Abstract
At crayfish neuromuscular junctions, cAMP increases transmitter released by action potentials by activating two effectors, hyperpolarization and cyclic nucleotide-activated channels (HCNCs) and a separate target that has been tentatively identified as exchange protein activated by cAMP (Epac). Intense electrical activity in the motor neuron induces a long-term facilitation (LTF) of transmitter release in which hyperpolarization from an electrogenic Na+-K+ exchanger activates HCNCs. The coupling of HCNCs to transmission involves actin. After LTF induction, cAMP further increases transmission in an HCNC-independent manner, activating the second target. This relaxation of the requirement for HCNC activation to enhance release is called temporal synaptic tagging. Tagging lasts at least 1 d but develops only in the 10 min period after electrical activity. The HCNCs are activated by the post-tetanic hyperpolarization occurring during this time. Both synaptic tagging and LTF induction depend on presynaptic Ca2+ accumulation during activity; both are blocked by EGTA-AM, and LTF is also prevented by stimulation in a low-[Ca2+] medium. Actin depolymerizers prevent induction of LTF and tagging, with little effect on HCNCs, whose sensitivity to cAMP and HCNC blockers is unaffected by tagging. Enhancement of actin polymerization can rescue tagging from HCNC block, suggesting that actin acts at a step after HCNC activation. These and other recent results suggest a model in which HCNC activation, followed by a process involving actin polymerization, acts cooperatively with [Ca2+] to induce tagging, after which only Epac activation is required for cAMP to further enhance transmission.
Keywords: synaptic transmission, synaptic tagging, calcium, HCN channels, actin, neuromuscular junction, crayfish
Introduction
Temporal synaptic tagging is a property of some crayfish neuromuscular junctions in which previous electrical activity changes the rules for regulation of transmission by the neuromodulator serotonin (Beaumont et al., 2002). Tagging refers to an interaction between two processes, the cAMP-dependent enhancement of transmission and long-term facilitation (LTF).
The leg-opener muscle is innervated by a single glutamatergic exciter motor neuron and a single inhibitory motor neuron (Wiersma, 1941). The number of glutamate-containing synaptic vesicles available for release by action potentials at the exciter is increased by the circulating neurohormone serotonin (Wang and Zucker, 1998) acting in part by production of the presynaptic second messenger cAMP (Beaumont and Zucker, 2000). This cAMP-dependent enhancement of transmission, which can be produced directly by stimulating adenylyl cyclase with forskolin, involves the activation of presynaptic hyperpolarization and cyclic nucleotide-activated channels (HCNCs) by cAMP as well as the integrity of the actin cytoskeleton; any of three HCNC blockers (ZD 7288, DK-AH 269, or Cs+) or depolymerization of actin by cytochalasin D, latrunculin B, or swinholide A greatly reduce the forskolin-induced enhancement of transmission with little effect on the HCNCs.
Prolonged electrical activity in the motor neuron results in a long-lasting activity-dependent form of synaptic plasticity called long-term facilitation (Atwood and Wojtowicz, 1986). This enhancement of transmitter release, which lasts for hours, also involves HCNC activation during the tetanus, in this case by hyperpolarization arising from the electrogenic Na+-K+ pump removing internal Na+ ions that accumulated during the activity, and is also sensitive to actin depolymerizers (Beaumont et al., 2002). It also requires Ca2+ influx during the conditioning activity, and its expression is dependent on local presynaptic protein synthesis and on the activity of a number of protein kinases (MAP kinase, phosphatidylinositol 3-OH kinase, rapamycin-sensitive kinase) as well as the phosphatase calcineurin (Beaumont et al., 2001).
After induction of LTF, transmission is further enhanced by the subsequent elevation of cAMP (by forskolin or serotonin), but now this enhancement is insensitive to HCNC blockers or actin depolymerizers (Beaumont et al., 2002). LTF induction apparently “tagged” the synapses so that enhancement of transmission by cAMP no longer appeared to operate on HCNCs. It is as if when HCNCs are activated, they do not need to be activated again, and cAMP operates on some other target. Recent evidence implicates exchange protein activated by cAMP (Epac) as that target (our unpublished observations). Thus, we believe cAMP enhances transmission by activating two targets, HCNCs and Epac. After tagging by high levels of activity, only the second HCNC-independent target needs to be activated by cAMP, and responses are unaffected by actin depolymerization. Synaptic tagging also requires Ca2+ influx during conditioning activity but differs from LTF expression in not being dependent on kinase or phosphatase activity and not requiring protein synthesis.
In this study, we explore the temporal characteristics of synaptic tagging, its Ca2+ dependence, and the nature of actin-HCNC interactions; we also check for effects of tagging on HCNC responses to cAMP.
Materials and Methods
Preparation and electrophysiology. First walking legs of crayfish (Procambarus clarkii) obtained locally (Niles Biological, Sacramento, CA) were prepared and mounted as described previously (Delaney et al., 1991; Zhong et al., 2001); they were continuously perfused with cooled saline containing (in mm) 195 NaCl, 13.5 CaCl2, 5.4 KCl, 2.6 MgCl2, and 10 Na-HEPES, pH 7.4, and held at 15-17°C. Basal transmission was assessed by stimulating the exciter motor neuron with a suction electrode in the meropodite at 2 Hz while recording excitatory junctional potentials (EJPs) from proximal muscle fibers with sharp microelectrodes (12-25 MΩ) filled with 3m KCl, using a Neuroprobe 1600 amplifier (A-M Systems, Everett, WA). In some experiments, presynaptic membrane potentials were recorded from primary or secondary nerve branches using beveled microelectrodes (25-45 MΩ). To induce LTF and temporal synaptic tagging, a 20 Hz tetanus lasting 10 min was used. Signals were filtered at 2 kHz, digitized at 5 kHz, stored on a personal computer using pClamp8 software, and analyzed off-line with Clampfit 6.05 (Axon Instruments, Foster City, CA). Effects on synaptic transmission were quantified as percentage changes in EJP amplitude from control levels, measured from stable recordings of averaged EJP amplitudes in the first 10-20 min of each experiment; effects on presynaptic potential were expressed as changes (in millivolts) from stable initial control levels. LTF was quantified as the percentage increase in average EJP amplitudes measured between 20 and 60 min after the tetanus, compared with average EJP amplitudes recorded 0-10 min before the tetanus (Beaumont et al., 2001). Data are presented as means ± SE, and statistical significance was evaluated using two-tailed Student's t tests.
