Abstract
We used the inositol 1,4,5-trisphosphate (IP3) biosensor, the pleckstrin homology (PH) domain of PLCδ1 (phospholipase C) tagged with enhanced green fluorescent protein (eGFP-PHPLCδ), to examine muscarinic acetylcholine (mACh) receptor regulation of phospholipase C/IP3 signaling in intact single hippocampal neurons in “real time.” Initial experiments produced a pharmacological profile consistent with the presence of a predominant M1 mACh receptor population coupled to the IP3 response. To investigate M1 mACh receptor regulation, neurons were stimulated with approximate EC50 concentrations of the mACh receptor agonist methacholine before (R1) and after (R2) a short (60 sec) exposure to a high concentration of agonist. This resulted in a marked attenuation in the R2 relative to R1 response. Inhibition of endogenous GRK6 (G-protein-coupled receptor kinase) activity, by the introduction of catalytically inactive K215RGRK6, partially reversed the attenuation of agonist-induced responsiveness, whereas overexpression of wild-type GRK6 increased receptor desensitization. Manipulation of endogenous GRK2 activity through introduction of either wild-type or catalytically inactive GRK2 (K220RGRK2) almost completely inhibited agonist-stimulated IP3 production, implying a phosphorylation-independent regulation of M1 mACh receptor signaling, most probably mediated by a GRK2 N-terminal RGS-like (regulator of G-protein signaling) domain interaction with GTP-bound Gαq/11. Together, our data suggest a role for both phosphorylation-dependent and -independent regulation of M1 mACh receptors in hippocampal neurons.
Keywords: G-protein-coupled receptor kinase; GRK; GRK2; GRK6; M1 muscarinic acetylcholine receptor; hippocampal neurons; inositol 1,4,5-trisphosphate; IP3 biosensor; confocal imaging
Introduction
Neuronal G-protein-coupled receptors (GPCRs) play important roles in many aspects of brain function. In addition to modulating neurotransmission at CNS synapses, GPCRs are also instrumental in different forms of synaptic plasticity and regulate processes such as gene transcription (Berridge, 1998; Greengard, 2001; Katz and Clemens, 2001). A fundamental property of GPCRs is an ability to adapt to different patterns of stimulation. Prolonged or recurrent activation of GPCRs results in a consequent attenuation of signaling. Classically, this type of GPCR regulation is initiated by phosphorylation of the receptor by agonist-dependent (homologous) or -independent (heterologous) mechanisms (Hausdorff et al., 1990; Ferguson, 2001), brought about by GRKs (G-protein-coupled receptor kinases) and second-messenger-regulated kinases, respectively. Receptor phosphorylation may attenuate signaling per se and/or may facilitate recruitment of arrestin proteins (Krupnick and Benovic, 1998). The phosphorylated receptor-arrestin complex can be internalized and may also act as an adaptor scaffold to recruit other signaling pathways (Ferguson, 2001).
GRKs and second-messenger-regulated kinases are expressed at high levels in neurons (Arriza et al., 1992; Erdtmann-Vourliotis et al., 2001; Grange-Midroit et al., 2002); however, we still know relatively little about whether GPCRs, endogenously expressed in neurons, are regulated by GRK-dependent mechanisms. McConalogue et al. (1998) used an immunocytochemical approach to provide evidence for a GRK2/3-dependent desensitization-internalization of NK1 neurokinin receptors in guinea pig myenteric neurons, whereas Kouznetsova et al. (2002) have reported that CB1 cannabinoid receptor desensitization can be attenuated by the expression of a dominant-negative GRK2 mutant in rat hippocampal neurons. Evidence for the involvement of GRK4, GRK5, and GRK6 in neuronal GPCR regulation has also been presented. Thus, De Blasi and colleagues have made a strong case for GRK4 involvement in the desensitization of type 1 metabotropic glutamate receptors in Purkinje cells (Sallese et al., 2000; Iacovelli et al., 2003), and targeted GRK5 (Gainetdinov et al., 1999) and GRK6 (Gainetdinov et al., 2003) gene knock-out by homologous recombination has provided evidence for an involvement of these GRKs in muscarinic acetylcholine (mACh) receptor and dopamine receptor regulation in vivo, respectively.
