paradigms demonstrate a critical role for the cAMP cascade in long-term mammalian memory (Abel et al., 1997). Most recently, in humans, the enhancement of memory by emotional arousal, previously shown to depend on the activation of central β-receptors (van Stegeren et al., 1998), improved long-term memory tested at 1 week but not short-term memory tested at 1 hr (Quevedo et al., 2003). The present pattern of results, after burst activation of the locus ceruleus, is consistent with a selective role for an NE and β-adrenergic receptor-mediated potentiation of synaptic circuitry in long-term memory.
Footnotes
This research was supported by the Natural Science and Engineering Research Council with a grant to C.W.H. (A9791). We also thank Drs. Richard S. Neuman and John H. Evans for their thoughtful comments on this manuscript and Jim Williams for his excellent technical assistance.
Correspondence should be addressed to Carolyn W. Harley, Behavioral Neuroscience, Department of Psychology, Memorial University of Newfoundland, St. John's, Newfoundland, Canada A1B 3X9. E-mail: charley@mun.ca.
Copyright © 2004 Society for Neuroscience 0270-6474/04/240598-07$15.00/0
References
- Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R (1997) Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell 88: 615-626. [DOI] [PubMed] [Google Scholar]
- Barea-Rodriguez EJ, Rivera DT, Jaffe DB, Martinez Jr JL (2000) Protein synthesis inhibition blocks the induction of mossy fiber long-term potentiation in vivo J Neurosci 20: 8528-8532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman DE, Dudai Y (2001) Memory extinction, learning anew, and learning the new: dissociations in the molecular machinery of learning in cortex. Science 291: 2417-2419. [DOI] [PubMed] [Google Scholar]
- Berman DE, Hasvi S, Neduva V, Dudai Y (2000) The role of identified neurotransmitter systems in the response of insular cortex to unfamiliar taste: activation of ERK1-2 and formation of a memory trace. J Neurosci 20: 7017-7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernabeu R, Schmitz P, Faillace MP, Izquierdo I, Medina JH (1996) Hippocampal cGMP and cAMP are differentially involved in memory processing of inhibitory avoidance learning. NeuroReport 31: 585-588. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Foote SL (1991) Effects of locus ceruleus activation on electroencephalographic activity in neocortex and hippocampus. J Neurosci 11: 3135-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD (2003) The locus ceruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev 42: 33-84. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Bacher SK, Sarvey JM (1997) LTP in the lateral perforant path is β-adrenergic receptor-dependent. NeuroReport 8: 719-724. [DOI] [PubMed] [Google Scholar]
- Brunelli M, Castellucci V, Kandel ER (1976) Synaptic facilitation and behavioral sensitization in Aplysia: possible role of serotonin and cyclic AMP. Science 194: 1178-1181. [DOI] [PubMed] [Google Scholar]
- Cahill L, Prins B, Weber M, McGaugh JL (1994) Beta-adrenergic activation and memory for emotional events. Nature 371: 702-704. [DOI] [PubMed] [Google Scholar]
- Clayton EC, Williams CL (2000) Posttraining inactivation of excitatory afferent input to the locus ceruleus impairs retention in an inhibitory avoidance learning task. Neurobiol Learn Mem 73: 127-140. [DOI] [PubMed] [Google Scholar]
- Dahl D, Li J (1994) Induction of long-lasting potentiation by sequenced applications of isoproterenol. NeuroReport 5: 657-660. [DOI] [PubMed] [Google Scholar]
- Dahl D, Sarvey JM (1989) Norepinephrine induces pathway-specific long-lasting potentiation and depression in the hippocampal dentate gyrus. Proc Natl Acad Sci USA 86: 4776-4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N, Kandel ER, Schacher S (1987) Serotonin produces long-term changes in the excitability of Aplysia sensory neurons in culture that depend on new protein synthesis. J Neurosci 7: 2232-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emptage NJ, Carew TJ (1993) Long-term synaptic facilitation in the absence of short-term facilitation in Aplysia neurons. Science 262: 253-256. [DOI] [PubMed] [Google Scholar]
- Frey U, Krug M, Reymann KG, Matthies H (1988) Anisomycin, an inhibitor of protein synthesis, blocks late phases of LTP phenomena in the hippocampal CA1 region in vitro. Brain Res 452: 57-65. [DOI] [PubMed] [Google Scholar]
- Harley CW, Milway JS (1986) Glutamate ejection in the locus ceruleus enhances the perforant path-evoked population spike in the dentate gyrus. Exp Brain Res 63: 143-150. [DOI] [PubMed] [Google Scholar]
