Abstract
Recent animal models suggest that disturbances in serotonin type-1A receptor (5-HT1AR) function may contribute to chronic anxiety, although it is not clear at all whether such models constitute relevant models for panic disorder (PD) in humans. The selective 5-HT1AR radioligand [18F]trans-4-fluoro-N-2-[4-(2-methoxyphenyl)piperazin-1-yl]ethyl]-N-(2-pyridyl)cyclohexanecarboxamide (FCWAY) permits in vivo assessment of central 5-HT1AR binding using positron emission tomography (PET). We studied 16 unmedicated symptomatic outpatients with PD and 15 matched healthy controls. Seven patients had an additional diagnosis of a current major depressive episode, however PD was the primary diagnosis. A 120 min PET study of 5-HT1AR binding was acquired using a GE Advance scanner in three-dimensional mode. Using quantitative PET image analysis, regional values were obtained for [18F]-FCWAY volume of distribution (DV), corrected for plasma protein binding, and K1, the delivery rate of [18F]-FCWAY from plasma to tissue. MRI scanning was performed using a GE Signa Scanner (3.0 Tesla) to provide an anatomical framework for image analysis and partial volume correction of PET data. PD patients showed lower DV in the anterior cingulate (t = 4.3; p < 0.001), posterior cingulate (t = 4.1; p < 0.001), and raphe (t = 3.1; p = 0.004). Comparing patients with PD, patients with PD and comorbid depression, and healthy controls revealed that DVs did not differ between PD patients and PD patients with comorbid depression, whereas both patient groups differed significantly from controls. These results provide for the first time in vivo evidence for the involvement of 5-HT1ARs in the pathophysiology of PD.
Keywords: anxiety, imaging, neuron, neurotransmitter, positron emission tomography, serotonin
Introduction
Panic disorder (PD) is a syndrome characterized by recurrent episodes of intense fear, thoughts of impending death, accelerated and more forceful heartbeat, chest discomfort, shortness of breath, dizziness, sweating, and tremulousness, that arise in the absence of identifiable precipitants. Recent animal models have suggested a role of serotonin type-1A receptors (5-HT1AR) in the development of chronic anxiety (Overstreet et al., 2003) and have helped to generate animal models of anxiety-related disorders. However, it is not clear at all whether these models constitute relevant models for PD in humans. The recent development of highly selective 5-HT1AR radioligands such as [18F]trans-4-fluoro-N-2-[4-(2-methoxyphenyl)piperazin-1-yl]ethyl]-N-(2-pyridyl)cyclohexanecarboxamide (FCWAY) has now made it possible to assess central 5-HT1AR binding in PD, using positron emission tomography (PET). In the present study we assess for the first time in vivo 5-HT1AR in a group of unmedicated symptomatic patient with PD and a group of matched healthy control subjects.
Materials and Methods
Patients. Sixteen unmedicated (nine naive to psychotropic drugs) symptomatic outpatients with a diagnosis of PD on the basis of the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, nonpatient version (First et al., 1996) (10 female, mean age 35.2 ± 9.6 years; range, 20-52 years), seven of whom also met criteria for a current, secondary major depressive episode (PD was temporally the first, or “primary” diagnosis) and 15 matched healthy controls (10 female, age 35.2 ± 9.5 years, range 22-50 years) were imaged. Information about family history of mental illness (mood and anxiety disorders, schizophrenia, and other psychotic disorders, substance abuse disorders) was obtained from the study participants for all first degree relatives using the Family Interview of Genetic Studies (Maxwell, 1992). PD patients with other axis I diagnoses in addition to PD and major depressive disorder were ineligible to participate. Subjects had no lifetime history of drug or alcohol abuse, and remained off psychotropic drugs during the 3 weeks before scanning. Controls were required to have no personal or family history of psychiatric disorders in first degree relatives. All participants were free of medical illness on the basis of history and results of physical examination, electrocardiogram, and laboratory tests, including liver and kidney function tests, hematologic profile, thyroid function tests, urinalysis, and toxicology. Pregnant and nursing women were ineligible to participate. All female subjects underwent plasma pregnancy tests at the time of screening and urine pregnancy tests before the imaging study. Premenopausal women were studied whenever possible during the follicular phase of the menstrual cycle. The menstrual phase was determined using plasma estradiol and progesterone concentrations, time since onset of last menses, and home urine ovulation kit to detect the midcycle luteinizing hormone (LH) surge (Clear Plan Easy; Whitehall Laboratories, Madison, NJ) to identify the time of ovulation within the index menstrual cycle. Clinical ratings, using the Panic Disorder Severity Scale (PDSS) (Shear et al., 1997) and the Montgomery-Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979) were obtained on the day of the PET study and showed significantly higher scores for the PD patients relative to the controls (values are mean ± SD; PDSS: 14.3 ± 4.8 vs 0 ± 0; MADRS: 18.5 ± 9.6 vs 0.5 ± 1.0). All subjects were entered into the study after full explanation of the purpose of the study and the study procedures were given to the participants, and after written consent had been obtained as approved by the National Institutes of Mental Health Institutional Review Board.