There is substantial evidence for an M1 mACh receptor population in the hippocampus and in particular a potential role of this GPCR subtype in mechanisms underlying longer-term regulation of synaptic function. In the present study, we examined potential roles of GRK2, GRK5, and GRK6 in the regulation of M1 mACh receptor signaling in single hippocampal neurons using an IP3 biosensor imaging approach (Stauffer et al., 1998; Nahorski et al., 2003). Our data reveal a potential role for both phosphorylation-dependent and -independent regulation of mACh receptor signaling on the basis of manipulations of GRK2 and GRK6 activities through the introduction of wild-type or dominant-negative GRK mutants.
Materials and Methods
Cell culture and transfections. Hippocampal neurons from 1-d-old Lister hooded rat pups were isolated as described previously (Schell et al., 2001). Briefly, isolated hippocampi were dissociated with Pronase E (0.5 mg/ml) and thermolysin (0.5 mg/ml) in HBSS (in mm: 130 NaCl, 10 HEPES, 5.4 KCl, 1.0 MgSO4, 25 glucose, and 1.8 CaCl2, pH 7.2) for 30 min. Tissue fragments were further dissociated by trituration in HBSS containing DNase I (40 μg/ml). After centrifugation and further trituration, cells were plated onto poly-d-lysine (50 μg/ml)-treated 25 mm glass coverslips. For the first 72 hr, cells were cultured in Neurobasal medium (Invitrogen, Paisley, UK), supplemented with B27 and 10% fetal calf serum. Cytosine arabinoside (5 μm) was added after 24 hr, and, after 72 hr, cells were transferred to serum-free medium. Cultured cells were transfected on day 5 with a 3:1 ratio of either vector control or GRK constructs, to eGFP-PHPLCδ, respectively using the Lipofectamine 2000 reagent (Invitrogen) according to the instructions of the manufacturer.
Measurement of IP3 in single cells and assessment of mACh receptor desensitization. Translocation of eGFP-PHPLCδ [the pleckstrin homology (PH) domain of PLCδ1 (phospholipase C) tagged with enhanced green fluorescent protein (eGFP)] was visualized using an Olympus Optical (Europa, UK) FV500 scanning laser confocal IX70 inverted microscope. Cells were incubated at 37°C using a temperature controller and microincubator (PDMI-2 and TC202A; Burleigh Instruments) and perfused at 5 ml/min with Krebs' buffer (in mm: 119 NaCl, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 4.2 NaHCO3, 10 HEPES, 11.7 glucose, and 1.3 CaCl2, pH 7.4). Images were captured using an oil immersion 100× objective and 4.5× optical zoom. Cytosolic IP3 levels were measured as the relative change in fluorescence detected in an area of interest as described previously (Nash et al., 2002). This approach detects IP3 accumulation rather than phosphatidylinositol 4,5-bisphosphate (PIP2) depletion, because selective removal of IP3 (by coexpression of the enzyme IP3 3-kinase) completely prevents translocation of eGFP-PHPLCδ (Nash et al., 2002; Nahorski et al., 2003). Drugs were applied via perfusion lines. Desensitization of the mACh receptor was assessed in hippocampal neurons transfected with eGFP-PHPLCδ on day 5 in vitro (DIV) and used experimentally between days 7 and 10 in vitro. All experiments were undertaken in the presence of tetrodotoxin (500 nm) to block action potential-dependent synaptic activity. Desensitization was assessed in single cells using a slightly modified protocol to that published previously for the M3 mACh receptor in SHSY5Y neuroblastoma cells (Willets et al., 2003a). Neurons were challenged with an approximate EC50 concentration of the mACh receptor agonist methacholine (MCh) for 30 sec (termed R1), followed by a 5 min washout to allow recovery of PIP2, [Ca2+]i, and eGFP-PHPLCδ fluorescence to basal levels. After this, a maximal concentration of MCh (100 μm) was applied for 1 min to induce receptor desensitization. The washout period after desensitization was varied before rechallenge with the same approximate EC50 concentration of MCh (termed R2). Receptor desensitization was determined as the reduction in peak IP3 formation in R2 when compared with R1.
Detection of endogenously expressed GRKs. After 7 d in culture, hippocampal neurons were lysed, and GRK expression was detected by Western blotting using specific rabbit polyclonal anti-GRK2, GRK3, GRK5, or GRK6 (Santa Cruz Biotechnology, Santa Cruz, CA) as described previously (Willets and Kelly, 2001).
Data analysis. Data were analyzed using one-way ANOVA (Excel 5.0; Microsoft, Redmond, WA), followed by Student's t test. Significance was accepted when p < 0.05.
