Abstract
Because 5-HT1A receptors located on the soma dendrites of serotonin (5-HT) neurons normally mediate an inhibition of 5-HT firing and release, the desensitization of these autoreceptors is essential for obtaining an enhancement of 5-HT transmission after treatment with 5-HT reuptake inhibitors (SSRIs). We have demonstrated previously, using immunoelectron microscopy with specific 5-HT1A antibodies, that an internalization of 5-HT1A autoreceptors is associated with their desensitization in rats given a single dose of the 5-HT1A receptor agonist 8-hydroxy-2-(di-n-propylamino)tetralin. Here, we examined the subcellular distribution of 5-HT1A receptors in dendrites from nucleus raphe dorsalis (NRD) (autoreceptors) and hippocampus (heteroreceptors) after acute treatment with the antidepressant SSRI, fluoxetine (10 mg/kg, i.p.). In parallel experiments, the kinetics of in vivo binding of the 5-HT1A positron emission tomography radioligand 4,2-(methoxyphenyl)-1-[2-(N-2-pyridinyl)-p-fluorobenzamido]ethylpiperazine ([18F]MPPF) was measured in these two brain regions by means of stereotaxically implanted β microprobes. One hour after treatment, there was a 36% decrease in 5-HT1A immunogold labeling of the plasma membrane of NRD dendrites, and a concomitant increase in their cytoplasmic labeling, without any change in hippocampal dendrites. In vivo binding of [18F]MPPF was reduced by 35% in NRD and unchanged in hippocampus. Both effects were blocked by pretreatment with the 5-HT1A receptor antagonist (N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridinyl) cyclohexane-carboxamide) (1 mg/kg, i.p.). In brain sections of NRD and hippocampus, [18F]MPPF autoradiographic labeling did not differ between fluoxetine- and saline-treated rats. These immunocytochemical results confirmed that internalization of 5-HT1A autoreceptors may account for their desensitization, and the microprobe results suggest that this prerequisite for antidepressant treatment efficacy could be amenable to brain imaging in humans.
Keywords: SSRI, fluoxetine, 5-HT1A autoreceptors, internalization, immunogold, [18F]MPPF
Introduction
There is substantial evidence that the therapeutic efficacy of antidepressants, and notably of selective serotonin [5-hydroxytryptamine (5-HT)] reuptake inhibitors (SSRIs) such as fluoxetine, citalopram, fluvoxamine, paroxetine, zimeldine, and sertraline, depends on an enhancement of 5-HT neurotransmission in key brain regions (Blier and de Montigny, 1994; Wong et al., 1995). Blockade of the neuronal 5-HT reuptake mechanism (plasma membrane transporter) increases the extracellular concentration of 5-HT, which ultimately leads to the beneficial increase in 5-HT neurotransmission.
At the onset of SSRI treatment, however, the increase in extracellular 5-HT activates 5-HT1A autoreceptors, located on the soma dendrites of 5-HT neurons, that inhibit the firing and release by these cells (Blier and de Montigny, 1987; Sprouse and Aghajanian, 1987; Hamon et al., 1988). It takes 2 or 3 weeks of treatment for clinical efficacy to be achieved, during which the firing activity in 5-HT neurons progressively returns to normal, because of a desensitization of 5-HT1A autoreceptors (Blier and de Montigny, 1994; Jolas et al., 1994; Le Poul et al., 1995, 2000; Czachura and Rasmussen, 2000). This allows for 5-HT neurotransmission to increase in the forebrain territories of 5-HT innervation, in which 5-HT1A heteroreceptors are not subject to desensitization and may thus contribute to the mediation of the therapeutic effects (Blier and de Montigny, 1994).
Using quantitative electron microscopic immunocytochemistry with specific anti-5-HT1A antibodies (El Mestikawy et al., 1990; Riad et al., 1991) and immunogold-labeled secondary antibodies, we have shown previously in rat that direct activation of 5-HT1A autoreceptors by the selective agonist 8-hydroxy-2(di-n-propylamino)tetralin (8-OH-DPAT) internalizes these receptors in the soma dendrites of nucleus raphe dorsalis (NRD) (autoreceptors) but not hippocampal neurons (heteroreceptors) (Riad et al., 2001). From these results, it was surmised that the internalization of 5-HT1A autoreceptors accounts for their desensitization. In the present study, we wanted to know whether an indirect activation of the 5-HT1A receptors by acute treatment with fluoxetine (Prozac) would have a similar effect. We therefore used the immunocytochemical approach to visualize and quantify the subcellular distribution of 5-HT1A receptors in dendrites of the NRD and hippocampus (CA3) of rats treated or not with a single dose of fluoxetine (10 mg/kg, i.p.).
Moreover, the possibility of eventually imaging 5-HT1A auto-receptor internalization in human patients treated for depression did not escape us (Zimmer et al., 2004). For this reason, we also set out to determine in parallel experiments whether fluoxetine-induced internalization of 5-HT1A autoreceptors would be associated with a lowering of the density of 4,2-(methoxyphenyl)-1-[2-(N-2-pyridinyl)-p-fluorobenzamido]ethylpiperazine ([18F]MPPF) binding in NRD (but not hippocampus). [18F]MPPF is a specific ligand of the 5-HT1A receptors amenable to brain imaging in human brain (Shiue et al., 1997; Passchier et al., 2000, 2001; Costes et al., 2002). To examine its kinetics of binding in the fluoxetine-treated rats, we used β-microprobes stereotaxically implanted next to the NRD and inside hippocampus, as in our previous study after 8-OH-DPAT treatment (Zimmer et al., 2004).
Materials and Methods
5-HT1A immunocytochemistry
Animals. Adult male Sprague Dawley rats (250 ± 50 gm body weight; Charles River, St. Constant, Quebec, Canada) were housed at a constant temperature (21°C) and humidity (60%) under a fixed 12 hr light/dark cycle and with ad libitum access to food and water. Procedures involving animals and their care were conducted in strict accordance with the Guide to the Care and Use of Experimental Animals (Ed 2) of the Canadian Council on Animal Care, and the experimental protocols were approved by the Comité de Déontologie pour l'Expérimentation sur des Animaux at the Université de Montréal.