In experiments requiring long-term viability, the preparation was washed for 30-60 min in Ringer's solution containing the antibiotic-fungicide mixture of 0.1 mg/ml gentamicin, 100 IU/ml penicillin, 0.1 mg/ml streptomycin, and 0.25 μg/ml fungizone (Grand Island Biological, Grand Island, NY), and then switched to Ringer's solution containing 1 mg/ml glucose and 1 μg/ml gentamicin (Invitrogen, Carlsbad, CA). This procedure (Egid and Lnenicka, 1993) kept the preparation alive for 24 hr.
Drugs. Forskolin was purchased from EMD Biosciences (Pasadena, CA), ZD 7288 was obtained from Tocris Cookson (Ballwin, MO), jasplakinolide was supplied by Molecular Probes (Eugene, OR), and the Epac agonist 8-(4-chloro-phenylthio)-2′-O-methyladenosine-3′, 5′-cyclic monophosphate (8CPT) was purchased from Axxora Biolog Biochemicals (San Diego, CA). To load cells with the Ca2+ buffer EGTA, EGTA-AM (Molecular Probes) was added to the bath with 1% Pluronic F-127 for 30 min, followed by an additional 30 min incubation to permit complete de-esterification. Forskolin, jasplakinolide, and EGTA-AM stock solutions were prepared in DMSO and dissolved before use in external media to a final concentration containing no more than 0.1% DMSO. Control experiments showed that synaptic transmission was not affected by this solvent.
Results
A delay in the initiation of temporal synaptic tagging
In “naive” preparations, cAMP-dependent enhancement of transmitter release involves the action of cAMP on two presynaptic targets, HCNCs and a second HCNC-independent target recently identified as Epac, and is disrupted by actin depolymerization (Beaumont and Zucker, 2000; our unpublished observations). Synaptic tagging refers to the effect of conditioning stimulation that induces LTF on subsequent responses to cAMP elevation, where now cAMP needs to act only on the HCN-independent target, without a need for additional HCNC activation or an intact actin cytoskeleton (Beaumont et al., 2002; our unpublished observations). Tagging is demonstrated by showing that forskolin-induced enhancement of EJPs is no longer blocked by HCNC inhibition. In experiments up until this point, synaptic tagging has been probed by testing the response to cAMP elevation by forskolin exposure at only one time point, 60-80 min after the end of the conditioning stimulation, or 30-50 min after the HCNC blockers ZD 7288 or DK-AH 269 or the actin depolymerizers cytochalasin D or latrunculin B had been added to the bath 30 min after the stimulation (Beaumont et al., 2002). Similar to ZD 7288 or DK-AH 269, Cs+ (1 mm) did not affect subsequent forskolin-induced enhancement after LTF induction (86.8 ± 28% enhancement of EJP amplitudes; n = 3). This is similar to the forskolin-induced enhancement of EJPs after LTF induction with no Cs+ (65.5 ± 4.7%; n = 6; p > 0.3). When no tetanus preceded exposure to forskolin, Cs+ reduced the forskolin-induced enhancement of EJPs by 40% (Beaumont and Zucker, 2000), Therefore, after LTF induction, forskolin-induced enhancement of transmission became resistant to all three blockers of HCNCs (Cs+, ZD 7288, and DK-AH 269). We have now explored in more detail when synaptic tagging begins and how long it lasts.
Because the hallmark of tagging is its insensitivity to HCNC blockers, we began by varying the time between the end of tetanic stimulation and the addition of ZD 7288 (30 μm), while the time of forskolin (30 μm) addition was held constant at 30 min after the tetanus (Fig. 1A). When forskolin-induced enhancement of EJPs was tested without ZD 7288, EJP amplitudes were enhanced 65.5 ± 4.7% (n = 6) (Fig. 1B, blue trace) compared with their level just before adding forskolin. If ZD 7288 was added immediately after the end of stimulation, there was no sign of synaptic tagging, in that responses to forskolin were much smaller (17 ± 6.3% enhancement; n = 6) (Fig. 1B, orange trace) (i.e., they were sensitive to HCNC block). This degree of block (73%) is nearly identical (p > 0.05) to the reduction in forskolin enhancement caused by ZD 7288 in the absence of any tetanic stimulation (67 ± 6%) (Beaumont and Zucker, 2000). Apparently, synaptic tagging develops with some delay.
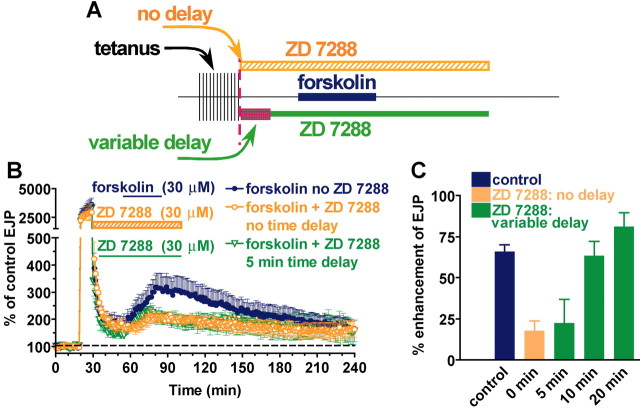
Figure 1.
Temporal synaptic tagging is induced only if HCNC block is delayed until 10 min after conditioning stimulation. After a tetanus (20 Hz for 10 min), the enhancement of EJP amplitudes by forskolin loses its sensitivity to HCNC blockers such as ZD 7288, which is the hallmark of synaptic tagging. However, ZD 7288 treatment right at the end of the tetanus is ineffective in blocking forskolin-induced enhancement, and tagging is not established. A, Outline of the experimental design in which no delay (orange bar) or a variable delay (0-20 min; green bar) is interposed between the end of stimulation and exposure to ZD 7288 (30 μm). Forskolin treatment remains at 30 min after the tetanus (blue bar). B, Sequence of changes in EJP amplitude from initial pretetanic level (dashed line) showing short-term enhancement of EJPs during the tetanus and effects of forskolin when ZD 7288 is applied immediately after the tetanus (orange symbols) or delayed by 5 min (green symbols). Blue symbols show the effect of forskolin in the absence of ZD 7288. C, Percentage enhancement of post-tetanic EJPs by forskolin alone, when ZD 7288 is added immediately after the tetanus, or after various delays. Color code is the same as that described above for B.
