Abstract
Locations of a distinctive mode of trans-endocytosis involving dendrites, axons, and glia were quantified through serial section electron microscopy. Short vesicular or long vermiform evaginations emerged from dendrites and axons and were engulfed by presynaptic or neighboring axons, astrocytes, and, surprisingly, a growth cone to form double-membrane structures called spinules. In total, 254 spinules were evaluated in 326 μm3 of stratum radiatum in area CA1 of mature rat hippocampus. Spinules emerged from spine heads (62%), necks (24%), axons (13%), dendritic shafts (1%), or nonsynaptic protrusions (<1%) and invaginated into axons (∼90%), astrocytic processes (∼8%), or a growth cone (∼1%). Coated pits occurred on the engulfing membrane at the tips of most spinules (69%), and double-membrane structures occurred freely in axonal and astrocytic cytoplasm, suggesting trans-endocytosis. Spinule locations differed among mushroom and thin spines. For mushroom spines, most (84%) of the spinules were engulfed by presynaptic axons, 16% by neighboring axons, and none by astrocytic processes. At thin spines, only 17% of the spinules were engulfed by presynaptic axons, whereas 67% were engulfed by neighboring axons and 14% by astrocytic processes. Spinules engulfed by astrocytic processes support the growing evidence that perisynaptic glia interact directly with synapses at least on thin spines. Spinules with neighboring axons may provide a mechanism for synaptic competition in the mature brain. Trans-endocytosis of spinules by presynaptic axons suggest retrograde signaling or coordinated remodeling of presynaptic and postsynaptic membranes to remove transient perforations and assemble the postsynaptic density of large synapses on mushroom spines.
Keywords: dendritic spines, exocytosis, endocytosis, coated vesicles, spinules, synapse, serial section electron microscopy, three-dimensional reconstructions
Introduction
Endocytosis is a process whereby a cell internalizes exogenous materials and components of its own plasma membrane. Neurons and glia, like other cells, manifest various distinct modes of endocytosis (Ghadially, 1982; Alberts et al., 2002). One mode involves pits or caveolae pinching off from the plasma membrane to form smooth-membraned vesicles or tubules that internalize fluids and disperse nonproteinaceous material. Another mode utilizes clathrin-coated vesicles to internalize proteinaceous material. Both of these modes involve the single plasma membrane of the cell.
Endocytosis is an important mechanism in synaptic plasticity at hippocampal dendrites and spines (Blanpied et al., 2002) that is regulated by developmental state (Blanpied et al., 2003). Endocytic organelles have been quantified in developing and mature hippocampal CA1 dendrites (Cooney et al., 2002). Sorting complexes contain an aggregation of endosomal organelles including coated and smooth vesicles, tubules with and without coated budding vesicles, and multivesicular bodies. Approximately one sorting complex per 10 μm occurs primarily in the dendritic shaft. Depending on age, one or more endosomal organelles occurs in 25-36% of CA1 dendritic spines. These organelles provide ultrastructural correlates of the large “hotspots” of endocytic activity observed with confocal microscopy, whereas planar arrays of clathrin provide sites of potential endocytosis in the plasma membrane of nearly every dendritic spine (Blanpied et al., 2002, 2003).
Coated pits occur in another mode of internalization characterized by double-membrane invaginations from dendritic spines into presynaptic axons (Westrum and Blackstad, 1962; Andres, 1964; Tarrant and Routtenberg, 1977, 1979; Boyne and Tarrant, 1982; Sorra et al., 1998). This structure has been referred to as the “synaptic spinule” (Tarrant and Routtenberg, 1977, 1979) or “spinule complex” (Westrum and Blackstad, 1962). In this mode, a vesicular or vermiform evagination from the dendritic spine is engulfed by the presynaptic axon. A similar mode of intercellular transfer among other cell types has been referred to as “trans-endocytosis” (Klueg and Muskavitch, 1999; Greco et al., 2001; Marston et al., 2003). Spinules also present a zone where extracellular diffusion could be restricted (Boyne and Tarrant, 1982). Based on observations of single thin sections, synaptic spinules have been thought to split the presynaptic axonal bouton in conjunction with the splitting of the postsynaptic density (PSD) and dendritic spine (Carlin and Siekevitz, 1983; Luscher et al., 2000). This hypothesis has been rejected, because three-dimensional reconstructions show that synaptic spinules are thin projections that do not divide the axon and that dendritic spines do not split (Jones and Harris, 1995; Sorra et al., 1998; Geinisman et al., 2001; Fiala et al., 2002).
Here, we provide the first detailed quantification of spinules in synaptic neuropil of the mature hippocampus. The findings suggest that spinules may be involved in both structural remodeling and intercellular signaling between dendritic spines, presynaptic axons, neighboring nonsynaptic axons, and glia.
Materials and Methods
Analyses were performed on serial section electron micrographs obtained during previous studies from the middle of stratum radiatum in hippocampal area CA1 (series K21 and K24) (Harris et al., 1992; Spacek and Harris, 1997). Intracardiac perfusion was performed on a Long-Evans adult male rat (310 gm) under deep pentobarbital (80 mg/kg) anesthesia with fixative containing 2% paraformaldehyde, 2.5% glutaraldehyde, and 2 mm CaCl2 in 0.1 m cacodylate buffer, pH 7.35, at 37°C and 4-φ backing pressure from compressed gas (95% O2, 5% CO2). Tissue blocks were processed by standard protocols as described by Harris et al. (1992), and serial sections were obtained at 50 nm and photographed in a JEOL (Peabody, MA) electron microscope. Negatives were scanned and then the serial sections were aligned, and components in them were reconstructed in three dimensions using personal computer-based software (Fiala and Harris; http://synapses.mcg.edu/tools/index.stm). Quantitative analysis of the double-membrane structures was made from two volumes of neuropil totaling 326 μm3 from stratum radiatum of hippocampal area CA1. All procedures followed National Institutes of Health guidelines for the care and use of animals in research.
Results
Ultrastructure and three-dimensional reconstructions of spinules
Spinules originated from mushroom-shaped dendritic spines in the vicinity of synaptic perforations and at the edges of the spine (Fig. 1). Typically, spinules on mushroom-shaped spines invaginated their own presynaptic axon. Some mushroom-shaped dendritic spines were deeply penetrated by their presynaptic axons, although these structures differed from spinules in that they had adhesion junctions at their base (Fig. 1f). Spinules with their double-walled membranes were readily distinguished from endosomal compartments, which have only a single coated or smooth plasma membrane (Fig. 2). Spinules originated from the heads of thin spines and penetrated neighboring axons (Fig. 3a,b,e), perisynaptic glia (Fig. 3a-d, f), and occasionally their presynaptic axons (data not shown). The necks of thin and branched spines gave rise to both short and long spinules that invaginated neighboring nonsynaptic axons (Fig. 4). Some spinules arose from axons and invaginated into other axons (Fig. 5a,b) or neighboring astrocytic processes (Fig. 5c). Two spinules from an axon were traced to their partner and surprisingly, during reconstruction, it was found to be a growth cone (Fig. 6). The growth cone was identified by classic ultrastructural features of multiple tubules and vesicles in the lamellar region (Peters et al., 1991).
Figure 1.