Drug treatments. Fluoxetine, N-[2-[4-(2-methoxyphenyl)-1-piperazinyl] ethyl]-N-(2-pyridinyl) cyclohexane-carboxamide (WAY 100635), and 8-OH-DPAT were purchased from Sigma (Saint Quentin, France; Oakville, Ontario, Canada). Four rats each were injected intraperitoneally with (1) the SSRI fluoxetine (10 mg/kg), (2) the 5-HT1A receptor antagonist WAY 100635 (1 mg/kg), 10 min before fluoxetine, (3) the 5-HT1A receptor agonist 8-OH-DPAT (0.5 mg/kg), or (4) fluoxetine plus 8-OH-DPAT (above dosages). All drugs were dissolved in saline and administered in a volume of 1 ml/kg. In each experiment on treated rats, tissue from one rat injected with 0.9% NaCl was simultaneously processed to be used as control (n = 5).
Antibodies. The production and characterization of the anti-5-HT1A antibodies raised against a segment of the third intracellular loop of rat 5-HT1A receptor have been fully described previously (El Mestikawy et al., 1990; Riad et al., 1991). These antibodies specifically react with rat 5-HT1A receptor and do not cross-react with other 5-HT receptor subtypes.
Tissue preparation. One or 24 hr after drug or saline injection, the rats were deeply anesthetized with sodium pentobarbital (80 mg/kg, i.p.) and perfused through the aortic arch with a solution of 3.5% acrolein, followed by 4% paraformaldehyde (PFA) in 0.1 m phosphate buffer (PB), pH 7.4, at room temperature. The brain was removed and postfixed by immersion for 2 hr in 4% PFA at 4°C and washed in phosphate buffered saline (PBS) (0.9% NaCl in 50 mm PB, pH 7.4). Vibratome sections, 50 μm thick, were then cut in cooled PBS, immersed in 0.1% sodium borohydride in PBS for 30 min (room temperature), and washed in this buffer before immunocytochemical processing.
Immunogold labeling. Free-floating sections were processed for 5-HT1A receptor immunohistochemistry with a pre-embedding immunogold technique described previously in detail (Riad et al., 2000, 2001). Briefly, sections were rinsed in PBS followed by a 60 min preincubation in a blocking solution of PBS containing 5% normal goat serum and 0.5% gelatin. Sections were then incubated overnight at room temperature in 1:1000 rabbit anti-5-HT1A receptor antiserum diluted in blocking solution, rinsed in PBS, and incubated for 2 hr in goat anti-rabbit IgGs conjugated to 1 nm colloidal gold particles (AuroProbe One, Amersham Biosciences, Oakville, Ontario, Canada) in blocking solution. The diameter of the immunogold particles was increased by using a silver enhancement kit (IntenSE, Amersham Biosciences) for 15 min.
Electron microscopy. Sections intended for electron microscopy were rinsed in PB, postfixed in 1% osmium tetroxide, and dehydrated in ascending concentrations of ethanol. They were then treated with propylene oxide, impregnated with resin overnight (Durcupan ACM; Sigma), mounted on glass slides, and cured at 60°C for 48 hr. Areas of interest (NRD and CA3 of hippocampus) were excised from the slides and glued at the tip of resin blocks. Ultrathin (silver-gold) sections were cut with an ultramicrotome (Sorvall Poter-Blum MT-2), collected on bare square-meshed copper grids, stained with lead citrate, and examined with the electron microscope (Philips CM100; 60 KV).
Quantitative analysis. Electron micrographs of immunoreactive NRD and hippocampal dendrites were taken at a uniform working magnification of 17,000×. A dendritic profile was considered as immunoreactive when showing at least three silver-intensified immunogold particles. The film negatives were scanned (Epson Perfection 3200) and converted into a positive picture at a final magnification of 8900×. Size measurements and counts of silver-intensified immunogold particles were obtained from an average of 35 labeled dendrites per region per rat, in three to five rats from each treated group and an equivalent number of saline controls. Dendritic size (diameter and perimeter) was measured with the aid of an image analysis system (NIH Image 1.60), and the immunogold particles were counted in two subcellular compartments: a narrow zone, 30 nm wide, at the periphery of each dendrite, corresponding to the plasma membrane, and the remaining surface of dendritic sections corresponding to cytoplasm. The surface of each compartment was calculated, and the results were expressed initially as density of labeling (number of immunogold particles per surface unit) for each compartment. They were ultimately expressed as percentage of control for each group, to take into account possible variations in intensity of labeling between experimental sessions. The mean values (±SEM) between groups were compared by a two-way ANOVA, followed by Student's t test, in an equal number of rats from each group (n = 3).
[18F]MPPF binding in vivo (dual β-microprobe)
Radiochemical.[18F]MPPF was synthesized with a radiochemical yield of 25% (decay corrected) in an automated synthesizer using the chemical pathway described previously (Le Bars et al., 1998). Specific activity ranged from 74 × 103 to 148 × 103 MBq/μmol (2-4 Ci/μmol).
Animals. Adult male Sprague Dawley rats (250 ± 50 gm body weight; Elevage Dépré, Saint Doulchard, France) housed under similar conditions as above were used. All procedures involving animals and their care abided by the regulations of the European Economic Commission (86/09/EEC) and were approved by the Animal Care Committee at the Claude Bernard University. As described previously in detail (Zimmer et al., 2002a, 2002), after anesthesia with urethane (1.7 gm/kg, i.p.), each rat was positioned in a stereotaxic frame on a thermostatically controlled heating blanket (37 ± 1°C), and a catheter was inserted into the tail vein to allow for subsequent injection of the radiotracer. Two β-microprobes (IPN prototype, Orsay, and Biospace, Paris, France) were then implanted under stereotaxic control: one close to the NRD [anteroposterior (A/P) -7.8, lateromedial (L/M) 1.0, and dorsoventral (D/V) -7.0 from λ) and the other in the hippocampus (A/P -5.2, L/M 5.0, and D/V -8.0 from bregma), according to Paxinos and Watson (1986). The sensitive end of the probe consisted of a plastic scintillating fiber that was 1 mm in length and 1 mm in diameter (Zimmer et al., 2002). The sensitivity of the two probes, characterized in vitro, is close to 0.55 measured counts per kilobecquerel per milliliter. According to previous Monte Carlo simulations, the probe detection volume is defined as a 1.3 mm radius sphere centered on the probe end corresponding to 90% of the detected signal (Zimmer et al., 2002a).