If ZD 7288 was added 5 min after stimulation, tagging still appeared to be undeveloped, in that responses in forskolin were still substantially reduced (22 ± 14.9% enhancement; n = 3) (Fig. 1B, green trace, C, green column, at 5 min) compared with the forskolin-induced enhancement of responses in the absence of ZD 7288 (66 ± 4.7%; n = 6; p < 0.05). However, if ZD 7288 was added 10 min after stimulation, tagging appeared, in that responses in forskolin remained large (63 ± 8.9% enhancement; n = 3) (Fig. 1C, green column, at 10 min), comparable with typical tagged responses (77 ± 8.7% enhancement; n = 6; p < 0.05) (Beaumont et al., 2002), and so were insensitive to HCNC block. Finally, waiting 20 min after the tetanus before adding ZD 7288 resulted in responses in forskolin that were even larger (81 ± 8.9% enhancement; n = 3) (Fig. 1C, green column, at 20 min), indicating that by now tagging was fully expressed, and responses were completely insensitive to HCNC block. In all cases, responses that we refer to as tagged were nearly identical to those without ZD 7288 (∼70% enhancement) but significantly larger than responses in ZD 7288 without conditioning stimulation (∼20% enhancement; p < 0.05).
The apparent 10 min delay in the development of tagging could have two explanations: (1) either tagging develops slowly after its induction at the end of the tetanus, or (2) it actually requires a delay after the tetanus for its induction. In the first case, prolonging the tetanus by 10 min would allow time for tagging to develop during the tetanus, and it would then be apparent at the end of the tetanus. Figure 2 E tests this prediction. Prolonging the tetanus from 10 to 20 min did not lead to tagging if ZD 7288 was applied immediately after the tetanus. In that case, the forskolin-induced enhancement of EJPs was still blocked, or the synapses were not tagged. Therefore, it is necessary to wait 10 min after the end of a tetanus before blocking HCNCs, or synapses will not be tagged.
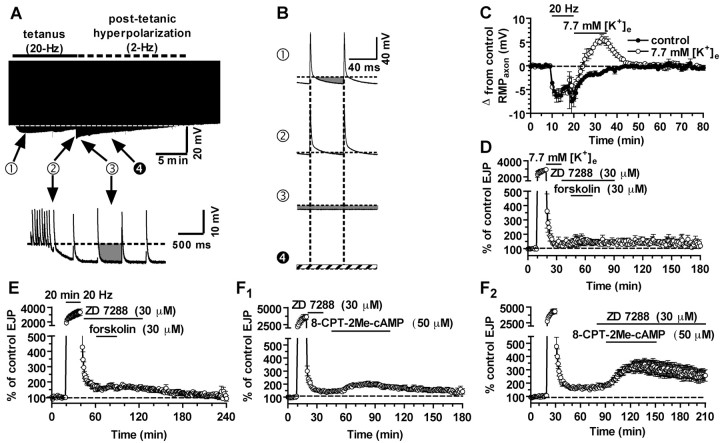
Figure 2.
Post-tetanic hyperpolarization is required to induce temporal synaptic tagging. A, Top, Envelope of presynaptic membrane potential changes before, during, and after tetanic stimulation. Action potential peaks are clipped, and the hyperpolarization shown represents the maximum hyperpolarization reached just before each action potential. Bottom, The end of the tetanus, with stimulation frequency reduced from 20 to 2 Hz, showing the large and persistent post-tetanic hyperpolarization. B, Examples of 120 msec segments from the presynaptic record. The amount of hyperpolarization (gray area) below the initial resting potential (dashed lines) is shown for typical 120 msec intervals at the times marked with filled circles in A. A, (1) Stimulation of the exciter axon at 20 Hz results in interspike membrane hyperpolarization, reaching its maximum within 1 min; (2), later in the tetanus, depolarizing afterpotentials intervene, and the membrane potential barely drops below the resting level before the next action potential, so that there is very little hyperpolarization during most of the tetanus; (3), immediately after the tetanus, the membrane potential is strongly and persistently hyperpolarized; (4) when normal saline was replaced with a high-[K+]e (7.7 mm) solution at the end of the tetanus [in a different preparation from that used in (1) through (3)], the post-tetanic hyperpolarization was converted to a depolarization, the amplitude of which is indicated by the cross-hatching. The cross-hatching represents post-tetanic depolarization in elevated [K+]. C, Peak hyperpolarization measured from the resting membrane potential that is reached before, during, and after tetanic stimulation in control solution (open circles) and when [K+] is elevated at the end of the tetanus (filled circles). D, Reversal of the post-tetanic hyperpolarization by [K+] elevation prevents induction of synaptic tagging: forskolin-induced enhancement of EJP amplitude is now blocked by ZD 7288. E, Prolonging tetanic stimulation to 20 min does not restore tagging when HCNCs are blocked immediately after the tetanus; activation of HCNCs in the post-tetanic period of hyperpolarization is critical for induction of tagging. F1, When tagging is prevented by blocking HCNCs at the end of the tetanus, the Epac agonist 8CPT (50 μm) enhances transmission only slightly. F2, Delaying exposure to ZD 7288 to induce tagging reveals a large enhancement of transmission by 8CPT. In this and all subsequent figures, dashed lines indicate presynaptic resting potential or postsynaptic control EJP amplitudes.
The delay arises from the post-tetanic period of strong hyperpolarization
This suggests that some process occurs in the first few minutes after a tetanus that is required to induce tagging. A clue to what is going on comes from recordings of the presynaptic potential during and after the tetanus. Figure 2 A shows the envelope of action potentials stimulated at 2 Hz before and after the tetanus and at 20 Hz during the tetanus. It is seen that early in the tetanus, the membrane potential hyperpolarizes to below the resting potential between action potentials, but this occurs only just before each action potential, and for only approximately the first 2 min of the tetanus (Fig. 2B, trace 1). This hyperpolarization is attributable to presynaptic Na+ accumulation and its removal by an electrogenic Na+-K+ exchange pump (Beaumont et al., 2002). However, as the tetanus proceeds and internal [Na+] ([Na+]i) rises, the action potentials broaden, and they are followed by a depolarizing afterpotential that grows until it replaces the hyperpolarizing phase after each spike (Fig. 2B, trace 2). Thus, by the end of the tetanus, stimulation no longer hyperpolarizes the membrane potential, except for a very brief period of a few milliseconds before each action potential. Thus, activation of the HCNCs occurs only at the beginning of the tetanus, and even then for only brief moments before each action potential. Prolonging the tetanus does not change this situation.
