Abstract
Intracellular amyloidβ peptide (iAβ1-42) accumulates in the Alzheimer's disease brain before plaque and tangle formation (Gouras et al., 2000) and is extremely toxic to human neurons (Zhang et al., 2002). Here, we investigated whether androgen and estrogen could prevent iAβ1-42 toxicity, because both these hormones have a wide range of neuroprotective actions. At physiological concentrations, 17-β-estradiol, testosterone, and methyl testosterone reduce iAβ1-42-induced cell death by 50% in neurons treated after the injection and by 80-90% in neurons treated 1 hr before the injection. The neuroprotective action of the hormones is mediated by receptors, because the estrogen receptor (ER) antagonist tamoxifen and the androgen receptor (AR) antagonist flutamide completely block the estrogen- and androgen-mediated neuroprotection, respectively. Transcriptional activity is required for the neuroprotective action, because dominant negative forms of the receptors that block the transcriptional activity of the ER and AR prevent estrogen- and androgen-mediated neuroprotection. Proteomics followed by Western blot analyses identified increased levels of heat shock protein 70 (Hsp70) in testosterone- and estrogen-treated human neurons. Comicroinjection of Hsp70 with the iAβ1-42 blocks the toxicity of iAβ1-42. We conclude that estrogen and androgens protect human neurons against iAβ1-42 toxicity by increasing the levels of Hsp70 in the neurons.
Keywords: intracellular amyloid, estrogen, androgen, neuroprotection, human neurons, Hsp70
Introduction
Intracellular amyloid β1-42 (iAβ1-42) accumulates in the hippocampus and the entorhinal cortex neurons of mildly cognitively impaired and Alzheimer's disease (AD) individuals (Gouras et al., 2000; D'Andrea et al., 2001, 2002; Takahashi et al., 2002), in Down's syndrome brain neurons (Gyure et al., 2001; Busciglio et al., 2002), and in inclusion body myositis muscle cells (Querfurth et al., 2001; Sugarman et al., 2002). In AD, the accumulation of iAβ1-42 precedes amyloid plaque formation (Gouras et al., 2000; D'Andrea et al., 2001). Cytosolically microinjected Aβ1-42 is selectively and highly toxic to human neurons in primary cultures (Zhang et al., 2002). In vivo, neuronal cytosolic Aβ1-42 transgenic expression induces neuronal loss in the cerebral cortex, hippocampus, and thalamus (LaFerla et al., 1995). Together, these results suggest that the accumulation of iAβ1-42 is an early event in AD that could result in the dysfunction and eventual loss of neurons. Therefore, it is of interest to find inhibitors of intracellular Aβ1-42-mediated toxicity that may be useful as an early treatment in AD patients. In the present manuscript, we investigate the potential of androgens and estrogens as inhibitors of intracellular Aβ1-42-mediated toxicity in human neurons.
Despite controversy in vivo, there is strong evidence that both estrogens and androgens are neuroprotective in cell culture. Testosterone protects primary human neurons against serum deprivation (Hammond et al., 2001), cultured rat hippocampal neurons against extracellular Aβ toxicity (Pike, 2001), rat neurons against heat shock-mediated hyperphosphorylation of tau by modulating glycogen synthase kinase 3β activation (Papasozomenos and Shanavas, 2002), cerebellar granule neurons against oxidative stress (Ahlbom et al., 1999, 2001), and rat hippocampal neurons against kainic acid-induced toxicity (Ramsden et al., 2003); testosterone also promotes neuritic extension in pheochromocytoma 12 cells (Lustig et al., 1994). Testosterone can protect through the androgen receptor (AR) rather than through aromatization into estrogen (Ahlbom et al., 2001; Hammond et al., 2001; Ramsden et al., 2003). Estrogen prevents caspase-6-mediated human neuronal cell death (Zhang et al., 2001), decreases Aβ toxicity in cultured rat primary hippocampal neurons and human neuroblastoma SK-N-SH cells (Goodman et al., 1996; Green et al., 1996; Pike, 1999), inhibits serum deprivation-induced cell death in SK-N-SH cells (Goodman et al., 1996; Green et al., 1997), and protects against oxidative stress in mouse hippocampal neurons and neuronal injury induced by hemoglobin, chemical hypoxia, and excitatory amino acids (Behl et al.,1995, 1997). These steroid sex hormones can protect through activation of transcriptional activity or via signaling of survival pathways (Driggers and Segars, 2002; Heinlein and Chang, 2002; Segars and Driggers, 2002).
In the present study, we demonstrate that physiological concentrations of androgens or estrogens completely protect human neurons in primary cultures against microinjected iAβ1-42 toxicity. Using AR and estrogen receptor (ER) antagonists and dominant negative forms of these receptors, we show that the neuroprotection occurs in a receptor- and transcription-dependent manner. Proteomics studies followed by Western blotting identify an increase in heat shock protein 70 (Hsp70) levels in testosterone- and estrogen-treated neurons. Hsp70 comicroinjected with iAβ1-42 also completely prevents iAβ1-42 toxicity. We conclude that testosterone and estrogen can protect against iAβ1-42 through Hsp70.
Materials and Methods
Aβ peptides, recombinant proteins, antibodies, and cDNAs
Aβ peptides (Bachem, King of Prussia, PA) were dissolved in sterile distilled water at 25 μm and incubated at 37°C for 5 d (Zhang et al., 2002). The peptide stock solutions were kept frozen in aliquots until use. The recombinant human Hsp70 protein and monoclonal anti-Hsp70 antibody were purchased from Stressgen Biotech (Victoria, British Columbia, Canada). The wild-type and mutant 12474 and 15579 human androgen receptor cDNAs have been described previously. The AR mutants, 12474 and 15579, contain a deletion of 579Val and 580Phe and of 614Cys and 615Arg in the DNA binding domain (DBD), respectively (Beitel et al., 1994). All cDNAs were cloned into the vector pcDNA3 under the cytomegalovirus promoter (Panet-Raymond et al., 2000). The human ER wild-type and mutant ΔDBD cDNAs were obtained from Dr. Sylvie Mader (University of Montreal, Montreal, Quebec, Canada). The ΔDBD ER construct contains a deletion of amino acids 185-251 (Mader et al., 1993). All ER cDNAs were cloned into the pSG5 vector under the simian virus 40 promoter (Rosenauer et al., 1998). The androgen responsive element (ARE) luciferase reporter vector pGL3-MMTV-(ARE)-Luc and the estrogen responsive element (ERE) luciferase reporter vector pGL3-(ERE)-Luc have been described previously (Panet-Raymond et al., 2000). The cDNAs were purified with the UltraClean-15 DNA Purification kit (MoBio Laboratories Inc., Solana Beach, CA).