Drug treatments and kinetics of [18F]MPPF binding. These experiments were always conducted in pairs of rats, one given the pharmacological treatment tested and one injected with saline. In five pairs each, fluoxetine (10 mg/kg, i.p.) was injected alone or 10 min after pretreatment with WAY 100635 (1 mg/kg, i.p.) in rats that had been implanted with β-microprobes 2 hr previously. Data acquisition from the probes was initiated at the time of drug or saline injection, integrating counts for successive 10 sec periods. One hour later, [18F]MPPF (37 MBq, i.e., 1 mCi, dissolved in 0.4 ml saline) was injected intravenously in 45 sec, and the amount of radioactivity was measured at the two recording sites over the next 90 min.
Data analysis. The raw data were expressed as mean number of disintegrations per 10 sec, averaged at 1 min intervals and corrected for radioactivity decay. After completion of all experiments, the rats were killed, and the anatomical localization of the microprobes was verified histologically. The mean values obtained from the comparison of a treated and a control animal at each time point (every minute) were analyzed by one-way ANOVA on repeated measurements followed by a post hoc Student's t test.
[18F]MPPF binding in vitro (autoradiography)
One hour after fluoxetine (10 mg/kg, i.p.) or saline injection, rats (n = 5 in each group) were anesthetized with urethane and killed by decapitation, and the brain was frozen in 2-methylbutane cooled to -40°C with liquid nitrogen and stored at -80°C. Cryostat sections (30 μm thick) were cut across the NRD and the dorsal hippocampus, thaw mounted on slides, and allowed to air dry for 30 min before storage at -80°C until use. Every slide contained two sections from each region, one from a treated and one from a control rat. On the day of the assay, the slides were allowed to reach room temperature, and the sections were incubated in 50 mm Tris-HCl, pH 7.5, containing 10 nm [18F]MPPF (2 μCi/ml; from 1 to 3 Ci/μmol). Adjacent sections were incubated in the same medium supplemented with 10 μm WAY 100635 for estimation of nonspecific binding. After 20 min of incubation, the slides were rapidly dipped in distilled water, dried, and juxtaposed to sensitive Micro-Imager plates (Biospace, Paris) overnight. Using the β-analysis software (β-Vision+, Biospace), the data were recorded as optical densities that are proportional to the radioactivity of the measured sample. Readings were obtained from at least three to five sections of both the NRD and the dorsal hippocampus. Nonspecific binding was subtracted, and the results were expressed as disintegrations per second per square millimeter of section (dps/mm2).
Results
Internalization of 5-HT1A receptors in nucleus raphe dorsalis (autoreceptors)
In keeping with our previous immunocytochemical descriptions (Riad et al., 2000, 2001), the immunogold labeling of 5-HT1A receptors predominated over the plasma membrane of NRD dendrites in normal or saline-injected rats (Fig. 1A). Eighty-eight percent of the silver-intensified immunogold particles were found to be associated with the plasma membrane (30.49 ± 3 particles per square micrometer), and only 12% were associated with the cytoplasm of these dendrites (0.29 ± 0.02 particles per square micrometer).
Figure 1.
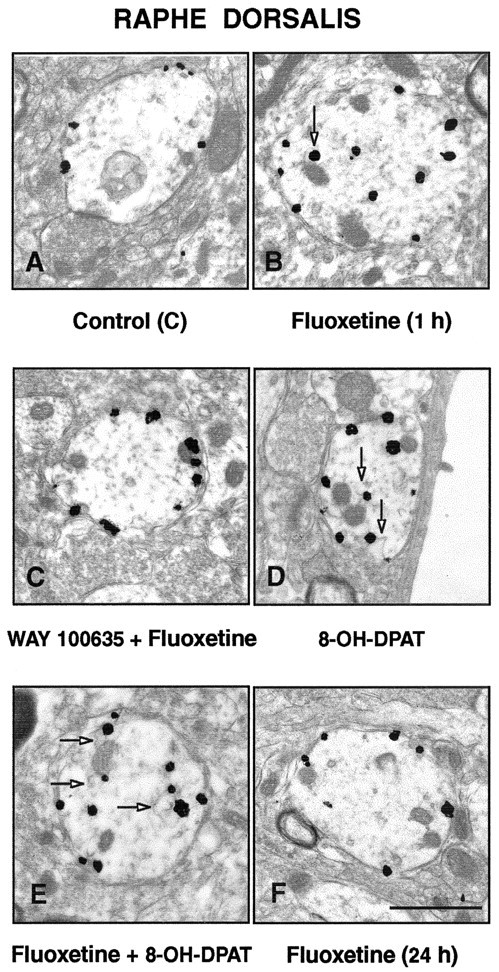
A-F, Electron micrographs illustrating the subcellular distribution of 5-HT1A immunoreactivity (immunogold labeling) in dendrites of the nucleus raphe dorsalis, 1 hr after the intraperitoneal injection of saline (A), 10 mg/kg fluoxetine (B), 1 mg/kg WAY 100635, 10 min before fluoxetine(C), 0.5 mg/kg 8-OH-DPAT (D), fluoxetine plus 8-OH-DPAT (E), and 24 hr after fluoxetine (F). Note the predilection of the immunogold particles for the plasma membrane of the labeled dendrites after injection of saline (A), WAY 100635 plus fluoxetine (C), and 24 hr after fluoxetine (F). In contrast, 1 hr after fluoxetine (B), 8-OH-DPAT (D), or fluoxetine plus 8-OH-DPAT (E), the particles are found mostly in the dendritic cytoplasm, often in association with endosome-like organelles (arrows). Scale bar: (in F) 1 μm.