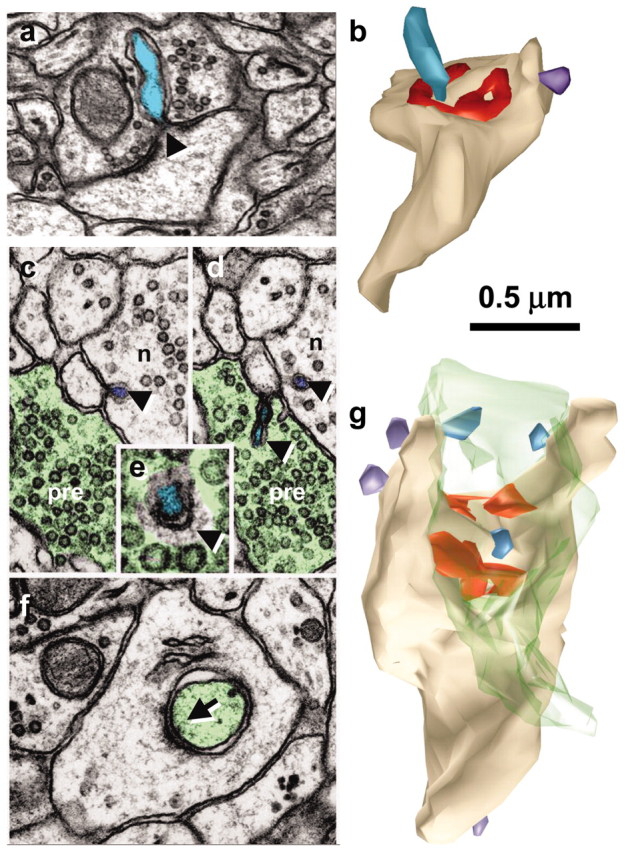
Spinules on mushroom dendritic spines. a, Micrograph through the center of a spinule (turquoise) emerging from a perforation (arrowhead) in the postsynaptic density into the presynaptic axon. b, Reconstruction of the spine illustrated in a with the spinule in turquoise and the PSD surface area in red. c, d, Serial sections through the presynaptic axon (pre; green) and a spinule (arrowhead; turquoise) emerging from the edge of a mushroom spine head into the presynaptic axon and another spinule also emerging from the edge of the spine head (arrowhead; lavender) but invaginating a neighboring axon (n). e, High magnification of serial section beyond d showing a coating along the cytoplasmic surface of the spinule on the side of the invaginated presynaptic axon (arrowhead). f, Later sections of the mushroom spine head showing where the presynaptic axon deeply invaginated the spine head in a vesicle-free zone adjacent to a cell-adhesion (arrow) that is adjacent to the postsynaptic density on subsequent serial sections. g, Three-dimensional reconstruction of the mushroom spine (beige) with perforated synapse (red) and several small spinules into the presynaptic axon (turquoise spinules) or neighboring axon (lavender spinules).
Figure 2.
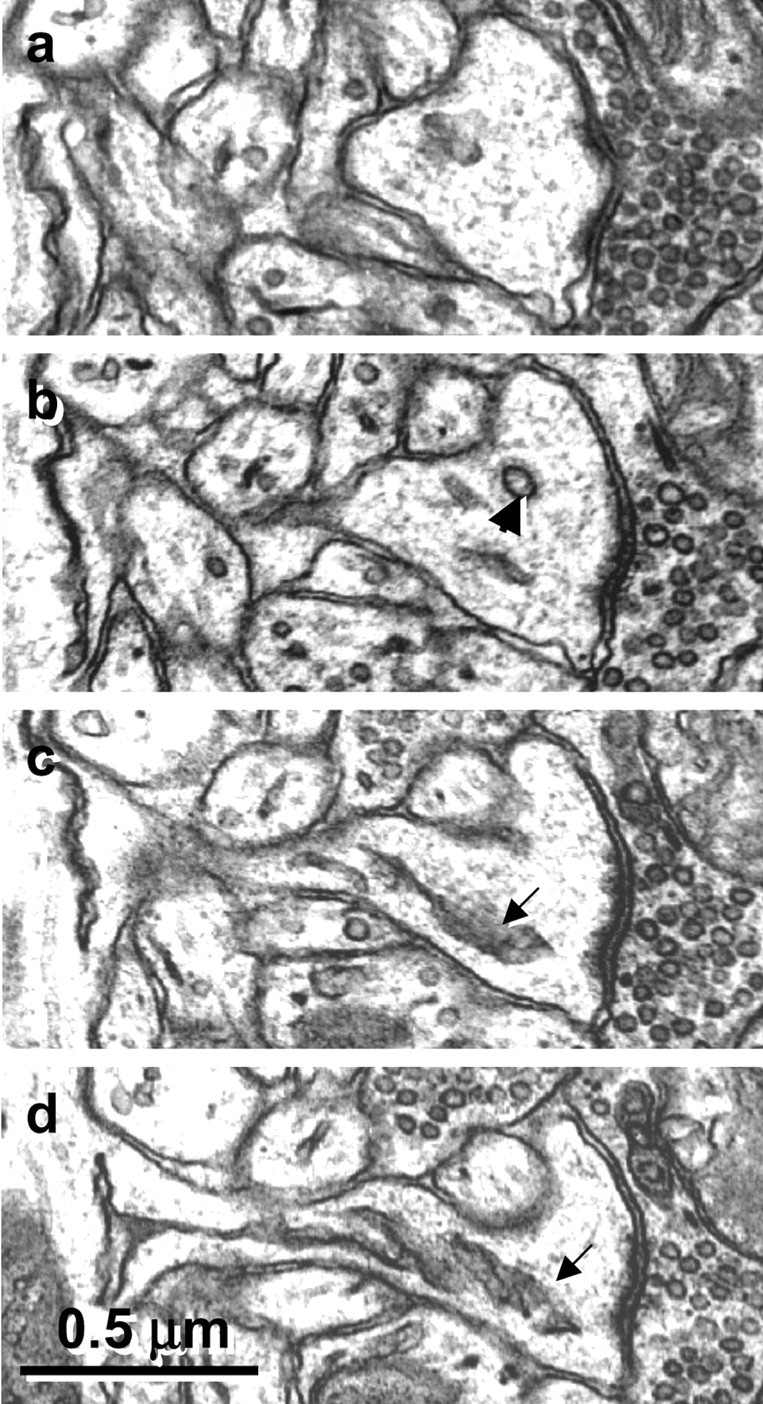
Serial sections through a mushroom spine show the clear distinction between single membrane-coated vesicles (b, arrowhead), the smooth endoplasmic reticulum of a spine apparatus (c, d, arrows), and the spinules illustrated in Figure 1.
Figure 3.