Results
Characterization of mACh receptor desensitization in hippocampal neurons
Transfection of 5 DIV hippocampal cultures yielded up to 5% of cells expressing eGFP-PHPLCδ, an example of which can be seen in Figure 1A. When stimulated with the mACh receptor agonist MCh (100 μm), the eGFP-PHPLCδ construct translocated to the cytoplasm, producing a rapid peak, followed by a slowly fading plateau phase that rapidly decreased to prestimulation levels after addition of atropine (1 μm) (Fig. 1B) or agonist washout. Concentration-dependent increases in cytosolic eGFPPLCδ fluorescence were seen for MCh (Fig. 1C). Pirenzepine (300 nm) and the M1-selective toxin MT7 (100 nm) (Fig. 1D) fully reversed the MCh-induced increase in IP3 in >75% cells investigated. These pharmacological data indicate the presence of a predominant or exclusive M1 mACh receptor population in the majority of neurons studied.
Figure 1.

Imaging IP3 in hippocampal neurons. A, Image of a hippocampal neuron expressing eGFP-PHPLCδ. B, Representative trace showing IP3 production, indicated by translocation of eGFP-PHPLCδ from the membrane to the cytoplasm, in response to MCh (100 μm) and subsequent atropine (1 μm) addition. C, Representative trace (and hippocampal images) showing concentration-dependent increases in IP3 generation in response to 30 sec additions of MCh (1, 3, 10, 30, and 100 μm) interspersed by 4 min washout periods. D, Representative traces showing MCh (100 μm, 30 sec)-stimulated IP3 generation, which is inhibited in the same hippocampal neuron after MT7 (100 nm, 30 min preincubation) treatment. Data are representative of 10 separate experiments.
Consistent with the minimal mACh receptor desensitization in the continuing presence of 100 μm MCh (Fig. 1B), repeated applications of MCh (100 μm) for 1 min, interspersed with 5 min washes, also resulted in only a slight attenuation of the IP3 response (Fig. 2A). The experimental protocol was modified to use a 30 sec application of a submaximal concentration of MCh before and after a near-maximal concentration of MCh (100 μm) applied for 1 min (Fig. 2B). Comparison of the responses before (R1) and after (R2) the 60 sec pulse of MCh revealed a clear reduction (∼50%) in the second response (Fig. 2B). Using R1 and R2 concentrations of MCh of 3, 10, or 30 μm produced similar degrees of desensitization (40-50%) (Fig. 2C). The extent of the reduction in mACh receptor signaling observed was also dependent on the washout period before the R2 challenge (Fig. 2C). Thus, the desensitization (R2/R1 ratio) was similar at 3 or 5 min after removal of 100 μm MCh but was decreased (i.e., R2 approaches R1 response) after 10 min of washout (Fig. 2C), suggesting that receptor resensitization can occur over this timescale. In all additional experiments, 5 min was used as the standard washout period between agonist additions.
Figure 2.

Assessment of mACh receptor desensitization in hippocampal neurons. A, Representative trace showing IP3 responses to repeated 1 min additions of 100 μm MCh (horizontal bars) interspersed by 5 min washout periods. B, A representative trace (and hippocampal images) showing how the response to an approximate EC50 concentration of MCh (R1/R2, 10 μm, 30 sec) is attenuated by the application of a near-maximal MCh addition (MChmax; 100 μm, 1 min). C, Mean data using the protocol shown in B. The R2/R1 ratio was calculated for experiments in which 3, 10, or 30 μm MCh was used as the approximate EC50 concentration. After the near-maximal MCh addition, the washout time before addition of the R2 pulse was varied (3, 5, or 10 min). Data are shown as means ± SEM for five to eight separate experiments on at least three different hippocampal preparations.
Detection of endogenous GRK expression
Western blot analysis of lysates prepared from 7 DIV hippocampal neurons showed the presence of immunoreactivity to GRK2, GRK3, GRK5, and GRK6 antibodies (Fig. 3A). A similar pattern of expression was observed in lysates prepared from hippocampus of 7- to 8-d-old rats (Fig. 3A).
Figure 3.