- Harley CW, Sara SJ (1992) Locus ceruleus bursts induced by glutamate trigger delayed perforant path spike amplitude potentiation in the dentate gyrus. Exp Brain Res 89: 581-587. [DOI] [PubMed] [Google Scholar]
- Harley CW, Milway JS, Lacaille J-C (1989) Locus ceruleus potentiation of dentate gyrus responses: evidence for two systems. Brain Res Bull 22: 643-650. [DOI] [PubMed] [Google Scholar]
- Huang YY, Kandel ER (1996) Modulation of both the early and the late phase of mossy fiber LTP by the activation of β-adrenergic receptors. Neuron 16: 611-617. [DOI] [PubMed] [Google Scholar]
- Huang YY, Nguyen PV, Abel T, Kandel ER (1996) Long-lasting forms of synaptic potentiation in the mammalian hippocampus. Learn Mem 3: 74-85. [DOI] [PubMed] [Google Scholar]
- Huang YY, Martin KC, Kandel ER (2000) Both protein kinase A and mitogen-activated protein kinase are required in the amygdala for the macromolecular synthesis-dependent late phase of long-term potentiation. J Neurosci 20: 6317-6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo I, Beamish DG, Anisman H (1979) Effect of an inhibitor of dopamine-β-hydroxylase on the acquisition and retention of four different avoidance tasks in mice. Psychopharmacology (Berl) 63: 173-178. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Medina JH, Izquierdo LA, Barros DM, de Souza MM, Souza T (1998) Short- and long-term memory are differentially regulated by monominergic systems in the rat brain. Neurobiol Learn Mem 69: 219-224. [DOI] [PubMed] [Google Scholar]
- Izquierdo LA, Barros DM, Vianna MR, Coitinho A, deDavid e Silva T, Choi H, Moletta B, Medina JH, Izquierdo I (2002) Molecular pharmacological dissection of short- and long-term memory. Cell Mol Neurobiol 22: 269-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kety SS (1970) The biogenic amines in the central nervous system: their possible roles in arousal, emotion and learning. In: The neurosciences: second study program (Schmidt FO, ed), pp 324-335. New York: Rockefeller Press.
- Klukowski G, Harley CW (1994) Locus ceruleus activation induces perforant path-evoked population spike potentiation in the dentate gyrus of awake rat. Exp Brain Res 102: 165-170. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Noda Y, Matsushita N, Nishii K, Sawada H, Nagatsu T, Nakahara D, Fukabori R, Yasoshima Y, Yamamoto T, Miura M, Kano M, Mamiya T, Miyamoto Y, Nabeshima T (2000) Modest neuropsychological deficits caused by reduced noradrenaline metabolism in mice heterozygous for a mutated tyrosine hydroxylase gene. J Neurosci 20: 2418-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh MT, Thiele TE, Bernstein IL (2002) Inhibition of protein kinase A activity interferes with long-term, but not short-term, memory of conditioned taste aversions. Behav Neurosci 116: 1070-1074. [PubMed] [Google Scholar]
- Krug M, Lossner B, Ott T (1984) Anisomycin blocks the late phase of long-term potentiation in the dentate gyrus of freely moving rats. Brain Res Bull 13: 39-42. [DOI] [PubMed] [Google Scholar]
- Lacaille J-C, Harley CW (1985) The action of norepinephrine in the dentate gyrus: beta-mediated facilitation of evoked potentials in vitro. Brain Res 358: 210-220. [DOI] [PubMed] [Google Scholar]
- Mauelshagen J, Sherff CM, Carew TJ (1998) Differential induction of long-term synaptic facilitation by spaced and massed applications of serotonin at sensory neuron synapses of Aplysia californica. Learn Mem 5: 246-256. [PMC free article] [PubMed] [Google Scholar]
- McIntyre CK, Hatfield T, McGaugh JL (2002) Amygdala norepinephrine levels after training predict inhibitory avoidance retention performance in rats. Eur J Neurosci 16: 1223-1226. [DOI] [PubMed] [Google Scholar]
- Montarolo PG, Goelet P, Castellucci VF, Morgan J, Kandel ER, Schacher S (1986) A critical period for macromolecular synthesis in long-term heterosynaptic facilitation in Aplysia. Science 234: 1249-1254. [DOI] [PubMed] [Google Scholar]
- Neuman RS, Harley CW (1983) Long-lasting potentiation of the dentate gyrus population spike by norepinephrine. Brain Res 273: 162-165. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Kandel ER (1996) A macromolecular synthesis-dependent late phase of long-term potentiation requiring cAMP in the medial perforant pathway of rat hippocampal slices. J Neurosci 16: 3189-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PV, Kandel ER (1997) Brief theta-burst stimulation induces a transcription-dependent late phase of LTP requiring cAMP in area CA1 of the mouse hippocampus. Learn Mem 4: 230-243. [DOI] [PubMed] [Google Scholar]
- Nielson KA, Jensen RA (1994) Beta-adrenergic receptor antagonist antihypertensive medications impair arousal-induced modulation of working memory in elderly humans. Behav Neural Biol 62: 190-200. [DOI] [PubMed] [Google Scholar]