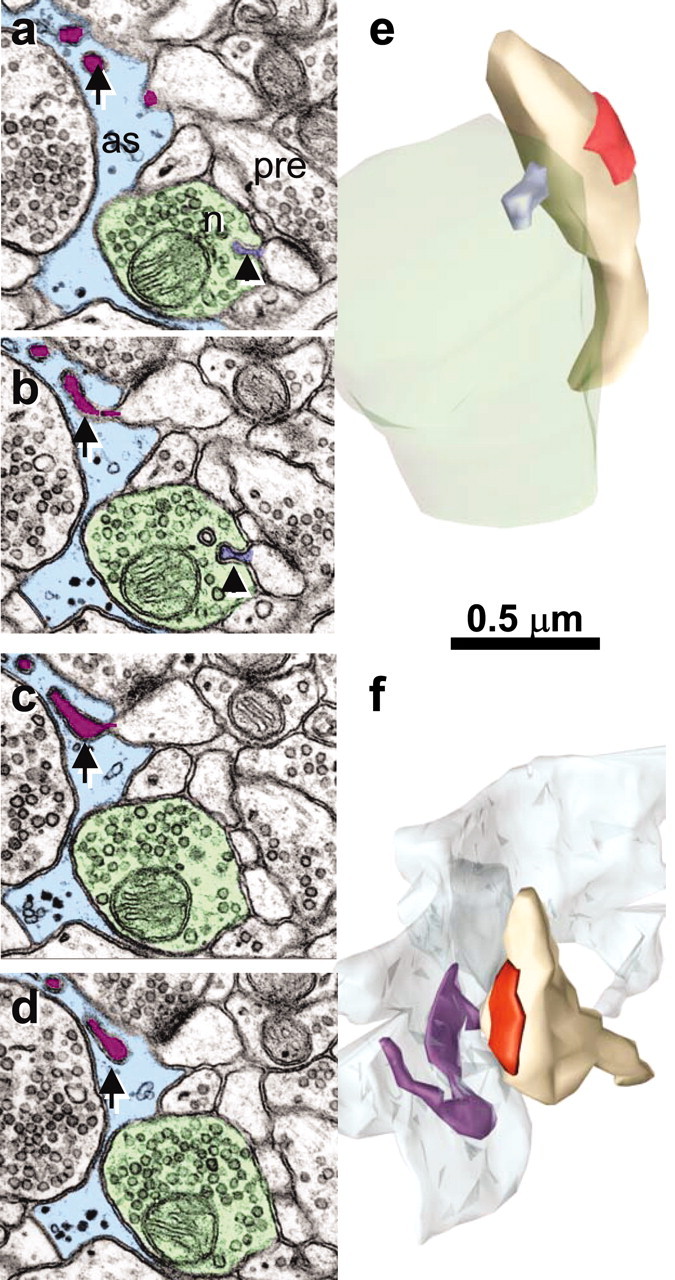
Spinules from thin spine heads into a neighboring axon or an astrocytic process. a, b, Serial sections though a small spinule (arrowhead; lavender) emerging from a thin spine head into a neighboring axon (n; green). In serial sections a-d, portions of a long spinule (arrow; purple) emerge from a thin spine head into an astrocytic process (as, light blue). e, Reconstruction of the small spinule (lavender) from the thin spine neck into the neighboring axon (green) as illustrated in a and b; postsynaptic density on the spine is red. f, Reconstruction of a thin spine with long spinule emerging from its head (purple) into an astrocytic process (light blue) as illustrated in a-d.
Figure 4.
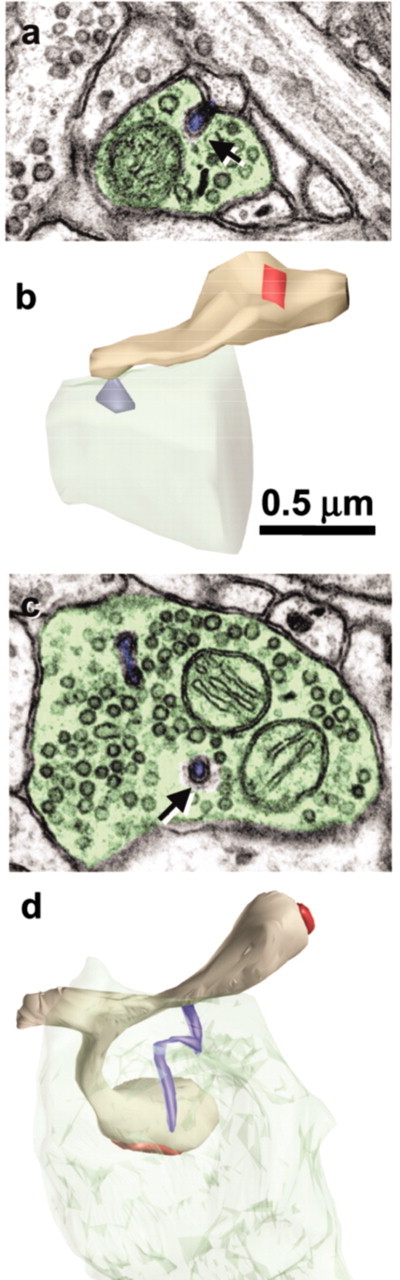
Spinules from thin and branched spine necks into neighboring axons. a, Lavender spinule (arrow) from thin spine neck into neighboring axon (green) is reconstructed in b. c, Lavender spinule with coated tip (arrow) from a branched spine neck into a neighboring axon (green) is reconstructed in d.
Figure 5.
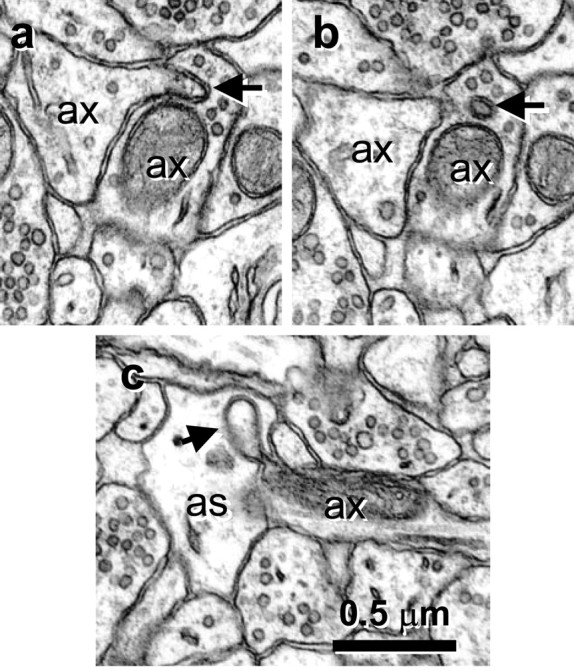
Spinules between neighboring axons and between axons and astrocytic process. a, Origin of spinule (arrow) emerging from the left axon (ax) into a neighboring axon (ax). b, Adjacent serial section with a illustrating the coat at the tip of the axonal spinule. c, Spinule emerging from an axon (ax; arrow) into an astrocyte (as).
Figure 6.