After the end of the tetanus, when stimulation is reduced to 2 Hz, the membrane potential repolarizes to -6 mV more negative than the resting potential, because of Na+-K+ transport (Fig. 2A,B, trace 3). This hyperpolarization lasts for ∼15 min (Fig. 2C), approximately as long as we must wait for tagging to become fully activated. During this post-tetanic period, the hyperpolarization is only occasionally interrupted by action potentials (at 2 Hz). If several minutes of strong and nearly continuous hyperpolarization are required to activate the HCNCs sufficiently to induce tagging, then the post-tetanic period of hyperpolarization may be essential. To test this idea, we devised the following procedure to eliminate the post-tetanic hyperpolarization. At the end of the tetanus, the [K+] in the Ringer's solution was increased from 5.4 to 7.7 mm for 15 min. This replaced the post-tetanic hyperpolarization with a depolarization of several millivolts and also eliminated the induction of tagging. Now, ZD 7288 blocked forskolin-induced enhancement, even when it was applied 15 min after the tetanus, when tagging is normally fully established.
We have shown previously that tagged synapses respond to cAMP independently of HCNC activation by the action of cAMP on Epac (our unpublished observations). After tetanic stimulation, a 50 μm concentration of the Epac agonist 8CPT (Enserink et al., 2002) doubled EJP amplitudes (94% increase), whereas this agonist increased EJPs in naive synapses by only 39% (our unpublished observations). We propose that synapses are only tagged if HCNCs are adequately activated during the post-tetanic period of uninterrupted hyperpolarization. This hypothesis predicts that if ZD 7288 is present immediately after the tetanus, the untagged synapses will respond only weakly to the Epac agonist 8CPT, like naive synapses. Figure 2 F1 confirms this prediction: 8CPT enhanced transmission by only 43 ± 5.8% (n = 4), comparable with the 39% enhancement induced by 8CPT in naive synapses (p > 0.05). However, when ZD 7288 was added >15 min after the tetanus, the synapses were tagged and 8CPT potentiated EJPs by 94 ± 8.7% (n = 4; p < 0.05, compared with the responses when ZD 7288 was applied right after the tetanus) (Fig. 2F2).
Our results indicate that LTF induction was also impeded when HCNCs were not activated at the end of the conditioning tetanus. In Figure 2F2, the level of response before addition of 8-8CPT showed an LTF of 69 ± 6.6% (n = 8; including data without ZD 7288 not shown in Fig. 2F2). When ZD 7288 was present at the end of the tetanus, LTF was reduced to 44 ± 9.4% (n = 4; p = 0.05) (Fig. 2F1). When [K+] was elevated at the end of the tetanus, LTF was also reduced (to 28 ± 7.3%; n = 3; p < 0.05) (Fig. 2D).
Synaptic tagging lasts for 24 hr
In addition to examining the time of initiation of tagging, we have studied its duration. In one set of experiments, we increased the interval between tetanic stimulation and testing with 30 μm forskolin to 4 hr. EJPs were enhanced by 60 ± 23.3% (n = 6) at this time, and the responses in forskolin applied for 30 min were insensitive to 30 μm ZD 7288 added 30 min before forskolin and left in the bath for 1 hr (62 ± 9.1% enhancement). Prolonging the interval between stimulation and testing to 24 hr yielded similar results; responses in forskolin were increased by 72 ± 9.4% (n = 5) even in the presence of ZD 7288, indicating that the cAMP continued to act in an HCNC-independent manner to enhance transmission a full day after previous induction of tagging by tetanic stimulation.
Tagging requires a rise in presynaptic internal [Ca2+]
We have reported previously that stimulating in a Ca2+-free medium induces neither LTF nor synaptic tagging (Beaumont et al., 2001, 2002). We interpreted this result as implying that presynaptic Ca2+ influx is required for the induction of both processes. A limitation of this result is that transmitter release was also blocked during stimulation in a Ca2+-free medium. Thus it could be argued that some process downstream of Ca2+ influx specifically associated with transmitter release was responsible for inducing LTF and tagging, rather than presynaptic Ca2+ itself.
To address this possibility, we have examined the effects of reducing the presynaptic internal [Ca2+] ([Ca2+]i) accumulation in a tetanus with the membrane-permeant Ca2+ chelator EGTA-AM. This is an ester form of EGTA, which becomes trapped intracellularly by virtue of its hydrolysis to the membrane-impermeant native buffer form. EGTA has little effect on evoked release by single action potentials, which depend on the rapidly forming and dissipating microdomains of Ca2+ near Ca2+ channel mouths, but it reduces short-term synaptic enhancement such as facilitation and augmentation by reducing the residual Ca2+ after neuronal activity (Zucker and Regehr, 2002).
Figure 3 A shows the effects of a 30 min treatment with 1% Pluronic F-127 with and without 50 mm EGTA-AM on baseline (2 Hz) EJP amplitudes. Pluronic alone had no effect, whereas adding EGTA-AM reduced 2 Hz transmission by 36% [to 62 ± 6% of initial amplitude (n = 4) compared with 96 ± 3% of initial amplitude in vehicle controls (n = 3)]. During a tetanus, EJP amplitudes increased ∼50-fold, representing the summed effects of facilitation, augmentation, and potentiation (Delaney and Tank, 1994). These large EJPs were also reduced (by ∼50%) during tetanic 20 Hz stimulation for 10 min in preparations loaded with EGTA-AM (Fig. 3B). The larger reduction of tetanic EJPs than control EJPs by EGTA-AM is consistent with previous results indicating that short-term synaptic plasticity is dependent on accumulation of tetanic calcium (Delaney and Tank, 1994). LTF expression was abolished in these preparations [11 ± 6% enhancement (n = 5; p < 0.05) compared with 50 ± 12% enhancement in controls (n = 5)] (Fig. 3B).
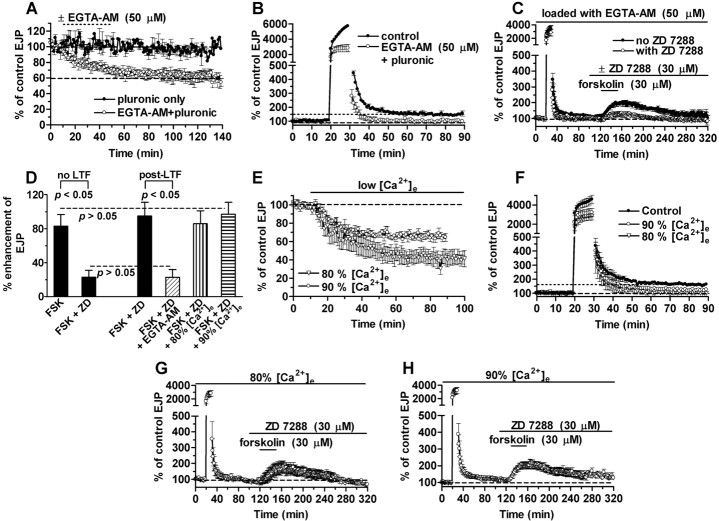
Figure 3.