Effects of wild-type and dominant-negative GRK5 and GRK6 constructs on mACh receptor desensitization in hippocampal neurons. A, Western blot detection of GRK2, GRK3, GRK5, and GRK6 protein in 7 DIV hippocampal cultures (lane 1) or hippocampal homogenates (lane 2) prepared from 7- to 8-d-old rats. B, Representative trace of hippocampal neurons cotransfected with a 1:3 ratio of eGFP-PHPLCδ and K215RGRK6. Additions (shown by the horizontal bars) were 10 μm MCh for 30 sec (R1), 100 μm MCh for 1 min (MChmax), and 10 μm MCh for 30 sec (R2), interspersed by 5 min washout periods. C, Desensitization of mACh receptor-mediated IP3 production in single cells transfected with the vector control (pcDNA3; n = 21 cells), K215RGRK5 (DN5; n = 21), wild-type GRK5 (GRK5; n = 6), K215RGRK6 (DN6; n = 18), or wild-type GRK6 (GRK6; n = 8). Data are shown as means ± SEM for the number of neurons indicated above taken from least three different hippocampal preparations. K215RGRK6 expression significantly attenuated desensitization (**p < 0.01), whereas overexpression of GRK6 caused a significantly greater change in the R2/R1 ratio compared with controls (*p < 0.05).
Effects of inhibiting GRK5 and GRK6 activities on mACh receptor desensitization
Hippocampal neurons were transfected with catalytically inactive, dominant-negative GRK5 and GRK6 constructs, which are mutated at a conserved lysine residue (K215R) required for ATP binding at the catalytic domain (Willets et al., 2002). To determine whether K215RGRK5 and K215RGRK6 were expressed, hippocampal neurons were transfected with eGFP-tagged versions and detected by confocal microcopy. Expression could still be detected 10 d after transfection (data not shown). Transfection with empty vector (pcDNA3), K215RGRK5, or K215RGRK6 had no effect on acute MCh-stimulated IP3 production. Transfection of hippocampal neurons with pcDNA3 did not affect the extent of mACh receptor desensitization (Fig. 3C). In K215RGRK6-transfected neurons, the R2/R1 ratio difference was reduced, indicating that inhibition of endogenous GRK6 in hippocampal neurons attenuates mACh receptor desensitization (Fig. 3B,C). However, transfection with the closely related K215RGRK5 had no effect on mACh receptor responsiveness (Fig. 3C). Wild-type GRK6 was also coexpressed in neurons, and this resulted in a small but statistically significant increase in the R2/R1 ratio, suggesting that overexpression of GRK6 could further increase agonist-driven mACh receptor desensitization (Fig. 3C). In contrast, coexpression of wild-type GRK5 had no effect on M1 mACh receptor desensitization (Fig. 3C).
Involvement of GRK2 in mACh receptor regulation
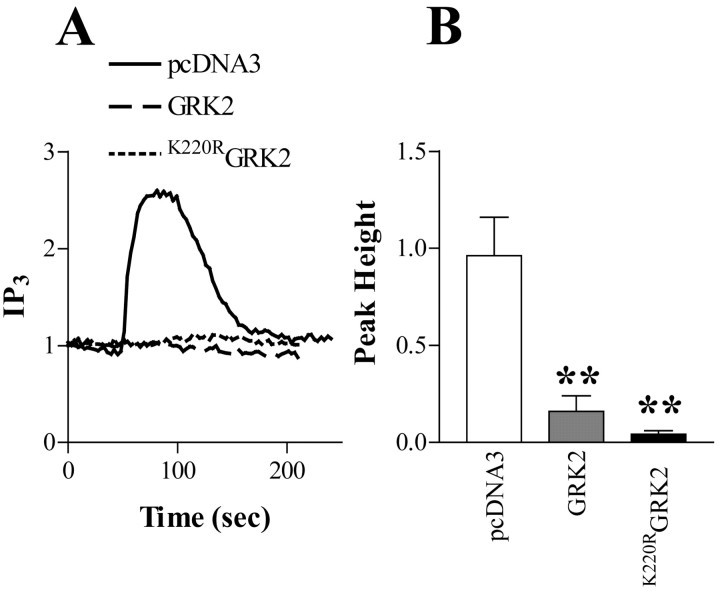
We and others have reported that GRK2 and GRK3 are able to inhibit receptor-mediated IP3 formation independent of their receptor kinase activity (Carman et al., 1999; Willets et al., 2001, 2003a) but dependent on direct binding of the RGS-like (regulator of G-protein signaling) domain of GRK2/3 to Gαq-GTP. To date, most of this work has been undertaken in cell lines with overexpressed kinases and/or receptors. In this study, we examined whether a similar phenomenon can be observed in hippocampal neurons. Transfection of wild-type GRK2 into hippocampal neurons markedly inhibited MCh-stimulated eGFP-PHPLCδ translocation (Fig. 4). An equally marked inhibition of signaling was seen in neurons transfected with the catalytically inactive, dominant-negative K220RGRK2 (Fig. 4). These data indicate that the GRK2-mediated inhibition of mACh receptor signaling is not dependent on receptor phosphorylation.