Human primary cell cultures and treatments
Primary cultures. Primary cultures of human neurons were prepared from 12- to 16-week-old fetal brains collected as approved by the McGill University Institutional Review Board and according to Canadian Institute of Health Research regulations (LeBlanc, 1995). In brief, fetal cortical brain tissues were dissected free of meninges and blood vessels in PBS (in m: 0.14 NaCl, 0.003 KCl, 0.01 Na2HPO4, and 0.002 KH2PO4, pH 7.2) and dissociated with 0.25% trypsin (Invitrogen, Burlington, Ontario, Canada). The trypsin was inactivated with 10% decomplemented fetal bovine serum (FBS; HyClone, Logan, UT), and released DNA was removed with DNase I (0.1 mg/ml; Sigma, Oakville, Ontario, Canada). The cells were filtered successively through 130 and 70 μm sterilized filters, washed with PBS, and washed with minimal essential medium (MEM) with Earle's balanced salt solution containing 0.225% sodium bicarbonate, 1 mm sodium pyruvate, 2 mm l-glutamine, 0.1% dextrose, 1× antibiotic Pen-Strep (all from Invitrogen), and 5% decomplemented FBS. Cells were then plated on poly-l-lysine-coated plates, flasks, or Aclar coverslips (33°C, 5 mm; Allied Chemical Inc., Minneapolis, MN) at a density of 3 × 106 cells/ml. The cultures were incubated at 37°C with 5% CO2, and the medium was changed every 48 hr. The neurons attached within 24 hr and developed extensive neuritic extensions within 3 d of plating. In general, the cultures contained 90-95% neurons and 5-10% astrocytes (LeBlanc, 1995).
Microinjection of Aβ peptides and cDNA constructs in primary cultures of human neurons. Microinjections were performed 11 d after plating the neurons with the Eppendorf (Fishers, NY) Microinjector 5246 and the three-dimensional Burleigh (Fishers, NY) Micromanipulator MIS-5000 (Zhang et al., 2002). Microinjections were performed with a glass needle with a tip diameter of ∼0.5 μm pulled from 1.0 mm outer diameter and 0.5 mm inner diameter thin-walled glass capillaries with microfilament (borosilicate with filament MTW100F-4; World Precision Instruments, Sarasota, FL) with a Flaming/Brown micropipette puller (model P-87; Sutter Instruments, Novato, CA). Human neurons were injected at an injection pressure of 100 hPa, a compensation pressure of 50 hPa, and an injection time of 0.1 sec. The injected volume was 25 pl. Aβ (10 nm), cDNAs (30 ng/ml), and Hsp70 (5 μg/ml) were coinjected with 100 μg/ml fluorescent marker dye Dextran Texas Red (DTR) (MW3000; Molecular Probes, Eugene, OR) into the cytosolic area of the neuron (Zhang et al., 2002). Approximately 90% of neurons survived the injection. Microinjections were done in 200 neurons per preparation in three independent neuron preparations, for a total of 600 injected neurons.
Treatment of human neurons with estrogen, androgen, tamoxifen, or flutamide. 17-α-Estradiol, 17-β-estradiol, BSA-17-β-estradiol, testosterone enanthate, and epitestosterone were purchased from Sigma. Methyl testosterone was bought from Pharmacopeia (Rockville, MD). Hormones were dissolved in 100% ethanol at a 5 mm concentration and diluted to 2-10 nm with culture medium before use (Hammond et al., 2001). Tamoxifen (TMX; Sigma) and flutamide (Flut; Sigma) were dissolved in sterile distilled water at 10 and 20 mm and serially diluted to the indicated concentration (2-10 nm) with MEM before treatment of neurons (Hammond et al., 2001). For the postincubation experiments, cells were microinjected with peptides or cDNAs and incubated at 37°C with hormones in the presence or absence of hormone receptor antagonists for 24 hr. For the preincubation experiments, cells were incubated at 37°C with hormones and hormone receptor antagonists for 1 hr before the microinjection of peptide followed by incubation with hormones in the presence or absence of hormone receptor antagonists for 24 hr.
Proteomics analyses and Western blot. Neurons were treated with 10 nm testosterone for 1 hr. The cells were washed in 1× PBS and collected in 10 mm Tris-HCl, pH 7.4, and 5 mm MgCl2 for two-dimensional gel analysis. Cells were lysed with two freeze-thaw cycles in liquid nitrogen and extracts treated with 1000 U of DNase (Promega, Madison, WI) and 60 μg of RNase A for 10 min on ice. Proteins were precipitated with methanol-chloroform and solubilized in 9.5 m urea, 2.8% 3-[(3-cholamido-propyl)dimethylammonio]-1-propanesulfonate, 20 mm Tris-Cl, pH 7.4, 50 mm DTT, bromophenol blue, and 1% immobilized pH gradient buffer (Amersham Biosciences, Quebec, Canada). Proteins (100 μg) were separated by isoelectric focusing on IPGPhor IEF-13 cm strips (Amersham Biosciences). After isoelectric focusing separation, the gel strips were equilibrated with 50 mm Tris-Cl, pH 8.8, 6 m urea, 30% glycerol, 2% SDS, and 65 mm DTT buffer and placed on a 10% SDS-polyacrylamide gel for protein separation. The separated proteins were visualized by silver staining compatible with mass spectrometric analysis (Yan et al., 2000). Proteins that increased or decreased compared with untreated neurons were sent for mass spectrometric analysis at the Southern Alberta Mass Spectrometry Center in Calgary, Canada. To confirm increased levels of Hsp70 in human neurons exposed to estrogen and testosterone, 30 μg of proteins extracted from neurons treated with 10 nm estrogen or testosterone for 6 hr were immunoblotted with the monoclonal Hsp70 antibody, and the immunoreactivity was revealed with ECL.
Cell line cultures and treatments
MCF-7 and AR24 cell cultures. Human breast cancer MCF-7 cells (American Type Culture Collection, Manassas, VA) were cultured in RPMI 1640 medium (Invitrogen) with 10% FBS. AR24 cells were created by stably expressing human androgen receptors in rat motor neuron hybrid cells and cultured in DMEM (Invitrogen) with 5% FBS (Brooks et al., 1998). Cells were incubated at 37°C with 5% CO2. The culture media was changed every 48 hr.
Transfection of MCF-7 and AR24 cells. Eighty percent confluent MCF-7 and AR24 cell cultures were washed and incubated at 37°C for 6 hr with 3 μg/ml cDNA and 6 μg/ml Lipofectamine-2000 (Invitrogen) in OPTIMEM (Invitrogen) without FBS. After the incubation, the cells were washed with DMEM-5% FBS and incubated at 37°C for 24 hr in the presence or absence of 10 nm estrogen or androgen.