LTF and synaptic tagging are Ca2+ dependent. A, Effect of EGTA-AM loading (open circles) or vehicle alone (filled circles) on EJP amplitude. B, Elimination of LTF by EGTA-AM (open circles) compared with controls (filled circles). C, Block of tagging by EGTA-AM: post-tetanic EJP enhancement by forskolin (filled circles) is blocked by ZD 7288 (open circles).D, Post-tetanic reduction of forskolin (FSK)-induced enhancement by ZD 7288 (ZD) after EGTA-AM treatment (oblique hatched bar) resembles reduction of unconditioned responses in forskolin, indicating block of tagging. Eighty and 90% [Ca2+]e do not affect tagging (vertical and horizontal hatched bars, respectively). E, Effects of 10 or 20% reduction of [Ca2+]e (90 or 80% [Ca2+]e are indicated by circles and triangles, respectively) on EJPs at 2 Hz. F, Effects of 90 and 80% [Ca2+]e (circles and triangles, respectively) on LTF compared with controls in normal [Ca2+]e (filled circles). G, H, Ninety and 80% [Ca2+]e leave synaptic tagging intact: forskolin-enhanced responses are unblocked by ZD 7288 (compare with C). In this and subsequent figures, dotted lines indicate LTF levels.
EGTA-AM also eliminated tagging. Post-tetanic EJPs in forskolin (85 ± 6.8% enhancement; n = 5; identical to normal) were sensitive to HCNC block (23 ± 9% enhancement; n = 5) (Fig. 3C); the effect of forskolin was significantly reduced (p < 0.05) by the same amount as unconditioned responses (Fig. 3D).
We also examined the effects of reducing external [Ca2+] ([Ca2+]e) on LTF induction and temporal synaptic tagging. EJP amplitudes at 2 Hz were reduced to 66 ± 3.5 and 44 ± 7.5% of control values in 90 and 80% of normal [Ca2+] (12.2 and 10.8 mm), respectively (Fig. 3E), and tetanic EJPs were similarly reduced. LTF appeared to be somewhat reduced in 90% [Ca2+] (28 ± 7% EJP enhancement; n = 7; p = 0.12 compared with LTF in normal medium), whereas it was abolished in 80% [Ca2+] (6 ± 7% EJP enhancement; n = 5; p < 0.02) (Fig. 3F-H). Synaptic tagging was resistant to these treatments; post-tetanic responses in forskolin remained enhanced by 86 ± 15% (n = 7) in 90% [Ca2+] (Fig. 3G) and by 97 ± 14% (n = 5) in 80% [Ca2+] (Fig. 3H) in the presence of 30 μm ZD 7288 (Fig. 3D). These enhancements were similar (p ≥ 0.3) to that seen in normal medium (77 ± 8.7%; n = 6). Thus LTF appeared somewhat more sensitive to reduction of tetanic Ca2+ influx than did synaptic tagging.
HCNC activation precedes actin action in inducing tagging
Previous work showed a tight association between the functional integrity of the actin cytoskeleton and HCNC induction of synaptic enhancement. Three different HCNC inhibitors and three actin depolymerizers blocked the cAMP-dependent enhancement of synaptic transmission and the induction of LTF. Moreover, either treatment prevented the establishment of a synaptic tag (Beaumont et al., 2002), so that both HCNC activation and an integral actin cytoskeleton were required for all cAMP- and activity-dependent enhancement and tagging. The relationship between actin and HCNCs remains unclear. Activation of HCNCs alone (by cAMP, by hyperpolarization caused by electrical activity, or by the HCNC activator lamotrigine) was insufficient to induce synaptic enhancement or tagging (Beaumont et al., 2002; our unpublished observations). Furthermore, actin depolymerization, especially by latrunculin B, was able to prevent tagging as well as both forms of synaptic enhancement without affecting HCNC function. These results suggested that HCNC activation might precede some action dependent on actin in the sequence of events leading to synaptic enhancement.
This issue was examined further by exploring the effect of the actin polymerization activator jasplakinolide on synaptic transmission. At a concentration of 1 μm, jasplakinolide had no effect on basal EJP amplitude (Fig. 4A). EJP enhancement by forskolin (92 ± 9.6% increase; n = 4), its reduction by ZD 7288 (to 28 ± 11% increase; n = 4), and the magnitude of LTF (56 ± 7% enhancement; n = 4) (Fig. 4B) were all normal. However, jasplakinolide did appear to substitute for HCNC activation in inducing synaptic tagging. Normally, if ZD 7288 is present throughout an experiment, there is no LTF induction by a tetanus and no tagging, in that subsequent enhancement by forskolin remains sensitive to block by ZD 7288 (Beaumont et al., 2002). However, if jasplakinolide was present during the tetanus, the subsequent enhancement of responses by forskolin was unaffected by ZD 7288 (104 ± 22%; n = 7) (Fig. 4C). If jasplakinolide was added after the tetanus, responses in forskolin were strongly reduced by ZD 7288 (28 ± 12% enhancement; n = 3) (Fig. 4D). When compared with EJPs in forskolin in identical experiments, with ZD 7288 present throughout but no jasplakinolide (12 ± 15% enhancement; n = 3) (Beaumont et al., 2002), the responses with jasplakinolide present during the tetanus were significantly larger (p < 0.05), whereas those with jasplakinolide added afterward were not (p > 0.5). Thus, jasplakinolide acted during the tetanus to induce tagging, rather than afterward to affect its expression.