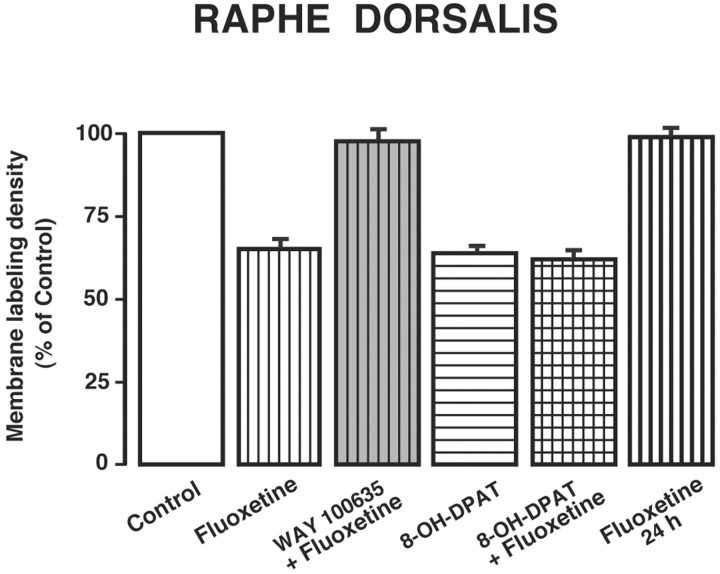
As illustrated in Figure 1B, 1 hr after a single injection of fluoxetine (10 mg/kg, i.p.), the proportion of immunogold particles associated with the plasma membrane of NRD dendrites was markedly decreased, having fallen from 88 to 56% (19.89 ± 2 particles per square micrometer). In contrast, the labeling of dendritic cytoplasm was then considerably stronger than in controls, rising from 12 to 44% (0.92 ± 0.04 particles per square micro-meter). As shown in Figure 2, when normalized to a 100% control density, this internalization corresponded to a 36% decrease in density of 5-HT1A receptors on the dendritic plasma membrane, whereas the increase over the much lower density of the dendritic cytoplasm amounted to 243%. As documented previously after 8-OH-DPAT treatment (Riad et al., 2001), some of this cytoplasmic labeling was clearly associated with endosome-like organelles (Fig. 1B,D,E).
Figure 2.
Histograms displaying the density of 5-HT1A plasma membrane labeling in NRD dendrites under the same conditions as in Figure 1. The number of immunogold particles per square micrometer of membrane compartment (density) is given as percentage of control. Means ± SEM from three to five rats in each treated group. ***p < 0.001 by one-way ANOVA followed by Student's t test.
Pretreatment of the rats with the 5-HT1A receptor antagonist WAY 100635 (1 mg/kg, i.p.) completely prevented this fluoxetine-induced effect (Figs. 1C, 2). The proportion of plasma membrane-associated gold particles in NRD dendrites was then the same as in controls. It should be noted that treatment with WAY 100635 alone has no measurable effects of its own on the subcellular distribution of 5-HT1A receptors (Riad et al., 2001).
Treatment with 8-OH-DPAT (0.5 mg/kg, i.p.) induced a decrease similar to that of fluoxetine in the density of plasma membrane-associated gold particles (-38%) and an increase in the cytoplasm (Figs. 1D, 2). Combined treatment with fluoxetine plus 8-OH-DPAT had no cumulative effect on the internalization of 5-HT1A autoreceptors (Figs. 1E, 2). Twenty-four hours after fluoxetine injection, the 5-HT1A labeling of NRD dendrites was indistinguishable from that seen in saline-treated controls (Figs. 1F, 2).
Immutability of 5-HT1A receptors in hippocampus (heteroreceptors)
As described previously (Riad et al., 2000, 2001), the immunogold labeling of 5-HT1A heteroreceptors in rat hippocampus (CA3) also predominated on the plasma membrane in dendrites of pyramidal cells (Fig. 3A). In striking contrast to the redistribution of 5-HT1A autoreceptors observed in NRD dendrites, however, the injection of fluoxetine had no apparent effect on the density of 5-HT1A heteroreceptors associated with the membrane of hippocampal dendrites (Fig. 3B).
Figure 3.
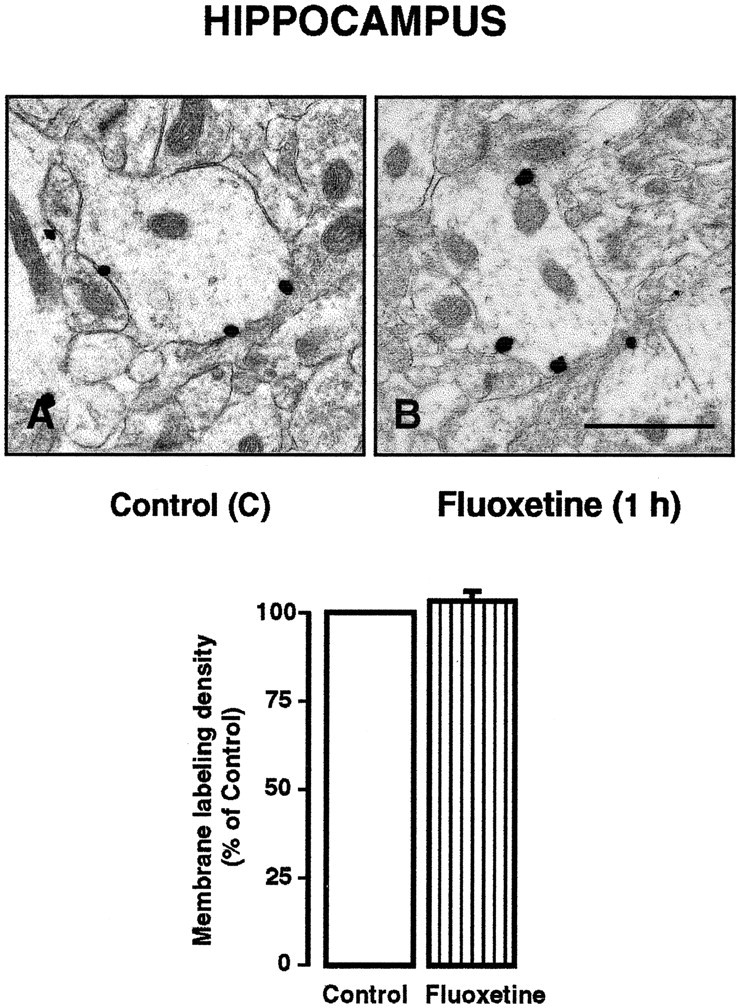
A, B, 5-HT1A immunoreactivity in hippocampal dendrites (CA3) of saline-treated (A) and fluoxetine-treated (B) rats. Note the predominance of the immunogold particles on the dendritic membrane in both cases. Scale bar: (in B) 1 μm. In the corresponding histograms, note the equal density of dendritic membrane labeling in the hippocampus (CA3) of saline controls and fluoxetine-treated rats. Data are from four rats in each group (mean ± SEM for the treated group).