Luciferase reporter system is used to measure androgen and estrogen receptor activity. The Dual-Luciferase Reporter Assay System kit (E1910; Promega) was used to investigate the dominant negative effects of the mutant AR and ER. The constructs with the firefly (Photnus pyralis) luciferase gene downstream of the ERE or ARE [pGL3-MMTV-(ARE)-Luc and pGL3-(ERE)-Luc] were cotransfected with the wild-type or mutant ER or AR constructs into estrogen receptor-positive MCF-7 or androgen receptor-positive AR24 cells, respectively. After treatment with 10 nm estrogen or testosterone, firefly luminescence was detected as indicated by the manufacturer. To control for transfection efficiency, the Renilla reniformis luciferase construct was cotransfected into cells. The activity was corrected for the protein concentration of each sample and expressed as [(firefly/renilla luciferase) × 10,000]. The luminescence is expressed as relative light units.
Measurement of cell death. Cells were fixed in fresh 4% paraformaldehyde and 4% sucrose in PBS for 20 min at room temperature and permeabilized in 0.1% Triton X-100 and 0.1% sodium citrate in PBS for 2 min on ice. Terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling (TUNEL) was performed using the In Situ Cell Death Detection Kit I as described by the manufacturer (Roche, Quebec, Canada). The TUNEL-stained coverslips were then washed once in distilled water for 5 min and mounted on glass slides. The percentage of cell death was determined by the ratio of the number of DTR-TUNEL-double-positive cells to the total number of DTR-positive cells. All of the coverslips were counted blindly.
Statistical evaluation
One-way ANOVA with post hoc tests (Statview 5.01; SAS Institute, Cary, NC) determined the statistical significance of the difference between treatments. The Sheffé's test was applied as the post hoc analysis comparing data between each treatment group. A p value of <0.05 was taken as the criteria for statistical significance.
Results
Physiological concentrations of estrogen and androgen protect human neurons in primary culture against intracellular Aβ1-42-induced cell death
Human fetal brains express estrogen receptor α and β subtypes and androgen receptor β subtype (Wilson and McPhaul, 1996; Takeyama et al., 2001). Microinjection of Aβ1-42 into the cytosol of neurons induces ∼60% cell death within 24 hr (Fig. 1A,B) (Zhang et al., 2002). Immediate incubation of the injected neurons with 2, 4, or 10 nm 17-β-estradiol, testosterone, and methyl testosterone significantly decreases cell death by 50% as measured by TUNEL and morphological assessments (Fig. 1A,B,D). A 1 hr preincubation with these hormones before the microinjection of Aβ1-42 into neurons completely protects against cell death (Fig. 1C). In contrast, the transcriptionally inactive isoform 17-α-estradiol, membrane-impermeable BSA-conjugated 17-β-estradiol, and the testosterone antagonist epitestosterone do not have any protective effects (Fig. 1A,B). These results show that physiological concentrations of estrogen and androgens (Wilson et al., 1978) protect human neurons against iAβ1-42. The increased protection with preincubation indicates that the hormones alter the state of neuronal susceptibility to the iAβ1-42.
Figure 1.
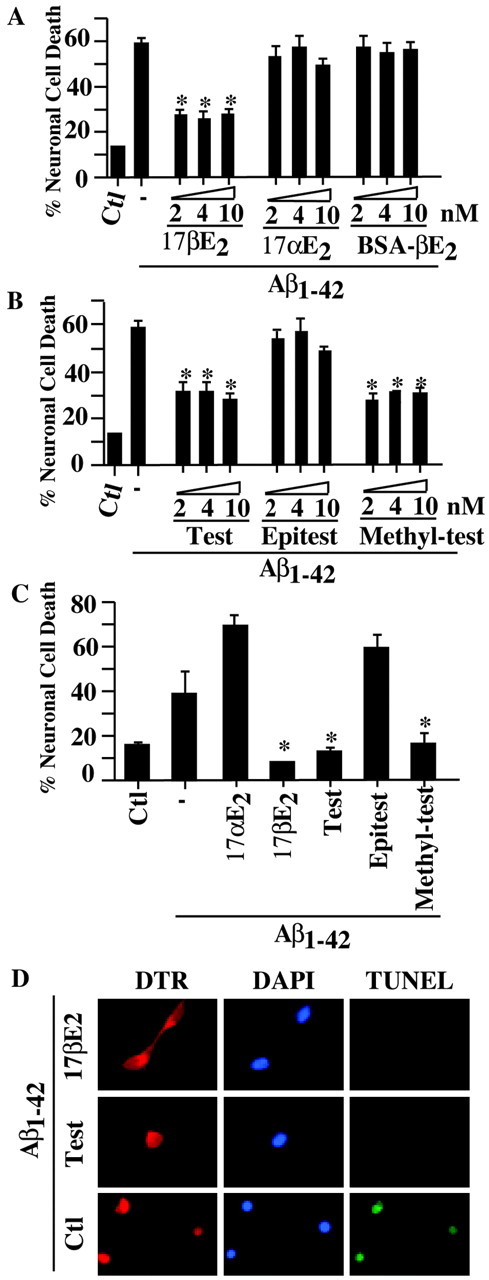
Estrogen and androgens protect human neurons against intracellular Aβ1-42 toxicity. A, B, TUNEL-positive cell death in human neurons in primary cultures injected with 10 nm Aβ1-42 and incubated with 2, 4, and 10 nm 17-β-estradiol (17βE2), 17-α-estradiol (17αE2), BSA-17-β-estradiol (BSA-βE2), testosterone (Test), epitestosterone (Epitest), or methyl testosterone (Methyl-test). Data represent the mean ± SEM of three independent experiments. *p < 0.002 compared with Aβ1-42. C, TUNEL-positive cell death in human neurons preincubated with 10 nm estrogen or androgens for 1 hr before microinjections of iAβ1-42 and further incubated with hormones for 24 hr after the injection. Data represent the mean ± SEM of three independent experiments. *p < 0.001 compared with iAβ1-42 alone. D, Immunofluorescence micrographs of neurons injected with Aβ1-42 and DTR and either left untreated (control) or treated with 2 and 4 nm 17βE2 or testosterone (Test), respectively. The panels show the DTR-injected neurons stained with the DNA stain 4′,6′-diamidino-2-phenylindole (DAPI) or the cell death marker TUNEL. Ctl, Control.