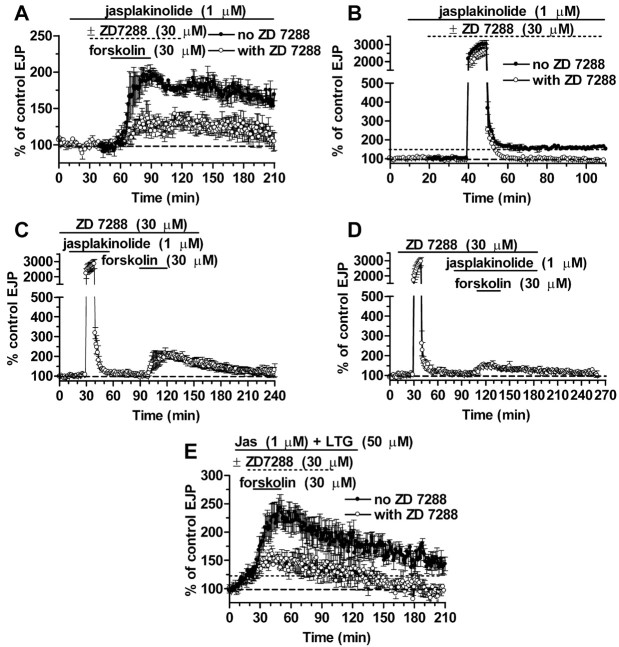
Figure 4.
Enhancing actin polymerization with jasplakinolide substitutes for HCNC activation to induce tagging. A, Jasplakinolide has no effect on synaptic enhancement by forskolin (filled circles) or its block by ZD 7288 (open circles). B, Induction of LTF (filled circles) and its block by ZD 7288 (open circles) are also unaffected. C, Exposure to jasplakinolide during tetanization rescues synaptic tagging from ZD 7288 block. D, Jasplakinolide is ineffective in blocking the expression of tagging once it has been induced. E, Forskolin induced a large increase in EJP amplitudes in the presence of jasplakinolide (Jas) and lamotrigine (LTG). This coactivation of actin polymerization and HCNCs without Ca2+ influx did not induce tagging, because the enhancement was reduced by 77% in ZD 7288.
Together, the lack of effect of latrunculin B on HCNCs, the ability of actin depolymerization to block effects of HCNC activation, the inability of HCNC activation alone to produce effects, and the ability of jasplakinolide to “rescue” the block of tagging by ZD 7288 suggest that, at least with respect to synaptic tagging, actin exerts its effects on synaptic transmission at a step downstream from HCNC activity.
Interestingly, although jasplakinolide was able to induce tagging without HCNC activation, it did not induce LTF without HCNC activation (Fig. 4B,C). Post-tetanic EJPs showed no enhancement (change of -0.75 ± 2.4%; n = 8) when jasplakinolide and ZD 7288 were both present in the tetanus. Thus, although jasplakinolide was able to rescue the block of tagging by ZD 7288, it could not rescue the block of LTF.
In the previous section, we concluded that a presynaptic Ca2+ accumulation is essential for the induction of tagging. We expected, then, that simultaneous activation of HCNCs with enhancement of actin polymerization would not induce tagging without concurrent presynaptic [Ca2+]i elevation. To test this prediction, we used jasplakinolide to augment actin polymerization and lamotrigine to activate HCN channels without either cAMP elevation or tetanic stimulation. Lamotrigine produces a hyperpolarizing shift in HCN activation, increasing the magnitude of HCN current at resting potentials (Poolos et al., 2002).
Our prediction was confirmed by the experiments of Figure 4E, showing that forskolin-induced enhancement of EJPs was still sensitive to ZD 7288 in synapses pretreated with 1 μm jasplakinolide plus 50 μm lamotrigine. This pretreatment caused a modest increase in EJP amplitude (38 ± 9.6%; n = 4), comparable with the effects of lamotrigine alone, as a result of HCNC activation (our unpublished observations). Subsequent addition of forskolin produced an additional 90 ± 16% enhancement (n = 4) attributable to recruitment of the HCNC-independent pathway activated by cAMP elevation (our unpublished observations). The enhancement of responses in forskolin was cut to an increase of only 21 ± 7% when ZD 7288 was also present (n = 4), so that the synapses were not tagged (p < 0.01). Apparently, jasplakinolide can substitute for HCNC activation in generating a tag but not for a rise in presynaptic [Ca2+]i. Thus, it can rescue the induction of tagging from ZD 7288 blockade, but tetanic stimulation is still required to elevate [Ca2+]i.
HCNCs are unaltered after induction of synaptic tagging
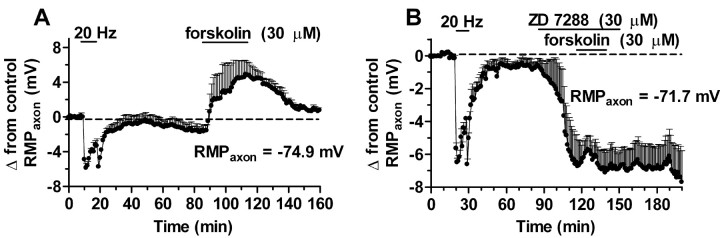
In synaptic tagging, tetanic stimulation that activates HCNCs renders the subsequent cAMP-dependent enhancement of transmission independent of additional HCNC activation. Thus, forskolin or 8-bromo-cAMP (8-Br-cAMP) enhancement of EJPs becomes insensitive to HCNC blockers such as ZD 7288, DK-AH 260, or Cs+ and to actin depolymerizers such as latrunculin B and cytochalasin D (Beaumont et al., 2002). It seemed possible that the abolishment of the requirement for additional HCNC activation might reflect some alteration in the behavior of HCNCs, particularly in their response to cAMP. To test for this possibility, we recorded the effect of forskolin on presynaptic membrane potential after tetanic stimulation. We found that forskolin induced the same presynaptic depolarization (5.8 ± 1.5 mV; n = 3) (Fig. 5A) after induction of tagging as the depolarization seen without previous tetanization (6 mV) (Beaumont and Zucker, 2000). This depolarization is attributable to activation of HCNCs, because it is blocked by ZD 7288 (Fig. 5B), just as it is without previous tetanization (Beaumont and Zucker, 2000). Finally, ZD 7288 hyperpolarizes presynaptic terminals after a tetanus by 6.1 ± 1 mV (n = 3) (Fig. 5B) by blocking the resting activity of HCNCs, just as it does without tetanization (Beaumont and Zucker, 2000). These results make it unlikely that synaptic tagging is the consequence of some alteration in the sensitivity of HCNCs to cAMP.
Figure 5.
Responses of HCNCs to forskolin and ZD 7288 after tetanization are normal. A, After a 10 min, 20 Hz tetanus that hyperpolarizes the presynaptic terminals, forskolin depolarizes them by ∼6 mV. B, ZD 7288 blocks this effect, and the drug itself induces a 6 mV hyperpolarization as a result of resting activation of HCNCs. These effects are identical to those seen without tetanization (Beaumont and Zucker, 2000). RMP, Resting membrane potential.