Figure 4.
Effects of wild-type and dominant-negative GRK2 on mACh receptor-stimulated IP3 production. A, Representative traces of hippocampal neurons cotransfected with a 1:3 ratio of eGFP-PHPLCδ and empty vector (pcDNA3), wild-type GRK2 (GRK2), or dominant-negative/kinase-dead GRK2 (K220RGRK2). The response to a single addition of MCh (100 μm, 60 sec) is shown.B, Inhibitory effects of GRK2 and K220RGRK2 overexpression on mACh receptor-mediated IP3production in single neurons. Data are shown as means ± SEM for 6-11 neurons taken from at least three different hippocampal preparations. Both GRK2 and K220RGRK2 expression significantly attenuated the response to MCh (**p < 0.01) compared with controls.
Discussion
The cholinergic innervation of the hippocampus is widespread and derives mainly from the septal nuclei (Rouse et al., 1999). Lesions to this pathway, or blockade of mACh receptors, can lead to memory and attentional deficits (Bartus et al., 1982; Power et al., 2003). Likewise, there is evidence for extensive expression of mACh receptors, particularly of the M1 subtype, in both neuronal soma and dendrites in rat (Levey et al., 1995) and human (Shiozaki et al., 2001) hippocampal pyramidal cells. The M1 mACh receptor subtype preferentially couples via Gαq/11-proteins to activation of phospholipase C, and recent studies have revealed that agonist-stimulated Gαq/11-[35S]GTPγS binding is abolished in hippocampal membranes from M1 mACh receptor knock-out mice (Porter et al., 2002). Furthermore, activation of mACh receptors on CA1 pyramidal neurons leads to IP3-dependent Ca2+ waves that propagate from the dendrites to the soma, in which they invade the nucleus (Power and Sah, 2002). This action, and indeed the activation of extracellular signal-regulated kinases selectively by the M1 mACh receptor (Berkeley and Levey, 2003), could be significant in cholinergic-induced changes in hippocampal synaptic plasticity.
We used a fluorescent biosensor, eGFP-PHPLCδ, to image for the first time IP3 generation stimulated by M1 mACh receptor activation in single hippocampal neurons in culture. In particular, we focused on the potential regulation of mACh receptors by GRKs, and this has been facilitated by the ability to cotransfect specific GRK constructs with the biosensor and thus allow GRK/biosensor coexpressing neurons to be imaged. Continuous or repeated maximal stimulation of M1 mACh receptors only led to a slight desensitization. However, in view of the receptor reserve observed for this subtype in hippocampus with respect to agonist-stimulated Gαq/11-[35S]GTPγS binding (Porter et al., 2002), we adopted a different protocol comparing submaximal agonist responses assessed before and after a 60 sec maximal stimulation. Under these conditions, we observed a significant attenuation of IP3 generation in hippocampal neurons during rechallenge.
Our hippocampal cultures express GRK2, GRK3, GRK5, and GRK6, and manipulation of the expression-activity of these kinases had profound effects on M1 mACh receptor-PLC signaling. Perhaps surprisingly, overexpression of GRK2 and the catalytically inactive K220RGRK2 mutant both markedly suppressed mACh receptor-mediated IP3 generation. This strongly implies a phosphorylation-independent mechanism for this kinase in hippocampal neurons, consistent with several examples of such regulation at a number of GPCRs (Willets et al., 2003b). Currently, the most likely mechanism relates to the direct binding of GRK2 to activated GTP-bound Gαq/11 through an RGS-like domain present in the N terminus of GRK2 (Sallese et al., 2000; Sterne-Marr et al., 2003). Recent findings using mutations in the RGS domain have revealed a novel sequence in GRK2/3, termed the C-site, which avidly binds Gαq/11 and is absent from other GRKs (Sterne-Marr et al., 2003). Indeed, the crystallographic structure of GRK2 reveals three domains occupying the vertices of an essentially equilateral triangle with spacing such that each of the three domains could potentially interact simultaneously with the GPCR, Gαq/11, and Gβγ subunits (Lodowski et al., 2003).