Estrogen and androgen protect against intracellular Aβ through their respective receptors
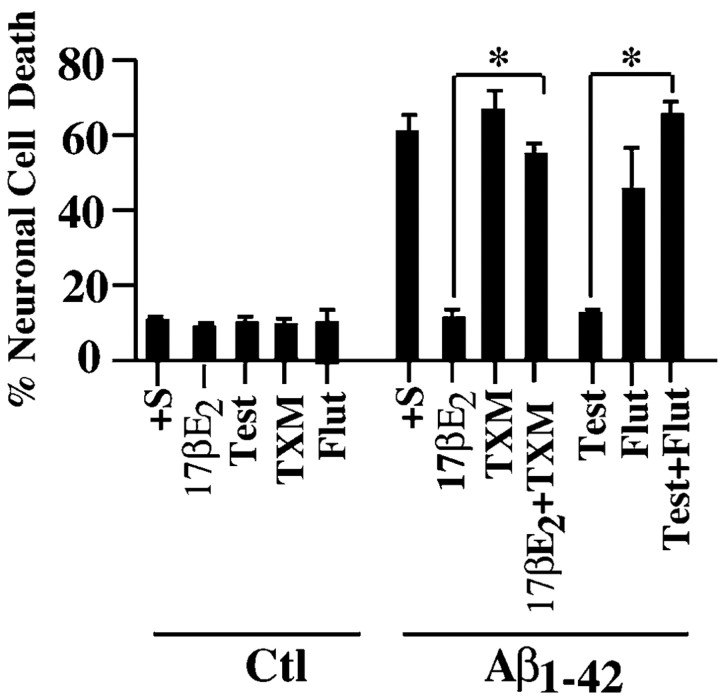
Both estrogen and androgen receptors exist in the primary cultures of human neurons (Hammond et al., 2001; Zhang et al., 2001). However, androgens can also be aromatized into estrogen (Finkelstein et al., 1981). To determine whether each hormone is neuroprotective through its respective receptor, the effect of antagonists on hormone-mediated neuroprotection was evaluated. The estrogen receptor antagonist tamoxifen and the androgen receptor antagonist flutamide completely blocked the protection induced by estrogen and androgen, respectively (Fig. 2). Tamoxifen and flutamide treatments alone do not induce cell death in human neurons. These results show that both hormones are neuroprotective through their respective receptors.
Figure 2.
Estradiol and testosterone protect against iAβ1-42 through their respective receptors. TUNEL-positive cell death in human neurons in primary cultures injected with Aβ1-42 and incubated with TMX and Flut in the absence or presence of 10 nm 17-β-estradiol (17βE2) or testosterone (Test) is shown. Data represent the mean ± SEM of three independent experiments. *p < 0.001 compared with the respective hormonal treatment. Ctl, Control.
Estrogen- and androgen-mediated neuroprotection against iAβ1-42 requires transcriptional activity
To investigate whether the estrogen protection requires gene transcription, we first sought to identify a dominant negative form of the ER using the ERE-luciferase reporter assay in ERα-positive MCF-7 cells. In the absence of 17-β-estradiol, there is a baseline level of estrogen receptor activity in MCF-7 cells that may be induced by trace amounts (20 pm) of estrogen in the culture serum (Fig. 3A). In the presence of 10 nm 17-β-estradiol for 24 hr, luciferase activity increases threefold, consistent with the level found by others in this cell line (Khan et al., 2003). The cotransfected ΔDBD mutant ER construct abolishes estrogen-mediated gene transcription of the ERE-luciferase reporter gene and thus shows a dominant negative effect. Comicroinjection of the ΔDBD mutant, but not the wild-type ER, with Aβ1-42 in human neurons also abolishes the estrogen-mediated neuroprotection against iAβ1-42 (Fig. 3B). Similarly, two AR DBD mutants, 12474 and 15579, were tested as dominant negatives in androgen-responsive AR24 cells (Brooks et al., 1998). These cells were stably transfected with the AR subtype β. After a 24 hr treatment with 10 nm testosterone, the AR activity increases threefold (Fig. 4A), similar to the induction levels observed with the same cDNA constructs in cultured human skin fibroblasts (Nguyen et al., 2001). The mutant 12474 decreases the androgen response by only 30-40%, whereas 15579 completely abolishes the androgen response (Fig. 5A). When injected into human neurons with Aβ1-42, wild-type AR but not mutant ARs decrease Aβ toxicity in untreated or epitestosterone-treated neurons, again indicating a response to serum hormone (Fig. 4B). In neurons treated with testosterone and methyl testosterone, mutant 15579 completely inhibits and 12474 partially inhibits androgen-mediated neuroprotection against iAβ1-42. Therefore, estrogen-and androgen-mediated transcription is necessary for both estrogen- and androgen-regulated neuroprotection against iAβ1-42.
Figure 3.
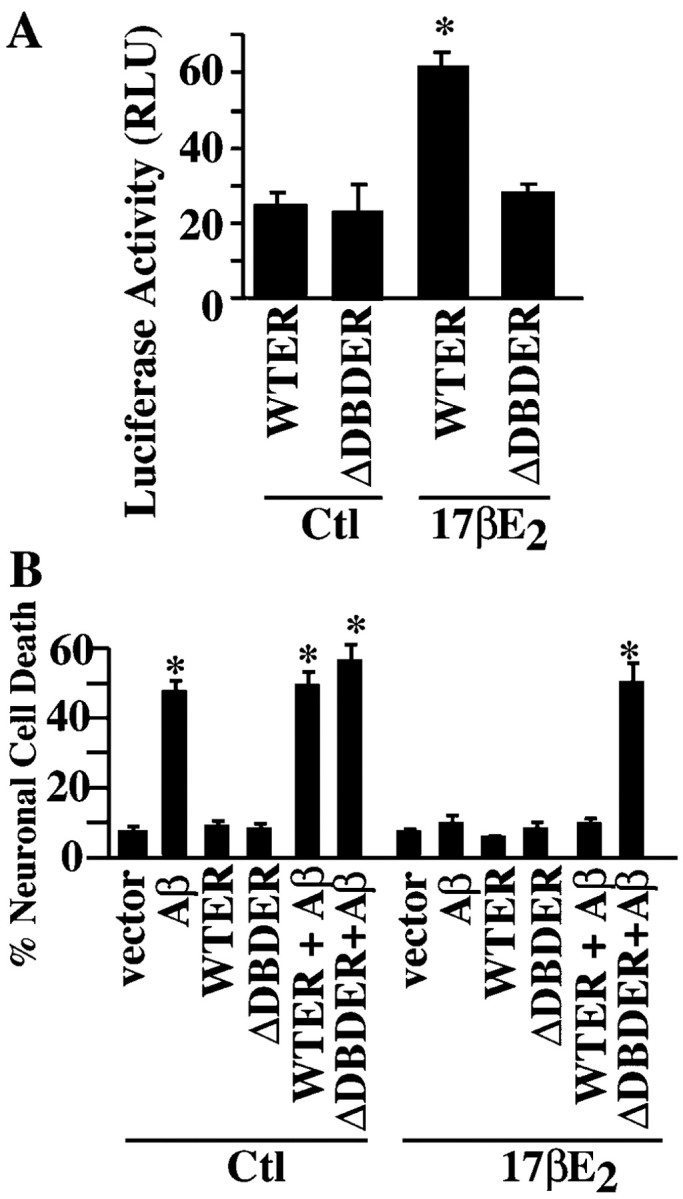
Estrogen requires transcriptional activation to protect against Aβ1-42. A, Estrogen transcriptional activity assay using a luciferase reporter system in MCF-7 cells transfected with wild-type (WTER) or mutant (ΔDBDER) ER and treated without (control) or with 10 nm 17-β-estradiol (17βE2) for 24 hr. Data represent the mean ± SEM of three independent experiments. *p < 0.001 compared with control values. B, TUNEL-positive cell death in primary neurons microinjected with iAβ1-42 and WT-ER or ΔDBD-ER and treated without (control) or with 17βE2. Data represent the mean ± SEM of three independent experiments. *p < 0.02 compared with vector-injected cells. Ctl, Control; RLU, relative light units.