Image acquisition and analysis. A 120 min PET study of 5-HT1AR binding was acquired using a GE Advance PET scanner in three-dimensional (3D) mode [35 contiguous slices, 4.25 mm plane separation; reconstructed resolution = 7 mm full-width at half-maximum (FWHM) in all planes], bolus intravenous injection of 8 mCi of high-specific activity [18F]FCWAY, and arterial blood sampling (Carson et al., 2000). Magnetic resonance images (MRI), obtained for each subject using a GE Signa Scanner (3.0 Tesla) and a 3D MPRAGE sequence (echo time, 2.982 msec; repetition time, 7.5 msec; inversion time, 725 msec; voxel size, 0.9 × 0.9 × 1.2 mm), were coregistered to the PET images to provide an anatomical framework for analysis and to perform partial volume correction of the PET images. Regional 5-HT1AR binding was measured in regions-of-interest (ROI) defined a priori on the MRI images (or directly on the PET image in the case of the raphe) and transferred to the coregistered PET images using MEDx software (Sensor Systems, Sterling, VA). The ROI were localized to brain structures that contained abundant postsynaptic 5-HT1AR concentrations in the anterior cingulate cortex, posterior cingulate cortex, anterior insula, mesiotemporal cortex (hippocampus plus amygdala), anterior temporopolar cortex, and midbrain raphe. The raphe ROI is centered over the raphe nuclei, collectively evident in FCWAY images because of their high 5-HT1AR density relative to that of the surrounding tissues. The left and right mesiotemporal cortices are defined to encompass the hippocampal formation, amygdala, and adjacent parahippocampal and periamygdaloid cortex using conventions for MRI analyses (Bronen and Cheung, 1991; Watson et al., 1992). The left ventral anterior cingulate ROI encompasses the anterior cingulate cortex ventral to the genu of the corpus callosum (i.e., subgenual PFC), bilaterally, extending ventrally to include three horizontal planes corresponding to the cingulate gyrus. The anterior temporopolar cortex ROI is defined on the anterior temporal cortex ventral to the amygdala. To correct data for free and nonspecifically bound radiotracer, a reference tissue ROI was defined in the cerebellum, which is devoid of 5-HT1AR. The cerebellar ROI is defined in cerebellar cortex, bordered anteromedially by the peduncles, and extending posteriorly to no closer than twice the FWHM distance from the edge of the occipital and temporal cortex (to reduce spilling in of radioactivity from the occipital and temporal cortex). Because the signal-to-noise ratio of image data decreases in the most dorsal and ventral slices of PET images acquired in 3D mode, cerebellar activity is not sampled in the lowest planes. The regional FCWAY volume of distribution (DV; milliliters of plasma per milliliter of brain), corrected for plasma protein binding, and K1, the delivery rate of FCWAY from plasma to tissue (in milliliter per minute per minute) were obtained using quantitative tracer kinetic modeling. The mean DV values were compared between groups using unpaired t tests, with p values corrected (Bonferroni) for comparisons in six ROI. The specificity for receptor-specific binding was assessed post hoc by comparing regional binding potentials (BP) as (DVROI/DVcerebellum - 1) to factor out the influence of free and nonspecifically bound radiotracer. The influences of comorbid depression and past medication exposure were assessed using three-group ANOVAs with Tukey post hoc tests.