To our knowledge, our data provide the first report of phosphorylation-independent regulation of Gq/11 signaling in neurons by GRKs. Although GRK2 is thus able to suppress PLC-coupled receptor signaling independently of receptor phosphorylation, we cannot discount the fact that endogenous GRK2 may also phosphorylate the M1 mACh receptor. Suppression of endogenous GRK2 through antisense or RNA interference techniques should allow additional assessment of the role of GRK2 in M1 mACh receptor regulation in hippocampal neurons. However, the dramatic and rapid suppression of signaling by GRK2 independently of its kinase activity remains a powerful and potential regulator of GPCR action in vivo. Such a phenomenon may extend beyond the GRK2/3 subfamily, because a recent paper by Perroy et al. (2003) revealed a similar phosphorylation-independent regulation of Gi/o-coupled GABAB receptor signaling mediated by GRK4 in cerebellar granule neurons.
In addition to a GRK2-mediated phosphorylation-independent suppression of M1 mACh receptor signaling, we show here that manipulations of GRK6 activity can also affect mACh receptor responsiveness. Indeed, inhibition of endogenous GRK5 or GRK6 by dominant-negative constructs provided evidence only for GRK6 playing a role in M1 mACh receptor signaling in the hippocampus. Considering that neither GRK5 nor GRK6 possess a C-site RGS domain, and are thus unable to bind Gαq, we reason that endogenous GRK6 is likely to regulate M1 mACh receptor signaling through a phosphorylation-dependent mechanism. This conclusion is consistent with our previous findings on the regulation of the endogenously expressed M3 mACh receptor in SH-SY5Y neuroblastoma cells by GRK6-dependent receptor phosphorylation (Willets et al., 2001, 2002, 2003a). Unfortunately, because of the heterogeneous nature of hippocampal cultures and difficulties in culturing sufficient neurons, direct examination of GRK6- and GRK2-mediated phosphorylation of the M1 mACh receptor will have to be performed in cell lines endogenously or recombinantly expressing the M1 mACh receptor. The relatively small changes induced by GRK6 and K215RGRK6 suggest the potential involvement of other kinases in the regulation of M1 mACh receptor signaling; however, pharmacological inhibition or downregulation of PKC activities fail to affect agonist-induced M1 mACh receptor desensitization (data not shown).
In conclusion, this study has revealed regulation of a GPCR in hippocampal neurons using the eGFP-PHPLCδ biosensor to follow “real time” changes in IP3 in individual neurons. Furthermore, we showed the potential for phosphorylation-dependent and -independent regulation of M1 mACh receptor signaling in hippocampal neurons through selective actions of GRK6 and GRK2, respectively. Whether the actions of these GRKs play a role in the acute regulation of M1 mACh receptor function in the hippocampus or whether they also initiate longer-term changes in synaptic activity, perhaps associated with cholinergic activation of mitogen-activated kinases, remain to be established.
Footnotes
This work was supported by Wellcome Trust (UK) Grant 062495.
Correspondence should be addressed to Jonathon M. Willets, Department of Cell Physiology and Pharmacology, University of Leicester, Maurice Shock Medical Sciences Building, University Road, Leicester LE1 9HN, UK. E-mail: jmw23@le.ac.uk.