Figure 4.
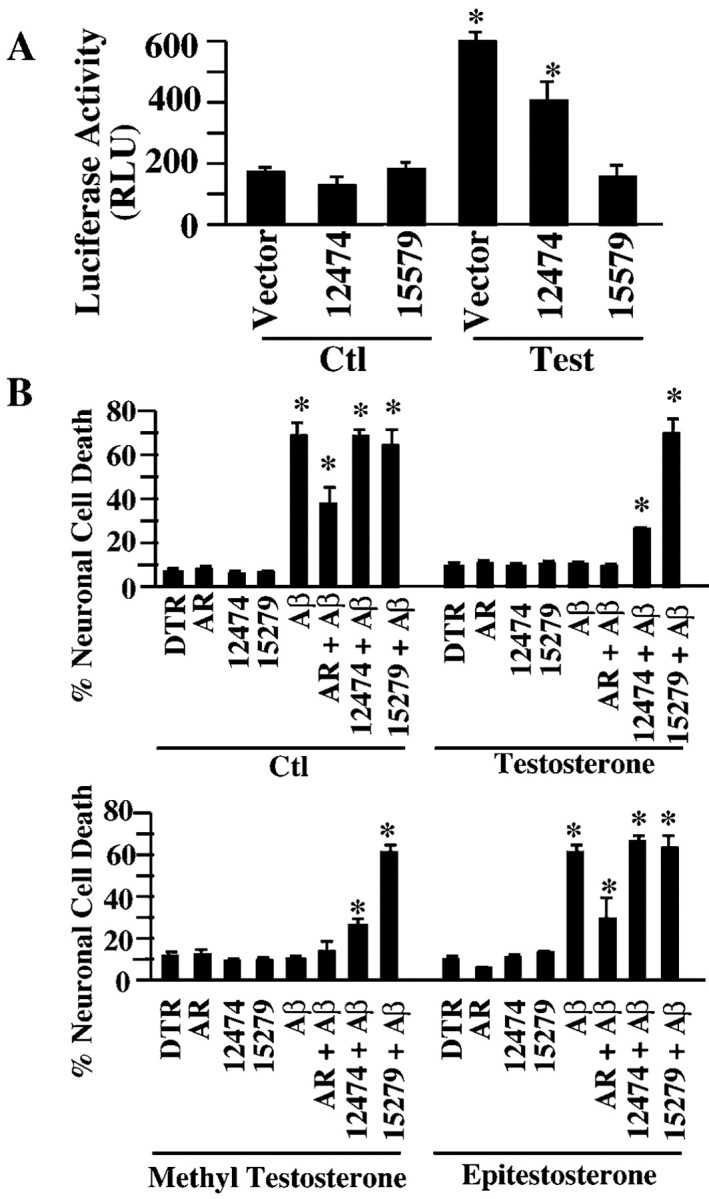
Testosterone requires transcriptional activation to protect against Aβ1-42. A, Luciferase assay for androgen responsive element activity in AR24 cells cotransfected with the AR mutants 15579 and 12474 or empty pcDNA3 vector and treated with 10 nm testosterone for 24 hr. Data represent the mean ± SEM of three independent experiments. *p < 0.01 compared with control. B, TUNEL-positive cell death in neurons comicroinjected with Aβ1-42 and wild-type AR (AR), 12474 AR, or 15279 AR and treated with 10 nm testosterone, methyl testosterone, or epitestosterone. *p < 0.001 compared with DTR-microinjected cells. Ctl, Control.
Figure 5.
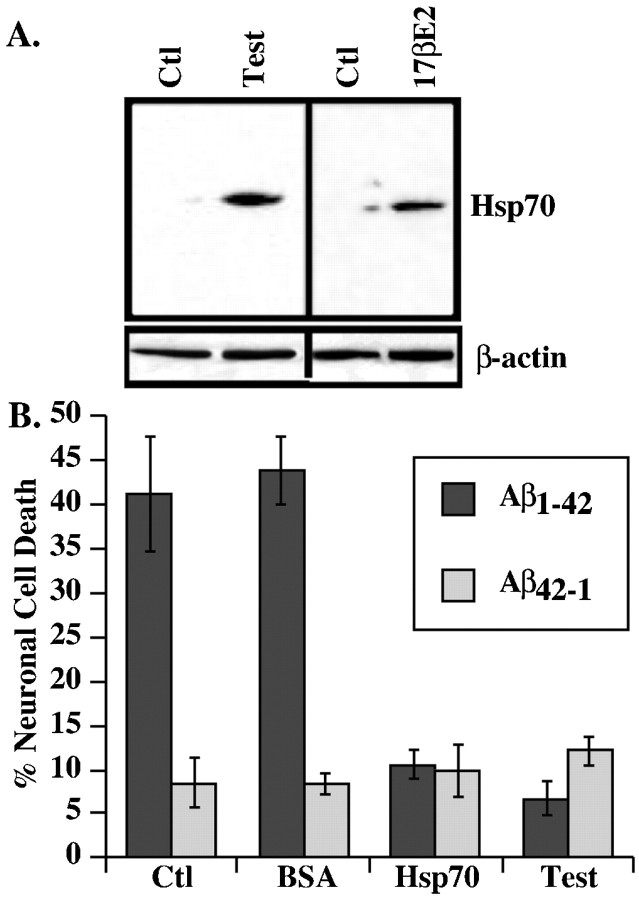
Hsp70 is increased in estrogen- and testosterone-treated neurons and protects against iAβ1-42. A, Western blot analysis of Hsp70 andβ-actin in 30 μg protein extracts from estrogen- or testosterone-treated neurons. B, TUNEL-positive neuronal cell death in neurons comicroinjected with iAβ1-42 and recombinant Hsp70 or control BSA protein compared with testosterone treatment. Ctl, Control.