Kinetics of [18F]MPPF binding in vivo (dual β-microprobes)
As monitored with β-microprobes stereotaxically implanted into the NRD and the hippocampus of anesthetized rats, the time-radioactivity curves of [18F]MPPF binding obtained from control rats were highly reproducible (coefficient of variation ≤10%) (Fig. 4A,B) and similar to those reported previously (Zimmer et al., 2002b). In fluoxetine-treated rats, the magnitude of [18F]MPPF binding was significantly reduced in NRD (-35% by comparison with control; p < 0.05 by one-way ANOVA followed by Student's t test) over a period of ∼65 min after the injection of [18F]MPPF (Fig. 4A). In the hippocampus, the kinetics of [18F]MPPF binding were identical in fluoxetine-treated and control rats (Fig. 4C) and not significantly different from that in the NRD after injection of saline.
Figure 4.
A-D, β-microprobe measurements of the kinetics of [18F]MPPF binding in the NRD and the hippocampus of rats treated or not with 10 mg/kg fluoxetine (A, C), or 1 mg/kg WAY 100635, 10 min before fluoxetine (B, D), as described in Materials and Methods. Oblique arrows on each graph indicate the time of saline or drug administration and the beginning of data acquisition. Vertical arrows point at the time of [18F]MPPF injection. The amount of radioactivity (becquerel) is displayed as mean ± SEM from five rats in each group.
Pretreatment with WAY 100635 (1 mg/kg, i.p.) prevented the fluoxetine-induced decrease in [18F]MPPF binding within the NRD, and rats given both drugs displayed time-radioactivity curves comparable with those of controls in both NRD (Fig. 4B) and hippocampus (Fig. 4D). Our previous study has shown that, on its own, WAY 100635 (1 mg/kg) has no measurable effects on [18F]MPPF binding in either the NRD or the hippocampus (Zimmer et al., 2004).
[18F]MPPF binding to rat brain sections
In autoradiographs of tissue sections (Fig. 5A-D), the amount of [18F]MPPF binding was not significantly modified by fluoxetine administration in either NRD (2.37 ± 0.67 and 2.52 ± 0.60 dps/mm2 in treated and control rats; means ± SEM; n = 5, respectively) or hippocampus (1.50 ± 0.55 and 1.31 ± 0.53 dps/mm2 in treated and control rats; means ± SEM; n = 4, respectively).
Figure 5.
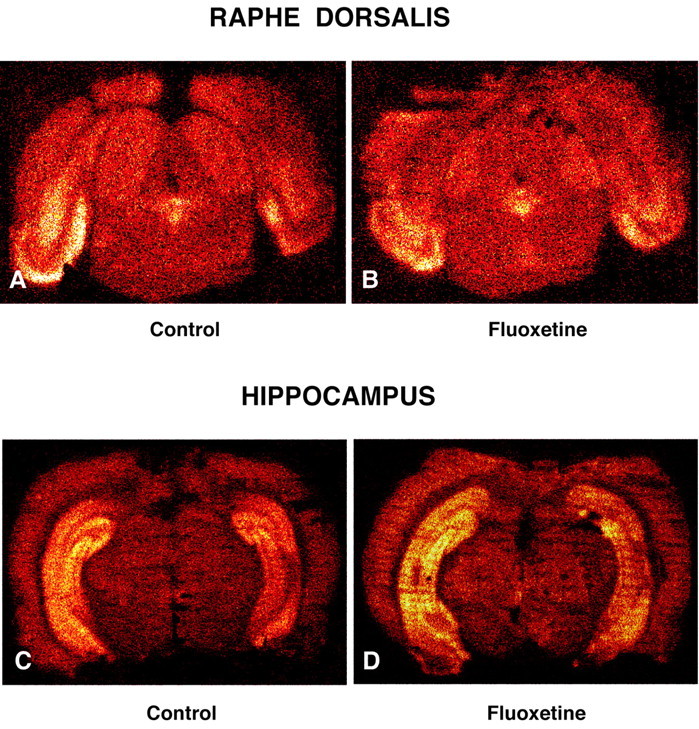
A-D, Color-coded autoradiographs of [18F]MPPF binding to 5-HT1A receptors in tissue sections across nucleus raphe dorsalis (A, B) and hippocampus (C, D) of saline control (A, C) and fluoxetine-treated (B, D) rats. Note the comparable density of binding in both regions in the treated and control rats.