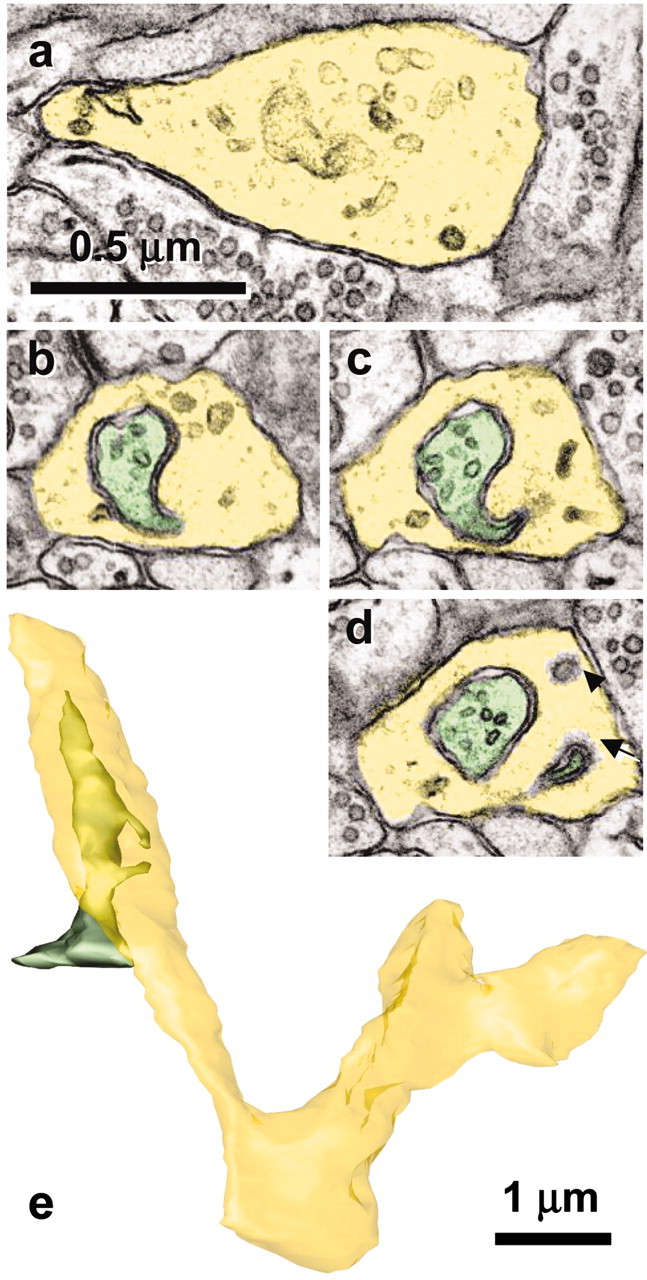
Growth cone in mature hippocampus receives spinules from an axon. a, The lamellar junction (yellow) between two branch points shows numerous tubulo-vesicular components characteristic of growth cones. b-d, Serial sections through one of the spinules emerging from an axon (green) that has a coated tip on the cytoplasmic side of the growth cone invagination (d, arrow) and that is distinguished from a single membrane-coated vesicle (d, arrowhead). e, Reconstruction of the growth cone (yellow) and axon (green) that forms spinules with it. Scale bar in a applies to a-d.
Most of the cytoplasmic surface of the invaginated membrane was smooth, but the tip was often coated (Figs. 1e, 4c), supporting the hypothesis of trans-endocytosis via spinules.
Frequency of spinules in different locations
Two volumes of hippocampal neuropil were systematically evaluated for spinules along all profiles. Series K21 had 162 spinules in 113 μm3, and series K24 had 92 spinules in 213 μm3 of neuropil for a total of 254 spinules. Overall, 86% of the spinules emerged from dendritic spines, 12% emerged from axons, three emerged from dendritic shafts, and one emerged from a nonsynaptic protrusion (Table 1). More than 90% of the spinules invaginated axons, 8% invaginated astrocytes, two invaginated the growth cone, and one invaginated a mushroom spine head (Table 1). Coats occurred on the cytoplasmic side of 69% of the invaginated axons, astrocytic processes, or growth cone (Table 1).
Table 1.
Locations of spinules in the middle of stratum radiatum of mature rat hippocampus (area CA1)
|
|
Emerging from |
Engulfed by |
Engulfing membrane coated |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Spinule |
Number |
Percentage |
Number |
Percentage |
Number |
Percentage |
|||
| Dendritic spine | 219 | 86.2% | 1 | 0.4% | 1 | 100% | |||
| Axon | 31 | 12.2% | 229 | 90.2% | 162 | 71% | |||
| Dendritic shaft | 3 | 1.2% | 0 | 0.0% | 0 | 0% | |||
| Nonsynaptic protrusion | 1 | 0.4% | 0 | 0.0% | 0 | 0% | |||
| Astrocytic process | 0 | 0.0% | 21 | 8.3% | 8 | 38% | |||
| Growth cone | 0 | 0.0% | 3 | 1.2% | 3 | 100% | |||
| Total |
254 |
100.0% |
254 |
100.0% |
174 |
69% |
|||
Spinules occurred on all types of dendritic spines. Among the 254 spinules in the volume sample, 37.4% emerged from thin spine heads, 33.3% emerged from mushroom spine heads, and 26.5% emerged from thin spine necks. A few spinules arose from mushroom spine necks (1.4%), branched spines (0.9%), or stubby spines (0.5%). Six dendritic segments were accessible for reconstruction in each series for a total of 12 dendrites and 139 complete dendritic spines (Table 2). All of these were reconstructed to determine what percentage of dendritic spines has spinules, to specify further the structures that engulf spinules, and to measure the spinules. Spinules emerged from 45 (32%) of these spines, with a maximum of six spinules per spine, for a total of 63 spinules. Among these 24% of thin spines, 91% the mushroom spines, two of the six heads on three branched spines, and one of the four stubby spines had spinules (Table 2). Spinules on spines involved five locations: emerging from a spine head into a presynaptic axon, from a spine head or neck into a neighboring nonsynaptic axon, and from a spine head or neck into an astrocytic process. The distribution of spinules differed markedly between thin and mushroom spines. Of the 35 spinules emerging from thin spines, only 17% were with the presynaptic axon; the other 83% were with the neighboring axonal boutons or astrocytic processes. In contrast, 84% of the spinules on mushroom spines were with their presynaptic axons, 16% were with neighboring axons, and none were observed with astrocytic processes (Table 2).
Table 2.
Spinules on reconstructed dendritic spines
|
|
Total |
Thin |
Mushroom |
Branched |
Stubby |
|---|---|---|---|---|---|
| Reconstructed spines | 139 | 121 | 11 | 3 | 4 |
| Number of spines with spinules | 45 | 30 | 10 | 2 | 1 |
| Maximum number of spinules per spine | 6 | 3 | 6 | 1 | 1 |
| Total number of spinules | 63 | 35 | 25 | 2 | 1 |
| Spinule locations | |||||
| Spine head into presynaptic axon | 17.1% | 84.0% | |||
| Spine head into neighboring axon | 31.4% | 8.0% | 50.0% | ||
| Spine neck into neighboring axon | 37.1% | 8.0% | |||
| Spine head into astrocytic process | 11.4% | 50.0% | 100.0% | ||
| Spine neck into astrocytic process |
|
2.9% |
|
|
|
Note that three branched spines had two branches each.
Spinule diameters ranged from a nearly complete constriction (<8 nm) to 80 nm at the base and from 27 to 150 nm along their length. The lengths of spinules emerging from dendritic spines ranged from 33 to 765 nm. There were also 31 complete spinules emerging from axons that ranged in length from 40 to 240 nm.
The 71 spinules located on mushroom spine heads in the volume samples were analyzed with respect to perforations in the postsynaptic density (Table 3). Of these, ∼40% were in the center of a perforation (Fig. 1a,b). In addition, 32% were located at the edge of the postsynaptic density. Only 17% emerged from the spine head into the presynaptic axon distant from the postsynaptic density. Neighboring axons accounted for <10% of the spinule partners on mushroom spines.