Androgen and estrogen increase Hsp70 in human neurons, and Hsp70 is sufficient to inhibit iAβ1-42-mediated neurotoxicity
Mass spectrometric analysis of proteins from neurons incubated with testosterone identified Hsp70 with an expectation value of 4 × 10-9. Because Hsp70 has been found to interact with both Aβ1-42 (Fonte et al., 2002) and p53 (King et al., 2001), which is activated by iAβ1-42 in neurons (Zhang et al., 2002), we pursued the potential role of Hsp70 in hormone-mediated neuroprotection. Western blot analyses confirm the increased levels of Hsp70 in estrogen- and androgen-treated neurons (Fig. 5A). Microinjection of recombinant human Hsp70 with iAβ1-42 into primary neurons abolishes iAβ1-42-mediated neurotoxicity to the same extent as with testosterone and estrogen treatments (Fig. 5B). We conclude from these experiments that androgen- and estrogen-mediated increases in Hsp70 cellular levels are sufficient to modulate estrogen- and androgen-mediated protection against iAβ1-42.
Discussion
In this study, we show that: (1) physiological concentrations of both estrogen and androgen completely protect human primary neurons against iAβ1-42 toxicity, (2) estrogen and androgens are neuroprotective through their respective receptors, (3) estrogen-and androgen-mediated protection against iAβ1-42 depends on transcriptional activation, and (4) Hsp70, which is increased in estrogen- and testosterone-treated neurons, completely protects against iAβ1-42-mediated toxicity.
The neuroprotective role of physiological concentrations of androgens and estrogens against iAβ1-42 neurotoxicity is an important finding, because iAβ1-42 accumulation in AD neurons precedes other pathological hallmarks and is an early event that likely leads to neuronal dysfunction and cell death (Gouras et al., 2000; Takahashi et al., 2002; Zhang et al., 2002). Except for inhibitors of β- or γ-secretases to prevent the production of amyloid, most efforts to inhibit or remove amyloid toxicity, including vaccines, protease treatments, and anti-fibril agents, are directed at the extracellular amyloid but not intracellular amyloid toxicity. Our results indicate that sex steroids could play an important role in the early treatment of AD by preventing potential intracellular amyloid toxicity. There is presently much controversial evidence on the use of sex steroids against neurodegeneration. However, the strong neuroprotective role of these hormones shown in this study justifies additional investigations on their potential use in neurodegenerative diseases. Our results indicate that potential problems incurred in clinical trials may be avoided. First, the use of physiological levels of hormones would likely be sufficient for the protective action and may avoid undue complications caused by pharmacological doses of the hormones. Second, because both estrogens and androgens protect equally well through their respective receptors, both men and women can be treated with lower side effects. Third, understanding the underlying molecular mechanism of the action of these hormones may help target a pathway that is involved in neuroprotection against iAβ1-42 toxicity while avoiding the multiple potentially detrimental actions of these hormones.
Increased levels of Hsp70 are not unique to estrogen- and androgen-treated neurons. Several studies have found increased expression of Hsp70 in various hormone-treated cell types (Tang et al., 1995; Jones et al., 2000; Lu et al., 2002; Gehring, 2004). The fact that Hsp70 can completely prevent iAβ1-42-mediated toxicity indicates only that Hsp70 is sufficient for neuroprotection against iAβ1-42 toxicity but does not rule out the possibility that other effects of the steroid hormones on neurons could also be neuroprotective. However, our results corroborate those showing that Hsp70 can protect against polyglutamine toxicity (Warrick et al., 1998; Jana et al., 2000; Cummings et al., 2001), α-synuclein toxicity (Auluck et al., 2002), and iAβ1-42 toxicity (Fonte et al., 2002; Magrane et al., 2004) in flies, cell cultures, and mice, respectively. Most importantly, prevention by Hsp70 of a progressive paralysis phenotype caused by overexpression of iAβ1-42 in muscle cells of Caenorhabditis elegans indicates a functional protection against iAβ1-42 (Fonte et al., 2002).
The molecular mechanism underlying Hsp70 neuroprotection against neurodegenerative conditions is not clear. In our system, we have demonstrated previously that iAβ1-42 induces human neuronal apoptosis through the activation of p53 and Bax (Zhang et al., 2002). Hsp70 could sequester p53 in the cytosol and prevent its translocation to the nucleus and activation of apoptosis (King et al., 2001; Zylicz et al., 2001). An alternative possibility is that Hsp70 interacts directly with iAβ1-42. Fonte et al. (2002) have shown interaction between iAβ1-42 and Hsp70 in C. elegans that is consistent with the ability of Hsp70 to bind peptides and denatured proteins. Finally, Hsp70 has been implicated recently in steroid-mediated transcriptional activation by forming a complex with Bag-1 and steroids (Gehring, 2004). Hsp70 may enhance steroid-mediated transcriptional activation of prosurvival genes. These are issues that will be difficult to resolve in human neurons considering the natural predisposition of Aβ1-42 to form aggregates that can interact nonspecifically with proteins and the resistance of human neurons in culture to infections or transfections, thereby limiting our studies to single-cell analyses (our unpublished observations).
In summary, we have shown that physiological concentrations of estrogen and androgens protect human neurons from iAβ1-42-mediated toxicity and increase Hsp70 in these neurons. Hsp70 completely protects against iAβ1-42-mediated toxicity. This study therefore identifies in Hsp70 a common downstream target of estrogen and androgen that is involved in neuroprotection.
Footnotes
This work was funded by the Canadian Institute for Health Research, the National Institutes of Health, the Fond de Rechercheen Santé du Québec (A.L.), and the Alzheimer Society of Canada (Y.Z.).We are grateful for the technical support of Jennifer Hammond pertaining to cell cultures. We thank Dr. Sylvie Mader for the ER wild-type and mutant constructs.
Correspondence should be addressed to Dr. Andréa LeBlanc, The Bloomfield Center for Research in Aging, Lady Davis Institute for Medical Research, The Sir Mortimer B. Davis Jewish General Hospital, 3755 Chemin Côte Ste-Catherine, Montréal, Québec, Canada, H3T 1E2. E-mail:andrea.leblanc@mcgill.ca.