Discussion
Synaptic tagging lasts for >1 d and is produced by the post-tetanic activation of HCNCs. Only in the post-tetanic period does the electrogenic Na+-K+ pump, driven by the tetanic accumulation of [Na]i (Beaumont et al., 2002), hyperpolarize the presynaptic membrane by a substantial amount (∼5 mV) for a substantial period (several minutes) without continual interruption by action potentials and their depolarizing afterpotentials during the tetanus. Thus, preventing this post-tetanic activation of HCNCs, either by blocking the HCNCs immediately after the tetanus or by reversing the post-tetanic hyperpolarization with elevated [K+], blocks the induction of tagging, and subsequent responses in forskolin remain sensitive to HCNC inhibition.
Experiments using EGTA-AM showed that tagging as well as LTF induction depend on the accumulation of presynaptic Ca2+ in a tetanus, and that a modest reduction in this accumulation is sufficient to prevent tagging. These results extend previous observations (Beaumont et al., 2002) that tetanic stimulation in a Ca2+-free medium induces no tagging, but in the present results there was only a modest reduction in transmitter release during the tetanus, by ∼40%, in EGTA-AM. Thus, it is not some step related to secretion that is required for tagging but rather some other Ca2+-dependent process that remains to be identified.
We also showed that tagged synapses that are insensitive to HCNC block in responding to a cAMP elevation respond normally to activation by cAMP and inhibition by ZD 7288, indicating that tagging is not caused by some major alteration in the pharmacological properties of HCNC channels.
Tagging requires the concurrence of three events: an activation of HCNCs, presynaptic Ca2+ influx in a tetanus sufficient to elevate [Ca2+]i to some threshold level, and the integrity of the actin cytoskeleton. Thus, reducing [Ca2+]i accumulation, blocking HCNC activation, or depolymerization of actin filaments each prevents tagging, and forskolin is unable to induce tagging because it does not produce a significant [Ca2+]i elevation (Beaumont et al., 2002). We have now found that enhancement of actin polymerization by jasplakinolide can substitute for HCNC activation, but not for presynaptic [Ca2+]i elevation, in generating a temporal synaptic tag. Because some actin depolymerizers that block tagging have no effect on HCNC activation and because actin polymerizers can substitute for HCNC activation [although HCNC activation cannot substitute for actin polymerization (Beaumont et al., 2002)], we believe that some actin-dependent step, probably involving the turnover of actin filaments, follows the step of HCNC activation in the induction of tagging.
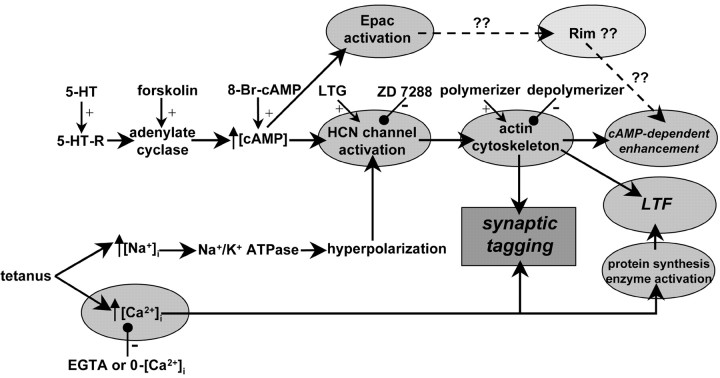
Figure 6 presents our current hypothetical scheme for the roles of Ca2+ elevation and HCNC activation in a tetanus plus actin polymerization in generating a temporal synaptic tag. Tagged synapses no longer require cAMP to activate HCNCs but instead require a separate action of cAMP on an HCN-independent target to produce an effect of forskolin. In tagged synapses, the HCN- and actin-dependent switch has already been set, and only Epac activation by cAMP is required for enhancement of transmission (our unpublished observations). In contrast, in untagged or naive synapses, cAMP must activate both HCNCs and Epac to fully potentiate transmitter release to action potentials.
Figure 6.
A schematic of the steps involved in induction of serotonergic (cAMP-dependent) enhancement of transmission, tetanic activation of LTF, and synaptic tagging. cAMP enhances transmission by activating Epac and HCNCs, the latter acting through an actin-dependent step. LTF induction involves tetanic elevation of [Na+]i, hyperpolarization of Na+-K+ exchange, and HCNC activation, the latter again probably acting through an actin-dependent step, as well as a rise in presynaptic [Ca2+]i, protein synthesis, and a number of enzymatic phosphorylation and dephosphorylation pathways. Tagging represents the previous activation of HCNCs by conditioning stimulation with [Ca2+]i elevation that renders subsequent effects of cAMP independent of HCN activation and actin, so that only activation of Epac is required for cAMP to further enhance transmission. 5-HT-R, 5-HT (serotonin) receptor; LTG, lamotrigine.
Figure 6 also shows that tetanic activation of HCNCs and [Ca2+]i elevation result in the induction of actin-dependent LTF of transmitter release. This process displays a number of differences from tagging and the cAMP-dependent enhancement of transmission. Only LTF requires local presynaptic protein synthesis, and only LTF expression depends on the actions of a number of kinases (phosphatidylinositol 3-OH kinase, MAP kinase, and rapamycin-sensitive kinase) and Ca2+-dependent phosphatase (Beaumont et al., 2001). Also, jasplakinolide substitutes for HCNC activation in generating a tag but not in inducing LTF. Finally, LTF is blocked in Ca2+-free medium, by EGTA-AM loading, and also by a 10 or 20% reduction in external [Ca2+], whereas tagging is abolished in Ca2+-free medium or by EGTA-AM loading but is resistant to reduction of [Ca2+] by 10-20%. It appears that tagging is less sensitive to a modest reduction in [Ca2+]i accumulation than is LTF.
The downstream targets of HCNC channels and Epac remain to be determined. A strong candidate for Epac is a Rab3 interacting molecule (Rim), because Epac binds to Rim proteins (Ozaki et al., 2000), and Rim2 appears to mediate the regulation of insulin secretion by Epac (Kashima et al., 2001). How HCNC activation regulates secretion remains a mystery. The end result of the cAMP-dependent activation of Epac and HCNCs appears to be an increase in the number of vesicles available for release (Wang and Zucker, 1998).