Table 3.
Spinule locations relative to perforations in the PSD on mushroom spines
|
Spinule locations |
Number |
Percentage |
|---|---|---|
| Total at perforated synapses | 71 | |
| Center of the perforation into presynaptic axon | 29 | 40.8% |
| At the edge of the PSD into presynaptic axon | 23 | 32.4% |
| Distant from the PSD into the presynaptic axon | 12 | 16.9% |
| Into a neighboring axon |
7 |
9.9% |
All of the spinules in the volume of series K21 are illustrated in Figure 7. Spinules emerging from spines on a dendritic segment passing through the volume are illustrated as royal blue (Fig. 7) on the beige dendrite reconstruction and on an enlarged spine. All of the other spinules in the volume are represented schematically as small light blue spheres (Fig. 7).
Figure 7.
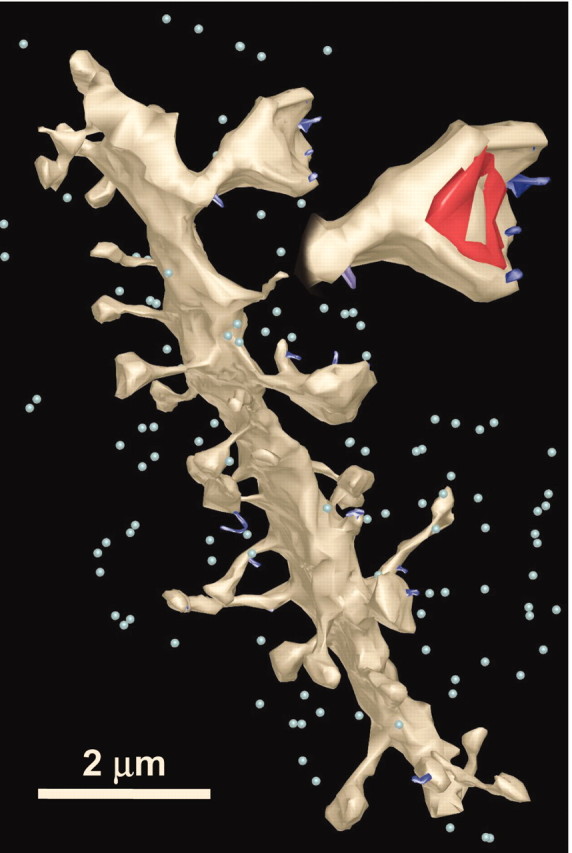
Reconstructed locations of spinules (dark blue protrusions) on a dendrite and enlarged spine. Other spinules in the surrounding neuropil are marked with light blue spheres to give a visual guide for their relative densities.
Free double-walled vesicles
The shape of the spinules ranged from double-membrane pits to longer vermiform double-membrane structures. The relatively constricted base gave the appearance that the invaginated structure was about to “engulf” or “pinch off” the evaginating structure. Occasionally, free double-walled structures were found in axons, in support of the hypothesis that spinules are undergoing trans-endocytosis (Fig. 8) [Sorra et al. (1998), their Fig. 10d].
Figure 8.
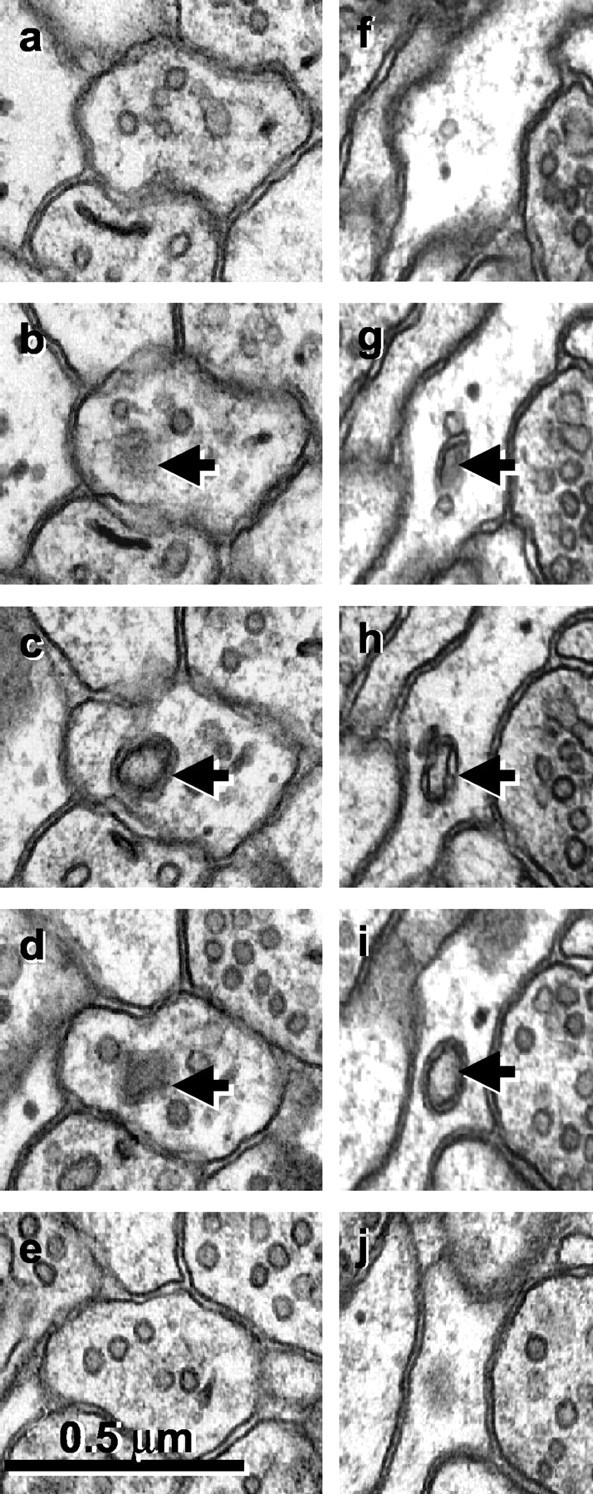
a-j, Serial sections through free double-walled vesicles (arrows) in an axonal bouton (a-e) and an astrocytic process (f-j). Sections at the beginning and end of each series illustrate that these apparent spinules are not connected to neighboring structures but are free within the cytoplasm of the axon or astrocyte.
Discussion
Spinules occurred in the mature hippocampus on all parts of dendritic spines in conjunction with their presynaptic axons, neighboring axons, and perisynaptic astrocytic glia. Most of the spinules were between dendritic spines and axons or glia. Axons also formed spinules with axons and glia. Few spinules arose from dendritic shafts or a nonsynaptic dendritic protrusion. Three-dimensional reconstructions and quantitative analyses demonstrated some spinules to be double-walled vesicles or tubules, whereas others were longer vermiform projections. The term trans-endocytosis is apt in light of coated pits on most of the structures engulfing spinules. These findings provide evidence for substantial exchange of cytoplasmic and membrane materials among neurons and with glia. Whether the emerging or engulfing structures initiate spinule formation remains to be determined and may differ depending on their location.