Copyright © 2004 Society for Neuroscience 0270-6474/04/245315-07$15.00/0
References
- Ahlbom E, Grandison L, Bonfoco E, Zhivotovsky B, Ceccatelli S (1999) Androgen treatment of neonatal rats decreases susceptibility of cerebellar granule neurons to oxidative stress in vitro Eur J Neurosci 11: 1285-1291. [DOI] [PubMed] [Google Scholar]
- Ahlbom E, Prins GS, Ceccatelli S (2001) Testosterone protects cerebellar granule cells from oxidative stress-induced cell death through a receptor mediated mechanism. Brain Res 892: 255-262. [DOI] [PubMed] [Google Scholar]
- Auluck PK, Chan HY, Trojanowski JQ, Lee VM, Bonini NM (2002) Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson's disease. Science 295: 865-868. [DOI] [PubMed] [Google Scholar]
- Behl C, Widmann M, Trapp T, Holsboer F (1995) 17-beta estradiol protects neurons from oxidative stress-induced cell death in vitro. Biochem Biophys Res Commun 216: 473-482. [DOI] [PubMed] [Google Scholar]
- Behl C, Skutella T, Lezoualc'h F, Post A, Widmann M, Newton C, Holsboer F (1997) Neuroprotection against oxidative stress by estrogens: structure-activity relationship. Mol Pharmacol 51: 535-541. [PubMed] [Google Scholar]
- Beitel LK, Prior L, Vasiliou DM, Gottlieb B, Kaufman M, Lumbroso R, Alvarado C, McGillivray B, Trifiro M, Pinsky L (1994) Complete androgen insensitivity due to mutations in the probable alpha-helical segments of the DNA-binding domain in the human androgen receptor. Hum Mol Genet 3: 21-27. [DOI] [PubMed] [Google Scholar]
- Brooks BP, Merry DE, Paulson HL, Lieberman AP, Kolson DL, Fischbeck KH (1998) A cell culture model for androgen effects in motor neurons. J Neurochem 70: 1054-1060. [DOI] [PubMed] [Google Scholar]
- Busciglio J, Pelsman A, Wong C, Pigino G, Yuan M, Mori H, Yankner BA (2002) Altered metabolism of the amyloid beta precursor protein is associated with mitochondrial dysfunction in Down's syndrome. Neuron 33: 677-688. [DOI] [PubMed] [Google Scholar]
- Cummings CJ, Sun Y, Opal P, Antalffy B, Mestril R, Orr HT, Dillmann WH, Zoghbi HY (2001) Over-expression of inducible HSP70 chaperone suppresses neuropathology and improves motor function in SCA1 mice. Hum Mol Genet 10: 1511-1518. [DOI] [PubMed] [Google Scholar]
- D'Andrea MR, Nagele RG, Wang HY, Peterson PA, Lee DH (2001) Evidence that neurons accumulating amyloid can undergo lysis to form amyloid plaques in Alzheimer's disease. Histopathology 38: 120-134. [DOI] [PubMed] [Google Scholar]
- D'Andrea MR, Nagele RG, Wang HY, Lee DH (2002) Consistent immunohistochemical detection of intracellular beta-amyloid42 in pyramidal neurons of Alzheimer's disease entorhinal cortex. Neurosci Lett 333: 163-166. [DOI] [PubMed] [Google Scholar]
- Driggers PH, Segars JH (2002) Estrogen action and cytoplasmic signaling pathways. Part II: the role of growth factors and phosphorylation in estrogen signaling. Trends Endocrinol Metab 13: 422-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein M, Weidenfeld J, Ne'eman Y, Samuni A, Mizrachi Y, Ben-Uzilio R (1981) Comparative studies of the aromatization of testosterone and epitestosterone by human placental aromatase. Endocrinology 108: 943-947. [DOI] [PubMed] [Google Scholar]
- Fonte V, Kapulkin V, Taft A, Fluet A, Friedman D, Link CD (2002) Interaction of intracellular beta amyloid peptide with chaperone proteins. Proc Natl Acad Sci USA 99: 9439-9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring U (2004) Biological activities of HAP46/BAG-1. EMBO Rep 5: 148-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman Y, Bruce AJ, Cheng B, Mattson MP (1996) Estrogens attenuate and corticosterone exacerbates excitotoxicity, oxidative injury, and amyloid beta-peptide toxicity in hippocampal neurons. J Neurochem 66: 1836-1844. [DOI] [PubMed] [Google Scholar]
- Gouras GK, Tsai J, Naslund J, Vincent B, Edgar M, Checler F, Greenfield JP, Haroutunian V, Buxbaum JD, Xu H, Greengard P, Relkin NR (2000) Intraneuronal Abeta42 accumulation in human brain. Am J Pathol 156: 15-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green PS, Gridley KE, Simpkins JW (1996) Estradiol protects against beta-amyloid (25-35)-induced toxicity in SK-N-SH human neuroblastoma cells. Neurosci Lett 218: 165-168. [DOI] [PubMed] [Google Scholar]
- Green PS, Bishop J, Simpkins JW (1997) 17 α-estradiol exerts neuroprotective effects on SK-N-SH cells. J Neurosci 17: 511-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyure KA, Durham R, Stewart WF, Smialek JE, Troncoso JC (2001) Intraneuronal Aβ-amyloid precedes development of amyloid plaques in Down syndrome. Arch Pathol Lab Med 125: 489-492. [DOI] [PubMed] [Google Scholar]
- Hammond J, Le Q, Goodyer C, Gelfand M, Trifiro M, LeBlanc A (2001) Testosterone-mediated neuroprotection through the androgen receptor in human primary neurons. J Neurochem 77: 1-9. [DOI] [PubMed] [Google Scholar]
- Heinlein CA, Chang C (2002) The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol Endocrinol 16: 2181-2187. [DOI] [PubMed] [Google Scholar]
- Jana NR, Tanaka M, Wang G, Nukina N (2000) Polyglutamine length-dependent interaction of Hsp40 and Hsp70 family chaperones with truncated N-terminal huntingtin: their role in suppression of aggregation and cellular toxicity. Hum Mol Genet 9: 2009-2018. [DOI] [PubMed] [Google Scholar]
- Jones KJ, Alexander TD, Brown TJ, Tanzer L (2000) Gonadal steroid enhancement of facial nerve regeneration: role of heat shock protein 70. J Neurocytol 29: 341-349. [DOI] [PubMed] [Google Scholar]
- Khan S, Abdelrahim M, Samudio I, Safe S (2003) Estrogen receptor/Sp1 complexes are required for induction of cad gene expression by 17beta-estradiol in breast cancer cells. Endocrinology 144: 2325-2335. [DOI] [PubMed] [Google Scholar]
- King FW, Wawrzynow A, Hohfeld J, Zylicz M (2001) Co-chaperones Bag-1, Hop and Hsp40 regulate Hsc70 and Hsp90 interactions with wild-type or mutant p53. EMBO J 20: 6297-6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFerla FM, Tinkle BT, Bieberich CJ, Haudenschild CC, Jay G (1995) The Alzheimer's Aβ peptide induces neurodegeneration and apoptotic cell death in transgenic mice. Nat Genet 9: 21-30. [DOI] [PubMed] [Google Scholar]