Discussion
The present study provides the first demonstration that acute treatment with the antidepressant SSRI fluoxetine internalizes 5-HT1A receptors in NRD (autoreceptors) but not hippocampus (heteroreceptors) and this 5-HT1A autoreceptor internalization is associated with a reduction of [18F]MPPF binding in the living brain. The decreased density of 5-HT1A immunoreactivity on the plasma membrane of NRD dendrites and parallel reduction in [18F]MPPF binding were observed after a single intraperitoneal injection of fluoxetine at a dose (10 mg/kg) that has been shown to induce in the rat a degree of 5-HT transporter blockade roughly similar to that achieved by clinically efficient doses in man (Czachura and Rasmussen, 2000). The fact that the selective 5-HT1A receptor antagonist WAY 100635 completely prevented both the internalization of 5-HT1A autoreceptors and the decrease in [18F]MPPF binding demonstrated the dependence of both effects on an indirect activation of 5-HT1A auto-receptors by fluoxetine.
Internalization of 5-HT1A autoreceptors
As documented in previous biochemical, electrophysiological, and behavioral studies in the rat, there is a rapid but transient desensitization of 5-HT1A autoreceptors after acute administration of 8-OH-DPAT (Kennett et al., 1987; Beer et al., 1990; Seth et al., 1997). Such a desensitization is not observed in the hippocampus and other territories of 5-HT projection (Beer et al., 1990), and it coincides with the internalization of plasma membrane 5-HT1A receptors in NRD but not hippocampus, suggesting a causal relationship between the desensitization and internalization processes (Riad et al., 2001).
The regional selectivity of the internalization of 5-HT1A receptors after acute treatment with fluoxetine or 8-OH-DPAT is not explained entirely. Somatodendritic 5-HT1A autoreceptors (NRD) have been documented to display a pharmacological profile distinct from that of somatodendritic 5-HT1A heteroreceptors (hippocampus) (de Montigny and Aghajanian, 1977; Millan et al., 1994; Newman-Tancredi et al., 1997). Differences in G-protein coupling between 5-HT1A autoreceptors and heteroreceptors have been hypothesized (Blier et al., 1993). Recent studies performed with subtype-specific anti-G-protein antibodies have indicated that native 5-HT1A receptors are coupled to Gαi3 in NRD and to Gαo in hippocampus (Mannoury la Cour et al., 2001).
It has been shown previously (Riad et al., 2000, 2001) and confirmed in the present study that most if not all 5-HT1A auto-receptors on the plasma membrane of NRD soma-dendrites are located extrasynaptically. The mobilization of these receptors after fluoxetine treatment suggests that they might be normally activated by 5-HT accumulated outside synaptic clefts, after release either from serotoninergic soma-dendrites or from the rare and predominantly asynaptic serotoninergic axon varicosities present within the nucleus (Descarries et al., 1982).
As in our previous study with 8-OH-DPAT (Riad et al., 2001), the decrease in plasma membrane density of 5-HT1A autoreceptors induced by fluoxetine was associated with an increased density of immunoparticles in the dendritic cytoplasm and the formation of endosomes (data not shown). There was no indication of a significant amount of 5-HT1A receptors in other vesicular compartments and especially in lysosomes, favoring the hypothesis of recycling rather than new synthesis of the receptors after their internalization, to account for their return to normal plasma membrane density 24 hr after the treatment.
[18F]MPPF does not bind to internalized 5-HT1A receptor in vivo
The selectivity of the WAY 100635 analog [18F]MPPF for the 5-HT1A receptor has been demonstrated repeatedly (Shiue et al., 1997; Le Bars et al., 1998). The decrease in [18F]MPPF binding observed in NRD but not hippocampus after fluoxetine treatment was of similar magnitude and displayed the same kinetics as that observed previously after 8-OH-DPAT treatment (Zimmer et al., 2004). It was also of comparable magnitude and compatible in duration with the reduced density of plasma membrane labeling of 5-HT1A receptors measured in NRD.
In contrast, the density of [18F]MPPF binding in both NRD and hippocampus was the same in rats treated or not with fluoxetine, when measured in autoradiographs of whole-brain sections. This indicated that fluoxetine treatment did not reduce the total number of 5-HT1A receptor binding sites in either region, confirming at the same time that the decrease in density of [18F]MPPF binding detected in vivo with the β-microprobe in the NRD of treated rats was indeed caused by receptor internalization.
The reason(s) for the lack of binding of [18F]MPPF to internalized 5-HT1A autoreceptors in vivo could not be determined. The relatively low lipophilicity of [18F]MPPF was not a likely explanation, because this tracer readily penetrates the intact blood-brain barrier. A conformational change of internalized 5-HT1A receptors, with occlusion of the MPPF binding site, is more likely. It must be emphasized that combined treatment with both fluoxetine and 8-OH-DPAT had no cumulative reducing effect on the in vivo binding of [18F]MPPF in NRD. This suggests that there is a mobilizable pool of the 5-HT1A autoreceptors and that this same pool is mobilized by direct activation with the agonist and indirect activation by SSRI (via serotonin).
The fact that the decrease in [18F]MPPF binding in vivo associated with the internalization of 5-HT1A autoreceptors induced by fluoxetine in the NRD was of similar magnitude as that in the local density of membrane-bound 5-HT1A receptors measured by immunoelectron microscopy was also noteworthy. Indeed, these data are hardly compatible with the possibility that this decrease in [18F]MPPF binding in vivo is caused, at least in large part, by a competition between [18F]MPPF and 5-HT. Moreover, no measurable decrease in the in vivo binding of [18F]MPPF was observed in the hippocampus, in which extracellular 5-HT levels also rise after the acute administration of 10 mg/kg of fluoxetine (Hervás and Artigas, 1998; our unpublished results). The association between the internalization of 5-HT1A autoreceptors and the decreased in vivo binding of [18F]MPPF within the NRD was also in keeping with the previous observation of a comparable reduction in local [18F]MPPF binding after 8-OH-DPAT treatment. It must be emphasized that both 8-OH-DPAT and fluoxetine internalize 5-HT1A autoreceptors, in sharp contrast with the antagonist WAY 100635, which does not trigger this process although it displays an even greater affinity than 8-OH-DPAT or MPPF for 5-HT1A receptors (Zimmer et al., 2004). A regionally selective reduction in blood flow, and hence in bioavailability of the radiotracer, could also be ruled out as an eventual explanation of the fluoxetine-induced reduction of the in vivo [18F]MPPF binding in dorsal raphe nucleus. Indeed, microdialysis measurements indicated equal extracellular concentrations of this compound in NRD and hippocampus under conditions similar to those of the present experiments (Zimmer et al., 2004).