Of particular interest were the distinct locations of spinules on mushroom and thin spines. Most of the spinules on mushroom spines were engulfed by their presynaptic axons. These spinules may remodel mushroom spines or provide a retrograde signaling mechanism. In contrast, most of the spinules emerging from thin spines were engulfed by neighboring axons and glia. These observations suggest that thin spines have a less exclusive relationship with their presynaptic axons. Perhaps the membrane of thin spines remains permissive to competition between different axons for synaptic sites.
Structural remodeling and retrograde signaling at mushroom spines via spinules
Spinules between mushroom spine heads and their presynaptic axons usually occurred in the perforation or at the edge of the postsynaptic density. The number of postsynaptic perforations and spinules increases on mushroom spines after repeated frequency facilitation in the hippocampus (Applegate and Landfield, 1988), during intense stimulation with potassium chloride of the frog forebrain (Van Harreveld and Trubatch, 1975), and after tetanic or theta burst stimulation that induces long-term potentiation (LTP) (Schuster et al., 1990; Toni et al., 1999). Such perforations are thought to mark especially effective synapses (Greenough et al., 1978; Edwards, 1995; Geinisman et al., 1995; Jones and Harris, 1995). It has been a mystery how perforations form in the postsynaptic density. Cell adhesion molecules span the synaptic junction with links to the underlying actin cytoskeleton. Recent evidence shows that the influx of calcium during repeated activation might cause the underlying actin cytoskeleton to “contract” (Matus et al., 2000), which might cause perforation and segmentation of the postsynaptic density. The cell adhesion molecules disassemble during LTP, which may weaken the links and allow perforations to form (Tang et al., 1998; Murase et al., 2002). Alternatively, the addition of new material to enlarge the PSD of synapses during LTP might initially be a chaotic process involving vesicular insertion of preassembled molecular complexes. Insertion might leave gaps between the old and new portions of the synapse.
The postsynaptic perforations and spinules are transient, lasting <1 hr after induction of LTP (Toni et al., 1999). Postsynaptic perforations persist if presynaptic endocytosis is blocked, suggesting their removal is synchronized with presynaptic endocytosis (Shupliakov et al., 1997). The coats at the invaginated tips of spinules indicate that the presynaptic axon is actively engaged in removing part of the spine membrane. Proteins associated with the engulfed spine membrane may provide retrograde signaling similar to that which occurs during trans-endocytosis in other systems including Notch (Klueg and Muskavitch, 1999; Parks et al., 2000), ephrins (Marston et al., 2003; Zimmer et al., 2003), and Wingless (Greco et al., 2001). As the spinules are trans-endocytosed, pieces of the postsynaptic density may slide back together (Fig. 9a).
Figure 9.
Models of spinule functions. a, Process whereby trans-endocytosis of spinules removes excess presynaptic and postsynaptic plasma membrane after substantial activation results in transient perforated or segmented synapses on mushroom spines. This process could also provide retrograde signaling. b, Neighboring axons vying for synapses via spinules on the necks and heads of thin spines. c, Intercellular signaling between thin spines and perisynaptic glia via spinules.
What might be the source of postsynaptic membrane in the spinule? Large mushroom spines contain vesicles, tubules, smooth endoplasmic reticulum, and a laminated membranous spine apparatus, each a potential source of membrane (Spacek and Harris, 1997; Cooney et al., 2002). During LTP, vesicular exocytosis inserts new receptors into the spine adding the vesicular membrane (Shi et al., 1999, 2001; Malinow and Malenka, 2002). Postsynaptic receptors move freely in the neuronal plasma membrane but slow down or stop at the postsynaptic density (Choquet and Triller, 2003). Perforations may open new slots for glutamatergic receptors, and attachment of scaffolding proteins would stabilize them, fill in the perforation, and enlarge the postsynaptic density (Harris et al., 2003). Thus, perforations appear after activation of large synapses and trans-endocytosis via spinules or addition of synaptic signaling, or scaffolding proteins could reassemble the postsynaptic density.
Competition among axons for synaptic sites and signaling via spinules on thin spines
Spinules might also mediate competition between neighboring axons for synapses especially on thin spines (Fig. 9b). This competition could be similar to that occurring between neighboring axons at the developing neuromuscular junction (Buffelli et al., 2003) or cortical synapses (Katz and Shatz, 1996). Double-membrane-coated structures occur more frequently in developing than adult cerebellum (Eckenhoff and Pysh, 1979). If spinules were randomly located on any available membrane surface, the larger mushroom spines should form spinules more often with neighboring axons than the smaller thin spines, but the converse is true. The larger synapses on mushroom spines might instead be functionally consolidated and exclude or at least discourage competition from other axons. These findings are consistent with the hypothesis that thin spines are less mature and less active as also evidenced by their lower density of AMPA-like glutamatergic receptors (Takumi et al., 1999; Racca et al., 2000). Thus, spinules with neighboring axons might reflect an ongoing process of competition between axons for immature synaptic sites in the mature brain.
Spinules and perisynaptic glia
Astrocytes have long been known to phagocytose degenerating axonal boutons (Ronnevi, 1978; Deller et al., 1997; Seil, 1997) or whole apoptotic neurons (Freeman et al., 2003). Recent studies show that astrocytes are also crucial for maintaining newly formed synapses on developing neurons in culture (Barres, 1991; Pfrieger and Barres, 1997; Drake-Baumann and Seil, 1999; Ullian et al., 2001). The form of trans-endocytosis or microphagocytosis taking place via spinules does not resemble traditional astrocytic phagocytosis of degenerating or apoptotic neurons, which have very dark and condensed cytoplasm. Instead, the relationship occurs in a healthy mature brain on spines that have clear cytoplasm and well defined postsynaptic densities and are associated with healthy axonal boutons filled with vesicles. The spinules are thin and vermiform and often have a coat and are similar to those interacting with axons (Fig. 9c). These spinules provide new evidence for direct communication between spines and perisynaptic glia. They could present important signals to the astrocyte or reflect an ongoing role for perisynaptic glia to support newly formed synapses especially on thin spines.
Trans-endocytosis via spinule-like processes elsewhere in the nervous system
Spinule-like structures between growth cones of axons induce coated pits, suggesting that trans-endocytosis may be an important process during growth-cone-mediated axon guidance (Bastiani and Goodman, 1984). Double-walled vesicles similar to spinules also occur frequently at other CNS neurons during development (Eckenhoff and Pysh, 1979; Altman, 1993) and are thought to be an important step in information exchange during synaptogenesis (Vaughn, 1989). We unexpectedly identified one growth cone, to our knowledge for the first time, in the mature brain. It received spinules from an axon, and its ultrastructural appearance favors that of a dendritic growth cone (compare Fig. 3.12 of Peters et al., 1991). It seems unlikely that this growth cone arose from a newly formed neuron, because little or no neurogenesis has been reported in hippocampal area CA1 of the mature rat brain (Rietze et al., 2000; Gage, 2002; Song et al., 2002). The spinules on horizontal cell terminals of cone pedicles in fish retinas that cycle during the light-dark periods all have tiny synapses on their heads (Wagner, 1980) and, hence, are more like small thin spines or filopodia than spinules. Spinules like those reported here have been observed elsewhere in the brain in dentate gyrus of the hippocampus (Tarrant and Routtenberg, 1977, 1979; Boyne and Tarrant, 1982), on the claw endings of cerebellar granule cells (Eccles et al., 1967), on perforated synapses in frontal cortex (Cadete-Leite et al., 1986), in sensorimotor cortex (Bozhilova-Pastirova and Ovtscharoff, 1999), and on CA3 thorny excrescences in both rat (Chicurel and Harris, 1992) and human brain (Zhang and Houser, 1999). These observations suggest that spinules provide a general mechanism for signaling and remodeling throughout the brain.