It is important to keep in mind the distinction between what we call temporal synaptic tagging and processes observed at mammalian hippocampal and Aplysia synapses. In the hippocampus, a weakly stimulated input can “capture” the enhancing effects of a separate strongly stimulated input to a postsynaptic cell, and this “spatial tagging” of the weak input occurs without local protein synthesis at that input (Frey and Morris, 1997). In Aplysia, a process exists in which a weakly stimulated terminal of a presynaptic neuron can capture the enhancing effects of a different strongly stimulated terminal onto another postsynaptic cell; the immediate “marking” of the weakly stimulated synapse was also independent of protein synthesis (Martin et al., 1997). Because in both cases transcription and translation are required for the effects of the strong stimulation, which were captured by the weak stimulus, it was proposed that the weak stimulus produced a post-translational protein alteration that captured a protein product of the strong stimulation to sensitize the weak synapse. Although these phenomena have some similarities to temporal synaptic tagging (for example in the initial independence of the tagging or marking process of protein synthesis), they are unlikely to involve the same mechanisms as in our preparation. In our experiments, there is no opportunity for transcription at all, because we work with neurons isolated from their nuclei, so no analog to the protein product of strong stimulation is likely to exist. We are also not dealing with a process known to mark synapses for synapse-specificity of plasticity, which is what spatial tagging or marking is about.
However, it is also unlikely that the processes we are studying are unique to crayfish. For example, Epac has been implicated recently (Sakaba and Neher, 2003) in altering the recruitment of vesicles into the releasable pool in a way that appears similar to what is occurring at crayfish neuromuscular junctions, and it has been suggested that HCNCs regulate transmission at retinal bipolar cells (Muller et al., 2003) and cerebellar inhibitory inter-neurons (Southan et al., 2000).
Footnotes
This work was supported by National Science Foundation Grant 0074842 and National Institutes of Health Grant NS15114. We thank Dr. Kevin Staras for valuable discussion.
Correspondence should be addressed to Robert S. Zucker, University of California, Molecular and Cell Biology Department, 111 Life Sciences Addition, Berkeley, CA 94720-3200. E-mail: zucker@socrates.berkeley.edu.
Copyright © 2004 Society for Neuroscience 0270-6474/04/244205-08$15.00/0
References
- Atwood HL, Wojtowicz JM (1986) Short-term and long-term plasticity and physiological differentiation of crustacean motor synapses. Int Rev Neurobiol 28: 275-362. [DOI] [PubMed] [Google Scholar]
- Beaumont V, Zucker RS (2000) Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic Ih channels. Nat Neurosci 3: 133-141. [DOI] [PubMed] [Google Scholar]
- Beaumont V, Zhong N, Fletcher R, Froemke RC, Zucker RS (2001) Phosphorylation and local presynaptic protein synthesis in calcium- and calcineurin-dependent induction of crayfish long-term facilitation. Neuron 32: 489-501. [DOI] [PubMed] [Google Scholar]
- Beaumont V, Zhong N, Froemke RC, Ball RW, Zucker RS (2002) Temporal synaptic tagging by Ih activation and actin: involvement in long-term facilitation and cAMP-induced synaptic enhancement. Neuron 33: 601-613. [DOI] [PubMed] [Google Scholar]
- Delaney K, Tank DW, Zucker RS (1991) Presynaptic calcium and serotonin-mediated enhancement of transmitter release at crayfish neuromuscular junction. J Neurosci 11: 2631-2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney KR, Tank DW (1994) A quantitative measurement of the dependence of short-term synaptic enhancement on presynaptic residual calcium. J Neurosci 14: 5885-5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egid K, Lnenicka GA (1993) Regeneration from crayfish phasic and tonic motor axons in vitro. J Neurobiol 24: 1111-1120. [DOI] [PubMed] [Google Scholar]
- Enserink JM, Christensen AE, de Rooij J, van Triest M, Schwede F, Genieser HG, Doskeland SO, Blank JL, Bos JL (2002) A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol 4: 901-906. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RG (1997) Synaptic tagging and long-term potentiation. Nature 385: 533-536. [DOI] [PubMed] [Google Scholar]
- Kashima Y, Miki T, Shibasaki T, Ozaki N, Miyazaki M, Yano H, Seino S (2001) Critical role of cAMP-GEFII-Rim2 complex in incretin-potentiated insulin secretion. J Biol Chem 276: 46046-46053. [DOI] [PubMed] [Google Scholar]
- Martin KC, Casadio A, Zhu H, Yaping E, Rose JC, Chen M, Bailey CH, Kandel ER (1997) Synapse-specific, long-term facilitation of Aplysia sensory to motor synapses: a function for local protein synthesis in memory storage. Cell 91: 927-938. [DOI] [PubMed] [Google Scholar]
- Muller F, Scholten A, Ivanova E, Haverkamp S, Kremmer E, Kaupp UB (2003) HCN channels are expressed differentially in retinal bipolar cells and concentrated at synaptic terminals. Eur J Neurosci 17: 2084-2096. [DOI] [PubMed] [Google Scholar]
- Ozaki N, Shibasaki T, Kashima Y, Miki T, Takahashi K, Ueno H, Sunaga Y, Yano H, Matsuura Y, Iwanaga T, Takai Y, Seino S (2000) cAMP-GEFII is a direct target of cAMP in regulated exocytosis. Nat Cell Biol 2: 805-811. [DOI] [PubMed] [Google Scholar]
- Poolos NP, Migliore M, Johnston D (2002) Pharmacological upregulation of h-channels reduces the excitability of pyramidal neuron dendrites. Nat Neurosci 5: 767-774. [DOI] [PubMed] [Google Scholar]
- Sakaba T, Neher E (2003) Direct modulation of synaptic vesicle priming by GABAB receptor activation at a glutamatergic synapse. Nature 424: 775-778. [DOI] [PubMed] [Google Scholar]
- Southan AP, Morris NP, Stephens GJ, Robertson B (2000) Hyperpolarization-activated currents in presynaptic terminals of mouse cerebellar basket cells. J Physiol (Lond) 526: 91-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Zucker RS (1998) Regulation of synaptic vesicle recycling by calcium and serotonin. Neuron 21: 155-167. [DOI] [PubMed] [Google Scholar]
- Wiersma CAG (1941) The inhibitory nerve supply of the leg muscles of different decapod crustaceans. J Comp Neurol 74: 63-79. [Google Scholar]
- Zhong N, Beaumont V, Zucker RS (2001) Roles for mitochondrial and reverse mode Na+/Ca2+ exchange and the plasmalemma Ca2+ ATPase in post-tetanic potentiation at crayfish neuromuscular junctions. J Neurosci 21: 9598-9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG (2002) Short-term synaptic plasticity. Annu Rev Physiol 64: 355-405. [DOI] [PubMed] [Google Scholar]