- LeBlanc A (1995) Increased production of 4 kDa amyloid β peptide in serum-deprived human primary neuron cultures: possible involvement of apoptosis. J Neurosci 15: 7837-7846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A, Ran RQ, Clark J, Reilly M, Nee A, Sharp FR (2002) 17-beta-estradiol induces heat shock proteins in brain arteries and potentiates ischemic heat shock protein induction in glia and neurons. J Cereb Blood Flow Metab 22: 183-195. [DOI] [PubMed] [Google Scholar]
- Lustig RH, Hua P, Smith LS, Wang C, Chang C (1994) An in vitro model for the effects of androgen on neurons employing androgen receptor-transfected PC12 cells. Mol Cell Neurosci 5: 587-596. [DOI] [PubMed] [Google Scholar]
- Mader S, Chambon P, White JH (1993) Defining a minimal estrogen receptor DNA binding domain. Nucleic Acids Res 21: 1125-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrane J, Smith RC, Walsh K, Querfurth HW (2004) Heat shock protein 70 participates in the neuroprotective response to intracellularly expressed β-amyloid in neurons. J Neurosci 24: 1700-1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen D, Steinberg SV, Rouault E, Chagnon S, Gottlieb B, Pinsky L, Trifiro M, Mader S (2001) A G577R mutation in the human AR P box results in selective decreases in DNA binding and in partial androgen insensitivity syndrome. Mol Endocrinol 15: 1790-1802. [DOI] [PubMed] [Google Scholar]
- Panet-Raymond V, Gottlieb B, Beitel LK, Pinsky L, Trifiro MA (2000) Interactions between androgen and estrogen receptors and the effects on their transactivational properties. Mol Cell Endocrinol 167: 139-150. [DOI] [PubMed] [Google Scholar]
- Papasozomenos S, Shanavas A (2002) Testosterone prevents the heat shock-induced overactivation of glycogen synthase kinase-3 beta but not of cyclin-dependent kinase 5 and c-Jun NH2-terminal kinase and concomitantly abolishes hyperphosphorylation of tau: implications for Alzheimer's disease. Proc Natl Acad Sci USA 99: 1140-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike CJ (1999) Estrogen modulates neuronal Bcl-xL expression and beta-amyloid-induced apoptosis: relevance to Alzheimer's disease. J Neurochem 72: 1552-1563. [DOI] [PubMed] [Google Scholar]
- Pike CJ (2001) Testosterone attenuates beta-amyloid toxicity in cultured hippocampal neurons. Brain Res 919: 160-165. [DOI] [PubMed] [Google Scholar]
- Querfurth HW, Suhara T, Rosen KM, McPhie DL, Fujio Y, Tejada G, Neve RL, Adelman LS, Walsh K (2001) Beta-amyloid peptide expression is sufficient for myotube death: implications for human inclusion body myopathy. Mol Cell Neurosci 17: 793-810. [DOI] [PubMed] [Google Scholar]
- Ramsden M, Shin TM, Pike CJ (2003) Androgens modulate neuronal vulnerability to kainate lesion. Neuroscience 122: 573-578. [DOI] [PubMed] [Google Scholar]
- Rosenauer A, Nervi C, Davison K, Lamph WW, Mader S, Miller Jr WH (1998) Estrogen receptor expression activates the transcriptional and growth-inhibitory response to retinoids without enhanced retinoic acid receptor alpha expression. Cancer Res 58: 5110-5116. [PubMed] [Google Scholar]
- Segars JH, Driggers PH (2002) Estrogen action and cytoplasmic signaling cascades. Part I: membrane-associated signaling complexes. Trends Endocrinol Metab 13: 349-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugarman MC, Yamasaki TR, Oddo S, Echegoyen JC, Murphy MP, Golde TE, Jannatipour M, Leissring MA, LaFerla FM (2002) Inclusion body myositis-like phenotype induced by transgenic overexpression of beta APP in skeletal muscle. Proc Natl Acad Sci USA 99: 6334-6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi RH, Milner TA, Li F, Nam EE, Edgar MA, Yamaguchi H, Beal MF, Xu H, Greengard P, Gouras GK (2002) Intraneuronal Alzheimer abeta42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am J Pathol 161: 1869-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeyama J, Suzuki T, Inoue S, Kaneko C, Nagura H, Harada N, Sasano H (2001) Expression and cellular localization of estrogen receptors alpha and beta in the human fetus. J Clin Endocrinol Metab 86: 2258-2262. [DOI] [PubMed] [Google Scholar]
- Tang PZ, Gannon MJ, Andrew A, Miller D (1995) Evidence for oestrogenic regulation of heat shock protein expression in human endometrium and steroid-responsive cell lines. Eur J Endocrinol 133: 598-605. [DOI] [PubMed] [Google Scholar]
- Warrick JM, Paulson HL, Gray-Board GL, Bui QT, Fischbeck KH, Pittman RN, Bonini NM (1998) Expanded polyglutamine protein forms nuclear inclusions and causes neural degeneration in Drosophila Cell 93: 939-949. [DOI] [PubMed] [Google Scholar]
- Wilson CM, McPhaul MJ (1996) A and B forms of the androgen receptor are expressed in a variety of human tissues. Mol Cell Endocrinol 120: 51-57. [DOI] [PubMed] [Google Scholar]
- Wilson JD, Foster DW, Kronenberg HM, Larsen PR (1978) Williams textbook of endocrinology, Ed 9. Philadelphia: Saunders.
- Yan JX, Wait R, Berkelman T, Harry RA, Westbrook JA, Wheeler CH, Dunn MJ (2000) A modified silver staining protocol for visualization of proteins compatible with matrix-assisted laser desorption/ionization and electrospray ionization-mass spectrometry. Electrophoresis 21: 3666-3672. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Tounekti O, Akerman B, Goodyer CG, LeBlanc A (2001) 17-β-estradiol induces an inhibitor of active caspases. J Neurosci 21: RC176(1-6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, McLaughlin R, Goodyer C, LeBlanc A (2002) Selective cytotoxicity of intracellular amyloid β peptide1-42 through p53 and Bax in cultured primary human neurons. J Cell Biol 156: 519-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylicz M, King FW, Wawrzynow A (2001) Hsp70 interactions with the p53 tumour suppressor protein. EMBO J 20: 4634-4638. [DOI] [PMC free article] [PubMed] [Google Scholar]