Clinical implications
The present results also have potential impact in the clinic. Chronic administration of SSRI appears to be required for achieving maximal desensitization of 5-HT1A autoreceptors (Blier and de Montigny, 1994; Le Poul et al. 1995, 2000; Czachura et al., 2000) and consequent return to normal firing of 5-HT neurons in the NRD. The fact that the internalization (and associated desensitization) (Kennett et al., 1987; Beer et al., 1990) of 5-HT1A autoreceptors induced by acute fluoxetine administration is only partial and transient is consistent with the long delay (3-4 weeks) observed in the therapeutic response to SSRI antidepressants. It is possible that chronic fluoxetine treatment will result in a sustained or even greater internalization of 5-HT1A autoreceptors. Because [18F]MPPF binding is quantifiable by positron emission tomography in the human NRD (Costes et al., 2002), the eventual decrease in [18F]MPPF binding associated with internalization of 5-HT1A autoreceptors might be detectable by brain imaging. The ability to image 5-HT1A receptor internalization as a condition of SSRI treatment efficacy would represent a major advance for current and future research on depression and its treatment, as well as its management.
Footnotes
Correspondence should be addressed to Dr. Laurent Descarries, Département de Pathologie et Biologie Cellulaire, Faculté deMédecine, Université de Montréal, CP 6128, Succursale Centre-ville, Montréal, Quebec, Canada H3C 3J7. E-mail: laurent.descarries@umontreal.ca.
This research was supported by Canadian Institutes of Health Research Grant NRF 3544 to L.D. and the French program Centre National de la Recherche Scientifique-Commissariat á l'Energie Atomique-Institut National de la Santé et de la Recherche Médicale “Imagerie du Petit Animal” to L.Z.
Copyright © 2004 Society for Neuroscience 0270-6474/04/245420-07$15.00/0
References
- Beer M, Kennett GA, Curzon G (1990) A single dose of 8-OH-DPAT reduces raphe binding of [3H]8-OH-DPAT and increases the effect of raphe stimulation on 5-HT metabolism. Eur J Pharmacol 178: 179-187. [DOI] [PubMed] [Google Scholar]
- Blier P, de Montigny C (1987) Modifications of 5-HT neuron properties by sustained administration of 5-HT1A agonist gepirone: electrophysiological studies in the rat brain. Synapse 1: 470-480. [DOI] [PubMed] [Google Scholar]
- Blier P, de Montigny C (1994) Current advances in the treatment of depression. Trends Pharmacol Sci 15: 220-226. [DOI] [PubMed] [Google Scholar]
- Blier P, Lista A, de Montigny C (1993) Differential properties of pre- and postsynaptic 5-hydroxytryptamine1A receptors in the dorsal raphe and hippocampus. II. Effects of pertussis and cholera toxins. J Pharmacol Exp Ther 265: 16-23. [PubMed] [Google Scholar]
- Costes N, Merlet I, Zimmer L, Lavenne F, Cinotti L, Delforge J, Luxen A, Pujol JF, Le Bars D (2002) Modeling [18F]MPPF positron emission tomography kinetics for the determination of 5-hydroxytryptamine-1A receptor concentration with multiinjection. J Cereb Blood Flow Metab 22: 753-765. [DOI] [PubMed] [Google Scholar]
- Czachura JF, Rasmussen K (2000) Effects of acute and chronic administration of fluoxetine on the activity of serotonergic neurons in the dorsal raphe nucleus of the rat. Naunyn Schmiedebergs Arch Pharmacol 362: 266-275. [DOI] [PubMed] [Google Scholar]
- de Montigny C, Aghajanian GK (1977) Preferential action of 5-methoxy-tryptamine and 5-methoxydimethyltryptamine on presynaptic serotonin receptors: a comparative iontophoretic study with LSD and serotonin. Neuropharmacology 16: 811-818. [Google Scholar]
- Descarries L, Watkins KC, Garcia S, Beaudet A (1982) The serotonin neurons in nucleus raphe dorsalis of adult rat: a light and electron microscope radioautographic study. J Comp Neurol 207: 239-254. [DOI] [PubMed] [Google Scholar]
- El Mestikawy S, Riad M, Laporte AM, Vergé D, Daval G, Gozlan H, Hamon M (1990) Production of specific anti-5-HT1A receptor antibodies in rabbits injected with a synthetic peptide. Neurosci Lett 118: 189-192. [DOI] [PubMed] [Google Scholar]
- Hamon M, Fattaccini CM, Adrien J, Gallissot MC, Martin P, Gozlan H (1988) Alterations of central serotonin and dopamine turnover in rats treated with ipsapirone and other 5-hydroxytryptamine1A agonists with potential anxiolytic properties. J Pharmacol Exp Ther 246: 745-752. [PubMed] [Google Scholar]
- Hervás I, Artigas F (1998) Effects of fluoxetine on extracellular 5-hydroxytryptamine in rat brain. Role of 5-HT autoreceptors. Eur J Pharmacol 358: 9-18. [DOI] [PubMed] [Google Scholar]
- Jolas T, Haj Dahmane S, Kidd EJ, Langlois X, Lanfumey L, Fattaccini CM, Vantalon V, Laporte AM, Adrien J, Gozlan H, Hamon M (1994) Central pre- and postsynaptic 5-HT1A receptors in rats treated chronically with a novel antidepressant, cericlamine. J Pharmacol Exp Ther 268: 1432-1443. [PubMed] [Google Scholar]