Copyright © 2004 Society for Neuroscience 0270-6474/04/244157-06$15.00/0
References
- Arriza JL, Dawson TM, Simerly RB, Martin LJ, Caron MG, Snyder SH, Lefkowitz RJ (1992) The G-protein-coupled receptor kinases βARK1 and βARK2 are widely distributed at synapses in rat brain. J Neurosci 12: 4045-4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, Beer B, Lippa AS (1982) The cholinergic hypothesis of geriatric memory dysfunction. Science 217: 408-414. [DOI] [PubMed] [Google Scholar]
- Berkeley JL, Levey AI (2003) Cell-specific extracellular signal-regulated kinase activation by multiple G protein-coupled receptor families in hippocampus. Mol Pharmacol 63: 128-135. [DOI] [PubMed] [Google Scholar]
- Berridge MJ (1998) Neuronal calcium signaling. Neuron 21: 13-26. [DOI] [PubMed] [Google Scholar]
- Carman CV, Parent JL, Day PW, Pronin AN, Sternweis PM, Wedegaertner PB, Gilman AG, Benovic JL, Kozasa T (1999) Selective regulation of Gαq/11 by an RGS domain in the G protein-coupled receptor kinase, GRK2. J Biol Chem 274: 34483-34492. [DOI] [PubMed] [Google Scholar]
- Erdtmann-Vourliotis M, Mayer P, Ammon S, Riechert U, Höllt V (2001) Distribution of G-protein-coupled receptor kinase (GRK) isoforms 2, 3, 5 and 6 mRNA in the rat brain. Mol Brain Res 95: 129-137. [DOI] [PubMed] [Google Scholar]
- Ferguson SSG (2001) Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev 53: 1-24. [PubMed] [Google Scholar]
- Gainetdinov RR, Bohn LM, Walker JKL, Laporte SA, Macrae AD, Caron MG, Lefkowitz RJ, Premont RT (1999) Muscarinic supersensitivity and impaired receptor desensitization in G-protein-coupled receptor kinase 5-deficient mice. Neuron 24: 1029-1036. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Bohn LM, Sotnikova TD, Cyr M, Laakso A, Macrae AD, Torres GE, Kim K-M, Lefkowitz RJ, Caron MG, Premont RT (2003) Dopaminergic supersensitivity in G-protein-coupled receptor kinase 6-deficient mice. Neuron 38: 291-303. [DOI] [PubMed] [Google Scholar]
- Grange-Midroit M, García-Sevilla JA, Ferrer-Alcón M, La Harpe R, Walzer C, Guimón J (2002) G protein-coupled receptor kinases, β-arrestin-2 and associated regulatory proteins in the human brain: post-mortem changes, effect of age and subcellular distribution. Mol Brain Res 101: 39-51. [DOI] [PubMed] [Google Scholar]
- Greengard P (2001) The neurobiology of slow synaptic transmission. Science 294: 1024-1030. [DOI] [PubMed] [Google Scholar]
- Hausdorff WP, Caron MG, Lefkowitz RJ (1990) Turning off the signal: desensitization of β-adrenergic receptor function. FASEB J 4: 2881-2889. [PubMed] [Google Scholar]
- Iacovelli L, Salvatore L, Capobianco L, Picascia A, Barletta E, Storto M, Mariggiò S, Sallese M, Porcellini A, Nicoletti F, De Blasi A (2003) Role of G protein-coupled receptor kinase 4 and β-arrestin 1 in agonist-stimulated metabotropic glutamate receptor 1 internalization and activation of mitogen-activated protein kinases. J Biol Chem 278: 12433-12442. [DOI] [PubMed] [Google Scholar]
- Katz PS, Clemens S (2001) Biochemical networks in nervous systems: expanding neuronal information capacity beyond voltage signals. Trends Neurosci 24: 18-25. [DOI] [PubMed] [Google Scholar]
- Kouznetsova M, Kelley B, Shen M, Thayer SA (2002) Desensitization of cannabinoid-mediated presynaptic inhibition of neurotransmission between hippocampal neurons in culture. Mol Pharmacol 61: 477-485. [DOI] [PubMed] [Google Scholar]
- Krupnick JG, Benovic JL (1998) The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu Rev Pharmacol Toxicol 38: 289-319. [DOI] [PubMed] [Google Scholar]
- Levey AI, Edmunds SM, Koliatsos V, Wiley RG, Heilman CJ (1995) Expression of M1-M4 muscarinic acetylcholine receptor proteins in rat hippocampus and regulation by cholinergic innervation. J Neurosci 15: 4077-4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodowski DT, Pitcher JA, Capel WD, Lefkowitz RJ, Tesmer JJG (2003) Keeping G proteins at bay: a complex between G protein-coupled receptor kinase 2 and Gβγ. Science 300: 1256-1262. [DOI] [PubMed] [Google Scholar]