Results
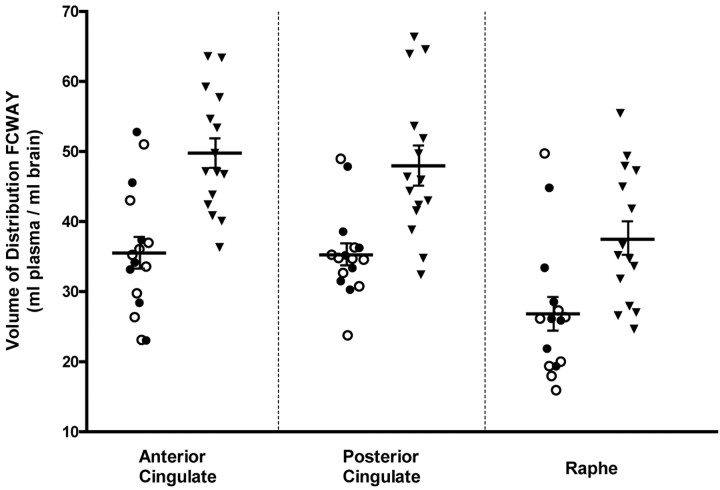
The mean 5-HT1AR DV was lower in the PD group relative to the control group in the anterior cingulate (mean ± SD = 35.9 ± 9.3 and 49.6 ± 8.6, respectively; t = 4.3; p < 0.001) (Fig. 1), posterior cingulate (35.6 ± 6.2 vs 47.9 ± 10.5; t = 4.1; p < 0.001), and raphe (26.9 ± 9.5 vs 37.7 ± 9.6; t = 3.1; p = 0.004). These differences were also significant (p < 0.001 in each region) when comparing the raw DV values (i.e., uncorrected for protein binding) and the 5-HT1AR binding potential. No between-group differences were found in the anterior insula, mesiotemporal cortex, and the anterior temporopolar cortex. Tracer delivery (K1) did not differ between groups in any region.
Figure 1.
Scatter histograms of the 5-HT1A receptor DV values for the panic disorder (PD) and control groups (▾). Within the PD sample, distinct symbols designate the pure PD (○) and the PD plus comorbid depression (•) subgroups. Mean (±SEM) bars indicate the significant differences between PD and controls groups, whereas no differences were found between the PD subgroup
Lower 5-HT1AR DV was evident in both the pure PD and the PD plus comorbid depression subgroups relative to the control group (anterior cingulate, F(2,28) = 8.88; p < 0.001), posterior cingulate (F(2,28) = 7.97; p = 0.002), and raphe (F(2,27) = 4.89; p = 0.015), and these two PD subgroups did not significantly differ from each other (Fig. 1). The mean DV was also decreased in both the drug-naive and the previously medicated subgroups relative to the control group (p < 0.001 and p < 0.01, respectively in all three ROI), and these two subgroups did not significantly differ from each other.
Discussion
Using PET, we show in a group of PD patients a marked reduction of cerebral 5-HT1AR binding in the anterior and posterior cingulate cortices, which have been implicated in the modulation of anxiety by electrophysiological and functional brain-mapping studies in human and nonhuman primates (Charney and Drevets, 2002) and from conditional and tissue-specific 5-HT1AR knock-out studies in mice (Gross et al., 2002), and the midbrain raphe, an area where 5-HT1AR stimulation regulates serotonin synthesis and release.
The reduction of cerebral 5-HT1AR binding in patients with PD relative to healthy controls was not accounted for by previous exposure to psychotropic drugs or comorbid depression. Although reduced 5-HT1AR binding was previously reported in primary major depressive disorder (Drevets et al., 1999), the magnitude of the difference in depression in the anterior and posterior cingulate cortices was less pronounced than that seen in PD. In contrast, reduction of 5-HT1AR binding in the raphe was similar (41% in major depressive disorder versus ∼34% in PD with the use of different ligands).
The anatomical extent of the abnormal 5-HT1AR binding as well as the mechanisms underlying reduced binding in PD remain unclear. Because [18F]FCWAY binds receptors in various affinity states, the reduced DV likely reflects a reduction in 5-HT1AR density. The regulation of 5-HT1AR density is insensitive to serotonin concentrations, but in experimental animals the genetic expression of 5-HT1AR is decreased by the glucocorticoid hormone secretion associated with repeated stress (Lopez et al., 1999). However, glucocorticoid (i.e., cortisol) secretion is generally not elevated in PD and does not increase during panic attacks (Charney and Drevets, 2002).