Conclusions
Differences in the locations, sizes, and frequency of spinules will provide new insights into the relative states of synaptic plasticity. Our findings are the first to distinguish spinule locations on mushroom and thin spines, and they increase awareness of possible differences in the functional states of these spines. Future quantitative analysis of the distinctions between structures involved in spinules will clarify whether particular spinules are involved in competition for synaptic sites, remodeling of consolidated synapses, or shaping of interactions between spines and their perisynaptic glia during plasticity in the immature and mature brain.
Footnotes
This work was supported by National Institute of Neurological Disorders and Stroke (NINDS)-National Institutes of Health (NIH) Grants NS21184 and NS33574 and by National Institute of Biomedical Imaging and Bioengineering-NIH-National Institute on Aging-NINDS Grant EB002170. We thank Ben Barres, Frances Edwards, Fred Gage, Corey Goodman, and the anonymous reviewers for helpful comments on this manuscript.
Correspondence should be addressed to Kristen M. Harris, Medical College of Georgia, Institute of Molecular Medicine and Genetics, 1120 15th Street, CB-2803, Augusta, GA 30912-2630. E-mail: kharris@mcg.edu.
Copyright © 2004 Society for Neuroscience 0270-6474/04/244233-09$15.00/0
References
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002) Molecular biology of the cell, Ed 4, pp 742-757. New York: Garland.
- Altman J (1993) Postnatal development of the cerebellar cortex in the rat II. Phases in the maturation of Purkinje cells and of the molecular layer. J Comp Neurol 145: 399-464. [DOI] [PubMed] [Google Scholar]
- Andres K (1964) Mikropinozytose im zentralnervensystem. In: Zietschrift four zellforschung und mikroskopische anatomie (Zellforsch Z, ed), pp 63-73. Vienna: Springer. [PubMed]
- Applegate MD, Landfield PW (1988) Synaptic vesicle redistribution during hippocampal frequency potentiation and depression in young and aged rats. J Neurosci 8: 1096-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA (1991) New roles for glia. J Neurosci 11: 3685-3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiani MJ, Goodman CS (1984) Neuronal growth cones: specific interactions mediated by filopodial insertion and induction of coated vesicles. Proc Natl Acad Sci USA 81: 1849-1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpied TA, Scott DB, Ehlers MD (2002) Dynamics and regulation of clathrin coats at specialized endocytic zones of dendrites and spines. Neuron 36: 435-449. [DOI] [PubMed] [Google Scholar]
- Blanpied TA, Scott DB, Ehlers MD (2003) Age-related regulation of dendritic endocytosis associated with altered clathrin dynamics. Neurobiol Aging 24: 1095-1104. [DOI] [PubMed] [Google Scholar]
- Boyne AF, Tarrant SB (1982) Pseudopodial interdigitations between abutted nerve terminals: diffusion traps which occur in several nuclei of the rat limbic system. J Neurosci 2: 463-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozhilova-Pastirova A, Ovtscharoff W (1999) Intramembranous structure of synaptic membranes with special reference to spinules in the rat sensorimotor cortex. Eur J Neurosci 11: 1843-1846. [DOI] [PubMed] [Google Scholar]
- Buffelli M, Burgess RW, Feng G, Lobe CG, Lichtman JW, Sanes JR (2003) Genetic evidence that relative synaptic efficacy biases the outcome of synaptic competition. Nature 424: 430-434. [DOI] [PubMed] [Google Scholar]
- Cadete-Leite A, Tavares MA, Paula-Barbosa MM, Gray EG (1986) “Perforated” synapses in frontal cortex of chronic alcohol-fed rats. J Submicrosc Cytol 18: 495-499. [PubMed] [Google Scholar]
- Carlin RK, Siekevitz P (1983) Plasticity in the central nervous system: do synapses divide? Proc Natl Acad Sci USA 80: 3517-3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicurel ME, Harris KM (1992) Three-dimensional analysis of the structure and composition of CA3 branched dendritic spines and their synaptic relationships with mossy fiber boutons in the rat hippocampus. J Comp Neurol 325: 169-182. [DOI] [PubMed] [Google Scholar]
- Choquet D, Triller A (2003) The role of receptor diffusion in the organization of the postsynaptic membrane. Nat Rev Neurosci 4: 251-265. [DOI] [PubMed] [Google Scholar]
- Cooney JR, Hurlburt JL, Selig DK, Harris KM, Fiala JC (2002) Endosomal compartments serve multiple hippocampal dendritic spines from a widespread rather than a local store of recycling membrane. J Neurosci 22: 2215-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deller T, Haas CA, Naumann T, Joester A, Faissner A, Frotscher M (1997) Up-regulation of astrocyte-derived tenascin-C correlates with neurite outgrowth in the rat dentate gyrus after unilateral entorhinal cortex lesion. Neuroscience 81: 829-846. [DOI] [PubMed] [Google Scholar]
- Drake-Baumann R, Seil FJ (1999) Influence of functional glia on the electrophysiology of Purkinje cells in organotypic cerebellar cultures. Neuroscience 88: 507-519. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Ito MSJ, Szentágothai J (1967) The cerebellum as a neuronal machine, pp 127-130. NY: Springer.
- Eckenhoff MF, Pysh JJ (1979) Double-walled coated vesicle formation: evidence for massive and transient conjugate internalization of plasma membranes during cerebellar development. J Neurocytol 8: 623-638. [DOI] [PubMed] [Google Scholar]
- Edwards FA (1995) LTP-a structural model to explain the inconsistencies. Trends Neurosci 18: 250-255. [DOI] [PubMed] [Google Scholar]
- Fiala JC, Allwardt B, Harris KM (2002) Dendritic spines do not split during hippocampal LTP or maturation. Nat Neurosci 5: 297-298. [DOI] [PubMed] [Google Scholar]
- Freeman MR, Delrow J, Kim J, Johnson E, Doe CQ (2003) Unwrapping glial biology: Gcm target genes regulating glial development, diversification, and function. Neuron 38: 567-580. [DOI] [PubMed] [Google Scholar]
- Gage FH (2002) Neurogenesis in the adult brain. J Neurosci 22: 612-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geinisman Y, DeToledo-Morrell L, Morrell F, Heller RE (1995) Hippocampal markers of age-related memory dysfunction: behavioral, electrophysiological and morphological perspectives. Prog Neurobiol 45: 223-252. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, Berry RW, Disterhoft JF, Power JM, van der Zee EA (2001) Associative learning elicits the formation of multiple-synapse boutons. J Neurosci 21: 5568-5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghadially FN (1982) Ultrastructural pathology of the cell, pp 1207-1293. London: Butterwords.