- McConalogue K, Corvera CU, Gamp PD, Grady EF, Bunnett NW (1998) Desensitization of the neurokinin-1 receptor (NK1-R) in neurons: effects of substance P on the distribution of NK1-R, Gαq/11, G-protein receptor kinase-2/3, and β-arrestin-1/2. Mol Biol Cell 9: 2305-2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahorski SR, Young KW, Challiss RAJ, Nash MS (2003) Visualizing phosphoinositide signaling in single neurons gets the green light. Trends Neurosci 26: 444-452. [DOI] [PubMed] [Google Scholar]
- Nash MS, Schell MJ, Atkinson PJ, Johnston NJ, Nahorski SR, Challiss RAJ (2002) Determinants of metabotropic glutamate receptor-5-mediated Ca2+ and inositol 1,4,5-trisphosphate oscillation frequency: receptor density versus agonist concentration. J Biol Chem 277: 35947-35960. [DOI] [PubMed] [Google Scholar]
- Perroy J, Adam L, Qanbar R, Chénier S, Bouvier M (2003) Phosphorylation-independent desensitization of GABAB receptor by GRK4. EMBO J 22: 3816-3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter AC, Bymaster FP, DeLapp NW, Yamada M, Wess J, Hamilton SE, Nathanson NM, Felder CC (2002) M1 muscarinic receptor signaling in mouse hippocampus and cortex. Brain Res 944: 82-89. [DOI] [PubMed] [Google Scholar]
- Power AE, Vazdarjanova A, McGaugh JL (2003) Muscarinic cholinergic influences in memory consolidation. Neurobiol Learn Mem 80: 178-193. [DOI] [PubMed] [Google Scholar]
- Power JM, Sah P (2002) Nuclear calcium signaling evoked by cholinergic stimulation in hippocampal CA1 pyramidal neurons. J Neurosci 22: 3454-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse ST, Marino MJ, Potter LT, Conn PJ, Levey AI (1999) Muscarinic receptor subtypes involved in hippocampal circuits. Life Sci 64: 501-509. [DOI] [PubMed] [Google Scholar]
- Sallese M, Salvatore L, D'Urbano E, Sala G, Storto M, Launey T, Nicoletti F, Knöpfel T, De Blasi A (2000) The G-protein-coupled receptor kinase GRK4 mediates homologous desensitization of metabotropic glutamate receptor 1. FASEB J 14: 2569-2580. [DOI] [PubMed] [Google Scholar]
- Schell MJ, Erneux C, Irvine RF (2001) Inositol 1,4,5-trisphosphate 3-kinase A associates with F-actin and dendritic spines via its N terminus. J Biol Chem 276: 37537-37546. [DOI] [PubMed] [Google Scholar]
- Shiozaki K, Iselki E, Hino H, Kosaka K (2001) Distribution of M1 muscarinic acetylcholine receptors in the hippocampus of patients with Alzheimer's disease and dementia with Lewy bodies: an immunohistochemical study. J Neurosci 193: 23-28. [DOI] [PubMed] [Google Scholar]
- Stauffer TP, Ahn S, Meyer T (1998) Receptor-induced transient reduction in plasma membrane PtdIns(4,5)P2 concentration monitored in living cells. Curr Biol 12: 343-346. [DOI] [PubMed] [Google Scholar]
- Sterne-Marr R, Tesmer JJ, Day PW, Stracquatanio RP, Cilente JA, O'Connor KE, Pronin AN, Benovic JL, Wedegaertner PB (2003) G protein-coupled receptor kinase 2/Gαq/11 interaction. J Biol Chem 278: 6050-6058. [DOI] [PubMed] [Google Scholar]
- Willets JM, Kelly E (2001) Desensitization of endogenously expressed δ-opioid receptors: no evidence for involvement of G protein-coupled receptor kinase 2. Eur J Pharmacol 431: 133-141. [DOI] [PubMed] [Google Scholar]
- Willets JM, Challiss RAJ, Kelly E, Nahorski SR (2001) GRK3 and GRK6 utilize different pathways to desensitize the endogenous M3 muscarinic acetylcholine receptor in human SH-SY5Y cells. Mol Pharmacol 60: 321-330. [DOI] [PubMed] [Google Scholar]
- Willets JM, Challiss RAJ, Nahorski SR (2002) Endogenous G protein-coupled receptor kinase 6 regulates M3 muscarinic acetylcholine receptor phosphorylation and desensitization in human SH-SY5Y neuroblastoma cells. J Biol Chem 277: 15523-15529. [DOI] [PubMed] [Google Scholar]
- Willets JM, Mistry R, Nahorski SR, Challiss RAJ (2003a) Specificity of G protein-coupled receptor kinase 6-mediated phosphorylation and regulation of single-cell M3 muscarinic acetylcholine receptor signaling. Mol Pharmacol 64: 1059-1068. [DOI] [PubMed] [Google Scholar]
- Willets JM, Challiss RAJ, Nahorski SR (2003b) Non-visual GRKs: are we seeing the whole picture? Trends Pharmacol Sci 24: 626-633. [DOI] [PubMed] [Google Scholar]