- Quevedo J, Sant' Anna MK, Madruga M, Lovato I, de Paris F, Kapczinski F, Izquierdo I, Cahill L (2003) Differential effects of emotional arousal in short-term and long-term memory in healthy adults. Neurobiol Learn Mem 79: 132-135. [DOI] [PubMed] [Google Scholar]
- Roullet P, Sara S (1998) Consolidation of memory after its reactivation: involvement of beta noradrenergic receptors in the late phase. Neural Plast 6: 63-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ, Roullet P, Przybyslawksi J (1999) Consolidation of memory for odor-reward association: beta-adrenergic receptor involvement in the late phase. Learn Mem 6: 88-96. [PMC free article] [PubMed] [Google Scholar]
- Sarvey JM, Burgard EC, Decker G (1989) Long-term potentiation: studies in the hippocampal slice. J Neurosci Methods 28: 109-124. [DOI] [PubMed] [Google Scholar]
- Schacher S, Castellucci VF, Kandel ER (1988) cAMP evokes long-term facilitation in Aplysia sensory neurons that requires new protein synthesis. Science 240: 1667-1669. [DOI] [PubMed] [Google Scholar]
- Schulz S, Siemer H, Krug M, Hollt V (1999) Direct evidence for biphasic cAMP responsive element-binding protein phosphorylation during long-term potentiation in the rat dentate gyrus in vivo J Neurosci 19: 5682-5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenbecher T, Reymann KG, Balschun D (1997) Post-tetanic time window for the reinforcement of long-term potentiation by appetitive and aversive stimuli. Proc Natl Acad Sci USA 94: 1494-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherff CM, Carew TJ (1999) Coincident induction of long-term facilitation in Aplysia: cooperativity between cell bodies and remote synapses. Science 285: 1911-1914. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S (1998) CREB science memory. Annu Rev Neurosci 21: 127-148. [DOI] [PubMed] [Google Scholar]
- Stone EA, Zhang Y, Carr KD (1995) Massive activation of c-fos in forebrain after mechanical stimulation of the locus ceruleus. Brain Res Bull 36: 77-80. [DOI] [PubMed] [Google Scholar]
- Straube T, Frey JU (2003) Involvement of β-adrenergic receptors in protein synthesis-dependent late long-term potentiation (LTP) in the dentate gyrus of freely moving rats: the critical role of the LTP induction strength. Neuroscience 119: 473-479. [DOI] [PubMed] [Google Scholar]
- Straube T, Korz Z, Balshun D and Frey JU (2003) Requirement of β-adrenergic receptor activation and protein synthesis for LTP-reinforcement by novelty in rat dentate gyrus. J Physiol (Lond), 552: 953-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Sagar HJ (1991) Double dissociation of short-term and long-term memory for nonverbal material in Parkinson's disease and global amnesia. A further analysis. Brain 114: 893-906. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DM, Leon M (1989) Norepinephrine and learning-induced plasticity in infant rat olfactory system. J Neurosci 9: 3998-4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Stackenwalt G, Nasr F, Lemon C, Wilson DA (2000) Association of an odor with activation of olfactory bulb noradrenergic β-receptors or locus ceruleus stimulation is sufficient to produce learned approach responses to that odor in neonatal rats. Behav Neurosci 114: 957-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vankov A, Hervé-Minvielle A, Sara SJ (1995) Response to novelty and its rapid habituation in locus ceruleus neurons of the freely exploring rat. Eur J Neurosci 7: 1180-1187. [DOI] [PubMed] [Google Scholar]
- van Stegeren AH, Everaerd W, Cahill L, McGaugh JL, Gooren LJ (1998) Memory for emotional events: differential effects of centrally versus peripherally acting β-blocking agents. Psychopharmacology (Berl) 138: 305-310. [DOI] [PubMed] [Google Scholar]
- Warrington EK (1979) Neuropsychological evidence for multiple memory systems. Ciba Found Symp 153-166. [DOI] [PubMed]
- Washburn M, Moises HC (1989) Electrophysiological correlates of presynaptic α2-receptor-mediated inhibition of norepinephrine release at locus ceruleus synapses in dentate gyrus. J Neurosci 9: 2131-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Harley CW, Bruce JC, Darby-King A, Mclean JH (2000) Isoproterenol increases CREB phosphorylation and olfactory nerve-evoked potentials in normal and 5-HT-depleted olfactory bulbs in rat pups only at doses that produce odor preference learning. Learn Mem 7: 413-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Harley CW, Mclean JH (2003) Mitral cell β1 and 5-HT2A receptor colocalization and cAMP coregulation: a new model of norepinephrine-induced learning in the olfactory bulb. Learn Mem 10: 5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]