Another possibility is that reduced 5-HT1AR expression is associated with a genetic risk factor for PD. It is noteworthy that a 5-HT1AR polymorphism regulating 5-HT1AR transcription has recently been linked to major depression and suicide (Lemonde et al., 2003). In mice reduced 5-HT1AR gene expression during the postnatal period results in exaggerated anxiety responses which persists into adulthood (Gross et al., 2002).
The finding of no difference in reduced 5-HT1AR binding between PD patients with and without a concurrent major depressive episode could be construed as support for the large twin literature, suggesting that depression and anxiety disorders have important genetic overlaps (Kendler et al., 2003).
The present results suggest that 5-HT1ARs may be a source of vulnerability in humans, and that abnormal function of these receptors appears to specifically impact the cortical circuitry involved in the regulation of anxiety.
Footnotes
We appreciate the excellent assistance of Marilla Geraci for nursing support and Jerry I. Jacobs and the other PET technicians for technical support during the PET studies.
Correspondence should be addressed to Dr. Alexander Neumeister, National Institutes of Health, National Institute of Mental Health, Mood and Anxiety Disorders Program, North Drive, Building 15K, Room 200, Bethesda, MD 20892-2670. E-mail: neumeisa@intra.nimh.nih.gov.
Copyright © 2004 Society for Neuroscience 0270-6474/04/240589-03$15.00/0
D.S.C. and W.C.D. contributed equally to this work.
References
- Bronen RA, Cheung G (1991) Relationship of hippocampus and amygdala to coronal MRI landmarks. Magn Reson Imaging 9: 449-457. [DOI] [PubMed] [Google Scholar]
- Carson RE, Lang L, Watabe H, Der MG, Adams HR, Jagoda E, Herscovitch P, Eckelman WC (2000) PET evaluation of [(18)F]FCWAY, an analog of the 5-HT(1A) receptor antagonist, WAY-100635. Nucl Med Biol 27: 493-497. [DOI] [PubMed] [Google Scholar]
- Charney DS, Drevets WC (2002) Neurobiological basis of anxiety disorders. In: Neuropsychopharmacology: the fifth generation of progress (Davis KL, Charney DS, Coyle JT, Nemeroff C, eds), pp 901-930. Philadelphia: Lippincott Williams and Wilkins.
- Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, Huang Y, Gautier C, Mathis C (1999) PET imaging of serotonin 1A receptor binding in depression. Biol Psychiatry 46: 1375-1387. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW (1996) Structured clinical interview for DSM-IV axis I disorders: nonpatient edition (SCID-I/NP). New York: Biometrics Department, New York State Psychiatric Institute.
- Gross C, Zhuang X, Start K, Ramboz S, Oosting R, Kirby L, Santarelli L, Beck S, Hen R (2002) Serotonin1A receptor acts during development to establish normal anxiety-like behavior in the adult. Nature 416: 396-400. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC (2003) The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry 60: 929-937. [DOI] [PubMed] [Google Scholar]
- Lemonde S, Turecki G, Bakish D, Du L, Hrdina PD, Bown CD, Sequeira A, Kushwaha N, Morris SJ, Basak A, Ou XM, Albert PR (2003) Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J Neurosci 23: 8788-8799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez JF, Liberzon I, Vazquez DM, Young EA, Watson SJ (1999) Serotonin 1A receptor messenger RNA regulation in the hippocampus after acute stress. Biol Psychiatry 45: 934-937. [DOI] [PubMed] [Google Scholar]
- Maxwell ME (1992) Manual for the family interview for genetic studies (FIGS). In: Clinical neurogenetics branch, intramural research program. National Institute of Mental Health, Bethesda MD.
- Montgomery SA, Asberg M (1979) A new depression scale designed to be sensitive to change. Br J Psychiatry 134: 382-389. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Commissaris RC, De La Garza II R, File SE, Knapp DJ, Seiden LS (2003) Involvement of 5-HT1A receptors in animal tests of anxiety and depression: evidence from genetic models. Stress 6: 101-110. [DOI] [PubMed] [Google Scholar]
- Shear MK, Brown TA, Barlow DH, Money R, Sholomskas DE, Woods SW, Gorman JM, Papp LA (1997) Multicenter collaborative panic disorder severity scale. Am J Psychiatry 154: 1571-1575. [DOI] [PubMed] [Google Scholar]
- Watson C, Andermann F, Gloor P, Jones-Gotman M, Peters T, Evans A, Olivier A, Melanson D, Leroux G (1992) Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurology 42: 1743-1750. [DOI] [PubMed] [Google Scholar]