- Kennett GA, Marcou M, Dourish CT, Curzon G (1987) Single administration of 5-HT1A agonists decreases 5-HT1A presynaptic, but not postsynaptic receptor-mediated responses: relationship to antidepressant-like action. Eur J Pharmacol 138: 53-60. [DOI] [PubMed] [Google Scholar]
- Le Bars D, Lemaire C, Ginovart N, Plenevaux A, Aerts J, Brihaye C, Hassoun W, Leviel V, Mekhsian P, Weissmann D, Pujol JF, Luxen A, Comar D (1998) High-yield radiosynthesis and preliminary in vivo evaluation of p-[18F]MPPF, a fluoro analog of WAY-100635. Nucl Med Biol 25: 343-350. [DOI] [PubMed] [Google Scholar]
- Le Poul E, Laaris N, Doucet E, Laporte AM, Hamon M, Lanfumey L (1995) Early desensitization of somato-dendritic 5-HT1A autoreceptors in rats treated with fluoxetine and paroxetine. Naunyn Schmiedebergs Arch Pharmacol 352: 141-148. [DOI] [PubMed] [Google Scholar]
- Le Poul E, Boni C, Hanoun N, Laporte AM, Laaris N, Chauveau J, Hamon M, Lanfumey L (2000) Differential adaptation of brain 5-HT1A and 5-HT1B receptors and 5-HT transporter in rats treated chronically with fluoxetine. Neuropharmacology 39: 110-122. [DOI] [PubMed] [Google Scholar]
- Mannoury la Cour C, El Mestikawy S, Rumajogee P, Bernard R, Miquel MC, Hamon M, Lanfumey L (2001) Identification of G proteins coupled to 5-HT1A receptors in the rat brain. Fundam Clin Pharmacol 15: 71. [Google Scholar]
- Millan MJ, Canton H, Gobert A, Lejeune F, Rivet JM, Bervoets K, Brocco M, Widdowson P, Mennini T, Audinot V, Honoré P, Renuard A, Le Marouille-Girardon S, Verriele L, Gressier H, Peglion JL (1994) Novel benzodioxopiperazines acting as antagonists at postsynaptic 5-HT1A receptors and as agonists at 5-HT1A autoreceptors: a comparative pharmacological characterization with proposed 5-HT1A antagonists. J Pharmacol Exp Ther 268: 337-352. [PubMed] [Google Scholar]
- Newman-Tancredi A, Conte C, Chaput C, Verriele L, Millan MJ (1997) Agonist and inverse agonist efficacy at human recombinant serotonin 5-HT1A receptors as a function of receptor: G-protein stoichiometry. Neuropharmacology 36: 451-459. [DOI] [PubMed] [Google Scholar]
- Passchier J, Van Waarde A, Pieterman RM, Elsinga PH, Pruim J, Hendrikse HN, Willemsen AT, Vaalburg W (2000) In vivo delineation of 5-HT1A receptors in human brain with [18F]MPPF. J Nucl Med 41: 1830-1835. [PubMed] [Google Scholar]
- Passchier J, Van Waarde A, Vaalburg W, Willemsen AT (2001) On the quantification of [18F]MPPF binding to 5-HT1A receptors in the human brain. J Nucl Med 42: 1025-1031. [PubMed] [Google Scholar]
- Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates, Ed 2. Sydney: Academic. [DOI] [PubMed]
- Riad M, El Mestikawy S, Vergé D, Gozlan H, Hamon M (1991) Visualization and quantification of central 5-HT1A receptors with specific antibodies. Neurochem Int 19: 413-423. [Google Scholar]
- Riad M, Garcia S, Watkins KC, Jon N, Langlois X, El Mestikawy S, Hamon M, Descarries L (2000) Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J Comp Neurol 417: 181-194. [PubMed] [Google Scholar]
- Riad M, Watkins KC, Doucet E, Hamon M, Descarries L (2001) Agonist-induced internalization of serotonion-1A receptors in the dorsal raphe nucleus (autoreceptors) but not hippocampus (heteroreceptors). J Neurosci 21: 8378-8386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth P, Gajendiran M, Ganguly DK (1997) Desensitization of spinal 5-HT1A receptors to 8-OH-DPAT: an in vivo spinal reflex study. NeuroReport 8: 2489-2493. [DOI] [PubMed] [Google Scholar]
- Shiue CY, Shiue GG, Mozley PD, Kung MP, Zhuang ZP, Kim HJ, Kung HF (1997) P-18F-MPPF: a potential radioligand for PET studies of 5-HT1A receptors in humans. Synapse 25: 147-154. [DOI] [PubMed] [Google Scholar]
- Sprouse JS, Aghajanian GK (1987) Electrophysiological responses of serotoninergic dorsal raphe neurons to 5-HT1A and 5-HT1B agonists. Synapse 1: 3-9. [DOI] [PubMed] [Google Scholar]
- Wong DT, Bymaster FP, Engleman EA (1995) Prozac (fluoxetine, Lilly 110140), the first selective serotonin reuptake inhibitor and an antidepressant drug: twenty years since its first publication. Life Sci 57: 411-441. [DOI] [PubMed] [Google Scholar]
- Zimmer L, Pain F, Mauger G, Plenevaux A, Le Bars D, Mastrippolito R, Pujol JF, Renaud B, Laniece P (2002a) The potential of the beta-microprobe, an intracerebral radiosensitive probe, to monitor the [18F]MPPF binding in the rat dorsal raphe nucleus. Eur J Nucl Med Mol Imaging 29: 1237-1247. [DOI] [PubMed] [Google Scholar]
- Zimmer L, Mauger G, Le Bars D, Bonmarchand G, Luxen A, Pujol JF (2002b) Effect of endogenous serotonin on the binding of the 5-HT1A PET ligand [18F]MPPF in the rat hippocampus: kinetic beta measurements combined with microdialysis. J Neurochem 80: 278-286. [DOI] [PubMed] [Google Scholar]
- Zimmer L, Riad M, Rbah L, Belkacem-Kahlouli A, Le Bars D, Renaud B, Descarries L (2004) Toward brain imaging of serotonin 5-HT1A autoreceptor internalization. NeuroImage, in press. [DOI] [PubMed]