- Greco V, Hannus M, Eaton S (2001) Argosomes: a potential vehicle for the spread of morphogens through epithelia. Cell 106: 633-645. [DOI] [PubMed] [Google Scholar]
- Greenough WT, West RW, De Voogd TJ (1978) Subsynaptic plate perforations: changes with age and experience in the rat. Science 202: 1096-1098. [DOI] [PubMed] [Google Scholar]
- Harris KM, Jensen FE, Tsao B (1992) Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. J Neurosci 12: 2685-2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Fiala JC, Ostroff L (2003) Structural changes at dendritic spine synapses during long-term potentiation. Philos Trans R Soc Lond B Biol Sci 358: 745-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DG, Harris RJ (1995) An analysis of contemporary morphological concepts of synaptic remodelling in the CNS: perforated synapses revisited. Rev Neurosci 6: 177-219. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ (1996) Synaptic activity and the construction of cortical circuits. Science 274: 1133-1138. [DOI] [PubMed] [Google Scholar]
- Klueg KM, Muskavitch MA (1999) Ligand-receptor interactions and trans-endocytosis of Delta, Serrate and Notch: members of the Notch signalling pathway in Drosophila J Cell Sci 112: 3289-3297. [DOI] [PubMed] [Google Scholar]
- Luscher C, Nicoll RA, Malenka RC, Muller D (2000) Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nat Neurosci 3: 545-550. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC (2002) AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci 25: 103-126. [DOI] [PubMed] [Google Scholar]
- Marston DJ, Dickinson S, Nobes CD (2003) Race-dependent trans-endocytosis of ephrinBs regulates Eph-ephrin contact repulsion. Nat Cell Biol 5: 879-888. [DOI] [PubMed] [Google Scholar]
- Matus A, Brinkhaus H, Wagner U (2000) Actin dynamics in dendritic spines: a form of regulated plasticity at excitatory synapses. Hippocampus 10: 555-560. [DOI] [PubMed] [Google Scholar]
- Murase S, Mosser E, Schuman EM (2002) Depolarization drives beta-Catenin into neuronal spines promoting changes in synaptic structure and function. Neuron 35: 91-105. [DOI] [PubMed] [Google Scholar]
- Parks AL, Klueg KM, Stout JR, Muskavitch MA (2000) Ligand endocytosis drives receptor dissociation and activation in the Notch pathway. Development 127: 1373-1385. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster HD (1991) The fine structure of the nervous system: the neurons and supporting cells, pp 98-100. Philadelphia: Saunders.
- Pfrieger FW, Barres BA (1997) Synaptic efficacy enhanced by glial cells in vitro Science 277: 1684-1687. [DOI] [PubMed] [Google Scholar]
- Racca C, Stephenson FA, Streit P, Roberts JD, Somogyi P (2000) NMDA receptor content of synapses in stratum radiatum of the hippocampal CA1 area. J Neurosci 20: 2512-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietze R, Poulin P, Weiss S (2000) Mitotically active cells that generate neurons and astrocytes are present in multiple regions of the adult mouse hippocampus. J Comp Neurol 424: 397-408. [PubMed] [Google Scholar]
- Ronnevi LO (1978) Origin of the glial processes responsible for the spontaneous postnatal phagocytosis of boutons on cat spinal motoneurons. Cell Tissue Res 189: 203-217. [DOI] [PubMed] [Google Scholar]
- Schuster T, Krug M, Wenzel J (1990) Spinules in axospinous synapses of the rat dentate gyrus: changes in density following long-term potentiation. Brain Res 523: 171-174. [DOI] [PubMed] [Google Scholar]
- Seil FJ (1997) Serial changes in granuloprival cerebellar cultures after transplantation with granule cells and glia: a timed ultrastructural study. Neuroscience 77: 695-711. [DOI] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Esteban JA, Malinow R (2001) Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell 105: 331-343. [DOI] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R (1999) Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science 284: 1811-1816. [DOI] [PubMed] [Google Scholar]
- Shupliakov O, Low P, Grabs D, Gad H, Chen H, David C, Takei K, De Camilli P, Brodin L (1997) Synaptic vesicle endocytosis impaired by disruption of dynamin-SH3 domain interactions. Science 276: 259-263. [DOI] [PubMed] [Google Scholar]
- Song HJ, Stevens CF, Gage FH (2002) Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nat Neurosci 5: 438-445. [DOI] [PubMed] [Google Scholar]
- Sorra KE, Fiala JC, Harris KM (1998) Critical assessment of the involvement of perforations, spinules, and spine branching in hippocampal synapse formation. J Comp Neurol 398: 225-240. [PubMed] [Google Scholar]
- Spacek J, Harris KM (1997) Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendritic spines of the immature and mature rat. J Neurosci 17: 190-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takumi Y, Ramirez-Leon V, Laake P, Rinvik E, Ottersen OP (1999) Different modes of expression of AMPA and NMDA receptors in hippocampal synapses. Nat Neurosci 2: 618-624. [DOI] [PubMed] [Google Scholar]
- Tang L, Hung CP, Schuman EM (1998) A role for the cadherin family of cell adhesion molecules in hippocampal long-term potentiation. Neuron 20: 1165-1175. [DOI] [PubMed] [Google Scholar]
- Tarrant SB, Routtenberg A (1977) The synaptic spinule in the dendritic spine: electron microscopic study of the hippocampal dentate gyrus. Tissue Cell 9: 461-473. [DOI] [PubMed] [Google Scholar]
- Tarrant SB, Routtenberg A (1979) Postsynaptic membrane and spine apparatus: proximity in dendritic spines. Neurosci Lett 11: 289-294. [DOI] [PubMed] [Google Scholar]
- Toni N, Buchs PA, Nikonenko I, Bron CR, Muller D (1999) LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature 402: 421-425. [DOI] [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Christopherson KS, Barres BA (2001) Control of synapse number by glia. Science 291: 657-661. [DOI] [PubMed] [Google Scholar]
- Van Harreveld A, Trubatch J (1975) Synaptic changes in frog brain after stimulation with potassium chloride. J Neurocytol 4: 33-46. [DOI] [PubMed] [Google Scholar]
- Vaughn JE (1989) Fine structure of synaptogenesis in vertebrate central nervous system. Synapse 3: 255-285. [DOI] [PubMed] [Google Scholar]
- Wagner H-J (1980) Light-dependent plasticity of the morphology of horizontal cell terminals in cone pedicles of fish retinas. J Neurocytol 9: 573-590. [DOI] [PubMed] [Google Scholar]
- Westrum LE, Blackstad T (1962) An electron microscopic study of the stratum radiatum of the rat hippocampus (regio superior, CA1) with particular emphasis on synaptology. J Comp Neurol 119: 281-309. [DOI] [PubMed] [Google Scholar]
- Zhang N, Houser CR (1999) Ultrastructural localization of dynorphin in the dentate gyrus in human temporal lobe epilepsy: a study of reorganized mossy fiber synapses. J Comp Neurol 405: 472-490. [PubMed] [Google Scholar]
- Zimmer M, Palmer A, Kohler J, Klein R (2003) EphB-ephrinB bidirectional endocytosis terminates adhesion allowing contact mediated repulsion. Nat Cell Biol 5: 869-878. [DOI] [PubMed] [Google Scholar]